Abstract
Background
We evaluated urine free light chains (FLC) as a potential biomarker for acute kidney allograft injury (AKAI).
Methods
Urine κ and λ FLC were compared with urine β-2 microglobulin (β2-M), RBP, KIM-1, NGAL and microalbuminuria (MAB) in biopsy-confirmed acute rejection (AR) and ATN. Healthy volunteers (Normal) and transplant recipients with normal allograft function (Control) were used as references.
Results
Compared to Control or Normal group (N=15), urine FLC, MAB and RBP were higher in ATN (N=29) and AR (N=41) groups (p<0.05). There was no difference in KIM-1, NGAL or β2-M between 4 groups. In AR group, urine κFLC demonstrated the highest predictive value with sensitivity of 95.12% and specificity of 87.5% (p<0.0001). Urine κFLC also performed best with a sensitivity of 96.55% and specificity of 93.33% (p<0.0001) in ATN group. The AUC by ROC analysis is greatest in urine RBP (100%) and FLC (99%), and lowest in KIM-1 (53.5%), then NGAL (71.5%) in AR group. The AUC is also greatest in urine FLC (100%) and RBP (99%), and lowest in urine KIM-1 (55.6%) and NGAL (69.9%) in ATN group.
Conclusions
Urine FLC appears sensitive for both AR and ATN, and it may be a novel AKAI biomarker.
Keywords: urine free light chains, kidney transplant, acute kidney injury, acute rejection, acute tubular necrosis, biomarker
Introduction
Acute kidney allograft injury (AKAI) after kidney transplant is a common and complicated clinical problem. The etiology includes acute rejection (AR), acute tubular necrosis (ATN) and a variety of others. It may have a negative impact on long term graft function and graft survival (1-3). Clinical diagnosis of AKAI, similar to acute kidney injury (AKI) in native kidneys, is usually based on renal function as measured by serum creatinine (Cr). However, it is well recognized that serum Cr is neither a sensitive nor a specific marker for AKI and it does not correlate well with the severity of tissue damage (4,5). Kidney biopsy is the gold standard for diagnosing AKI, but it is invasive, costly and associated with complications (6,7).
There is a great interest to identify non-invasive biomarkers for AKI(8-16). The urinary biomarkers studied in AKI may be classified as 1). the enzymes released from damaged tubular cells, such as gamma-glutamyltranspeptidase, glutathione S transferase and nacetyl-beta-d-glucosaminidase (4,15); 2). low-molecular-weight proteins, such as beta-2 microglobulin (β2-M), retinol-binding protein (RBP), alpha-1 microglobulin and cystatin C (12,16,17); 3). injury-induced proteins, such as kidney injury molecule 1 (KIM-1), neutrophil gelatinase associated lipocalin (NGAL) and interleukin 18 (9-14); and 4). tubular structural and functional proteins, such as F-actin, Na+/H+ exchange isoform 3 (4,15). To our knowledge, there is no report comparing these biomarkers in biopsy-confirmed AKAI after successful kidney transplantation and no study of urine polyclonal immunoglobulin free light chains (FLC) in transplant patients.
The purpose of this study is to evaluate the urinary excretion of FLC as a potential biomarker for AKAI. We compared urinary kappa (κ) and lambda (λ) FLC with RBP, β2-M, NGAL, KIM-1 and microalbuminuria (MAB) in kidney transplant patients with biopsy-confirmed AKAI.
Materials and Methods
We prospectively evaluated adult kidney transplant patients who had renal allograft biopsies for the workup of acute allograft dysfunction during the year of 2010. Acute graft dysfunction necessary for kidney biopsy was defined as elevation of serum Cr by 20% above the baseline value for each individual patient clinically without pre-renal (volume depletion) or post-renal (obstruction) causes. The study protocol was approved by the Tulane University Human Research Protection Program and IRB. In our center, all transplant candidates were screened with serum protein electrophoresis and immunofixation (SPEP-IF) during the pre-transplant work-up. The patients diagnosed multiple myeloma (MM), monoclonal gammopathy of undetermined significance (MGUS), light or heavy chain deposition disease (LCDD, HCDD) were excluded from kidney transplant.
Fresh urine samples (20 to 100 cc) were collected approximately 2 hours before kidney biopsy. Urine samples were centrifuged immediately and the supernatants were stored at −70°C until assay. A latex-enhanced immunonephelometric assay was used to measure urine κ and λ FLC concentration (Freelite®, The Binding Site, San Diego, CA). Urinary excretion of selected biomarkers, including RBP (Arbor Assays, Ann Arbor, MI), MAB and β2-M (Orgentec Diagnostic, Mainz, Germany) as well as KIM-1 and NGAL (R&D Systems, Minneapolis, MN) were quantified by competitive solid phase enzyme immunoassay in accordance with the manufacturer's instructions in our laboratory. Our renal pathologist was blinded to all biomarker levels.
