Abstract
Mammalian cells contain the cyclic pyrimidine nucleotides cCMP and cUMP. It is unknown whether these tentative new second messenger molecules occur in vivo. We used high performance liquid chromatography quadrupole tandem mass spectrometry to quantitate nucleoside 3′,5′-cyclic monophosphates. cCMP was detected in all organs studied, most notably pancreas, spleen and the female reproductive system. cUMP was not detected in organs, probably due to the intrinsically low sensitivity of mass spectrometry to detect this molecule and organ matrix effects. Intratracheal infection of mice with recombinant Pseudomonas aeruginosa harboring the nucleotidyl cyclase toxin ExoY massively increased cUMP in lung. The identity of cCMP and cUMP in organs was confirmed by high performance liquid chromatography quadrupole time of flight mass spectrometry. cUMP also appeared in serum, urine and faeces following infection. Taken together, this report unequivocally shows for the first time that cCMP and cUMP occur in vivo.
Keywords: Cyclic pyrimidine nucleotides, ExoY, Pseudomonas aeruginosa, Mass spectrometry
1. Introduction
cAMP and cGMP are well established second messengers [1–4]. Recent studies, taking advantage of the most advanced HPLC-MS/ MS and HPLC-MS/TOF methods have unequivocally demonstrated the presence of the cCMP and cUMP in numerous mammalian cell lines and primary cells [5–7]. Moreover, studies with membrane-permeable cNMP analogs point to unique signal transduction roles of cCMP and cUMP [8]. The bicarbonate-stimulated sAC is responsible for maintaining basal cCMP and cUMP levels in certain cells [6]. The current knowledge on cCMP and cUMP has been recently reviewed [9,10].
The presence of cAMP and cGMP in vivo is well documented [11], but it is still unknown whether cCMP and cUMP occur in intact organisms. The identification of cCMP and cUMP in vivo is critical for establishing their roles as new second messenger molecules, but technically, this is not trivial. Specifically, matrix effects in organ extracts, resulting in signal suppression, are an inherent problem of HPLC-MS/MS studies with complex biological samples [12,13]. Additionally, among all four cNMPs considered here, cUMP is detected with the lowest sensitivity so that low organ cUMP levels are likely below the LLOQ, i.e. 0.4 pmol/ sample [13]. As important experimental tool, we used the Pseudomonas aeruginosa NC toxin ExoY that generates large quantities of cUMP and, to a lesser extent, cCMP in various mammalian cells [14].
2. Materials and methods
2.1. Animal experiments
Animal experiments were approved by the local government. Female C57BL/6 mice (8–10 weeks old, 20 g, Elevage Janvier, Le Genest-Saint-Isle, France) were fed with with standard diet and tap water and housed at constant temperature (22 ºC) under a cycle of 12 h light and 12 h darkness. Faeces was collected between 11 a.m. and 7 p.m. Mice were intratracheally instilled with P. aeruginosa strains PA103ΔexoUexoT::Tc pUCPexoY or PA103ΔexoUexoT::Tc pUCPexoY K81M [15], respectively, as described in Ref. [16]. Both P. aeruginosa strains, maintained on Vogel–Bonner-medium (VBM), were streaked out on VBM plates containing 400 μg/mL carbenicillin and incubated at 37 ºC overnight. The next day, bacteria were harvested by washing the plates with sterile PBS and the number of colony-forming units (CFU)/mL was estimated by measuring the optical density (OD540 = 0.25 = 2 × 108 CFU/mL). Mice were infected with 1 × 106, 1 × 107 or 1 × 108 CFU in 50 μL PBS. Dilutions of the applied bacterial suspension were prepared to control retrospectively the number of CFU applied. During the infection procedure, the mice were anaesthetized by intraperitoneal injection of 0.1 mL/10 g body weight of a mixture of 1 mL ketamine (100 mg/mL) and 5 mL midazolame (5 mg/mL) and 4 mL of sterile NaCl solution (0.7%, m/v). Mice were sacrificed by an overdose of anesthetics. Blood was collected by cardiac puncture of the right ventricle and processed to serum using a Micro Tube 1.1 mL Z-Gel with clot activator (Sarstedt, Nümbrecht, Germany) according to manufacturer's instructions. Infected lungs were resected. For cNMP analysis the right lung was immediately frozen in liquid nitrogen. For determination of basal cNMP levels, 7 female and 7 male Balb/c mice (8–10 weeks old) were sacrificed by an overdose of CO2 and heart puncture. Tissues were resected and immediately frozen in liquid nitrogen.
