Abstract
Conventional antidepressants require 2–8 weeks for a full clinical response. In contrast, two rapidly acting antidepressant interventions, low-dose ketamine and sleep deprivation (SD) therapy, act within hours to robustly decrease depressive symptoms in a subgroup of major depressive disorder (MDD) patients. Evidence that MDD may be a circadian-related illness is based, in part, on a large set of clinical data showing that diurnal rhythmicity (sleep, temperature, mood and hormone secretion) is altered during depressive episodes. In a microarray study, we observed widespread changes in cyclic gene expression in six regions of postmortem brain tissue of depressed patients matched with controls for time-of-death (TOD). We screened 12 000 transcripts and observed that the core clock genes, essential for controlling virtually all rhythms in the body, showed robust 24-h sinusoidal expression patterns in six brain regions in control subjects. In MDD patients matched for TOD with controls, the expression patterns of the clock genes in brain were significantly dysregulated. Some of the most robust changes were seen in anterior cingulate (ACC). These findings suggest that in addition to structural abnormalities, lesion studies, and the large body of functional brain imaging studies reporting increased activation in the ACC of depressed patients who respond to a wide range of therapies, there may be a circadian dysregulation in clock gene expression in a subgroup of MDDs. Here, we review human, animal and neuronal cell culture data suggesting that both low-dose ketamine and SD can modulate circadian rhythms. We hypothesize that the rapid antidepressant actions of ketamine and SD may act, in part, to reset abnormal clock genes in MDD to restore and stabilize circadian rhythmicity. Conversely, clinical relapse may reflect a desynchronization of the clock, indicative of a reactivation of abnormal clock gene function. Future work could involve identifying specific small molecules capable of resetting and stabilizing clock genes to evaluate if they can rapidly relieve symptoms and sustain improvement.
INTRODUCTION
Circadian function is frequently disrupted during depressive episodes. When rhythms are dysregulated, the risk for disease1 including depression2 increases. Clinical findings suggest a relationship between the degree of desynchronization in circadian rhythms and the severity of depressive symptoms.3 As symptoms improve, normal rhythms are frequently restored.4 Low-dose ketamine and sleep deprivation therapy (SDT) can act within hours to robustly decrease symptoms of depression, in striking contrast to the 2–8 weeks required by conventional antidepressants for a full clinical response.5,6 It is proposed that low-dose ketamine7,8 and SDT9,10 act, in part, on circadian machinery to restore normal behavioral and physiological circadian rhythms.11
ABNORMAL CIRCADIAN RHYTHMS IN MAJOR DEPRESSIVE DISORDER (MDD)
A dysregulation in diurnal rhythmicity affecting sleep, mood, temperature and hormone secretion is reported in a subgroup of MDD patients.
Sleep
Sleep is regulated by homeostasis (sleep pressure) and circadian (diurnal timing of sleep) processes. Circadian regulation of sleep is reported to be independent of prior wakefulness, differentiating it from homeostasis, although data in humans and rodents suggest an interaction.12–15
Insomnia, a major symptom of depression affecting about 80% of patients, is characterized by difficulty in falling asleep, staying asleep, early morning awakening and/or shortened rapid eye movement latency—symptoms compatible with a shift in circadian phase.16 Chronic insomnia is associated with an increased risk for recurrent depressive episodes16–20 as well as suicidality.21 Normalization of sleep patterns may be an early predictor of antidepressant response.22–24
Mood
Diurnal variations in mood are normal in healthy controls. However, mood swings in depression can vary to extremes. For example, some patients awaken in early morning with severe psychotic symptoms and by evening, improve to an almost euthymic state.25,26 Marked patterns in mood swings can persist throughout the depressive episode.
Temperature
Thermoregulation has a critical role in biological function and as reported in human fibroblasts, as little as 1 °C change can significantly alter circadian gene expression.27 In healthy controls, core body temperature rises in early morning hours and decreases at night. Depressed patients often have ‘flattened’ diurnal rhythms as nocturnal temperatures remain high.28,29 Remission is usually associated with a decrease in nocturnal temperature and a restoration of normal rhythms.28,30
Hormone secretion
Corticosteroids
Depression is often associated with hypothalamic–pituitary–adrenal axis overactivation. Cortisol and its relevant peptides measured in saliva, cerebrospinal fluid and urine (24-h urinary cortisol and its major breakdown product, 17-hydroxycortisol-steroids) are often elevated. High cortisol levels may be sustained throughout the day—a finding most pronounced in psychotically depressed suicidal individuals.31 Morning cortisol peaks may also be phase-advanced by 2–3 h.32–35 In remission, evening cortisol levels may decrease to normal levels36 although not consistently.37
Melatonin
Melatonin, the primary hormone of the pineal gland, has been used as a marker of circadian phase. Secreted at night, the timing of melatonin release under dim light conditions (dim light melatonin onset) has been phase-delayed in depression.38–41
It should be noted that abnormalities in circadian function, although frequently reported in MDD can also be associated with bipolar disorder, psychosis and schizophrenia.31,42–44 In addition, some depressed patients may not have any symptoms of circadian dysregulation, while others have striking changes in daily rhythms. Chronotherapeutic antidepressant strategies (SDT, bright light therapy and sleep phase advance) are effective in 40–60% of severely depressed patients.4 The subset of patients who do not respond to chronotherapeutic treatment strategies may not have depression-related circadian abnormalities.
CIRCADIAN CLOCK GENES
Modulation of circadian rhythms
Circadian rhythms are generated, in part, by clock genes that are under the control of a small pair of nuclei in the anterior hypothalamus, the suprachiasmatic nucleus (SCN). The SCN receives extensive input from many brain regions and serves as a primary modulator of virtually all cellular clocks in the body. The SCN maintains synchrony by resetting circadian rhythms via photic and non-photic signaling.45,46 In addition, relevant to antidepressant medications, rhythmic release of 5-hydroxytryptamine is modulated by intrinsic feedback to the raphe,47 which can synchronize circadian rhythms in SCN and peripheral tissue.48,49
Circadian clock gene machinery
At the molecular level, a core set of clock genes (bmal1, clock, period (per1,2,3), cryptochrome (cry1,cry2) and their protein products have a central role in generating virtually all rhythms throughout the body. In the nucleus, BMAL1/CLOCK (or NPAS2—a paralog of CLOCK) form a heterodimer that binds to E-Box-containing promoters on per and cry genes. Per and Cry mRNAs move into the cytoplasm and are translated into their respective proteins to form heterodimers (PER/CRY). The PER/CRY heterodimers form a complex with casein kinase1ε enabling their translocation back into the nucleus to inhibit their own transcription by interacting with BMAL1/CLOCK.50 Multiple interlocking transcriptional–translational loops contain both positive and negative transcription factors that adjust rhythms to an approximate 24-h cycle. A subset of these loops includes orphan nuclear receptors that confer stability to the core circadian interactions. For example, Bmal1 rhythmicity is modulated by Rorα that activate Bmal1 transcription, while Rev-rbα represses Bmal1 transcription via common retinoid-related orphan receptor elements on the Bmal1 promoter. Conversely, BMAL1/CLOCK activates Rev-erbα, thus forming a link between the positive and negative loops. Additional clock genes, Dec1 and Dec2, repress transcription of BMAL1-enhanced promoter activity by binding directly to Enhancer boxes (E-boxes) (per and cry interact with the BMAL1/CLOCK heterodimer but cannot bind directly to E-boxes as they lack DNA binding domains).51,52 The high degree of complexity involved in the molecular architecture of circadian regulation has been reviewed elsewhere.53
HUMAN STUDIES OF CIRCADIAN CLOCK GENES
Pineal gland
Wu et al.54 were the first to demonstrate robust rhythmic expression of clock genes (Bmal1, Cry1 and Per1) in pineal gland in control subjects and its dysregulation in Alzheimer disease patients. Samples collected around the clock according to time-of-death (TOD) (that is, dawn, day, dusk and night) showed time-of-day-dependent effects. Peaks in gene expression occurred at dawn (Bmal1), dawn and dusk (Per1) and evening (Cry1). Ackermann et al.55 also analyzed pineal tissue in controls and although they did not replicate Wu et al.’s54 results, they discovered time-dependent nucleocytoplasmic shuttling of clock proteins (PER1, CRY1, CLOCK and BMAL1) (Clock proteins ‘shuttle’ between the nucleus and cytoplasm as part of the regulatory feedback loop to generate rhythms). Peak times for translocation of the circadian proteins occurred at dawn (PER1 and CLOCK), day (CRY1) and night (Bmal1).