Based on the kidney graft biopsy results, those diagnosed with ATN (ATN group) and AR, either acute cellular rejection or acute antibody-mediated rejection or both (AR group) without significant other overlap pathology were included in this study. Any biopsy indicating other co-existing pathology, such as chronic allograft nephropathy (CAN), donor nephrosclerosis, glomerular disease (either recurrent or de novo), infection (bacterial, viral or fungal infection) or thrombotic microangiopathy, was excluded from this study. BKV viremia and CMV antigenemia (or CMV viremia) were also routinely tested in patients with graft dysfunction, and patient with active viral infection was excluded from this study. For the control groups, urine samples were collected from 15 healthy medical professionals (Normal group) as well as 15 kidney transplant patients with normal allograft function, which was defined as no prior history of AKAI and serum Cr that remained stable at baseline level and below 1.3 mg/dl (Control group). Estimated glomerular filtration rate (eGFR) was calculated using the MDRD equation.
Statistical analysis
Results are expressed as mean ± SD. Prism 5.0c (GraphPad Software, La Jolla, CA) and SPSS Statistics 19 (IBM Corp, Somers, NY) were used for analyses. For continuous variables, a one-way ANOVA with Dunn's multiple comparison test was performed for comparison between two groups. When comparing more than two groups, a two-way ANOVA with the Bonferroni post-tests was used. Fisher's exact test was used for analysis of categorical variables in the AR and ATN vs. Control group. A P-value of < 0.05 was considered statistically significant. Receiver operating characteristic (ROC) curves were generated and the area under the ROC curves (AUC) were calculated as the measure of the utility for urine κFLC, λFLC, (κ+ λ) FLC, β2-M , RBP, KIM-1 and NGAL individually as AKAI biomarker.
Results
There are 41 patients with biopsy-confirmed AR (in AR group) and 29 patients with biopsy-confirmed ATN (in ATN group). None of them has significant other co-existing pathology. The demographic characteristics of Normal, AR, ATN and Control groups are described in Table 1. There is no statistical difference in race, gender or age in the 4 groups. Compared to the Normal group or Control group, both AR and ATN groups have significant kidney dysfunction as measured by serum Cr or eGFR (p<0.01). The transplanted related characteristics and immunosuppressive drugs of ATN, AR and Control groups are also summarized and there are not statistically significant between the 3 groups (Table 1). For perioperative induction therapy, all patients received methylprednisolone intravenously. In addition, the sensitized patients were given basiliximab and the retransplant patients (only 1 in ATN group and 2 in AR group) were given antithymocyte globulin.
Table 1.
Clinical characteristics of study subjects in each group
| Normal (n=15) | ATN (n=29) | AR (n=41) | Control (n=15) | |
|---|---|---|---|---|
| Male /female | 9/6 | 18/11 | 27/14 | 8/7 |
| Caucasian (%) | 40 | 41 | 34 | 47 |
| African American (%) | 27 | 59 | 61 | 53 |
| Others (%) | 33 | 0 | 5 | 0 |
| Age (yr)* | 42 ± 15.4 | 43 ± 16.2 | 46.5 ± 16 | 48 ± 17 |
| Body weight (kg)* | 75 ± 15 | 78 ± 17 | 77 ± 16 | 78 ± 16 |
| Serum Creatinine (mg/dL)* | 1.0 ± 0.1 | 3.9 ± 2.4** | 4.1 ± 3.0** | 1.2 ± 0.1 |
| Estimated GFR (ml/min)* | 73 ± 10 | 23 ± 11** | 21 ± 12** | 64 ± 15 |
| Cause of ESRD (%) | ||||
| HTN | 52 | 54 | 47 | |
| DM | 24 | 24 | 27 | |
| GN | 10 | 15 | 13 | |
| Others | 14 | 7 | 13 | |
| Previous transplant (%) | 21 | 22 | 13 | |
| Peak PRA (%)* | 20 ± 29 | 16 ± 31 | 17 ± 18 | |
| HLA mismatch* | 3.5 ± 1.1 | 4.2 ± 1.4 | 3.9 ± 1.2 | |
| Living donor (%) | 17 | 22 | 20 | |
| Deceased donor (%) | 83 | 78 | 80 | |
| Cold ischemia time (hrs)* | 16.4 ± 7 | 17.3 ± 6 | 16 ± 6.4 | |
| Immunosuppression (%) | ||||
| Baciliximab | 21 | 17 | 20 | |
| Antithymocyte globulin | 3.4 | 4.9 | 0 | |
| Tacrolimus | 62 | 59 | 53 | |
| Cyclosporine | 31 | 29 | 33 | |
| Mycophenolate | 90 | 80 | 93 | |
| Sirolimus | 10 | 12 | 7 | |