2.2. Sample preparations
Tissues or faeces (50–200 mg) were transferred to 2 mL Fast- Prep vials containing 200mg garnet matrix and one ¼-inch ceramic sphere (lysing matrix A). Eight hundred μL of organic extraction solvent (70/30 ethanol/water [v/v] containing 12.5 ng/mL of the internal standard tenofovir) were added and tissue was homogenized using a FastPrep-24 system (MP Biomedicals Santa Anna, CA, USA) at a speed of 5 m/s for 60 s. Phosphodiesterases were inactivated by heating the homogenate for 15 min at 95 ºC. After centrifugation (20,800 × g, 10 min, 4 ºC), 600 μL of the supernatant fluid were dried at 40 ºC under a gentle nitrogen stream. The residual pellet was resolved in 150 μL water and analyzed by HPLC-MS/ MS. cNMP analysis in serum samples was carried out by treating 50 μL serum with 200 μL of a mixture of acetonitrile/water (50/50, v/v). For phosphodiesterase inactivation, samples were heated for 15 min at 95 ºC. After cooling down, samples were centrifuged (20,800 × g,10 min, 4 ºC) and the supernatant fluid-was dried at 40 ºC under a gentle nitrogen stream. The residual pellet was resolved in 150 μL water containing 50 ng/mL of the internal standard (tenofovir).
2.3. HPLC-MS/MS
cNMP quantitation was performed via HPLC-MS/MS using a QTrap5500 triple quadrupole mass spectrometer (ABSCIEX, Foster City, CA, USA) [5–7,13]. cNMP analysis by HPLC-MS/TOF was performed as described [5]. cNMP identification was performed with an HPLC-MS/TOF system (TripleTOF 5600; ABSCIEX Foster City, CA, USA) equipped with an electrospray ionization source (ESI), operating in positive ionization mode and using an ion spray voltage of 4500 V. Further ESI parameters were: Curtain gas: 45 psi, gas 1: 60 psi, gas 2: 75 psi, source temperature: 400 ºC. The chromatographic separation of analytes was achieved on a Nexera UHPLC system (Shimadzu, Duisburg, Germany) using a Hypercarb column (30 × 4.6 mm; 5 μm particle size; Thermo Scientific, Wilmington, DE) and an injection volume of 50 μL. Using a flow rate of 1.2 mL/min the following gradient (solvent A: 10 mM ammonium acetate, pH 10 and solvent B: acetonitrile) was applied: 0–6 min, 4–46% B; 6–7 min, 46–95 % B, and 7–9 min 4% B. Analyst TF 1.5.1 software was used for data calculation. The LLOQ for standard cAMP was 0.04 pmol/sample, for standard cGMP 0.07 pmol per sample, for standard cCMP 0.07 pmol/sample, and for standard cUMP 0.4 pmol per sample [13].
2.4. Quantitative real-time PCR analysis
RNA was prepared using Nucleospin RNA (Macherey–Nagel, Düren, Germany). RNA was reverse-transcribed into cDNA and analyzed with TaqMan probes for the expression of maouse β-actin (mActb) (Mm00607939_s1, Life Technologies, Frankfurt/ Main, Germany), Adcy10 (AC10 or sAC) (Mm00557236_m1), Gucy1a2 (sGC α2 subunit) (Mm01253539_m1), Gucy1a3 (sGC α3 subunit) (Mm01220285_m1) and Gucy1b3 (sGC β3 subunit) (Mm00516926_m1) using the qPCRBIO Probe Mix (Nippon Genetics, Düren, Germany). To determine the mRNA expression of the genes ΔCt values were calculated according to the following equation: ΔCt = Ct (gene) – Ct (Actb).
2.5. Statistics
Data were analyzed with GraphPad Prism 5.01 (San Diego, CA, USA).