Pituitary
Although clock gene proteins are detectable in pituitary, Per1 was the only circadian gene to show robust diurnal rhythms (lowest levels at dusk). In contrast to other brain regions, clock genes and their proteins in pituitary are hypothesized to primarily modulate time-dependent hormone synthesis.56
First report of an abnormal clock gene in suicides vs non-suicides in postmortem brain tissue
Sequiera et al.57 analyzed the dorsolateral prefrontal cortex in suicide and non-suicide depressed patients and reported a significant downregulation in per1 in suicide victims. Although per1 was analyzed at only one time-point, to our knowledge this was the first study in brain to report that suicide may be associated with a dysregulation in a core clock gene critical to circadian function.
First evidence of a significant disruption in circadian clock genes in six brain regions in MDD patients contrasted with controls
We conducted a microarray study in brain tissue comparing circadian gene expression in control subjects and MDD patients. Six brain regions relevant to the psychopathology of MDD were selected for investigation. Microarray analyses including 12 000 transcripts identified genes with the most robust sinusoidal 24-h rhythms58 in anterior cingulate (ACC), dorsolateral prefrontal cortex, hippocampus, amygdala, nucleus accumbens and cerebellum as part of a multicenter Pritzker Neuropsychiatric Disorders Research Consortium study. High-quality postmortem brain tissue was collected—all cases had sudden death, pH values >6.5 (average pH = 6.87), and completion of a psychological autopsy (141 item questionnaire) with next-of-kin. Control cases were confirmed by family interviews with next-of-kin to rule out psychiatric illness in controls as well as in first-degree relatives. Diagnoses in MDD patients were confirmed by medical records, family interviews and reviewed by two psychiatrists. To discover the cyclic genes, we fit the expression values for each gene by a sinusoidal function of time using the method of least squares and fixing the period at 24 h. The statistical significance of the findings was evaluated by permutation, randomly assigning TOD data across subjects 1000 times. Peak levels of sinusoidal rhythms were determined (as described in Li et al.58).
Control subjects
Our data provide evidence that clock gene sinusoidal rhythms vary in synchrony over 24 h across six regions in control human brain. Results showed that Bmal1 ranked at the top of 12 000 transcripts in terms of consistency for time of peak expression across six brain regions. Period genes (per1, per2 and per3), Rev-erbα, Dbp, and Dec 2 ranked in the top 11 for having the most robust rhythms. Remarkably, the temporal expression pattern of per genes in our human data across all six brain regions was in agreement with that in rodent SCN59 whereby per1 levels peaked in early morning, per3 peaked 5 h later and per2 levels peaked 8 h later.
MDD patients
Results showed a significant and marked loss in rhythmicity in the top-ranked cyclic genes in the patients compared with controls and provide the first direct evidence of a disruption in clock gene expression in MDD. These findings were independent of the cause of death and medication (evaluated by toxicology screens).
Evidence that the anterior cingulate (ACC) may be a site for clock gene dysfunction
Converging data from neuroimaging, neuropathological and lesion studies implicate the ACC as important in the regulation of mood. The ACC is a major component of a large extended neural network including the hippocampus, striatum and amygdala. As reviewed by Drevets et al.,60 preclinical and clinical findings present convincing evidence to suggest that impairment in the ACC could contribute to symptoms of depression. Moreover, deep brain stimulation in subgenual ACC ameliorates symptoms of depression in treatment-resistant MDD patients.61 Structural abnormalities (for example, decreased gray matter volume) and reductions in cognitive performance are also associated with depression.62 A large body of functional brain imaging studies (for example, fMRI, PET, EEG, qEEG, SPECT and MEG) report increased activation in the ACC of depressed patients who respond to a wide range of therapies including conventional antidepressants63–69 and low-dose intravenous ketamine.63,70 Similarly, clinical response to non-pharmacological interventions such as SDT,71 ECT,72,73 rTMS,74,75 deep brain stimulation76 and cognitive behavioral therapy77 are also associated with elevated pretreatment ACC activation.
Evidence that clock gene expression is altered in the ACC comes from our microarray analyses of postmortem brain tissue from 34 MDD patients and 34 controls matched for TOD (Supplementary Material, Li et al.58). We found that 11 of the 12 top-ranked circadian genes in the ACC including core clock genes (Bmal1, Per1, Per2, Per3 and Dbp) had significant sinusoidal rhythmicity, whereas only 1 of 12 genes reached statistical significance in the MDDs (Table 1). We interpret these findings to suggest that in addition to other abnormalities in ACC in depression, a subgroup of MDDs may also have dysregulated clock gene expression. Our findings offer the potential for applying multilevel analyses in genomics to assess the critical role of circadian rhythm disruption in mood disorders.78 Thus, converging evidence from neuropathological investigations, brain imaging data and clock gene abnormalities support the ACC as a candidate region for future investigations.
Table 1.
Top-ranked circadian genes demonstrating significant sinusoidal rhythms in controls and disruption in MDD patients matched for TOD around the 24-h clock (N = 34 controls; 34 MDDs) (adapted from Li et al.58)
| Anterior cingulate | Controls (N = 34) | Matched MDDs (N = 34) |
|---|---|---|
| ARNTL (Bmal1) | 0.001 | 0.072 |
| Per2 | 0.006 | 0.083 |
| Per3 | 0.009 | 0.652 |
| NR1D1 (Rev-erbα) | 0.003 | 0.029 |
| DBP | 0.023 | 0.239 |
| SFPQ | 0.351 | 0.124 |
| ITIHS | 0.016 | 0.47 |
| LDLR | 0.026 | 0.385 |
| Per1 | 0.006 | 0.21 |
| Insig1 | 0.041 | 0.534 |
| SLC39A14 | 0.017 | 0.21 |
| NFIL3 | 0.049 | 0.326 |
Abbreviations: ACC, anterior cingulate; MDD, major depressive disorder; TOD, time-of-death. Numbers in bold indicate significant sinusoidal rhythmicity, P-values.