| Azathioprine | 7 | 10 | 13 | |
| Prednisone | 90 | 78 | 87 | |
Data are expressed as mean ± SD
P < 0.01 versus Normal and Control groups; ATN, acute tubular necrosis; AR, acute rejection; GFR, glomerular filtration rate; ESRD, end stage renal disease; HTN, hypertension; DM, diabetes mellitus; GN, glomerulonephritis; PRA, panel reactive antibody; HLA, human leukocyte antigen
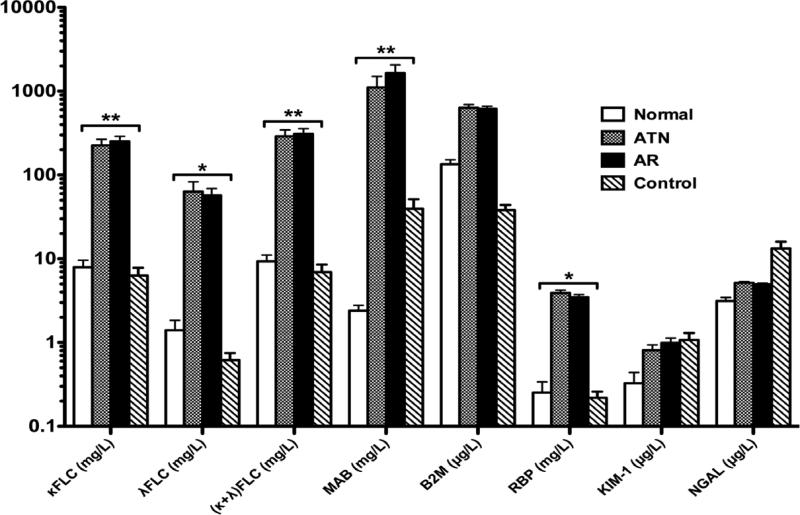
Urinary concentrations of κFLC, λFLC, (κ+ λ) FLC, MAB, β2-M, RBP, KIM-1, NGAL are summarized in Figure 1 (mean ± SE). Urine κFLC, λFLC, (κ+ λ) FLC, MAB and RBP are significantly higher in both ATN and AR groups than either Control or Normal group. However, there is no statistical difference in urine β2-M, KIM-1 or NGAL between ATN or AR group and Control or Normal group. There is no difference in each biomarker between the ATN and AR groups or between the Normal and Control groups.
Figure 1.
Urinary biomarker in Normal, ATN, AR and Control groups. Urinary concentrations of κFLC, λFLC, (κ+ λ) FLC, MAB and RBP are significantly higher in both ATN and AR groups than either Control or Normal group, **P < 0.01 or *P < 0.05. There is no difference in urinary concentrations of β2-M, KIM-1 and NGAL between ATN or AR group and Control or Normal group.
Based on the urinary concentration in the Normal group, we use the highest level as the upper “normal limit” for each biomarker, and the specific cut-off values are listed in Table 2. The positive case is defined by the urine concentration above this defined upper limit. The absolute number and the percentage of positive and negative cases for each biomarker are shown in Table 2. κFLC has the highest number of positive cases and lowest number of negative cases in both AR and ATN groups, which is followed by (κ+λ)FLC and RBP. KIM-1 has the lowest positive cases and highest negative cases in both AR and ATN groups, followed by NGAL and MAB.
Table 2.
Positive and negative cases of kidney dysfunction in each group
| Urine Biomarker | Normal (n=15) | ATN (n=29) | AR (n=41) | Control (n=15) | |
|---|---|---|---|---|---|
| κFLC (< 20 mg/L) | Positive | 0 (0%) | 28 (97%) | 39 (95%) | 1 (7%) |
| Negative | 15 (100%) | 1 (3%) | 2 (5%) | 14 (93%) | |
| λFLC (< 17 mg/L) | Positive | 0 (0%) | 17 (59%) | 25 (61%) | 0 (0%) |
| Negative | 15 (100%) | 12 (41%) | 16 (39%) | 15 (100%) | |
| (κ+λ) FLC (< 40 mg/L) | Positive | 0 (0%) | 26 (90%) | 36 (88%) | 0 (0%) |
| Negative | 15 (100%) | 3 (10%) | 5 (12%) | 15 (100%) | |
| MAB (< 40 mg/L) | Positive | 0 (0%) | 15 (52%) | 23 (56%) | 5 (33%) |
| Negative | 15 (100%) | 14 (48%) | 18 (44%) | 10 (67%) | |
| μ2-M (< 200 μg/L) | Positive | 0 (0%) | 24 (83%) | 34 (83%) | 0 (0%) |
| Negative | 15 (100%) | 5 (17%) | 7 (17%) | 15 (100%) | |
| RBP (< 0.56 mg/L) | Positive | 0 (0%) | 25 (86%) | 32 (78%) | 1 (7%) |
| Negative | 15 (100%) | 4 (14%) | 9 (22%) | 14 (93%) | |
| KIM-1 (< 2.15 μg/L) | Positive | 0 (0%) | 1 (3%) | 7 (17%) | 2 (13%) |
| Negative | 15 (100%) | 28 (97%) | 34 (83%) | 13 (87%) | |
| NGAL (< 5.3 μg/L) | Positive | 0 (0%) | 16 (55%) | 18 (44%) | 10 (67%) |
| Negative | 15 (100%) | 13 (45%) | 23 (56%) | 5 (33%) | |
Data are expressed as number (percentage)
The highest urine concentration in Normal group is used as the upper “normal limit” for each biomarker (left column)
The sensitivity and specificity of each individual biomarker in detecting AKAI in the AR group is summarized in Table 3. The Control group is used as the reference group for comparison in the Fisher's exact analysis. Urine κFLC performs best in both sensitivity (95.12%) and specificity (87.5%), which is followed by total (κ+ λ) FLC, then β2-M and RBP. Urine KIM-1 performs worst. Neither NGAL nor MAB significantly detects AR. The sensitivity and specificity of each biomarker in detecting AKAI in the ATN group are summarized in Table 4. The Control group is used as the reference group for comparison. Urine κFLC also performs best in both sensitivity (96.55%) and specificity (93.33%), followed by total (κ+ λ) FLC, then RBP and β2-M. Urine KIM-1, NGAL and MAB do not significantly detect ATN.
