Abstract
Background
Air pollution, especially emissions derived from traffic sources, is associated with adverse cardiovascular outcomes. However, it remains unclear how inhaled factors drive extrapulmonary pathology.
Objectives
Previously, we found that canonical inflammatory response transcripts were elevated in cultured endothelial cells treated with plasma obtained after exposure compared with pre-exposure samples or filtered air (sham) exposures. While the findings confirmed the presence of bioactive factor(s) in the plasma after diesel inhalation, we wanted to better examine the complete genomic response to investigate 1) major responsive transcripts and 2) collected response pathways and ontogeny that may help to refine this method and inform the pathogenesis.
Methods
We assayed endothelial RNA with gene expression microarrays, examining the responses of cultured endothelial cells to plasma obtained from 6 healthy human subjects exposed to 100 μg/m3 diesel exhaust or filtered air for 2 h on separate occasions. In addition to pre-exposure baseline samples, we investigated samples obtained immediately-post and 24h-post exposure.
Results
Microarray analysis of the coronary artery endothelial cells challenged with plasma identified 855 probes that changed over time following diesel exhaust exposure. Over-representation analysis identified inflammatory cytokine pathways were upregulated both at the 2 and 24 h condition. Novel pathways related to FOX transcription factors and secreted extracellular factors were also identified in the microarray analysis.
Conclusions
These outcomes are consistent with our recent findings that plasma contains bioactive and inflammatory factors following pollutant inhalation. The specific study design implicates a novel pathway related to inflammatory blood borne components that may drive the extrapulmonary toxicity of ambient air pollutants.
BACKGROUND
Air pollution, especially particulate matter (PM), is strongly correlated with the risk of death due to cardiovascular disease (Pope 1989, Dockery, Pope et al. 1993, Brook, Rajagopalan et al. 2010). Ambient PM concentrations associate with the overall risk of cardiovascular disease and it has been estimated that every 10 μg/m3 increase in PM increases cardiovascular disease risk by 0.6-1.1% (Le Tertre, Medina et al. 2002, Omori, Fujimoto et al. 2003, Analitis, Katsouyanni et al. 2006, Ostro, Broadwin et al. 2006, Zanobetti and Schwartz 2009). Additionally, acute cardiovascular events have been linked to PM exposures occurring just hours before myocardial infarction and inhaled toxicants represent a major proportion of events that can trigger acute myocardial infarctions (Nawrot et al, 2011). In the United States, around 25% of the mass of outdoor air pollution is comprised of diesel exhaust-derived PM, and diesel exhaust particle (DEP) levels are generally less than 3 μg/m3 ((EPA) 2002). However, much higher levels can be observed in “hotspots” or in occupational settings, with measured concentrations in excess of 200 μg/m3 (Shih, Lai et al. 2008, Zhang, Duan et al. 2014). With several recent analyses identifying traffic exposure as a major risk for triggering non-lethal myocardial infarction, understanding the pathophysiological mechanisms of combustion emission systemic toxicity remains an important knowledge gap that may help identify at-risk populations (Nawrot, Perez et al. 2011).
Our recent studies have noted changes in circulating bioactivity following exposure to various inhaled pollutants. The nature of this plasma- and serum-borne bioactivity remains poorly understood, in terms of the relevant compositional changes and the breadth of downstream responses; however, the endothelium, with homeostatic roles for vasodilation, vascular inflammation, and platelet aggregation, is the most clear intermediate target (Knuckles, Lund et al. 2008, Cherng, Paffett et al. 2011, Campen 2012). Following exposure to diesel exhaust or a major component thereof, nitrogen dioxide, healthy humans exhibited changes in the plasma bioactivity that led to increased expression of inflammatory adhesion molecules and chemokines in cultured endothelial cells (Channell, Paffett et al. 2012). Furthermore, serum from mice exposed to ozone (O3) or fresh engine emissions (combined diesel and gasoline) was capable of inhibiting endothelial-dependent vasodilation in vessels from unexposed mice (Robertson, Colombo et al. 2013, Campen, Robertson et al. 2014). The observed mechanistic role for CD36, a multi-ligand scavenger receptor, suggests that more than a single factor in the serum or plasma may be responsible for pathophysiological effects on systemic endothelial cells (Robertson, Colombo et al. 2013).
An important limitation of earlier studies on serum or plasma bioactivity was that, while using endothelial cells as biosensors of the whole serum or plasma “exposome” enable a holistic, functional capture of cumulative systemic inflammatory potential, the selection of gene/protein measures used to assess endothelial cell response was biased by a priori assumptions from canonical inflammatory pathways. The present study utilized the RNA from those prior studies in a straightforward genomic assessment to characterize the ontological nature of the whole genome response and examine the relative difference between immediate (2h post) and delayed (24h post) plasma bioactivity from healthy humans exposed to diesel emissions.
METHODS
Exposures
All human studies were approved by the EPA Institutional Review Board. Work on de-identified plasma samples was approved as a research exemption by the University of New Mexico Human Research Protections Office. RNA and plasma samples were banked from previous studies and exposures are described in detail elsewhere (Sobus, Pleil et al. 2008, Channell, Paffett et al. 2012). Healthy human volunteers (n = 6) were exposed to filtered air or diesel engine exhaust (DEE) at a level of 100 μg PM/m3 for two hours with periodic exercise on separate occasions separated by at least two weeks and in random order. DEE was generated by a Cummins engine operating at idle conditions using a certified commercial #2 fuel (ChevronPhillips). Idling conditions were specifically chosen to reflect urban near-roadway scenarios wherein pedestrians may have close contact with fresh emissions. Major components of the diesel exhaust atmosphere were quantified as follows: 106 ± 9 μg PM/m3; 4.7 ppm NOX; 0.8 ppm NO2; 2.8 ppm CO; and 2.4 ppm total hydrocarbons; the mass mean aerodynamic diameter of PM was 0.10 μm (Sobus, Pleil et al. 2008, Channell, Paffett et al. 2012). Plasma was obtained before (T0), after the two-hour exposure (T2), and 24 hours after exposures (T24).
Endothelial Cell Culture and RNA Purification
Cryopreserved primary human coronary artery endothelial cells (hCAECs) were obtained from a commercial supplier (Lonza) and maintained according to manufacturer's recommendations with complete microvascular endothelial growth medium-2 (EGM-2 MV) supplemented with 5% fetal bovine serum and antibiotics (gentamycin and amphotericin-B). After the fourth passage of the cells, hCAECs were plated on 48-well plates, grown to confluence, and serum-starved overnight. The plasma isolated from the volunteers was diluted to a final concentration of 10% with serum-free EGM-2 MV and added to the cells. 24 hours after the addition of conditioned media, the cells were washed with phosphate-buffered saline and total RNA was isolated from two identically treated wells per condition using RNeasy Mini Prep Kits (Qiagen). Isolation of RNA was conducted as previously described.