Animal models of depression are associated with alterations in circadian genes
Although our review is focused primarily on human clinical data, we briefly review relevant animal findings.
Three animal models of depression measuring clock gene expression were studied in rodents including chronic unpredictable stress (CUS), chronic restraint and social defeat. In rats, CUS administered for 28 days produced irreversible depressive-like behaviors. CUS-related changes in SCN included a transient decrease in Per2 amplitude, which normalized following termination of the stressors. Pretreatment with desipramine, blocked both depressive-like behavior and decreases in Per2.79 In hippocampus (CA1, CA3), CUS-related phase-shifts in Clock gene expression persisted at least 2 weeks beyond the termination of the stressors along with the depressive-like behavior. A follow-up investigation showed that knockdown of Clock in normal rats produced depressive-like behavior suggesting that dysregulated Clock gene expression in HC could contribute to CUS-related depressive behaviors.80
Other findings in rodents subjected to chronic restraint indicated that a downregulation in Per2 and a concomitant upregulation in glycogen synthase kinase 3 (GSK-3β) in SCN, HC and prefrontal cortex. Nighttime administration of lithium, normalized Per2 and reduced GSK-3β levels to control values.81
Landgraf et al.82 provide a critical review of animal studies relevant to the role of clock genes in mood disorders. They discuss limitations in current research including noncircadian effects of clock genes, the difficulty in identifying behavioral tests, which encompass the complex phenotypes of mood disorders. They also suggest that altering light–dark cycles in rodent mutant models could help identify circadian effects of modulation of mood and point out that current animal studies do not provide sufficient data to explain depressive- vs manic-like behavior. Importantly, it is suggested that studies of single-cell rhythms, amplitude and phase in brain areas related to mood could provide critical data describing the relationship of circadian rhythms and mood regulation.
Of interest is whether amphetamines, which do not have significant antidepressant effects, alter circadian clock gene expression. In striatum of rodents, chronic amphetamine shifted per1 and per2 from nocturnal to diurnal expression patterns whereas bmal1 expression patterns switched from diurnal to nocturnal.83 In other work, amphetamine restored abnormal locomotor rhythms in Clock mutant mice.84 Clinically, the high rate of comorbidity with addiction (that is, methamphetamine) makes it difficult to extrapolate these findings to MDD patients.
RAPID-ACTING ANTIDEPRESSANT TREATMENTS
Sleep deprivation therapy (SDT)
Keeping patients awake for approximately 36 h markedly relieves depressive symptoms in 40–60% of patients.9 Although relapse rates are high following recovery sleep, chronotherapeutic interventions such as morning bright light therapy (10 000 lux) and sleep phase advance (setting sleep time earlier and advancing bedtimes over subsequent nights) are effective in sustaining improvement for weeks to months.85–87 In Europe, SDT is often recommended as a first-line treatment for depression.10,87,88
Actions on clock gene machinery in animals
Studies of clock gene expression in mice suggest that sleep deprivation (SD) can produce rapid (within hours) alterations. Prolonged wakefulness elevated Per1 and Per2 levels in cerebral cortex, basal forebrain and hypothalamus, which returned to control values following recovery sleep.89,90 A possible mechanism of action could involve the alterations of DNA binding of the core-clock transcription factors to cis-regulatory sequences of target clock genes as evidenced by inhibition DNA binding of (CLOCK/BMAL1 or NPAS2).91 Further, SD had region-specific effects on immediate-early gene expression such that in SCN, clock gene expression was time-of-day dependent, whereas in neocortex, immediate-early gene expression was a function of time awake and/or cell type.92 Overall, SD suppresses approximately 80% of circadian genes in brain suggesting that many of the diurnal variations in circadian gene expression are likely sleep–wake dependent.93
A limitation to the interpretation of these findings relevant to MDD is that to our knowledge none of these studies were conducted in animal models of depression.
Possible role of GSK-3β, α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA), glutamate and mTOR on circadian rhythms
Glycogen synthase kinase 3β
It is reported that SDT response rates are higher in depressed patients who carry a gene promoter polymorphism (rs334558) for decreasing GSK-3β activation.94,95 GSK-3β, a key component of the Wnt pathway, is upstream of the mammalian target of rapamycin (mTOR) pathway94 and has a critical role in stabilizing and lengthening the period of biological rhythms.96,97 Multiple therapeutic interventions effective in treating depression including mood stabilizers such as lithium, serotonergic antidepressants, as well as low-dose ketamine increase GSK-3β phosphorylation suggesting that GSK-3β inhibition could be a candidate target for antidepressant interventions. In support of this hypothesis is a study showing that GSK-3β levels are elevated in prefrontal cortex of MDDs.98
AMPA
SD was found to significantly elevate AMPA levels in cerebral cortex.99,100 In mice, microinjections of AMPA into the SCN rapidly and robustly induced Per1 expression and phase delays in behavioral rhythms when administered during early subjective night. AMPA receptors are thought to be critical for entrainment by relaying photic information to the SCN.101
Glutamate
SD is reported in one study to increase N-methyl-D-aspartate receptor 2A (NMDAR) receptors in mouse cerebral cortex,102 while another suggested that SD inhibits NMDAR receptor trafficking in HC.103 It is hypothesized that both AMPA and N-methyl-D-aspartate (NMDA) signaling reciprocally regulate glutamatergic signaling and the magnitude of phase-shifts.101
Mammalian target of rapamycin
SD was shown to decrease total and phosphorylated mTOR in HC, an effect that was reversible with recovery sleep.104 mTOR exhibits robust rhythmicity in the SCN and is activated in a phase-dependent manner by light. Inhibition of mTOR decreases Per1 and Per2 expression and modulates behavioral shifts in animals in the SCN.105
Peripheral measures of clock genes in humans
Disruptions in sleep including restricting sleep, altering sleep times and SD can profoundly affect the temporal organization of the human blood transcriptome including clock gene expression and chromatin modification in healthy volunteers.106,107 A study conducted in 12 young healthy males assessing clock gene expression in leukocytes reported that after one night of SD, Bmal1 showed a 70% decrease in amplitude and suppression of rhythmicity whereby expression patterns of per 1,2,3 or Rev-erbα expression were not altered.108 Analyses of peripheral blood mononuclear cells in six healthy volunteers collected at 4-h intervals over 3 consecutive days (baseline, night of SD and recovery sleep) showed mixed results. Following SD, three subjects showed a loss in Per2 rhythmicity, whereas two subjects were phase shifted. In contrast to findings in leukocytes, SD did not affect Bmal1.109
To our knowledge, only one study focused on the effect of SD on clock genes in depressed patients. Lavebratt et al.110 measured Cry2 mRNA in peripheral blood mononuclear cells comparing bipolar depressed patients and healthy male controls. Cry2 mRNA levels were assessed approximately every 6 h in the controls over a period of 48 h. Results showed a marked diurnal rhythm in Cry2 expression. Sleep loss increased Cry2 mRNA levels by twofold in controls. A finding in depressed patients was that these individuals had significantly lower levels of Cry2 at baseline. SD had no significant effect on Cry2 levels in the depressed patients. Although this study was conducted in bipolar patients, the findings could have implications for MDD.