Table 3.
Performance of urinary markers in acute rejection
| AR group vs Control group | Specificity (%) | Sensitivity (%) | Likelihood Ratio | Odds Ratio | p-Value |
|---|---|---|---|---|---|
| κFLC | 87.50 | 95.12 | 7.80 | 273.00 | < 0.0001 |
| λFLC | 48.39 | 60.98 | 1.94 | 47.91 | < 0.0001 |
| (κ+λ)FLC | 75.00 | 87.80 | 4.00 | 205.70 | < 0.0001 |
| MAB | 35.71 | 56.10 | 1.28 | 2.56 | 0.2270 |
| β2-M | 68.18 | 82.93 | 3.14 | 142.60 | < 0.0001 |
| RBP | 60.87 | 78.05 | 2.48 | 49.78 | < 0.0001 |
| KIM-1 | 27.66 | 17.07 | 1.08 | 1.34 | 1.0000 |
| NGAL | 17.86 | 43.90 | 0.78 | 0.39 | 0.2270 |
Fisher's exact test with 95% confidence interval.
Table 4.
Performance of urinary markers in acute tubular necrosis
| ATN group vs Control group | Specificity (%) | Sensitivity (%) | Likelihood Ratio | Odds Ratio | p-Value |
|---|---|---|---|---|---|
| κFLC | 93.33 | 96.55 | 14.48 | 392.00 | < 0.0001 |
| λFLC | 55.56 | 58.62 | 2.25 | 43.40 | < 0.0001 |
| (κ+λ)FLC | 83.33 | 89.66 | 6.00 | 234.70 | < 0.0001 |
| MAB | 41.67 | 51.72 | 1.29 | 2.14 | 0.3420 |
| β2-M | 75.00 | 82.76 | 4.00 | 138.10 | < 0.0001 |
| RBP | 77.78 | 86.21 | 4.33 | 87.50 | < 0.0001 |
| KIM-1 | 31.71 | 3.45 | 0.49 | 0.234 | 0.2643 |
| NGAL | 27.78 | 55.17 | 0.85 | 0.62 | 0.5315 |
Fisher's exact test with 95% confidence interval.
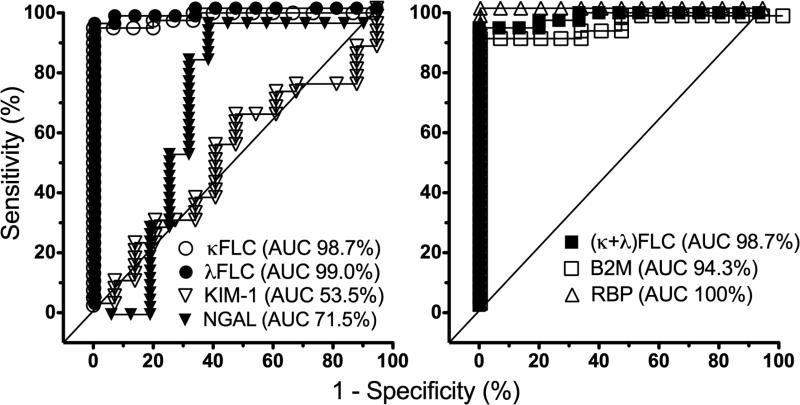
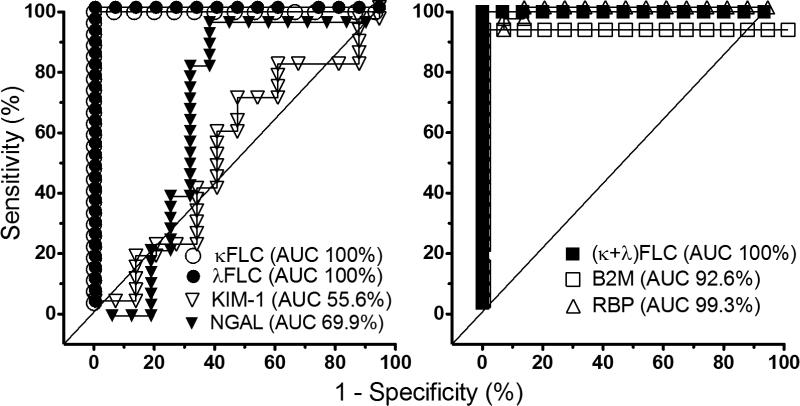
The AUC in the ROC analysis of each biomarker for AR is shown in Figure 2. The AUC is greatest in urinary RBP (100%), followed by κFLC, λFLC and (κ+ λ) FLC (about 99%). Urine KIM-1 has the lowest AUC (53.5%), then NGAL (71.5%). The AUC in the ROC analysis of biomarkers for ATN is shown in Figure 3. The AUC is greatest in urinary FLC, either κFLC, λFLC or (κ+ λ) FLC (100%), which is followed by RBP (99%). Again, urine KIM-1 has the lowest AUC (55.6%), then NGAL (69.9%).