Global Gene Expression Analysis
440 ng total RNA was used for in vitro transcription, overnight for 14h using biotin-11-dUTP for labeling of the cRNA product using the Illumina TotalPrep RNA amplification kit (Ambion). The cRNA yields were quantified with a spectrophotometer and the labeled cRNA (500 ng) was hybridized to HumanHT-12 V4 BeadChip™ arrays (Illumina) containing 47,231 transcripts (targeting approximate 31,000 annotated genes) at 55 °C overnight following staining with 1 μg/mL streptavidin-Cy3 (Amersham Biosciences) for visualization. Washing of the arrays was performed using Illumina high-stringency wash buffer for 30 min at 55 °C, followed by scanning according to standard Illumina protocols. Data for probe intensity and detection were captured with Illumina BeadStudio software using background correction and samples were quantile normalized (Schmid, Baum et al. 2010) and mean centered per chip (Kitchen, Sabine et al. 2010). Quality standards for hybridization, staining, labeling, and background signal, along with basal level of housekeeping gene expression for each chip were confirmed. The microarray data are deposited in NCBI's Gene Expression Omnibus and are accessible through GEO series accession number GSE63095.
Statistics
To identify sources of variation across all samples we used a 4-way ANOVA model using Method of Moments (Eisenhart 1947) including subject, condition, and time as factors, with chip number as a random effect. To identify patterns of gene expression that associate with diesel exposure, we used a 3-way ANOVA linear model on the diesel exposure subset including subject and time as factors, with chip number as a random effect: Yijkl = μ + Chip numberi+ Timej + Subject(Chip number)ik + εijkl. γijkl represents the lth observation on the ith Chip number jth Time kth Subject. μ is the common effect for the whole experiment. εijkl represents the random error present in the lth observation on the ith Chip jth Time kth Subject. The errors εijkl are assumed to be normally and independently distributed with mean 0 and standard deviation δ for all measurements. Additional statistical tests were performed as indicated in the methods, figure legends, and table legends.
RESULTS
Subject demographics, study design, and circulating lipids
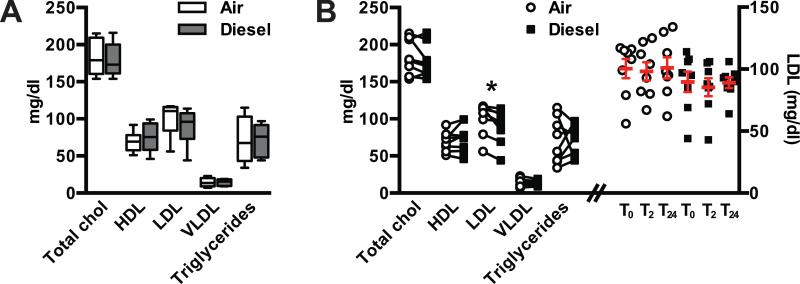
In order to study the transcriptional effects of diesel engine exhaust on coronary endothelial cell gene expression, plasma was obtained from a cohort of healthy individuals (Table 1) before, immediately after 2 h exposure, and 24 h after exposure to either filtered air or diesel engine exhaust (Fig. 1). The exposure conditions were randomized and a minimum of four weeks of time passed between either condition. Given the effect of circulating blood lipids on gene expression in cultured cells (Norata, Pirillo et al. 2003, Norata, Grigore et al. 2006) we measured lipids in the plasma samples obtained at baseline (T0) prior to either the filtered air or diesel exhaust exposure. There was no difference in total cholesterol, HDL, VLDL, or triglycerides (Fig. 2A, 2B) at T0 for either exposure on a per-subject basis using a paired t-test (p > 0.05). On average, LDL cholesterol was 11% lower (p = 0.01) in baseline samples when subjects were sampled prior to diesel exhaust exposure compared to the filtered air exposure (Fig. 2A, 2B). Given our primary comparison in this study is the effect of plasma from subjects post exposure over time, we measured the LDL levels across all three points using repeated measures ANOVA or by linear contrast and did not find any differences within each exposure (air and diesel), suggesting that time-dependent changes in gene expression are likely independent of LDL levels (Fig. 2B).
Table 1.
Subject demographics
| Study Subjects (n = 8) | Mean ± SEM |
|---|---|
| Age (y) | 24.9 ± 1.6 |
| Gender (Male/Female) | 3/5 |
| Ancestry (AA/CAU) | 2/6 |
| BMI (kg/m2) | 23.9 ± 1.9 |
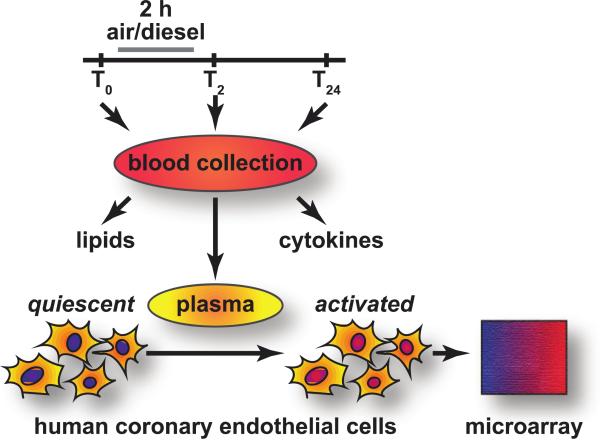
Figure 1. Exerimental design.
Blood was collected from subjects (T0) prior to exposure to either filtered air or diesel particles for two hours after which another blood sample was obtained (T2). The subjects then returned the next day for the final blood collection (T24). Lipid and cytokines were measured from each blood sample and plasma was extracted and frozen. After all samples were collected, the each plasma sample was to the culture media of human coronary endothelial cells.
Figure 2. Blood lipid profiles.
Total cholesterol (chol), high (HDL), low (LDL), and very low (VLDL) density lipoproteins, and triglycerides measured from blood samples. (A) grouped data from baseline samples represented by box and whiskers representing the min to max or (B, left) paired baseline samples (* p = 0.01 paired t-test comparing air to diesel conditions). LDL measured in each sample per time point in either the air or diesel exposure arms (open circle or filled square, respectively) and represented by the mean ± SEM (B, right).