Low-dose ketamine treatment for mood disorders
Intravenous low-dose ketamine as reported in 17 studies (see review Bunney and Bunney4) has rapid-acting antidepressant actions with robust efficacy in treatment-resistant depression. The time to response is usually within 2–4 h following ketamine administration. Approximately, one-third of responders have sustained improvement lasting 1–2 weeks.111 Low-dose ketamine is also a rapidly effective treatment for decreasing suicidality.112–116 Intramuscular ketamine,117 oral ketamine118 and intranasal routes of administration119 have been used successfully in open trials to decrease depressive symptoms.
Possible mechanisms of action involving clock genes and circadian-regulated GSK-3β, AMPA, glutamate and mTOR
NMDA antagonist
Ketamine is a non-competitive high-affinity NMDAR glutamate receptor antagonist with a half-life of 2–3 h when administered intravenously.120 It binds within the phencyclidine channel and increases presynaptic release of glutamate, which in turn activates post-synaptic glutamatergic sites including AMPA receptors. At low doses, ketamine increases synaptic plasticity most likely through its inhibitory actions on NMDA-mediated glutamatergic receptors.121,122
Ketamine’s effects on clock gene machinery in neuronal cell culture (preliminary data)
We addressed the question as to whether ketamine acts at the clock gene level to modify gene expression using neuronal cell culture (NG108-15).123 Our results showed that ketamine blunted the amplitude of the transcription of bmal1, per2 and cry1 in a dose-dependent manner. We next mutated the E-Box and found that ketamine’s effects on per2 and cry1 transcription were blocked, therefore suggesting that E-box elements may be essential to ketamine’s actions. Using chromatin immunoprecipitation (ChIP) analyses, we showed that ketamine altered the recruitment of the BMAL1/CLOCK complex on circadian promoters in a time-dependent manner. We tested various protein kinase inhibitors on the repressive effect of ketamine on the recruitment of BMAL1/CLOCK to its target promoter, and found that only the protein kinase that inhibited GSK-3 (SB 21673) significantly affected ketamine’s actions. To our knowledge, this was the first evidence that ketamine could produce robust changes in clock gene expression.123
Although these findings offer preliminary evidence to suggest that ketamine alters clock gene expression, it is premature to generalize from data in neuronal cell culture that these actions are relevant to the rapid antidepressant effects in man. As we were not investigating abnormal rhythms, we could not address whether ketamine normalizes dysregulated rhythms. Future work is needed to study the effects of ketamine on clock genes in brain regions relevant to mood disorders in animal models of depression.
Glycogen synthase kinase 3β
GSK-3β is a potent inhibitor of mTORC1 (rapamycin complex 1). Rodent studies demonstrate that inhibition of GSK-3β may have a role in the antidepressant actions of low-dose ketamine.97,124,125 The selective GSK-3β inhibitor, SB 21673, was shown to potentiate ketamine’s antidepressant actions in mice125 and as mentioned above, in our study,123 the actions of ketamine were specific to SB 21673. Moreover, in GSK-3 knockin mice, ketamine’s antidepressant effects in a learned helplessness model of depression were blocked.97
Mammalian target of rapamycin
Low-dose ketamine robustly activates mTOR, an effect that is thought to help sustain ketamine-related antidepressant effects beyond the bioavailability of ketamine—primarily due to its actions on AMPA, brain-derived neurotrophic factor and GSK-3β.8,121,122
AMPA
AMPA receptors mediate fast excitatory neurotransmission activation and increase synaptic strength. Upregulation of AMPA following ketamine administration is hypothesized to increase sensitivity to glutamate and stabilize synaptic plasticity.122 In SCN, AMPA receptors have a critical role in modulating phase-shifts to photic stimuli.101
Figure 1 summarizes evidence that low-dose ketamine and SDT may share common mechanisms of action. Both interventions may act on circadian clock gene machinery to alter expression of the core clock genes and modulate GSK-3β, mTOR and AMPA. Thus, their clinical antidepressant actions may converge on a number of molecular processes involving circadian-related pathways to accelerate clinical response.
Figure 1.
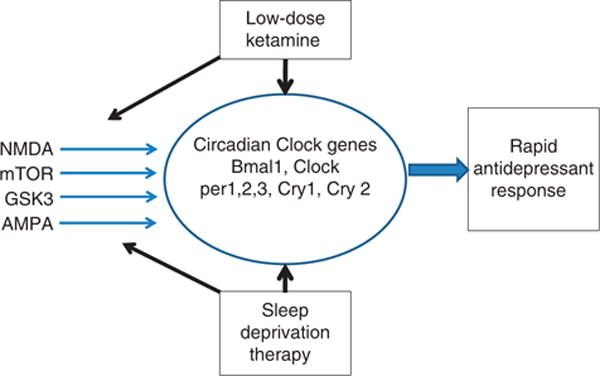
Low-dose ketamine and sleep deprivation therapy modulate clock gene machinery, which may represent an important mode of action leading to rapid treatment of depression. Evidence is reviewed that they share additional mechanisms of action involving N-methyl-D-aspartate (NMDA), mammalian target of rapamycin (mTOR), glycogen synthase kinase 3β (GSK-3β) and α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) all of which are modulated by clock genes.
Considerations and limitations
A limitation to the understanding of SDT and low-dose ketamine’s actions involving GSK-3, AMPA, NMDA and mTOR is that there is not enough evidence to identify a specific circadian clock mechanism or a specific circadian defect in MDD. Future research is needed to establish, which, if any of these effects have a critical role in the efficacy of rapid antidepressant responses or if their actions relate to resetting abnormal clock gene rhythms in MDD. Many of the results in circadian research are correlative in nature. Future research should emphasize the importance of direct measurements of circadian function.
DISCUSSION
Abnormal circadian rhythms (sleep, temperature, hormonal secretion and mood) have been consistently reported in a subset of MDD patients. The incidence of depression increases when circadian genes are dysregulated.1,2 The severity of depression correlates with the level of desychronization in circadian rhythms.3 As patients improve, abnormal rhythms circadian rhythms often normalize.4 It is hypothesized that rapid antidepressant actions involve the modulation of clock genes that control these circadian rhythms.4 Marked dysregulation in clock gene expression is reported in brain in MDD.58 SDT and low-dose ketamine rapidly reverses a core pathophysiological deficit in depressive symptomatology in a subset of MDD patients. Preliminary data in MDD and findings in animals show that SD alters circadian clock genes. Low-dose ketamine alters clock genes as reported in neuronal cell culture.123 It is, however, difficult to extrapolate from neuronal cell culture studies to in vivo rodent or human studies to MDD as to the precise modulation of clock genes. Therefore, at this stage in the research, we could not analyze what is similar and dissimilar in the effects of SD and ketamine on circadian clock genes in MDD patients.
Relatively, little is known about the actions of standard antidepressants on clock gene expression. One study showed an effect of escitalopram on circadian genes in MDD,126 while a rodent study127 presented data that SSRIs can modulate circadian rhythms. If clock gene modulation that can occur rapidly is relevant to the efficacy of antidepressant strategies, it is unclear why currently used antidepressant medications require 2–6 weeks to work, while ketamine and SD are effective within 24 h.