Figure 2.
The AUC in the ROC analysis of biomarkers for AR. The AUC is greatest in urinary FLC and RBP, and lowest in urinary KIM-1 then NGAL
Figure 3.
The AUC in the ROC analysis of biomarkers for ATN. The AUC is greatest in urinary FLC and RBP, and lowest in KIM-1 then NGAL.
Discussion
Immunoglobulin FLCs are small proteins with molecular weight of ~ 25 kD. They are freely filtered by the glomeruli and taken up by the proximal tubule cells via a receptor mediated pathway involving megalin and cubilin (18-20). In normal individuals, only about 1-10 mg of FLC is excreted every 24 hours in the urine. In patients without myeloproliferative disorders, increased urinary excretion of FLC reflects renal tubular injury (20,21). To our knowledge, this is the first pilot study testing urine FLC as a potential biomarker for AKAI in transplant patients.
Among the other tested biomarkers in our study, β2-M and RBP are also low-molecular-weight proteins (12,16,17). MAB historically thought to reflect the injured glomerular basement membrane, has now been shown to reflect alterations in the proximal tubular cell retrieval pathway(22). KIM-1 and NGAL are proteins specifically produced in response to AKI by the kidney. KIM-1 is a type-1 cell membrane glycoprotein receptor on renal epithelial cells, which may be related to tubular dedifferentiation and regeneration (9,13). NGAL is a 25 kD gelatinase-bound protein initially characterized in neutrophils, and late found in kidney and other tissues (11,14). Both KIM-1 and NGAL have been extensively studied for diagnosing AKI and for predicting severity and outcome of AKI in native kidneys (4,15). However, there is a weakness in these studies because of the use of serum Cr-defined AKI as the standard for injury (5,8,23). It is well known that AKI cases from pre-renal azotemia without tubular injury will not increase the AKI biomarkers. Also, patients with renal tubular damage and increased AKI biomarker may not develop serum Cr defined AKI. Therefore, the approach to AKI biomarker validation may be questioned by this limitation of serum Cr-defined AKI in some studies.
The current standard of care in diagnosing graft dysfunction calls for performance of kidney biopsy to establish the cause of AKAI. This clinical setting provides a unique opportunity to examine the utility of urinary biomarkers. In this study, we use kidney biopsy confirmed AR and ATN to study the biomarkers. Our results suggest that urine FLC, either κ, λ, or both, may be a novel biomarker for AKAI. Both AR and ATN groups have significantly higher urinary excretion of FLC than either Control group or Normal group. Compared to the Control and Normal groups, urine KIM-1 and NGAL did not reflect AKAI in either AR group or ATN group. In the AR group, urinary κFLC demonstrated the highest predictive value with sensitivity of 95.12% and specificity of 87.5 % (p<0.0001). In the ATN group, urine κFLC also exhibited the highest predictive value with sensitivity of 96.55% and specificity of 93.33 % (p<0.0001). AUC by ROC analyses further support that urine FLC is a more sensitive and specific AKAI biomarker than KIM-1 and NGAL in both AR and ATN groups.
Interestingly, KIM-1 and NGAL, the two most extensively investigated AKI biomarkers in native kidneys did not perform well in our study. Our study was not designed to investigate their use in the entire spectrum of AKAI. The setting and development of AKI are different between native kidneys and transplanted ones. The model of ischemia-associated AKI is usually used to study the biomarker in native kidneys. In transplant patients, the quality of donor kidney determines a baseline kidney function, which may not be normal. Over time, allograft function may slowly deteriorate from developing CAN, to which numerous immunologic and/or non-immunological insults contribute (24,25). So, the AKAI may develop on top of some degree of preexisting condition or CAN. This is demonstrated in our Control group. None in the Control group had any history of AKAI and all of them have normal serum Cr levels since transplant surgeries. But 5 of 15 (33%) patients in Control group have MAB. This suggests the presence of mild CAN in some of these patients, as we did not perform kidney biopsy to select patients for the Control group.