Diesel exhaust exposure did not affect plasma concentrations of IL-6 or TNF-α
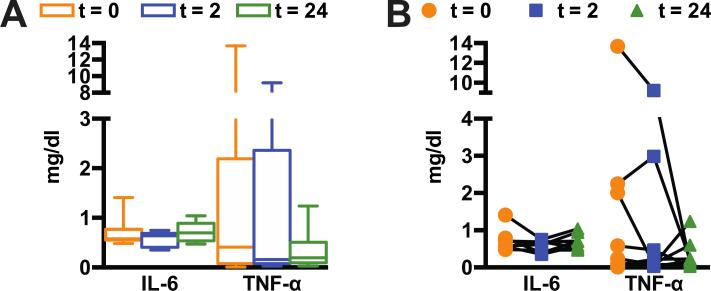
Previous studies on human exposure to diesel exhaust concluded that elevations of circulating cytokines are unlikely to be observed in a healthy individual exposed to diesel exhaust (Mills, Tornqvist et al. 2005). To confirm this in the present study, we measured circulating IL-6 and TNF-α levels in plasma samples before and after subjects were exposed to diesel exhaust. As anticipated, we did not observe any exposure-related change in either IL-6 or TNF-α analyzed by repeated measures ANOVA or by linear contrast (Fig. 3A, 3B) suggesting that IL-6 and TNF-α are not affected by the diesel exhaust exposures used in this study. Because these prototypical inflammatory markers were unchanged, we applied an unbiased, genomic approach to identify endothelial molecular signatures that may identify candidate pathways and mediators involved in the response to plasma.
Figure 3. Cytokine levels.
Interlukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) measured from blood samples at each collection time point from the diesel exposure arm. (A) grouped data from subjects represented by box and whiskers representing the min to max or (B) by individual.
Unsupervised analysis of the transcriptome identifies study subject as the primary source of the variance in differential gene expression
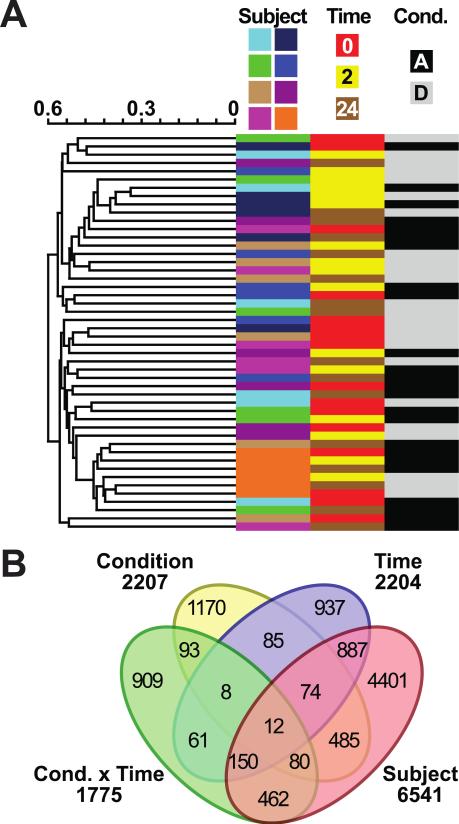
To identify changes in gene expression in coronary endothelial cells that correlate with diesel exposure, the plasma isolated at each collection point during the filtered air and diesel exhaust exposure was added to the culture medium of a single source of human coronary endothelial cells (Fig. 1). After 24 hours of culturing with collected plasma, the RNA from the coronary endothelial cells was purified and gene expression levels were measured using high-density BeadArray technology. The initial set of probes (47,231) was first filtered by removing probes identified as absent in ≥50% of samples per subject in at least two out of the eight subjects, reducing the number of detectable probes to 27,372 corresponding to 21,209 unique genes. Surprisingly, unsupervised hierarchical clustering (Fig. 4A) and ANOVA linear modeling (Fig. 4B) identified the study subjects as the primary sources of variance. These data suggest that subject-to-subject plasma components have a strong effect on endothelial gene expression and in our study, correlates to an effect three times greater than diesel exhaust exposure.
Figure 4. Global transcriptome analysis.
Probes that passed quality control (27,372) analyzed via (A) unsupervised hierarchical clustering of all arrays (represented by rows). Annotations of the array samples are represented by columns including subject number, time, or condition (Cond.) represented by “A” air exposure or “D” diesel exposure. (B) ANOVA linear modeling idendified differentially expressed probes per variable (Subject, Time, and Condition) including the intersection of condition and time variables (Cond. × Time). The Venn diagram indicates the overlap across the variables.
Transcriptional patterns associated with diesel exposure
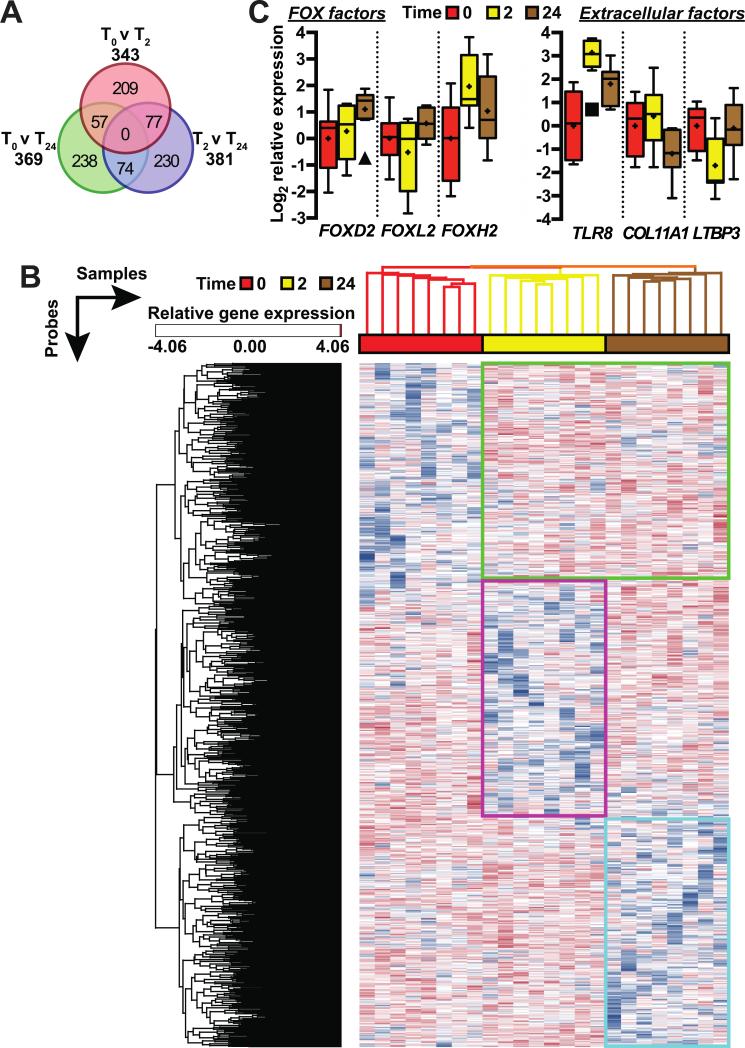
To identify patterns of gene expression that associate with diesel exposure, we used a 3-way ANOVA linear model on the diesel exposure subset (see Methods). Linear modeling identified unique 885 probes corresponding to 876 unique genes in comparing transcriptional responses of human coronary artery endothelial cells (hCAECs) cultured with plasma collected at either T0 vs. T2, T2 vs. T24, or T0 vs. T24 with an absolute fold change greater than two (p < 0.05). The overlap of each time point comparison is represented by Venn diagram (Figure 5A). Hierarchical clustering of the differentially expressed genes (Figure 5B) identified three main clusters of expression patterns: Cluster 1- persistently up-regulated genes in response to plasma obtained after either time point (T2 or T24) post diesel exhaust exposure (Fig. 5B, green), Cluster 2- down-regulated genes in response to plasma obtained at T2 that return to control exposure expression levels in cells cultured with T24 plasma (Fig. 5B, magenta), and Cluster 3- genes that change only in response to culturing with plasma obtained at T24 (Fig. 5B, cyan).