3. Results
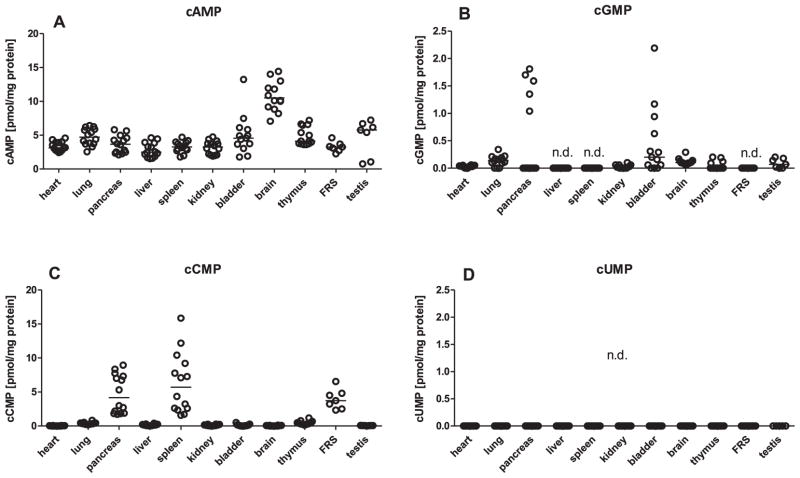
Basal cNMP levels organs from Balb/c mice (8–10 weeks old) were measured (Fig. 1). cAMP was detected at substantial levels in all organs examined, the cAMP levels in brain being particularly high. The highest cGMP levels were found in pancreas and bladder. The highest cGMP levels found among all mouse organs were about ten-fold lower than the highest cAMP levels. cGMP was not detected in liver, spleen and the FRS. In heart, lung, kidney, brain, thymus and testis, cGMP levels were just above LLOQ. In pancreas and the FRS, cCMP levels reached the corresponding cAMP levels, and spleen cCMP levels surpassed cAMP levels. In all other organs examined cCMP levels were close to LLOQ. Since very low levels of cGMP and cCMP are not readily visible in Fig. 1 due to the chosen scale of the y-axes, absolute cNMP values are reported in Table S1. In none of the organs, we could detect cUMP. This could have been due to the high LLOQ for cUMP [13]. In addition, quenching of MS signals by organ matrix components could contribute to our failure to detect cUMP [12]. To test this hypothesis, we compared MS signals of unquenched cUMP and cUMP quenched by lung extract (Fig. S1). In fact, addition of organ extract to purified cUMP reduced MS signals about four-fold. Thus, the overall sensitivity of HPLC-MS/MS to detect cUMP in vivo is low compared to other cNMPs.
Fig. 1.
Basal cNMP concentrations in Balb/c mouse organs. cNMP concentrations were determined using 50–200 mg organ tissues from 7 male and 7 female Balb/c mice (age, 8–10 weeks). Samples were processed for HPLC-MS/MS analysis as described in Materials and Methods. Data shown represent the organ cNMP concentrations for each individual mouse referred to protein concentration. Except for data on the reproductive system, data for male and female animals were pooled. Median cNMP values are indicated by the horizontal bar. FRS: female reproductive system, n.d.: not detected; i.e. cNMP values were below LLOQ.
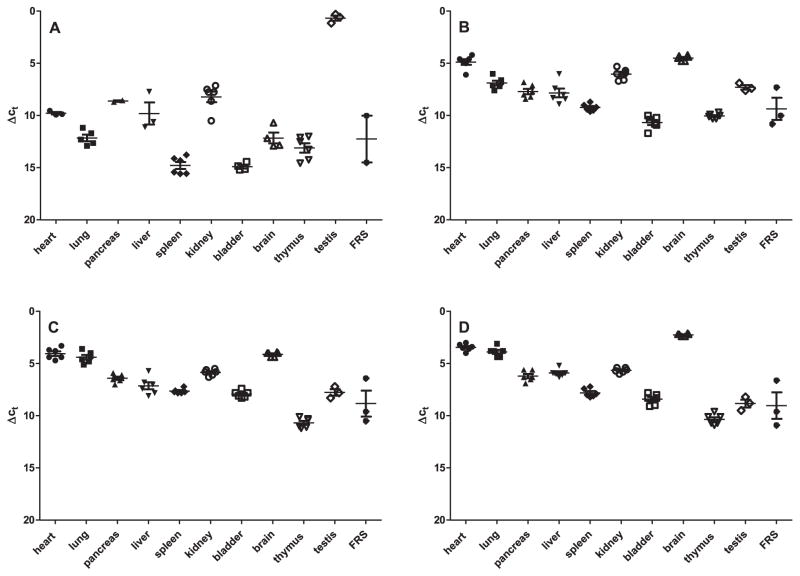
To obtain information about the source of basal cCMP in organs, we examined the expression of sAC at the mRNA level. As expected [17,18], sAC mRNA was highly abundant in testis (Fig. 2). In contrast, in all other organs sAC mRNA was barely detected as assessed by ΔCt values using actin as reference housekeeping gene. Soluble guanylyl cyclase (sGC) is another enzyme that can generate cCMP and cUMP, but high enzymatic activity is dependent on the presence of nitric oxide [5,19]. The highest expression of mRNA of sGC subunits was found in brain, followed by heart. In general, other organs contained lower sGC mRNA levels.