The mechanism of developing AKAI is also different in transplant patients from the AKI in native kidneys. In the cases of AR, immunological rather than ischemic injury predominates. Even in the cases of ATN, calcineurin inhibitor toxicity and compromised renal perfusion are the predominant causes in transplant patients. Different biomarkers have different patterns of elevation and decline during the course of AKI, and these patterns may not be the same in AKAI as in AKI. Transplant patients receive immunosuppressive medications, typically a combination of steroid, mycophenolic acid and a calcineurin inhibitor (either tacrolimus or cyclosporine) in our center. One or more immunosuppressive drugs may alter the expression and/or secretion of biomarkers. It is not known whether AKI biomarkers for native kidneys are equally useful in kidney transplant patients. Even the well-studied AKI biomarker NGAL and KIM-1 have not been verified in transplant recipients yet.
Hollmen et al reported that urinary NGAL level on post-transplant day 1 could predict prolonged delayed graft function (DGF) and worse 1-year graft survival in deceased donor kidney transplant (26). Serum NGAL level was shown as a predictive biomarker for DGF recovery after kidney transplant from donors after cardiac death (27). Schröppel et al studied KIM-1expression in preperfusion biopsies of both living and deceased donor kidneys. They found that tubular expression of KIM-1 was upregulated in deceased compared to living donor kidneys, but it failed to identify the allograft at risk for developing DGF after kidney transplant (28). Hall et al compared urinary NGAL, IL-18 and KIM-1 in the first 3 days after deceased donor kidney transplant. They found that only NGAL and IL-18, not KIM-1, predicted the need of dialysis within the first week of transplant as well as the 3-month recovery of graft function (29). It remains to be determined whether AKI biomarker can predict the development of AKAI rather than DGF in the posttransplant patients.
There has been great interest in searching for “immunological” biomarkers to predict AR in kidney transplant patients (30-36). Reported urine proteomics and biomarkers include HLA-DR, CXCL9, CXCL 10, mRNA levels of grazyme B, perforin, TIM-3 and FOXP3, and others (30-33). Enhanced expression of perforin, granzyme B, Fas ligand, HLA class-1 antigens in peripheral blood leukocytes or tissue have been linked to AR (34,35). As reviewed recently, current available information remains inconclusive and better designed multicenter studies are required to validate these “rejection–specific” biomarkers for clinical practice (36,37). Our study indicates that urine FLC predicts both AR and ATN. It does not differentiate AR from ATN. This is likely due to the fact that both AR and ATN are associated with significant tubular damage. An ideal AKAI biomarker would be one that can differentiate AR from ATN without the need for kidney biopsy. Recently, a three-gene signature of CD3ε mRNA, IP-10 mRNA and 18S rRNA levels in urinary cells has been reported to be diagnostic and prognostic of acute cellular rejection in kidney transplant grafts (38). It appears that this signature not only distinguishes acute cellular rejection from no rejection, but also separates acute cellular rejection from acute antibody mediated rejection and borderline rejection. It will be interesting to test whether the combination of urinary FLC with the three-gene mRNA levels can improve the diagnostic value for graft rejection. It is hoped that with further study of urine FLC in the development of different types of AKAI, we may further characterize the potential of urine FLC in predicting early AKAI before the rising of serum creatinine as well as in distinguishing the causes of AKAI, such as AR from ATN or graft infection.
This study is a pilot investigation of urine FLC as a potential AKI biomarker. It is limited by single center data, relative small sample size, highly selected patients, and lack of kidney biopsy in the Control group. Our data suggests that urinary excretion of FLC may be a novel biomarker of AKAI in kidney transplant patients. Further large studies are needed to investigate this potential. It will also be interesting to see whether urine FLC is a useful biomarker for DGF immediately after kidney transplant as well as for AKI of native kidneys.
Acknowledgments
This research was supported in part by Francisco M Gonzalez Research Fund of National Kidney Foundation of Louisiana, Akira Arimura Foundation, The Binding Site Inc and The DCI. We thank Dr. Shanker Japa for the measurement of urine free light chains.