Figure 5. Identifying transcriptional patterns associated with diesel exposure.
(A) The number of differentially expressed probes in cells treated with plasma obtained in the diesel exposure arm comparing either T0 vs T2, T2 vs T24, or T0 vs T24 conditions. The overlap of each subset of probes is shown in the Venn diagram. (B) Hierarchical clustering of both the 855 probes and array samples represented by rows and columns, respectively, segreated the samples based on time and identied three main clusters of gene expression: Cluster 1 (green) are persistently up-regulated genes in cells responding to T2 or T24 diesel plasma; Cluster 2 (magenta) are down-regulated genes in cells responding to T2 diesel plasma; and Cluster 3 (cyan) are down-regulated genes in cells responding to T24 diesel plasma. (C) Relative gene expression levels of the FOX transcription factors (left) or extracellular factors (right) identified via gene expression microarrays represented by boxplot (Tukey) with the mean indicated (+) and sample outliers in the T2 or T24 groupings identified by symbols (■□ and ▲, respectively).
Pathway analysis of transcriptional changes associated with diesel exposure
To characterize the differential expression patterns, each cluster was analyzed for over-representation in biological pathways (Table 2). Interestingly the persistently up-regulated genes in hCAECs cultured with T2 and T24 plasma (Cluster 1) were enriched for pathways associated with inflammation, including cytokine-mediated inflammation, angiotensin II stimulation, and TGF-β signaling. Whereas our candidate molecule screening of inflammatory mediators such as IL-6 and TNF-α did not associate with diesel exposure, these results suggest that there may be other molecules present in the diesel-exposed plasma that elicit inflammatory responses. When we analyzed the genes that are down-regulated in response to culturing with T24 plasma (Cluster 3) we identified genes involved in acetylcholine receptor signaling associated with anti-inflammation (STX16 and CHAT) and G-protein adapter proteins (SSR2, GNAL, ADCY5), suggesting that mediators found in T24 plasma may impact anti-inflammatory signaling. In the genes that are down-regulated in response to culturing only with T2 plasma (Cluster 2) we identified only one nondescript pathway involving targets of a bZIP transcription factor. Although overrepresentation and enrichment analysis did not reveal information into the underlying biology within Cluster 2, these approaches do have limitations (Glaab, Baudot et al. 2012) and as such, other approaches may be used in future studies to better interpret the down-regulation of genes in hCAECs in response to acute diesel-exposed plasma. However, the return to T0 gene expression levels at T24 suggest that this cluster captures early changes in gene expression that restore to T0 levels, at least by 24 hours. Although there was limited statistical enrichment for biological pathways within Cluster 2, genes such as BTNL are suggested to exacerbate asthma (Yamazaki, Goya et al. 2010) and SELP expression/activity is a target for asthma therapy (Schumacher 2007, Takyar, Vasavada et al. 2013) and associates with diesel exposure in other models (Wauters, Esmaeilzadeh et al. 2015). Additional genes from Cluster 2 include F7, a serum prothrombin conversion accelerator, that associates with enhanced disease risk associated with smoking (Redondo, Watzke et al. 1999, Ben-Hadj-Khalifa, Lakhal et al. 2013). It should be noted that there were 53 hypothetical or non-annotated genes within this cluster that may be useful candidates for more in-depth studies.
Table 2.
Pathway enrichment
| Pathway | Genes | p | FE |
|---|---|---|---|
| Cluster 1 (n = 156) | |||
| Inflammation mediated by chemokine and cytokine signaling pathway | *ARRB2, COL12A1, *,†PLCB2, PAK4, ALOX15 | 0.027 | 3.0 |
| Angiotensin II-stimulated signaling through G proteins and beta-arrestin | *ARRB2, *,†PLCB2, | 0.029 | 7.7 |
| TGF-beta signaling pathway | *,†MAPK10, FOXH1, GDF3 | 0.031 | 4.4 |
| Integrin signaling pathway | COL12A1, MAPK10, RND1, ‡ARF6 | 0.038 | 3.2 |
| 2-arachidonoylglycerol biosynthesis | *PLCB2 | 0.042 | 25 |
| Cluster 2 (n = 136) | |||
| Transcription regulation by bZIP transcription factor | CREB3L4, GTF2A1L | 0.037 | 6.7 |
| Cluster 3 (n = 124) | |||
| Muscarinic acetylcholine receptor 2 and 4 signaling pathway | #STX16, #CHAT | 0.025 | 8.3 |
| 2-arachidonoylglycerol biosynthesis | PLA1A | 0.034 | 33 |
| Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | SSR2, #GNAL, ADCY5 | 0.042 | 3.9 |
| Pyruvate metabolism | PCK1 | 0.049 | 20 |
The PANTHER (Protein ANalysis THrough Evolutionary Relationships) classification system (Mi, Muruganujan et al. 2013) was used to classify proteins (and their genes) via curated pathway analyses that explicitly specifies the relationships between the interacting molecules (Mi and Thomas 2009). The number of annotated genes for each Cluster are provided as well as the significant pathways identified, corresponding p values, and Fold Enrichment (FE) representing the number of genes found per pathway divided by the expected number of genes predicted based on the genes present on the microarray. Additionally, genes with promoter regions that contain binding sites for the transcription factors FOXO4 (*), FOXF2 (†), POU2F (‡), and MAZ (#) are identified (see Table 3).