Fig. 2.
Analysis of sAC and sGC gene expression in mouse organs. mRNA from the Balb/c mouse tissues shown in Fig. 1 was extracted, reverse-transcribed and analyzed by quantitative real-time PCR using TaqMan probes. Data were analyzed using the ΔCt method using actin as reference gene. A, sAC expression; B, sGC expression (α2 subunit); C, sGC expression (α3 subunit); D, sGC expression (β3 subunit). Note that the lower the numbers on the y-axis are, the higher is the expression of the respective gene.
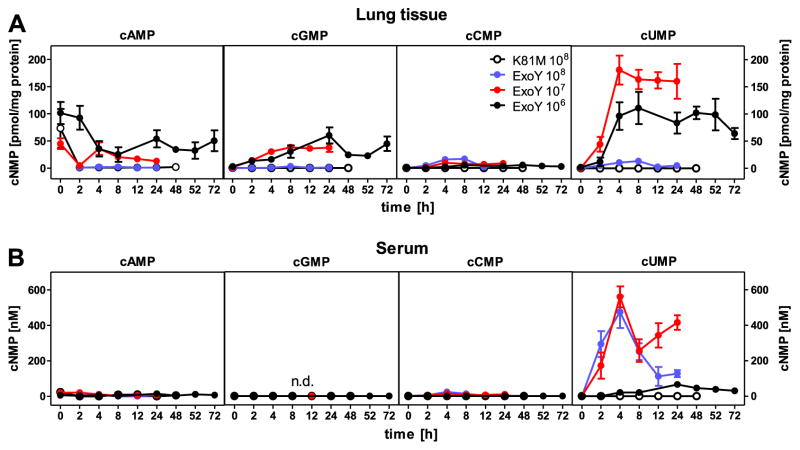
To detect cUMP in vivo, we infected female C57BL/6 mice (8–10 weeks old) intratracheally with recombinant P. aeruginosa harboring the NC toxin ExoY but no ExoT, or ExoU. These recombinant bacteria induce serious lung damage in rodents [20]. ExoY, but not the catalytically inactive mutant ExoY-K81M, induced a large time-dependent cUMP increase in lung, particularly with 1 × 107 CFU (Fig. 3). In marked contrast, ExoY did not increase cAMP and only moderately increased cGMP. ExoY also induced a small cCMP increase that is not readily visible due to the scale of the y-axis. cCMP and cUMP levels induced in lung by ExoY were sufficiently high to allow for HPLC-MS/TOF analysis. cCMP standard yielded two characteristic fragments that were readily identified in lung from P. aeruginosa-infected C57/BL6 mice (Fig. S2). cUMP standard yielded three typical fragments that were identified in lung. We confirmed the identity of cCMP in pancreas, spleen and FRS of Balb/c mice by HPLC-MS/TOF (Table S2).
Fig. 3.
cNMP concentrations in mouse lung and serum. Female C57/BL6 mice were infected with recombinant P. aeruginosa strain PA103ΔexoUexoT::Tc pUCPexoY (1 × 106–1 × 108 CFU) or recombinant P. aeruginosa strain PA103ΔexoUexoT::Tc pUCPexoY K81M (control, 1 × 108 CFU) and sacrificed at specific times after infection. Lung tissue and serum were then processed for HPLC-MS/MS analysis as described in Materials and Methods. (A) Data shown represent concentration of cNMPs in lung tissue referred to protein concentration 0–72 h after infection. (B) Data shown represent serum concentration of cNMPs 0–72 h after infection. Data shown are the means ± SEM of six animals. cGMP was not detected in serum. Note that after infection with 1 × 107–1 × 108 CFU of PA103ΔexoUexoT::Tc pUCPexoY, mice did after 24–48 h after infection.