Further clock gene research is needed to study effects of other fast-acting antidepressants such as scopolamine, which has clinical efficacy in MDD within a week.128 In rodents, scopolamine has been shown to activate the mTOR pathway and block muscarinic acetylcholine receptors. Further investigation could determine whether ketamine, SD and scopolamine share downstream targets.129
In addition, adjustments of human circadian rhythms following jet-lag can take a number of days, which contrasts with the rapid clinical response to ketamine and SD. However, the rapid normalization of abnormal mood in a depressed subject following SD or ketamine may represent an entirely different paradigm than jet-lag in a normal subject. Furthermore, it is reported that a subgroup of vulnerable individuals become severely depressed immediately following abrupt changes in time zones. These episodes are frequently associated with disturbances in sleep.130,131 Attempts to treat time-zone precipitated depressive episodes with ketamine or SD have not been documented and therefore it is unclear how rapidly these patients would respond to these interventions.
Future studies
Animal studies of ketamine and SD
A limited number of studies in animal models of depression leave a gap in our understanding of the mechanisms of action of rapid antidepressants. Further research is required in rodents to determine the effect of ketamine on clock genes in critical brain areas relevant to depression in wild-type mice, but most important, in animal models of depression. In addition, it may be helpful to establish that animals with depressive-like behavior have abnormal circadian rhythms (temperature, activity and hormonal secretion) associated with altered clock genes.
Clinical studies
Identify MDD patients with abnormal circadian rhythms (sleep, temperature, hormonal secretion and mood). Study peripheral clock genes to identify whether they are altered in this MDD subgroup. Test the effects of SD and ketamine to determine whether they ‘normalize’ abnormal clock gene rhythms in periphery. Also, assess circadian rhythms and peripheral clock genes in MDD responders and non-responders to SDT and/or ketamine.
We hope that this review will stimulate scientists to further explore the mechanisms of action of SDT and ketamine and provide insight into mechanisms for sustained rapid responses.
Further investigations could also contribute important data relevant to the hypothesis that SD and ketamine may act by resetting the disrupted clock gene machinery documented in human brain of MDD patients.
Recent research is providing detailed clues to mechanisms involved in phase shifting and resetting of cellular rhythms. As reviewed by Chen et al.,132 the identification of small molecules that modulate and rapidly reset cellular rhythms has motivated the evaluation of synthetic small molecules. These compounds allow more precise control of the circadian machinery and offer the advantage of allosterically altering clock gene proteins. High-throughput screening of 200 000 synthetic small molecules identified a number of molecules with a robust effect on clock gene machinery. These provide clues for lead compounds to target chronotherapeutic treatments of mood disorders and possibly reset abnormal clock gene machinery to normalize circadian rhythms and rapidly treat depression.
Acknowledgments
This work was supported by the Pritzker Neuropsychiatric Disorders Research Fund, National Institute of Mental Health (NIMH) Conte Center Grant P50 MH60398, the William Lion Penzner Foundation (WEB), the Della Martin Foundation (WEB), NIMH R01MH085801 (MPV), R01MH104261 (HA and SJW), Office of Naval Research Grants ONR- N00014-12-1-0366 (HA and SJW) and the Hope for Depression Research Foundation, HDRF (HA and SJW). JZL is supported by a National Alliance for Research on Schizophrenia and Depression Abramson Family Foundation Investigator Award and an International Mental Health Research Organization–Johnson & Johnson Rising Star Translational Research Award.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Masri S, Sassone-Corsi P. The circadian clock: a framework linking metabolism, epigenetics and neuronal function. Nat Rev Neurosci. 2013;14:69–75. doi: 10.1038/nrn3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edgar N, McClung CA. Major depressive disorder: a loss of circadian synchrony? Bioessays. 2013;35:940–944. doi: 10.1002/bies.201300086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasler BP, Buysse DJ, Kupfer DJ, Germain A. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: further evidence for circadian misalignment in non-seasonal depression. Psychiatry Res. 2010;178:205–207. doi: 10.1016/j.psychres.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunney BG, Bunney WE. Rapid-acting antidepressant strategies: mechanisms of action. Int J Neuropsychopharmacol. 2012;15:695–713. doi: 10.1017/S1461145711000927. [DOI] [PubMed] [Google Scholar]
- 5.Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatr Serv. 2009;60:1466–1467. doi: 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- 6.Rush AJ, Trivedi M, Fava M. Depression, IV: STAR*D treatment trial for depression. Am J Psychiatry. 2003;160:237. doi: 10.1176/appi.ajp.160.2.237. [DOI] [PubMed] [Google Scholar]
- 7.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 8.Mathews DC, Zarate CA., Jr Current status of ketamine and related compounds for depression. J Clin Psychiatry. 2013;74:516–517. doi: 10.4088/JCP.13ac08382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147:14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- 10.Benedetti F, Barbini B, Colombo C, Smeraldi E. Chronotherapeutics in a psychiatric ward. Sleep Med Rev. 2007;11:509–522. doi: 10.1016/j.smrv.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Zunszain PA, Horowitz MA, Cattaneo A, Lupi MM, Pariante CM. Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol Psychiatry. 2013;18:1236–1241. doi: 10.1038/mp.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dijk DJ, Duffy JF, Silva EJ, Shanahan TL, Boivin DB, Czeisler CA. Amplitude reduction and phase shifts of melatonin, cortisol and other circadian rhythms after a gradual advance of sleep and light exposure in humans. PLoS One. 2012;7:e30037. doi: 10.1371/journal.pone.0030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher SP, Foster RG, Peirson SN. The circadian control of sleep. Handb Exp Pharmacol. 2013;217:157–183. doi: 10.1007/978-3-642-25950-0_7. [DOI] [PubMed] [Google Scholar]
- 14.Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. Eur J Neurosci. 2009;29:1820–1829. doi: 10.1111/j.1460-9568.2009.06723.x. [DOI] [PubMed] [Google Scholar]
- 15.Borbely AA, Tobler I. Manifestations and functional implications of sleep homeostasis. Handb Clin Neurol. 2011;98:205–213. doi: 10.1016/B978-0-444-52006-7.00013-7. [DOI] [PubMed] [Google Scholar]
- 16.Mendlewicz J. Sleep disturbances: core symptoms of major depressive disorder rather than associated or comorbid disorders. World J Biol Psychiatry. 2009;10:269–275. doi: 10.3109/15622970802503086. [DOI] [PubMed] [Google Scholar]
- 17.Ohayon MM. Insomnia: a ticking clock for depression? J Psychiatr Res. 2007;41:893–894. doi: 10.1016/j.jpsychires.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23:571–585. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10:473–481. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997;146:105–114. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 21.Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73:e1160–e1167. doi: 10.4088/JCP.11r07586. [DOI] [PubMed] [Google Scholar]