Enrichment for specific transcription factor binding sites in diesel-responsive genes
We extended our expression profiling by analyzing the promoter regions of genes identified in each cluster for consensus transcription factor binding sites to identify factors that may mediate exposure-dependent signals. Within the Cluster 1 genes up-regulated in with exposure to T2 and/or T24 diesel plasma we identified binding sites for three transcription factors (Table 3) two of which are from the O and F classes of winged helix/forkhead transcription factor (FOX) family, FOXO4 and FOXF2, that have binding sites in 24 and 14 genes, respectively, within the 156 annotated genes in Cluster 1 (FDR < 1%) as well as several of the genes identified in the pathway enrichment (Table 2). Interestingly, we identified three FOX transcription factors within Cluster 1 (Figure 5C). FOXD2 and FOXL2 are over 2-fold higher with exposure to T24 diesel plasma compared to T0 control and T2 diesel plasma, whereas FOXH1 is up-regulated over 2-fold with exposure to either T2 and T24 diesel plasma compared to T0 control plasma. FOX transcription factors are associated with several inflammatory pathologies including ambient air pollution-based asthma (Nadeau, McDonald-Hyman et al. 2010), heart failure (Hannenhalli, Putt et al. 2006), and chronic inflammation (Peng 2010) suggesting that members of this family of transcriptional regulators may also play a role in the acute response to diesel emission exposure. FOX transcription factors are regulated in part by phosphorylation (Schisler, Willis et al. 2008, Ronnebaum and Patterson 2010) and our analysis is limited by not knowing the phosphorylation status of the various FOX factors identified in this study; however, the increases in FOX transcription factor expression (Figure 5C) are consistent with the increase in transcription of the genes in Cluster 1 (Table 2) with FOX binding sites (Table 3).
Table 3.
Transcription factor promoter analysis
| TF motif | # genes | q |
|---|---|---|
| Cluster 1 | ||
| *FOXO4 | 24 | 1.1 e−3 |
| †FOXF2 | 14 | 3.7 e−3 |
| ‡POU2F1 | 7 | 1.6 e−2 |
| Cluster 2 | ||
| TCF3 | 26 | 6.4 e−5 |
| MEF2A | 10 | 1.9 e−2 |
| NF1 | 10 | 1.9 e−2 |
| Cluster 3 | ||
| #MAZ | 22 | 2.0 e−3 |
The promoter regions of the genes within each cluster were analyzed for significant overlaps with the collection of transcription factor targets defined in the TRANSFAC database v7.4 (Wingender, Chen et al. 2000, Wingender, Chen et al. 2001). Listed are the number of genes identified for each transcription factor that was identified as significantly enriched via the hypergeometric test at a q value less than 0.05 (false discovery rate < 5%) (Subramanian, Tamayo et al. 2005).
Extracellular factors associated with diesel exposure
We also analyzed each cluster for genes that encode products located in the extracellular region of cells, including secreted factors that may contribute to diesel-dependent signaling (Table 4). Genes spanning all three gene clusters from this analysis including TLR8, COL11A1, and LTBP3 (Figure 5C) were previously associated in other studies ranging from smoking, ozone, particulate matter, asthma, and chronic obstructive pulmonary disease (Konigshoff, Kneidinger et al. 2009, Shi, Chen et al. 2009, Bauer, Diaz-Sanchez et al. 2012, Bezemer, Sagar et al. 2012, Sancini, Farina et al. 2014). The identification of extracellular signaling molecules within each cluster suggest a dynamic role for the extracellular matrix and the regulation of secretory molecules in response to diesel exposure.
Table 4.
List of extracellular gene products from each cluster of genes
| Cluster | Extracellular Gene Products |
|---|---|
| 1 | LTBP4, COL12A1, TRL8, FCN3, MMP25, WNT6 |
| 2 | COL11A1, FRAS1, C1QTNF9B, ADAMTS7, WNT16, MMP23A |
| 3 | ADAMTSL5, LTBP3, SLIT3, WNT2B |
The PANTHER classification system (Mi, Muruganujan et al. 2013) was used to classify proteins (and their genes) via functional classification. The genes from each cluster classified in the gene ontology cellular components of either extracellular region or extracellular matrix are provided.
DISCUSSION
Alteration of blood composition leading to vascular bioactivity has been shown in both human and animal studies following controlled pollutant exposure (Channell, Paffett et al. 2012, Robertson, Colombo et al. 2013). Cultured endothelial cells have been used in many of these studies as a biosensor of cumulative inflammatory potential of the circulation. The plasma can be viewed as a major part of the “exposome” for all endothelial cells in the body, and endothelial cell homeostasis is central to vascular health. By utilizing cultured endothelial cells in this manner and probing the transcriptional responses to the plasma from diesel-exposed human subjects, we observed a panel of responses consistent with inflammation and chronic vascular disease. The present study highlights both 1) the plausibility that inflammatory blood-borne factors arise after inhalation exposures and 2) the potential for such translational ex vivo bioactivity assays to help delineate the pathophysiological impacts of pollutant exposure.
Diesel emissions have been reported to induce vascular effects that are consistent with negative health outcomes. In healthy control volunteers, diesel exposure led to a loss of dilatory response to bradykinin, acetylcholine, and sodium nitroprusside (Mills, Tornqvist et al. 2005). In contrast, coronary artery disease patients with limited vasodilatory capacity exhibited substantial electrocardiographic deviations upon exercise with diesel exhaust exposure (Mills, Tornqvist et al. 2007). Rodent studies have found that nitric oxide pathways are negatively impacted following diesel exposures, in part due to uncoupling of the endothelial nitric oxide enzyme (Knuckles, Lund et al. 2008) and intracellular oxidative stress (Cherng, Paffett et al. 2011) and also nitric oxide bioavailability may be reduced by direct scavenging (Knuckles, Buntz et al. 2011, Langrish, Unosson et al. 2013). Chronically, diesel exhaust exposure leads to progression of atherosclerotic lesions, both in terms of size and complexity (Campen, Lund et al. 2010, Bai, Kido et al. 2011, Miller, McLean et al. 2013). A key facet of diesel exposure is the vascular influx of macrophages, which can be seen as early as a week of exposures (Lund, Lucero et al. 2011). Studies suggested that this inflammatory response to diesel was mediated via the LOX-1 receptor, suggesting that blood components interacting with endothelial cells and potentially circulating leukocytes may be central to such effects (Lund, Lucero et al. 2011). Importantly, such studies collectively implicate inflammatory activation of endothelial cells as a crucial intermediate process that drives both acute and chronic disease.