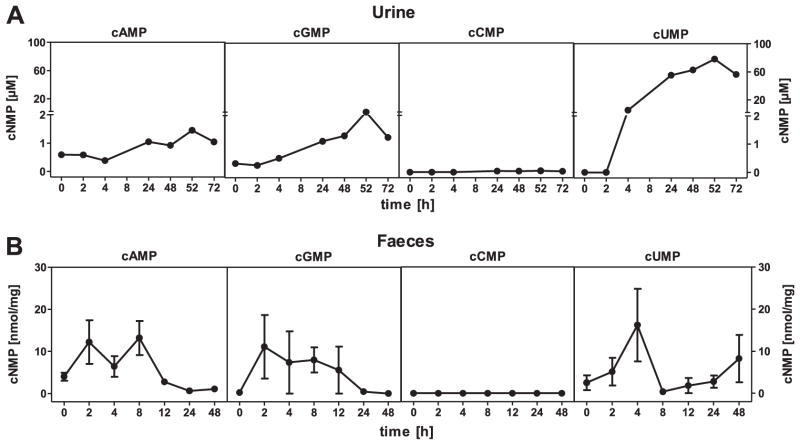
Multidrug resistance proteins constitute an important elimination pathway for cCMP and cUMP, i.e. isoforms 4 and 5 transport cUMP into the extracellular space, and MRP5 exports cCMP [21]. Therefore, we analyzed cNMP levels in serum from P. aeruginosa-infected mice. We found a massive cUMP increase, particularly with 1 × 107 CFU (Fig. 3), whereas cAMP and cGMP did not increase. We also observed a very small cCMP increase in serum. With the catalytically inactive mutant ExoY-K81M, no cNMP increases in serum were found. To further examine cNMP elimination pathways, we examined urine and faeces. ExoY induced only small cAMP and cGMP increases in urine and faeces, and cCMP was not detected at all (Fig. 4). In marked contrast, cUMP levels massively increased in urine. We also detected a transient cUMP increase in faeces.
Fig. 4.
cNMP concentrations in mouse urine and faeces. Female C57/BL6 mice were infected with recombinant P. aeruginosa strain PA103ΔexoUexoT::Tc pUCPexoY and sacrificed at specific times after infection. Urine and faeces were then processed for HPLC-MS/MS analysis as described in Materials and Methods. (A) Data shown represent molar concentrations of cNMPs in urine 0–72 h after infection with 1 × 106 CFU. (B) Data shown represent concentrations of cNMPs in faeces 0–48 h after infection with 1 × 107 CFU. Data shown are the means ± SEM of six animals. cCMP was not detected in faeces.
4. Discussion
We present the first unequivocal evidence that cCMP and cUMP occur in vivo. Two previous studies using an early mass spectrometry method claimed the identification of cCMP and cUMP in rat organs [22,23], but the method used was too insensitive and unspecific to unequivocally detect these cNMPs [9,10]. By analogy to cells [7], we observed specific cNMP distribution patterns in mouse organs, supporting the notion of a cNMP signaling code [7,10].
The high cCMP concentrations in spleen point to a role of cCMP in immune cell function. In support of this hypothesis, in human lymphocytes, cCMP is present [7]. Moreover, cCMP exerts biological effects in neutrophils, macrophages and leukemia cells [24–26]. The presence of cCMP at high levels in pancreas is an indication for a role of this cNMP in endocrine and/or exocrine function. In agreement with this notion, Capan1 pancreas tumor cells contain cCMP as well [7]. To address this question, the effects of membrane-permeable cCMP analogs on endocrine and exocrine pancreas function could be studied [8,27].
Based on the presence of cCMP in the FRS, a role of cCMP in female reproductive function is likely. We did not differentiate between ovary, oviduct and uterus because of limitations in organ availability, but it is noteworthy that Chinese hamster ovary (CHO) cells contain high cCMP concentrations [7]. A previous study provided evidence for a function of cCMP in blastocyst development [28]. The highest cCMP levels in organs surpass cGMP level by 10- fold and reach cAMP levels. Based on the unequivocal detection of cUMP in numerous mammalian cell lines and primary cells [7], it is likely that cUMP also occurs in intact organs, but the LLOQ is too low to detect low cUMP levels [13].
The overall high cAMP levels are consistent with the ubiquitous function of this cNMP as second messenger [1]. The high cAMP levels in brain fit to the high relevance of cAMP signaling for learning [29]. The high cGMP levels in bladder fit to the role of this cNMP in smooth muscle relaxation [30]. The identification of relatively high cGMP levels in pancreas was unexpected, but upregulation of cGMP signaling in pancreatitis has been reported [31]. Equally surprising was the finding that in lung, cGMP levels were rather low although cGMP plays an important role in lung function [32]. This dissociation between low cNMP level and established function of a cNMP demonstrates that low cNMP levels do not preclude an important function of a given cNMP. Subcellular cNMP compartmentation may be a key here [11]. An implication of these findings is that even in organs with very low cCMP levels, e.g. brain, cCMP could be functionally important [33].