- 22.Kupfer DJ, Foster FG, Reich L, Thompson SK, Weiss B. EEG sleep changes as predictors in depression. Am J Psychiatry. 1976;133:622–626. doi: 10.1176/ajp.133.6.622. [DOI] [PubMed] [Google Scholar]
- 23.Troxel WM, Kupfer DJ, Reynolds CF, 3rd, Frank E, Thase ME, Miewald JM, et al. Insomnia and objectively measured sleep disturbances predict treatment outcome in depressed patients treated with psychotherapy or psychotherapy-pharmacotherapy combinations. J Clin Psychiatry. 2012;73:478–485. doi: 10.4088/JCP.11m07184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buysse DJ, Monk TH, Kupfer DJ, Frank E, Stapf D. Circadian patterns of unintended sleep episodes during a constant routine in remitted depressed patients. J Psychiatr Res. 1995;29:407–416. doi: 10.1016/0022-3956(95)00021-v. [DOI] [PubMed] [Google Scholar]
- 25.Murray G. Diurnal mood variation in depression: a signal of disturbed circadian function? J Affect Disord. 2007;102:47–53. doi: 10.1016/j.jad.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Wirz-Justice A. Diurnal variation of depressive symptoms. Dialogues Clin Neurosci. 2008;10:337–343. doi: 10.31887/DCNS.2008.10.3/awjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saini C, Morf J, Stratmann M, Gos P, Schibler U. Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators. Genes Dev. 2012;26:567–580. doi: 10.1101/gad.183251.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avery DH, Shah SH, Eder DN, Wildschiodtz G. Nocturnal sweating and temperature in depression. Acta Psychiatr Scand. 1999;100:295–301. doi: 10.1111/j.1600-0447.1999.tb10864.x. [DOI] [PubMed] [Google Scholar]
- 29.Elsenga S, Van den Hoofdakker RH. Body core temperature and depression during total sleep deprivation in depressives. Biol Psychiatry. 1988;24:531–540. doi: 10.1016/0006-3223(88)90164-3. [DOI] [PubMed] [Google Scholar]
- 30.Souetre E, Salvati E, Wehr TA, Sack DA, Krebs B, Darcourt G. Twenty-four-hour profiles of body temperature and plasma TSH in bipolar patients during depression and during remission and in normal control subjects. Am J Psychiatry. 1988;145:1133–1137. doi: 10.1176/ajp.145.9.1133. [DOI] [PubMed] [Google Scholar]
- 31.Keller J, Flores B, Gomez RG, Solvason HB, Kenna H, Williams GH, et al. Cortisol circadian rhythm alterations in psychotic major depression. Biol Psychiatry. 2006;60:275–281. doi: 10.1016/j.biopsych.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 33.Banki CM, Bissette G, Arato M, O’Connor L, Nemeroff CB. CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry. 1987;144:873–877. doi: 10.1176/ajp.144.7.873. [DOI] [PubMed] [Google Scholar]
- 34.Bunney WE, Jr, Fawcett JA, Davis JM, Gifford S. Further evaluation of urinary 17-hydroxycorticosteroids in suicidal patients. Arch Gen Psychiatry. 1969;21:138–150. doi: 10.1001/archpsyc.1969.01740200010002. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter WT, Jr, Bunney WE., Jr Adrenal cortical activity in depressive illness. Am J Psychiatry. 1971;128:31–40. doi: 10.1176/ajp.128.1.31. [DOI] [PubMed] [Google Scholar]
- 36.Scharnholz B, Lederbogen F, Feuerhack A, Bach A, Kopf D, Frankhauser P, et al. Does night-time cortisol excretion normalize in the long-term course of depression? Pharmacopsychiatry. 2010;43:161–165. doi: 10.1055/s-0030-1248316. [DOI] [PubMed] [Google Scholar]
- 37.Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66:617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 38.Lewy AJ. Depressive disorders may more commonly be related to circadian phase delays rather than advances: time will tell. Sleep Med. 2010;11:117–118. doi: 10.1016/j.sleep.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Buckley TM, Schatzberg AF. A pilot study of the phase angle between cortisol and melatonin in major depression—a potential biomarker? J Psychiatr Res. 2010;44:69–74. doi: 10.1016/j.jpsychires.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Khaleghipour S, Masjedi M, Ahade H, Enayate M, Pasha G, Nadery F, et al. Morning and nocturnal serum melatonin rhythm levels in patients with major depressive disorder: an analytical cross-sectional study. Sao Paulo Med J. 2012;130:167–172. doi: 10.1590/S1516-31802012000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robillard R, Naismith SL, Rogers NL, Scott EM, Ip TK, Hermens DF, et al. Sleep-wake cycle and melatonin rhythms in adolescents and young adults with mood disorders: Comparison of unipolar and bipolar phenotypes. Eur Psychiatry. 2013;28:412–416. doi: 10.1016/j.eurpsy.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Lamont EW, Coutu DL, Cermakian N, Boivin DB. Circadian rhythms and clock genes in psychotic disorders. Isr J Psychiatry Relat Sci. 2010;47:27–35. [PubMed] [Google Scholar]
- 43.Zanini M, Castro J, Coelho FM, Bittencourt L, Bressan RA, Tufik S, et al. Do sleep abnormalities and misaligned sleep/circadian rhythm patterns represent early clinical characteristics for developing psychosis in high risk populations? Neurosci Biobehav Rev. 2013;37:2631–2637. doi: 10.1016/j.neubiorev.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez R, Tamminga CA, Tohen M, Suppes T. The relationship between affective state and the rhythmicity of activity in bipolar disorder. J Clin Psychiatry. 2014;75:e317–e322. doi: 10.4088/JCP.13m08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp Neurol. 2013;243:4–20. doi: 10.1016/j.expneurol.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarthy MJ, Welsh DK. Cellular circadian clocks in mood disorders. J Biol Rhythms. 2012;27:339–352. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- 47.Salomon RM, Cowan RL. Oscillatory serotonin function in depression. Synapse. 2013;67:801–820. doi: 10.1002/syn.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Oosterhout F, Lucassen EA, Houben T, vanderLeest HT, Antle MC, Meijer JH. Amplitude of the SCN clock enhanced by the behavioral activity rhythm. PLoS One. 2012;7:e39693. doi: 10.1371/journal.pone.0039693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mistlberger RE, Antle MC. Entrainment of circadian clocks in mammals by arousal and food. Essays Biochem. 2011;49:119–136. doi: 10.1042/bse0490119. [DOI] [PubMed] [Google Scholar]
- 50.Sahar S, Sassone-Corsi P. The epigenetic language of circadian clocks. Handb Exp Pharmacol. 2013;217:29–44. doi: 10.1007/978-3-642-25950-0_2. [DOI] [PubMed] [Google Scholar]
- 51.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 52.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, et al. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the ‘master clock’. FASEB J. 2006;20:1874–1876. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]
- 55.Ackermann K, Dehghani F, Bux R, Kauert G, Stehle JH. Day-night expression patterns of clock genes in the human pineal gland. J Pineal Res. 2007;43:185–194. doi: 10.1111/j.1600-079X.2007.00461.x. [DOI] [PubMed] [Google Scholar]
- 56.Wunderer F, Kuhne S, Jilg A, Ackermann K, Sebesteny T, Maronde E, et al. Clock gene expression in the human pituitary gland. Endocrinology. 2013;154:2046–2057. doi: 10.1210/en.2012-2274. [DOI] [PubMed] [Google Scholar]