The present findings suggest that plasma composition is altered by diesel exposure in a manner that activates inflammatory responses in endothelial cells, and we propose that this response reflects a cumulative inflammatory signal from numerous compositional changes in the plasma. Endothelial genomic responses were ontologically categorized via pathway enrichment as related to ligand-receptor interactions. The nature of the compositional change remains unclear, but is unlikely related to changes in inflammatory chemokines, as we found that IL-6 and TNF-α were unchanged in the present cohort. Mills and colleagues conducted a similar exposure and failed to see changes in circulating leukocytes, neutrophils, IL-6, TNF-α, CRP, or PAI-1 (Mills, Tornqvist et al. 2005). Subsequent human exposures studies confirmed this lack of effect, and added negative results for circulating CD40L, P-selectin, and ICAM-1 (Lucking, Lundback et al. 2011). Inflammation and cytokines may play an important role in the lung following pollutant exposure, in general, but systemic transfer of effects are clearly more nuanced. Complex compositional alterations in the serum are assured, given the complex chemistries of the gaseous and particulate phases of diesel emissions, as well as the secondary intermediates that may form on contact with the biological milieu. Establishing causality with the myriad potential biomolecular changes will be a major challenge for future research.
We have previously reported modest, significant changes in plasma nitrites / nitrates and plasma matrix metalloproteinase 9 concentration and activity in rodent studies, however (Lund, Lucero et al. 2009). While significant, the magnitude of such changes is unlikely to drive the biological effects seen in the present in vitro application. The value of the endothelial cell response as a biosensor lies in assessing a cumulative functional impact. Discrete assessment of chemokines or other plasma-borne factors may reveal changes in concentration – or not – but this change in concentration is rarely linked to a biological outcome. Furthermore, many other factors are left unaccounted for in all but the most thorough analytical chemistry approaches and such factors may have effects that would be predicted to offset or augment the biological impact of those measured factors. Measuring cumulative impacts of environmental exposures provides a more accurate consideration of the potential contribution to in vivo pathology.
The breadth of health-related outcomes associated with air pollution may share a common etiology related to the outcomes of the endothelial cell responses. Air pollution, especially particulate matter levels, associate with numerous inflammatory disease that frequently arise with comorbidities, such as atherosclerosis, metabolic diseases, and chronic obstructive pulmonary disease. More recently, studies have highlighted associations between various air pollutants (PM, SO2, NO2) and broader inflammatory syndromes, such as gastrointestinal diseases (Ananthakrishnan, McGinley et al. 2011, Beamish, Osornio-Vargas et al. 2011, Kaplan 2011), and arthritis and autoimmunity (Farhat, Silva et al. 2011). Vascular inflammation and metabolic syndrome plays a substantial role as a substrate for autoimmunity. The proatherogenic environment supports development of autoimmunity via TH17 differentiation, in a manner dependent on CD36 (Lim, Kim et al. 2014), a scavenger receptor that is activated by certain air pollutant exposures (Robertson, Colombo et al. 2013, Rao, Zhong et al. 2014). In the present study, we observed clusters of activated transcripts that control secreted proteins involved in common chronic diseases such as inflammatory bowel disease (WNT6), osteoarthritis (ADAMTS5, LTBP3), and connective tissue homeostasis (COL12A1). Though the present study is an acute, single exposure, we posit that repeated exposures can push a balance of inflammatory signaling that contributes, at least in a small way, to many of the above chronic condition. While speculative, the approach used in the present study could be expanded in terms of cohort, design, and analyses to better address the complexities of the relationships between air pollution and chronic inflammatory diseases.
One interesting observation from the current studies was that the subject factor played the greatest role in driving transcriptional differences, compared to exposure or time. This is consistent with our general appreciation that risk factors for cardiovascular disease include familial history, diet and lifestyle choices, with environmental factors playing a real, but lesser role. However, it is quite fascinating that the endothelial cells derived from the same lineage respond so uniquely to plasma from different individuals. While it is easy to assay for chemical differences in the plasma between subjects, it is worth noting that all plasma was supporting life in healthy subjects.
The present study has limitations that impact the strength of our conclusions. First, while the exposure was conducted under controlled conditions, it is considered a high concentration of diesel emissions for the United States and Europe, and the engine system is dated relative to new regulations regarding particle traps. The exposures are more typical in urban regions of less developed countries with older vehicle fleets. Furthermore, this single 2-h exposure is not unusual for many occupational settings. Importantly, the findings of the present study are better interpreted as pathobiological information regarding the transference of a generalized toxic signal from the lung to the vascular system, rather than a study specific to diesel emissions. Second, the cohort consisted of young, healthy individuals and may not reflect vulnerabilities conferred by age, diet, or pre-existing disease. Lastly, the dependence on genomic assessment and lack of mechanistic inquiry detracts from any conclusions related to cellular pathways. Probing the transcriptional responses provides clues as to what the compositionally altered plasma may activate in endothelial cells, but whether such changes are sufficient to impact homeostasis remains unclear. Based on recent findings of roles for multiligand receptors LOX-1 (Lund, Lucero et al. 2011), TLR4 (Kampfrath, Maiseyeu et al. 2011), and CD36 (Robertson, Colombo et al. 2013, Rao, Zhong et al. 2014), all of which could generate a portion of the inflammatory signaling responses observed in the plasma-treated endothelial cells (Silverstein and Febbraio 2009, Sikorski, Czerwoniec et al. 2011, Pirillo, Norata et al. 2013), ligands to such receptors are a likely intermediate that could be assessed in future research.
In summary, genomic analysis of endothelial responses to plasma obtained following diesel exhaust inhalation reveals substantial pro-inflammatory changes that could participate in the promotion of both acute events or progression of chronic inflammatory vascular disease. While a single exposure would not cause chronic systemic disease, scenarios of repeated exposure may contribute to or accelerate ongoing inflammatory conditions. Overall, this translational ex vivo assay platform may be valuable for both improved understanding of the pathophysiological link between inhalation exposures and vascular disease, as well as comparative studies of air pollution toxicity.
Acknowledgements
This study was funded by grants from the National Institutes of Health (ES014639) and the Environmental Protection Agency (RD-83479601-0). The views expressed in this document are solely those of the authors and the U.S. EPA does not endorse any products or commercial services mentioned in this publication.