Membranous adenylyl cyclases and particulate guanylyl cyclases do not generate cCMP [6,34]. sAC mediates high cCMP levels in certain cell types [6]. However, it is unlikely that sAC is responsible for the high cCMP levels in spleen, pancreas and FRS because these organs showed only very low or no sAC expression. Conversely, testis showed very high sAC expression but only very low cCMP concentration. The sGC expression pattern does also not fit to the cCMP pattern, and in addition cCMP formation by sGC is dependent on nitric oxide stimulation [5,19] which was not the case in our baseline studies. Genomic and biochemical approaches will have to be combined to answer the question which nucleotidyl cyclase is responsible for maintaining high cCMP levels in spleen, pancreas and FRS.
ExoY massively increased cUMP levels in lung. The in vivo data fit very well to the data obtained with ExoY in cell culture models [11]. Based on studies with cultured cell lines and primary cells, it is likely that both lung epithelial and endothelial cells contain cUMP [7]. The striking increases in cUMP in lung by ExoY with contrasting very low cAMP and cGMP levels (Fig. 4A) support the hypothesis that specific effector proteins for cUMP exist [8]. Affinity chromatography techniques will be applied to identify specific cUMP effector proteins [9]. ExoY increases endothelial permeability and induces lung damage [20,35]. Noteworthy, human endothelial cells do contain endogenous cUMP [7], pointing to a signaling role of this cNMP.
We could also provide in vivo-support for the in vitro-findings that cUMP is exported from cells [21]. In urine, cUMP levels approach 100 μM, exceeding serum levels by about 100-fold, suggesting that cUMP is actively secreted from kidney epithelial cells. cUMP has also been identified in Madin Darby canine kidney cells [7]. The identification of cUMP at concentrations in serum up to 0.6 μM raises the question whether extracellular cUMP could function as agonist at G-protein-coupled receptors. In support of a extracellular signaling role of cUMP is the finding that cAMP, cGMP and cCMP increased only marginally in lung, serum and urine. Moreover, cUMP induces chemotaxis in Dictyostelium discoideum [36] and behavioral effects in rat [33]. Extracellular cGMP regulates ligand-gated ion channels [37]. Intestinally localized cGMP modulates secretion and motility [38], and such a function is also feasible for intestinal cUMP.
In conclusion, we have demonstrated that cCMP and cUMP exist in the intact mammalian organism. It will be now important to systematically study cAMP, cGMP, cCMP and cUMP in various diseases. The identification of high cCMP concentrations in pancreas, spleen and the FRS was unexpected. Accordingly, no specific disease should be excluded for future in vivo studies.
Supplementary Material
Acknowledgments
This work was supported in part by grants of the Deutsche Forschungsgemeinschaft to RS and BT (SFB 587; projects A9 and B17).
Abbreviations
- cAMP
adenosine 3′,5′-cyclic monophosphate
- cGMP
guanosine 3′,5′-cyclic monophosphate
- cCMP
cytidine 3′,5′-cyclic monophosphate
- cNMP
nucleoside 3′,5′-cyclic monophosphate
- cUMP
uridine 3′,5′-cyclic monophosphate
- sAC
soluble adenylyl cyclase
- HPLC-MS/MS
high performance liquid chromatography quadrupole tandem mass spectrometry
- HPLC-MS/TOF
high performance liquid chromatography quadrupole time of flight mass spectrometry
- LLOQ
lower limit of quantitation
- NC
nucleotidyl cyclase
- sAC
soluble adenylyl cyclase
- sGC
soluble guanylyl cyclase
- FRS
female reproductive system
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.bbrc.2015.03.115.
Footnotes
Conflict of interest
None.
Transparency document
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.bbrc.2015.03.115.
Contributor Information
Heike Bähre, Email: baehre.heike@mh-hannover.de.
Christina Hartwig, Email: hartwig.christina@mh-hannover.de.
Antje Munder, Email: munder.antje@mh-hannover.de.
Sabine Wolter, Email: wolter.sabine@mh-hannover.de.
Tane Stelzer, Email: stelzer.tane@mh-hannover.de.
Bastian Schirmer, Email: Schirmer.bastian@mh-hannover.de.
Ulrike Beckert, Email: beckert-ulrike81@email.de.
Dara W. Frank, Email: frankd@mcw.edu.
Burkhard Tümmler, Email: tuemmler.burkhard@mh-hannover.de.
Volkhard Kaever, Email: kaever.volkhard@mh-hannover.de.
Roland Seifert, Email: seifert.roland@mhhannover.de.