- 57.Sequeira A, Morgan L, Walsh DM, Cartagena PM, Choudary P, Li J, et al. Gene expression changes in the prefrontal cortex, anterior cingulate cortex and nucleus accumbens of mood disorders subjects that committed suicide. PLoS One. 2012;7:e35367. doi: 10.1371/journal.pone.0035367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci USA. 2013;110:9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 60.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS spectrums. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 62.Gotlib IH, Sivers H, Gabrieli JD, Whitfield-Gabrieli S, Goldin P, Minor KL, et al. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- 63.Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 65.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunter AM, Korb AS, Cook IA, Leuchter AF. Rostral anterior cingulate activity in major depressive disorder: state or trait marker of responsiveness to medication? J Neuropsychiatry Clin Neurosci. 2013;25:126–133. doi: 10.1176/appi.neuropsych.11110330. [DOI] [PubMed] [Google Scholar]
- 67.Fu CH, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis. 2013;52:75–83. doi: 10.1016/j.nbd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 68.Rentzsch J, Adli M, Wiethoff K, Gomez-Carrillo de Castro A, Gallinat J. Pretreatment anterior cingulate activity predicts antidepressant treatment response in major depressive episodes. Eur Arch Psychiatry Clin Neurosci. 2013;264:213–223. doi: 10.1007/s00406-013-0424-1. [DOI] [PubMed] [Google Scholar]
- 69.Milak MS, Parsey RV, Lee L, Oquendo MA, Olvet DM, Eipper F, et al. Pretreatment regional brain glucose uptake in the midbrain on PET may predict remission from a major depressive episode after three months of treatment. Psychiatry Res. 2009;173:63–70. doi: 10.1016/j.pscychresns.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nugent AC, Diazgranados N, Carlson PJ, Ibrahim L, Luckenbaugh DA, Brutsche N, et al. Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord. 2013;16:119–128. doi: 10.1111/bdi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu JC, Gillin JC, Buchsbaum MS, Hershey T, Johnson JC, Bunney WE., Jr Effect of sleep deprivation on brain metabolism of depressed patients. Am J Psychiatry. 1992;149:538–543. doi: 10.1176/ajp.149.4.538. [DOI] [PubMed] [Google Scholar]
- 72.McCormick LM, Boles Ponto LL, Pierson RK, Johnson HJ, Magnotta V, Brumm MC. Metabolic correlates of antidepressant and antipsychotic response in patients with psychotic depression undergoing electroconvulsive therapy. J ECT. 2007;23:265–273. doi: 10.1097/yct.0b013e318150d56d. [DOI] [PubMed] [Google Scholar]
- 73.McCormick LM, Yamada T, Yeh M, Brumm MC, Thatcher RW. Antipsychotic effect of electroconvulsive therapy is related to normalization of subgenual cingulate theta activity in psychotic depression. J Psychiatr Res. 2009;43:553–560. doi: 10.1016/j.jpsychires.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Langguth B, Wiegand R, Kharraz A, Landgrebe M, Marienhagen J, Frick U, et al. Pre-treatment anterior cingulate activity as a predictor of antidepressant response to repetitive transcranial magnetic stimulation (rTMS) Neuro Endocrinol Lett. 2007;28:633–638. [PubMed] [Google Scholar]
- 75.Hernandez-Ribas R, Deus J, Pujol J, Segalas C, Vallejo J, Menchon JM, et al. Identifying brain imaging correlates of clinical response to repetitive transcranial magnetic stimulation (rTMS) in major depression. Brain Stimulation. 2013;6:54–61. doi: 10.1016/j.brs.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 76.Broadway JM, Holtzheimer PE, Hilimire MR, Parks NA, Devylder JE, Mayberg HS, et al. Frontal theta cordance predicts 6-month antidepressant response to subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. Neuropsychopharmacology. 2012;37:1764–1772. doi: 10.1038/npp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Konarski JZ, Kennedy SH, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, et al. Predictors of nonresponse to cognitive behavioural therapy or venlafaxine using glucose metabolism in major depressive disorder. J Psychiatry Neurosci. 2009;34:175–180. [PMC free article] [PubMed] [Google Scholar]
- 78.Li JZ. Circadian rhythms and mood: opportunities for multi-level analyses in genomics and neuroscience. Bioessays. 2014;36:305–315. doi: 10.1002/bies.201300141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang WG, Li SX, Zhou SJ, Sun Y, Shi J, Lu L. Chronic unpredictable stress induces a reversible change of PER2 rhythm in the suprachiasmatic nucleus. Brain Res. 2011;1399:25–32. doi: 10.1016/j.brainres.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 80.Jiang WG, Li SX, Liu JF, Sun Y, Zhou SJ, Zhu WL, et al. Hippocampal CLOCK protein participates in the persistence of depressive-like behavior induced by chronic unpredictable stress. Psychopharmacology (Berl) 2013;227:79–92. doi: 10.1007/s00213-012-2941-4. [DOI] [PubMed] [Google Scholar]
- 81.Kinoshita C, Miyazaki K, Ishida N. Chronic stress affects PERIOD2 expression through glycogen synthase kinase-3beta phosphorylation in the central clock. Neuroreport. 2012;23:98–102. doi: 10.1097/WNR.0b013e32834e7ec2. [DOI] [PubMed] [Google Scholar]
- 82.Landgraf D, McCarthy MJ, Welsh DK. The role of the circadian clock in animal models of mood disorders. Behav Neurosci. 2014;128:344–359. doi: 10.1037/a0036029. [DOI] [PubMed] [Google Scholar]
- 83.Wongchitrat P, Mukda S, Phansuwan-Pujito P, Govitrapong P. Effect of amphetamine on the clock gene expression in rat striatum. Neurosci Lett. 2013;542:126–130. doi: 10.1016/j.neulet.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 84.Masubuchi S, Honma S, Abe H, Nakamura W, Honma K. Circadian activity rhythm in methamphetamine-treated Clock mutant mice. Eur J Neurosci. 2001;14:1177–1180. doi: 10.1046/j.0953-816x.2001.01749.x. [DOI] [PubMed] [Google Scholar]
- 85.Wu JC, Kelsoe JR, Schachat C, Bunney BG, DeModena A, Golshan S, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66:298–301. doi: 10.1016/j.biopsych.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 86.Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry. 2013;73:1164–1171. doi: 10.1016/j.biopsych.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 87.Benedetti F, Colombo C. Sleep deprivation in mood disorders. Neuropsychobiology. 2011;64:141–151. doi: 10.1159/000328947. [DOI] [PubMed] [Google Scholar]
- 88.Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for Affective Disorders: A Clinician’s Manual for Light and Wake Therapy. Karger; Basel: 2009. [DOI] [PubMed] [Google Scholar]