ABBREVIATIONS
- PM
particulate matter
- DEP
diesel exhaust particles
- LDL
low density lipoprotein
- VLDL
very low density lipoprotein
- HDL
high density lipoprotein
- CD36
cluster of differentiation 36
- ANOVA
analysis of variance
- IL-6
interleukin 6
- TNF-α
tumor necrosis factor alpha
- hCAECs
human coronary artery endothelial cells
- TGF-β
transforming growth factor beta
- bZIP
basic leucine zipper domain
- FOX
winged helix/forkhead transcription factor
- FDR
false discovery rate
- LOX-1
lectin-like oxidized low-density lipoprotein (LDL) receptor-1
- CRP
c-reactive protein
- PAI-1
plasminogen activator inhibitor-1
- ICAM-1
intercellular adhesion molecule 1
- CD40L
CD40 ligand
- SO2
sulfur dioxide
- NO2
nitrogen dioxide
- TH17
T-helper 17 cells
- TLR4
toll-like receptor 4
- WNT6
wingless-type MMTV integration site family, member 6
- ADAMTS5
a disintegrin and metalloproteinase with thrombospondin motifs 5
- LTBP3
latent transforming growth factor beta binding protein 3
- COL12A1
collagen, type XII, alpha 1
- DEE
diesel engine exhaust
- EGM-2
endothelial growth medium-2
- GEO
gene expression omnibus
Footnotes
Competing Interests: The authors declare no financial conflicts of interest with the content of the present manuscript.
REFERENCES
- (EPA), U. S. E. P. A. Health Assessment Document for Diesel Engine Exhaust. U.S. Environmental Protection Agency; 2002. [Google Scholar]
- Analitis A, Katsouyanni K, Dimakopoulou K, Samoli E, Nikoloulopoulos AK, Petasakis Y, Touloumi G, Schwartz J, Anderson HR, Cambra K, Forastiere F, Zmirou D, Vonk JM, Clancy L, Kriz B, Bobvos J, Pekkanen J. Short-term effects of ambient particles on cardiovascular and respiratory mortality. Epidemiology. 2006;17(2):230–233. doi: 10.1097/01.ede.0000199439.57655.6b. [DOI] [PubMed] [Google Scholar]
- Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. Ambient air pollution correlates with hospitalizations for inflammatory bowel disease: an ecologic analysis. Inflamm Bowel Dis. 2011;17(5):1138–1145. doi: 10.1002/ibd.21455. [DOI] [PubMed] [Google Scholar]
- Bai N, Kido T, Suzuki H, Yang G, Kavanagh TJ, Kaufman JD, Rosenfeld ME, van Breemen C, Eeden SF. Changes in atherosclerotic plaques induced by inhalation of diesel exhaust. Atherosclerosis. 2011;216(2):299–306. doi: 10.1016/j.atherosclerosis.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer RN, Diaz-Sanchez D, Jaspers I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J Allergy Clin Immunol. 2012;129(1):14–24. doi: 10.1016/j.jaci.2011.11.004. quiz 25-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamish LA, Osornio-Vargas AR, Wine E. Air pollution: An environmental factor contributing to intestinal disease. J Crohns Colitis. 2011;5(4):279–286. doi: 10.1016/j.crohns.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Ben-Hadj-Khalifa S, Lakhal B, Nsiri B, Mahjoub T, Almawi WY. Factor VII levels, R353Q and -323P0/10 Factor VII variants, and the risk of acute coronary syndrome among Arab-African Tunisians. Mol Biol Rep. 2013;40(5):3793–3798. doi: 10.1007/s11033-012-2456-4. [DOI] [PubMed] [Google Scholar]
- Bezemer GF, Sagar S, van Bergenhenegouwen J, Georgiou NA, Garssen J, Kraneveld AD, Folkerts G. Dual role of Toll-like receptors in asthma and chronic obstructive pulmonary disease. Pharmacol Rev. 2012;64(2):337–358. doi: 10.1124/pr.111.004622. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux V, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr., Whitsel L, Kaufman JD, American E, Heart Association Council on, C. o. t. K. i. C. D. Prevention, P. A. Council on Nutrition and Metabolism Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Campen M, Robertson S, Lund A, Lucero J, McDonald J. Engine exhaust particulate and gas phase contributions to vascular toxicity. Inhal Toxicol. 2014;26(6):353–360. doi: 10.3109/08958378.2014.897776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen MJ. Vascular endothelium as a target of diesel particulate matter-associated toxicants. Arch Toxicol. 2012;86(4):517–518. doi: 10.1007/s00204-012-0806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen MJ, Lund AK, Knuckles TL, Conklin DJ, Bishop B, Young D, Seilkop S, Seagrave J, Reed MD, McDonald JD. Inhaled diesel emissions alter atherosclerotic plaque composition in ApoE(−/−) mice. Toxicol Appl Pharmacol. 2010;242(3):310–317. doi: 10.1016/j.taap.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channell MM, Paffett ML, Devlin RB, Madden MC, Campen MJ. Circulating factors induce coronary endothelial cell activation following exposure to inhaled diesel exhaust and nitrogen dioxide in humans: evidence from a novel translational in vitro model. Toxicol Sci. 2012;127(1):179–186. doi: 10.1093/toxsci/kfs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng TW, Paffett ML, Jackson-Weaver O, Campen MJ, Walker BR, Kanagy NL. Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ Health Perspect. 2011;119(1):98–103. doi: 10.1289/ehp.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr., Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Eisenhart C. The assumptions underlying the analysis of variance. Biometrics. 1947;3(1):1–21. [PubMed] [Google Scholar]
- Farhat SC, Silva CA, Orione MA, Campos LM, Sallum AM, Braga AL. Air pollution in autoimmune rheumatic diseases: a review. Autoimmun Rev. 2011;11(1):14–21. doi: 10.1016/j.autrev.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Glaab E, Baudot A, Krasnogor N, Schneider R, Valencia A. EnrichNet: network-based gene set enrichment analysis. Bioinformatics. 2012;28(18):i451–i457. doi: 10.1093/bioinformatics/bts389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannenhalli S, Putt ME, Gilmore JM, Wang J, Parmacek MS, Epstein JA, Morrisey EE, Margulies KB, Cappola TP. Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation. 2006;114(12):1269–1276. doi: 10.1161/CIRCULATIONAHA.106.632430. [DOI] [PubMed] [Google Scholar]
- Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, Kherada N, Brook RD, Reddy KM, Padture NP, Parthasarathy S, Chen LC, Moffatt-Bruce S, Sun Q, Morawietz H, Rajagopalan S. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. 2011;108(6):716–726. doi: 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G. Air pollution and the inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(5):1146–1148. doi: 10.1002/ibd.21449. [DOI] [PubMed] [Google Scholar]