Abbreviations
- ATN
acute tubular necrosis
- AKAI
acute kidney allograft injury
- AR
acute rejection
- β2-M
β-2 microglobulin
- CAN
chronic allograft nephropathy
- eGFR
estimated glomerular filtration rate
- FLC
free light chains
- MAB
microalbuminuria
- RBP
retinol-binding protein
- KIM -1
kidney injury molecule 1
- NGAL
neutrophil gelatinase associated lipocalin
- ESRD
end stage renal disease
- HTN
hypertension
- DM
diabetes mellitus
- GN
glomerulonephritis
- PRA
panel reactive antibody
- HLA
human leukocyte antigen
Footnotes
Conflict of Interest: all authors have no conflict of interest to report
Rubin Zhang – participated in research design, collection of data, data analysis and writing of paper
Min Li – participated in collection of data and data analysis
Kanwaljit K. Chouhan – Participated in collection of data
Eric E. Simon - participated in research design
L. Lee Hamm - participated in research design
Vecihil Batuman - participated in research design, data analysis and writing of paper
References
- 1.Nankivell BJ, Fenton-Lee CA, Kuypers DR, Cheung E, Allen RD, O'Connell PJ, Chapman JR. Effect of histological damage on long-term kidney transplant outcome. Transplant. 2001;71(4):515–23. doi: 10.1097/00007890-200102270-00006. [DOI] [PubMed] [Google Scholar]
- 2.Nickerson P, Jeffery J, Gough J, McKenna R, Grimm P, Cheang M, Rush D. Identification of clinical and histopathologic risk factors for diminished renal function 2 years posttransplant. J Am Soc Nephrol. 1998;9(3):482–7. doi: 10.1681/ASN.V93482. [DOI] [PubMed] [Google Scholar]
- 3.Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002;346(8):580–90. doi: 10.1056/NEJMra011295. [DOI] [PubMed] [Google Scholar]
- 4.Khan E, Batuman V, Lertora JJ. Emergence of biomarkers in nephropharmacology. Biomark Med. 2010;4(6):805–14. doi: 10.2217/bmm.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol Dial Transplant. 2009;24:3263–3265. doi: 10.1093/ndt/gfp428. [DOI] [PubMed] [Google Scholar]
- 6.Huraib S, Goldberg H, Katz A, Cardella CJ, deVeber GA, Cook GT, Uldall PR. Percutaneous needle biopsy of the transplanted kidney: technique and complications. Am J Kidney Dis. 1989;14(1):13–7. doi: 10.1016/s0272-6386(89)80087-3. [DOI] [PubMed] [Google Scholar]
- 7.Beckingham IJ, Nicholson ML, Bell PR. Analysis of factors associated with complications following renal transplant needle core biopsy. Br J Urol. 1994;73(1):13–5. doi: 10.1111/j.1464-410x.1994.tb07449.x. [DOI] [PubMed] [Google Scholar]
- 8.Coca SG, Parikh CR. Urinary biomarkers for acute kidney injury: perspectives on translation. Clin J Am Soc Nephrol. 2008;3:481–90. doi: 10.2215/CJN.03520807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 10.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43(3):405–14. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 12.Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, Grenet O, Pantano S, Moulin P, Wahl D, Mahl A, End P, Staedtler F, Legay F, Carl K, Laurie D, Chibout SD, Vonderscher J, Maurer G. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol. 2010;28(5):463–9. doi: 10.1038/nbt.1622. [DOI] [PubMed] [Google Scholar]
- 13.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118(5):1657–68. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaspari F, Cravedi P, Mandalà M, Perico N, de Leon FR, Stucchi N, Ferrari S, Labianca R, Remuzzi G, Ruggenenti P. Predicting cisplatin-induced acute kidney injury by urinary neutrophil gelatinase-associated lipocalin excretion: a pilot prospective case-control study. Nephron Clin Pract. 2010;115(2):c154–60. doi: 10.1159/000312879. [DOI] [PubMed] [Google Scholar]
- 15.Lisowska-Myjak B. Serum and urinary biomarkers of acute kidney injury. Blood Purif. 2010;29:357–65. doi: 10.1159/000309421. [DOI] [PubMed] [Google Scholar]
- 16.Valette JP, Nicot G, Charmes JP, Merle L, Benevent D, Leroux-Robert C. Low molecular weight proteins as urinary markers of aminoglycoside nephrotoxicity in man. Proc Eur Dial Transplant Assoc. 1979;16:597–602. [PubMed] [Google Scholar]
- 17.Bernard AM, Vyskocil AA, Mahieu P, Lauwerys RR. Assessment of urinary retinol binding protein as an index of proximal tubular injury. Clin Chem. 1987;33:775–9. [PubMed] [Google Scholar]
- 18.Batuman V, Verroust PJ, et al. Myeloma light chains are ligands for cubilin (gp280) Am J Physiol. 1998;275(2 Pt 2):F246–254. doi: 10.1152/ajprenal.1998.275.2.F246. [DOI] [PubMed] [Google Scholar]