- 89.Wisor JP, O’Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, et al. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wisor JP, Pasumarthi RK, Gerashchenko D, Thompson CL, Pathak S, Sancar A, et al. Sleep deprivation effects on circadian clock gene expression in the cerebral cortex parallel electroencephalographic differences among mouse strains. J Neurosci. 2008;28:7193–7201. doi: 10.1523/JNEUROSCI.1150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mongrain V, La Spada F, Curie T, Franken P. Sleep loss reduces the DNA-binding of BMAL1, CLOCK, and NPAS2 to specific clock genes in the mouse cerebral cortex. PLoS One. 2011;6:e26622. doi: 10.1371/journal.pone.0026622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thompson CL, Wisor JP, Lee CK, Pathak SD, Gerashchenko D, Smith KA, et al. Molecular and anatomical signatures of sleep deprivation in the mouse brain. Front Neurosci. 2010;4:165. doi: 10.3389/fnins.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maret S, Dorsaz S, Gurcel L, Pradervand S, Petit B, Pfister C, et al. Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci USA. 2007;104:20090–20095. doi: 10.1073/pnas.0710131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benedetti F, Dallaspezia S, Lorenzi C, Pirovano A, Radaelli D, Locatelli C, et al. Gene-gene interaction of glycogen synthase kinase 3-beta and serotonin transporter on human antidepressant response to sleep deprivation. J Affect Disord. 2012;136:514–519. doi: 10.1016/j.jad.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 95.Benedetti F, Bernasconi A, Lorenzi C, Pontiggia A, Serretti A, Colombo C, et al. A single nucleotide polymorphism in glycogen synthase kinase 3-beta promoter gene influences onset of illness in patients affected by bipolar disorder. Neurosci Lett. 2004;355:37–40. doi: 10.1016/j.neulet.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 96.Benedetti F, Serretti A, Colombo C, Lorenzi C, Tubazio V, Smeraldi E. A glycogen synthase kinase 3-beta promoter gene single nucleotide polymorphism is associated with age at onset and response to total sleep deprivation in bipolar depression. Neurosci Lett. 2004;368:123–126. doi: 10.1016/j.neulet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 97.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karege F, Perroud N, Burkhardt S, Fernandez R, Ballmann E, La Harpe R, et al. Protein levels of beta-catenin and activation state of glycogen synthase kinase-3beta in major depression. A study with postmortem prefrontal cortex. J Affect Disord. 2012;136:185–188. doi: 10.1016/j.jad.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 99.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 101.Mizoro Y, Yamaguchi Y, Kitazawa R, Yamada H, Matsuo M, Fustin JM, et al. Activation of AMPA receptors in the suprachiasmatic nucleus phase-shifts the mouse circadian clock in vivo and in vitro. PLoS One. 2010;5:e10951. doi: 10.1371/journal.pone.0010951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen C, Hardy M, Zhang J, LaHoste GJ, Bazan NG. Altered NMDA receptor trafficking contributes to sleep deprivation-induced hippocampal synaptic and cognitive impairments. Biochem Biophys Res Commun. 2006;340:435–440. doi: 10.1016/j.bbrc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 104.Vecsey CG, Peixoto L, Choi JH, Wimmer M, Jaganath D, Hernandez PJ, et al. Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiol Genomics. 2012;44:981–991. doi: 10.1152/physiolgenomics.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cao R, Robinson B, Xu H, Gkogkas C, Khoutorsky A, Alain T, et al. Translational control of entrainment and synchrony of the suprachiasmatic circadian clock by mTOR/4E-BP1 signaling. Neuron. 2013;79:712–724. doi: 10.1016/j.neuron.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moller-Levet CS, Archer SN, Bucca G, Laing EE, Slak A, Kabiljo R, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci USA. 2013;110:E1132–E1141. doi: 10.1073/pnas.1217154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Archer SN, Viola AU, Kyriakopoulou V, von Schantz M, Dijk DJ. Inter-individual differences in habitual sleep timing and entrained phase of endogenous circadian rhythms of BMAL1, PER2 and PER3 mRNA in human leukocytes. Sleep. 2008;31:608–617. doi: 10.1093/sleep/31.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ackermann K, Plomp R, Lao O, Middleton B, Revell VL, Skene DJ, et al. Effect of sleep deprivation on rhythms of clock gene expression and melatonin in humans. Chronobiol Int. 2013;30:901–909. doi: 10.3109/07420528.2013.784773. [DOI] [PubMed] [Google Scholar]
- 109.Kavcic P, Rojc B, Dolenc-Groselj L, Claustrat B, Fujs K, Poljak M. The impact of sleep deprivation and nighttime light exposure on clock gene expression in humans. Croat Med J. 2011;52:594–603. doi: 10.3325/cmj.2011.52.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lavebratt C, Sjoholm LK, Soronen P, Paunio T, Vawter MP, Bunney WE, et al. CRY2 is associated with depression. PLoS One. 2010;5:e9407. doi: 10.1371/journal.pone.0009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zarate C, Jr, Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18:293–303. doi: 10.3109/10673229.2010.511059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 114.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zigman D, Blier P. Urgent ketamine infusion rapidly eliminated suicidal ideation for a patient with major depressive disorder: a case report. J Clin Psychopharmacol. 2013;33:270–272. doi: 10.1097/JCP.0b013e3182856865. [DOI] [PubMed] [Google Scholar]
- 116.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harihar C, Dasari P, Srinivas JS. Intramuscular ketamine in acute depression: a report on two cases. Indian J Psychiatry. 2013;55:186–188. doi: 10.4103/0019-5545.111461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Gioannis A, De Leo D. Oral ketamine augmentation for chronic suicidality in treatment-resistant depression. Aust N Z J Psychiatry. 2014;48:686. doi: 10.1177/0004867414520754. [DOI] [PubMed] [Google Scholar]
- 119.Papolos DF, Teicher MH, Faedda GL, Murphy P, Mattis S. Clinical experience using intranasal ketamine in the treatment of pediatric bipolar disorder/fear of harm phenotype. J Affect Disord. 2013;147:431–436. doi: 10.1016/j.jad.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 120.Clements JA, Nimmo WS. Pharmacokinetics and analgesic effect of ketamine in man. Br J Anaesth. 1981;53:27–30. doi: 10.1093/bja/53.1.27. [DOI] [PubMed] [Google Scholar]
- 121.Browne CA, Lucki I. Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol. 2013;4:161. doi: 10.3389/fphar.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- 123.Bellet MM, Vawter MP, Bunney BG, Bunney WE, Sassone-Corsi P. Ketamine influences CLOCK:BMAL1 function leading to altered circadian gene expression. PLoS One. 2011;6:e23982. doi: 10.1371/journal.pone.0023982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19:51–60. doi: 10.1016/j.molmed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38:2268–2277. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li SX, Liu LJ, Xu LZ, Gao L, Wang XF, Zhang JT, et al. Diurnal alterations in circadian genes and peptides in major depressive disorder before and after escitalopram treatment. Psychoneuroendocrinology. 2013;38:2789–2799. doi: 10.1016/j.psyneuen.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 127.Cuesta M, Clesse D, Pevet P, Challet E. New light on the serotonergic paradox in the rat circadian system. J Neurochem. 2009;110:231–243. doi: 10.1111/j.1471-4159.2009.06128.x. [DOI] [PubMed] [Google Scholar]
- 128.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Monteggia LM, Kavalali ET. Scopolamine and ketamine: evidence of convergence? Biol Psychiatry. 2013;74:712–713. doi: 10.1016/j.biopsych.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 130.Srinivasan V, Singh J, Pandi-Perumal SR, Brown GM, Spence DW, Cardinali DP. Jet lag, circadian rhythm sleep disturbances, and depression: the role of melatonin and its analogs. Adv Ther. 2010;27:796–813. doi: 10.1007/s12325-010-0065-y. [DOI] [PubMed] [Google Scholar]
- 131.Katz G, Knobler HY, Laibel Z, Strauss Z, Durst R. Time zone change and major psychiatric morbidity: the results of a 6-year study in Jerusalem. Compr Psychiatry. 2002;43:37–40. doi: 10.1053/comp.2002.29849. [DOI] [PubMed] [Google Scholar]
- 132.Chen Z, Yoo SH, Takahashi JS. Small molecule modifiers of circadian clocks. Cell Mol Life Sci. 2012;70:2985–2998. doi: 10.1007/s00018-012-1207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