References
- 1.Gancedo JM. Biological roles of cAMP: variations on a theme in the different kingdoms of life. Biol Rev Camb Philos Soc. 2013;88:645–668. doi: 10.1111/brv.12020. [DOI] [PubMed] [Google Scholar]
- 2.Schlossmann J, Schinner E. cGMP becomes a drug target. Naunyn- Schmiedeberg's Arch Pharmacol. 2012;385:243–252. doi: 10.1007/s00210-012-0730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclases: multiplicities of signalling. Mol Interv. 2002;2:168–184. doi: 10.1124/mi.2.3.168. [DOI] [PubMed] [Google Scholar]
- 4.Tresguerres M, Levin LR, Buck J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 2011;79:1277–1288. doi: 10.1038/ki.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bähre H, Danker KY, Stasch JP, et al. Nucleotidyl cyclase activity of soluble guanylyl cyclase in intact cells. Biochem Biophys Res Commun. 2014;443:1195–1199. doi: 10.1016/j.bbrc.2013.12.108. [DOI] [PubMed] [Google Scholar]
- 6.Hasan A, Danker KY, Wolter S, et al. Soluble adenylyl cyclase accounts for high basal cCMP and cUMP concentrations in HEK293 and B103 cells. Biochem Biophys Res Commun. 2014;448:236–240. doi: 10.1016/j.bbrc.2014.04.099. [DOI] [PubMed] [Google Scholar]
- 7.Hartwig C, Bähre H, Wolter S, et al. cAMP, cGMP, cCMP and cUMP concentrations across the tree of life: high cCMP and cUMP levels in astrocytes. Neurosci Lett. 2014;529:183–187. doi: 10.1016/j.neulet.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Beckert U, Grundmann M, Wolter S, et al. cNMP-AMs mimic and dissect bacterial nucleotidyl cyclase toxin effects. Biochem Biophys Res Commun. 2014;451:497–502. doi: 10.1016/j.bbrc.2014.07.134. [DOI] [PubMed] [Google Scholar]
- 9.Seifert R. cCMP and cUMP: emerging second messengers. Trends Biochem Sci. 2015;40:8–15. doi: 10.1016/j.tibs.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Seifert R, Schneider EH, Bähre H. From canonical to non-canonical second messengers:pharmacological implications. Pharmacol Ther. 2015;148:154–184. doi: 10.1016/j.pharmthera.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Sprenger JU, Nikolaev VO. Biophysical techniques for detection of cAMP and cGMP in living cells. Int J Mol Sci. 2013;14:8025–8046. doi: 10.3390/ijms14048025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosetti F, Mazzucco E, Zampieri D, et al. Signal suppression/enhancement in high-performance liquid chromatography tandem mass spectrometry. J Chromatogr A. 2010;1217:3929–3937. doi: 10.1016/j.chroma.2009.11.060. [DOI] [PubMed] [Google Scholar]
- 13.Beckert U, Aw WY, Burhenne H, et al. The receptor-bound guanylyl cyclase DAF-11 is the mediator of hydrogen peroxide-induced cGMP increase in Caenorhabditis elegans. PLoS One. 2013;8:e72569. doi: 10.1371/journal.pone.0072569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckert U, Wolter S, Hartwig C, et al. ExoY from Pseudomonas aeruginosa is a nucleotidyl cyclase with preference for cGMP and cUMP formation. Biochem Biophys Res Commun. 2014;450:870–874. doi: 10.1016/j.bbrc.2014.06.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yahr TL, Vallis AJ, Hancock MK, et al. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci U S A. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munder A, Tümmler B. Assessing Pseudomonas virulence using mammalian models: acute infection models. Methods Mol Biol. 2014;1149:773–779. doi: 10.1007/978-1-4939-0473-0_59. [DOI] [PubMed] [Google Scholar]
- 17.Hess KC, Jones BH, Marquez B, et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie F, Garcia MA, Carlson AE, et al. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353–362. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 19.Beste KY, Burhenne H, Kaever V, et al. Nucleotidyl cyclase activity of soluble guanylyl cyclase α1β1. Biochemistry. 2012;51:194–204. doi: 10.1021/bi201259y. [DOI] [PubMed] [Google Scholar]
- 20.Stevens TC, Ochoa CD, Morrow KA, et al. The Pseudomonas aeruginosa exoenzyme Y impairs endothelial cell proliferation and vascular repair following lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;306:L915–L924. doi: 10.1152/ajplung.00135.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laue S, Winterhoff M, Kaever V, et al. cCMP is a substrate for MRP5. Naunyn-Schmiedeberg's Arch Pharmacol. 2014;387:893–895. doi: 10.1007/s00210-014-1018-9. [DOI] [PubMed] [Google Scholar]