- 19.Klassen RB, Allen PL, et al. Light chains are a ligand for megalin. J Applied Physiol. 2005;98(1):257–263. doi: 10.1152/japplphysiol.01090.2003. [DOI] [PubMed] [Google Scholar]
- 20.Batuman V. Proximal tubular injury in myeloma. Contributions to Nephrology. 2007;153:87–104. doi: 10.1159/000096762. [DOI] [PubMed] [Google Scholar]
- 21.Hutchison CA, Batuman V, et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nature Reviews: Nephrology. 2011;8(1):43–51. doi: 10.1038/nrneph.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 2007;71(6):504–13. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 23.Waikar SS, Betensky RA, Emerson SC, et al. Imperfect gold standards for kidney injury biomarker evaluation. Clin J Am Soc Nephrol. 2012;23:13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan E, Zhang R, Simon E, Hamm LL. Alloimmune injury in chronic allograft nephropathy. In: Monika G, editor. Chronic Kidney Disease. InTeck; 2012. pp. 401–414. ISBN 978-953-51-0171-0. [Google Scholar]
- 25.Zhang R, Kumar P, Ramcharan T, Reisin E. Kidney transplantation: the evolving challenges. Am J Med Sci. 2004;328(3):156–161. doi: 10.1097/00000441-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Hollmen ME, Kyllönen LE, Inkinen KA, Lalla ML, Salmela KT. Urine neutrophil gelatinase-associated lipocalin is a marker of graft recovery after kidney transplantation. Kidney Int. 2011;79(1):89–98. doi: 10.1038/ki.2010.351. [DOI] [PubMed] [Google Scholar]
- 27.Kusaka M, Kuroyanagi Y, Mori T, et al. Serum NGAL as a predictor of organ recovery form delayed graft function after kidney transplantation from donors after cardiac death. Cell Transplant. 2008;17:129–134. doi: 10.3727/000000008783907116. [DOI] [PubMed] [Google Scholar]
- 28.Schröppel B, Krüger B, Walsh L, Yeung M, Harris S, Garrison K, Himmelfarb J, Lerner SM, Bromberg JS, Zhang PL, Bonventre JV, Wang Z, Farris AB, Colvin RB, Murphy BT, Vella JP. Tubular expression of KIM-1 does not predict delayed function after transplantation. J Am Soc Nephrol. 2010;21(3):536–42. doi: 10.1681/ASN.2009040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21:189–197. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li B, Hartono C, Ding R, Sharma VK, Ramaswamy R, Qian B, Serur D, Mouradian J, Schwartz JE, Suthanthiran M. Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med. 2001;344(13):947–54. doi: 10.1056/NEJM200103293441301. [DOI] [PubMed] [Google Scholar]
- 31.O'Riordan E, Orlova TN, Mei JJ, Butt K, Chander PM, Rahman S, Mya M, Hu R, Momin J, Eng EW, Hampel DJ, Hartman B, Kretzler M, Delaney V, Goligorsky MS. Bioinformatic analysis of the urine proteome of acute allograft rejection. J Am Soc Nephrol. 2004;15(12):3240–8. doi: 10.1097/01.ASN.0000145241.83482.68. [DOI] [PubMed] [Google Scholar]
- 32.Renesto PG, Ponciano VC, Cenedeze MA, et al. High expression of Tim-3 mRNA in urinary cells from kidney transplant recipients with acute rejection. Am J Transplant. 2007;7:1661–6. doi: 10.1111/j.1600-6143.2007.01795.x. [DOI] [PubMed] [Google Scholar]
- 33.Metzger J, Chatzikyrkou C, Broecker V, Schiffer E, Jaensch L, Iphoefer A, Mengel M, Mullen W, Mischak H, Haller H, Gwinner W. Diagnosis of subclinical and clinical acute T-cell-mediated rejection in renal transplant patients by urinary proteome analysis. Proteomics Clin Appl. 2011;5:322–33. doi: 10.1002/prca.201000153. [DOI] [PubMed] [Google Scholar]
- 34.Heidt S, San Segundo D, Shankar S, et al. Peripheral blood sampling for the detection of allograft rejection: biomarker identification and validation. Transplant. 2011;92:1–9. doi: 10.1097/TP.0b013e318218e978. [DOI] [PubMed] [Google Scholar]
- 35.Flechner SM, Kurian SM, Head SR, et al. Kidney transplant rejection and tissue injury by gene profiling of biopsies and peripheral blood lymphocytes. Am J Transplant. 2004;4:1475–9. doi: 10.1111/j.1600-6143.2004.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohan DJ, Vella JP. Transplantation: acute rejection. NephSap. 2011;10(6):561–563. [Google Scholar]
- 37.Bestard O, Cruzado JM, Franquesa ML, Grinyo JM. Biomarkers in renal transplantation. Current Opinion in Organ Transplant. 2010;15:467–473. doi: 10.1097/MOT.0b013e32833b9ccb. [DOI] [PubMed] [Google Scholar]
- 38.Suthanthiran M, Schwartz JE, Ding R, et al. Urinary-cell mRNA and acute cellular rejection in kidney allografts. N Engl J Med. 2013;369:20–31. doi: 10.1056/NEJMoa1215555. [DOI] [PMC free article] [PubMed] [Google Scholar]