- 22.Newton RP, Salih SG, Salvage BJ, et al. Extraction, purification and identification of cytidine 3′,5′-cyclic monophosphate from rat tissues. Biochem J. 1984;221:665–673. doi: 10.1042/bj2210665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newton RP, Kingston EE, Hakeem NA, et al. Extraction, purification, identification and metabolism of 3′,5′-cyclic UMP, 3′,5′-cyclic IMP and 3′,5′-cyclic dTMP from rat tissues. Biochem J. 1986;236:431–439. doi: 10.1042/bj2360431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott GR, Lauwen AP, Bonta IL. Dibutyryl cytidine 3′:5′-cyclic monophosphate; an inhibitor of A23187-stimulated macrophage leukotriene B4 synthesis. Agents Actions. 1991;32:90–91. doi: 10.1007/BF01983323. [DOI] [PubMed] [Google Scholar]
- 25.Ervens J, Seifert R. Differential modulation by N4, 2′-O-dibutyryl cytidine 3′: 5′-cyclic monophosphate of neutrophil activation. Biochem Biophys Res Commun. 1991;174:258–267. doi: 10.1016/0006-291x(91)90514-8. [DOI] [PubMed] [Google Scholar]
- 26.Bloch A, Dutschman G, Maue R. Cytidine 3′,5′-monophosphate (cyclic CMP). II. Initiation of leukemia L-1210 cell growth in vitro. Biochem Biophys Res Commun. 1974;59:955–959. doi: 10.1016/s0006-291x(74)80072-0. [DOI] [PubMed] [Google Scholar]
- 27.Wolter S, Dove S, Golombek M, et al. N4-monobutyryl-cCMP activates PKA RIα and PKA RIIα more potently and with higher efficacy than PKG Iα in vitro but not in vivo. Naunyn-Schmiedeberg's Arch Pharmacol. 2014;387:1163–1175. doi: 10.1007/s00210-014-1042-9. [DOI] [PubMed] [Google Scholar]
- 28.Chan PJ. The effect of cyclic cytidine 3′,5′-monophosphate (cCMP) on the in vitro development, hatching and attachment of the mouse blastocyst. Experientia. 1987;43:929–930. doi: 10.1007/BF01951673. [DOI] [PubMed] [Google Scholar]
- 29.Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandner P, Neuser D, Bischoff E. Erectile dysfunction and lower urinary tract. Handb Exp Pharmacol. 2009;191:507–531. doi: 10.1007/978-3-540-68964-5_22. [DOI] [PubMed] [Google Scholar]
- 31.Buchwalow I, Schnekenburger J, Tiemann K, et al. L-Arginine-NO-cGMP signaling pathway in pancreatitis. Sci Rep. 2013;3:1899. doi: 10.1038/srep01899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sassi Y, Hulot JS. Pulmonary hypertension: novel pathways and emerging therapies inhibitors of cGMP and cAMP metabolism. Handb Exp Pharmacol. 2013;218:513–529. doi: 10.1007/978-3-642-38664-0_20. [DOI] [PubMed] [Google Scholar]
- 33.Brus R, Herman ZS, Juraszczyk Z, et al. Central action of cyclic 3′,5′-thymidine, 3′,5-uridine and 3′,5′-cytidine monophosphates in rat. Acta Med Pol. 1984;25:1–4. [PubMed] [Google Scholar]
- 34.Beste KY, Spangler CM, Burhenne H, et al. Nucleotidyl cyclase activity of particulate guanylyl cyclase A: comparison with particulate guanylyl cyclases E and F, soluble guanylyl cyclase and bacterial adenylyl cyclases CyaA and edema factor. PloS One. 2013;8:e70223. doi: 10.1371/journal.pone.0070223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayner SL, Frank DW, King J, et al. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res. 2004;95:196–203. doi: 10.1161/01.RES.0000134922.25721.d9. [DOI] [PubMed] [Google Scholar]
- 36.Chassy BM, Love LL, Krichevsky MI. The acrasin activity of 3′,5′-cyclic nucleotides. Proc Natl Acad Sci U S A. 1969;64:296–303. doi: 10.1073/pnas.64.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albrecht P, Henke N, Tien ML, et al. Extracellular cyclic GMP and ist derivatives GMP and guanosine protect from oxidative glutamate toxicity. Neurochem Int. 2013;62:610–619. doi: 10.1016/j.neuint.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Castro J, Harrington AM, Hughes PA, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3′,5′-monophosphate. Gastroenterology. 2013;145:1334–1346. doi: 10.1053/j.gastro.2013.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.