- Kitchen RR, Sabine VS, Sims AH, Macaskill EJ, Renshaw L, Thomas JS, van Hemert JI, Dixon JM, Bartlett JM. Correcting for intra-experiment variation in Illumina BeadChip data is necessary to generate robust gene-expression profiles. BMC Genomics. 2010;11:134. doi: 10.1186/1471-2164-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckles TL, Buntz JG, Paffett M, Channell M, Harmon M, Cherng T, Lucas SN, McDonald JD, Kanagy NL, Campen MJ. Formation of vascular S-nitrosothiols and plasma nitrates/nitrites following inhalation of diesel emissions. J Toxicol Environ Health A. 2011;74(13):828–837. doi: 10.1080/15287394.2011.570225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckles TL, Lund AK, Lucas SN, Campen MJ. Diesel exhaust exposure enhances venoconstriction via uncoupling of eNOS. Toxicol Appl Pharmacol. 2008;230(3):346–351. doi: 10.1016/j.taap.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Konigshoff M, Kneidinger N, Eickelberg O. TGF-beta signaling in COPD: deciphering genetic and cellular susceptibilities for future therapeutic regimen. Swiss Med Wkly. 2009;139(39-40):554–563. doi: 10.4414/smw.2009.12528. [DOI] [PubMed] [Google Scholar]
- Langrish JP, Unosson J, Bosson J, Barath S, Muala A, Blackwell S, Soderberg S, Pourazar J, Megson IL, Treweeke A, Sandstrom T, Newby DE, Blomberg A, Mills NL. Altered nitric oxide bioavailability contributes to diesel exhaust inhalation-induced cardiovascular dysfunction in man. J Am Heart Assoc. 2013;2(1):e004309. doi: 10.1161/JAHA.112.004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tertre A, Medina S, Samoli E, Forsberg B, Michelozzi P, Boumghar A, Vonk JM, Bellini A, Atkinson R, Ayres JG, Sunyer J, Schwartz J, Katsouyanni K. Short-term effects of particulate air pollution on cardiovascular diseases in eight European cities. J Epidemiol Community Health. 2002;56(10):773–779. doi: 10.1136/jech.56.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Kim YU, Sun H, Lee JH, Reynolds JM, Hanabuchi S, Wu H, Teng BB, Chung Y. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity. 2014;40(1):153–165. doi: 10.1016/j.immuni.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucking AJ, Lundback M, Barath SL, Mills NL, Sidhu MK, Langrish JP, Boon NA, Pourazar J, Badimon JJ, Gerlofs-Nijland ME, Cassee FR, Boman C, Donaldson K, Sandstrom T, Newby DE, Blomberg A. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation. 2011;123(16):1721–1728. doi: 10.1161/CIRCULATIONAHA.110.987263. [DOI] [PubMed] [Google Scholar]
- Lund AK, Lucero J, Harman M, Madden MC, McDonald JD, Seagrave JC, Campen MJ. The oxidized low-density lipoprotein receptor mediates vascular effects of inhaled vehicle emissions. Am J Respir Crit Care Med. 2011;184(1):82–91. doi: 10.1164/rccm.201012-1967OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AK, Lucero J, Lucas S, Madden MC, McDonald JD, Seagrave JC, Knuckles TL, Campen MJ. Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1-mediated pathways. Arterioscler Thromb Vasc Biol. 2009;29(4):511–517. doi: 10.1161/ATVBAHA.108.176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, Thomas PD. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 2013;41(Database issue):D377–386. doi: 10.1093/nar/gks1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Thomas P. PANTHER pathway: an ontology-based pathway database coupled with data analysis tools. Methods Mol Biol. 2009;563:123–140. doi: 10.1007/978-1-60761-175-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, McLean SG, Duffin R, Lawal AO, Araujo JA, Shaw CA, Mills NL, Donaldson K, Newby DE, Hadoke PW. Diesel exhaust particulate increases the size and complexity of lesions in atherosclerotic mice. Part Fibre Toxicol. 2013;10:61. doi: 10.1186/1743-8977-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, Boon NA, Donaldson K, Sandstrom T, Blomberg A, Newby DE. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357(11):1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112(25):3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, Tager I. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126(4):845–852. e810. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Nawrot TS, Perez L, Kunzli N, Munters E, Nemery B. Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet. 2011;377(9767):732–740. doi: 10.1016/S0140-6736(10)62296-9. [DOI] [PubMed] [Google Scholar]
- Norata GD, Grigore L, Raselli S, Seccomandi PM, Hamsten A, Maggi FM, Eriksson P, Catapano AL. Triglyceride-rich lipoproteins from hypertriglyceridemic subjects induce a pro-inflammatory response in the endothelium: Molecular mechanisms and gene expression studies. J Mol Cell Cardiol. 2006;40(4):484–494. doi: 10.1016/j.yjmcc.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Norata GD, Pirillo A, Callegari E, Hamsten A, Catapano AL, Eriksson P. Gene expression and intracellular pathways involved in endothelial dysfunction induced by VLDL and oxidised VLDL. Cardiovasc Res. 2003;59(1):169–180. doi: 10.1016/s0008-6363(03)00335-3. [DOI] [PubMed] [Google Scholar]
- Omori T, Fujimoto G, Yoshimura I, Nitta H, Ono M. Effects of particulate matter on daily mortality in 13 Japanese cities. J Epidemiol. 2003;13(6):314–322. doi: 10.2188/jea.13.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Broadwin R, Green S, Feng WY, Lipsett M. Fine particulate air pollution and mortality in nine California counties: results from CALFINE. Environ Health Perspect. 2006;114(1):29–33. doi: 10.1289/ehp.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SL. Forkhead transcription factors in chronic inflammation. Int J Biochem Cell Biol. 2010;42(4):482–485. doi: 10.1016/j.biocel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786. doi: 10.1155/2013/152786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA., 3rd Respiratory disease associated with community air pollution and a steel mill, Utah Valley. Am J Public Health. 1989;79(5):623–628. doi: 10.2105/ajph.79.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X, Zhong J, Maiseyeu A, Gopalakrishnan B, Villamena FA, Chen LC, Harkema JR, Sun Q, Rajagopalan S. CD36-Dependent 7-Ketocholesterol Accumulation in Macrophages Mediates Progression of Atherosclerosis in Response to Chronic Air Pollution Exposure. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.115.304666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo M, Watzke HH, Stucki B, Sulzer I, Biasiutti FD, Binder BR, Furlan M, Lammle B, Wuillemin WA. Coagulation factors II, V, VII, and X, prothrombin gene 20210G-->A transition, and factor V Leiden in coronary artery disease: high factor V clotting activity is an independent risk factor for myocardial infarction. Arterioscler Thromb Vasc Biol. 1999;19(4):1020–1025. doi: 10.1161/01.atv.19.4.1020. [DOI] [PubMed] [Google Scholar]
- Robertson S, Colombo ES, Lucas SN, Hall PR, Febbraio M, Paffett ML, Campen MJ. CD36 mediates endothelial dysfunction downstream of circulating factors induced by O3 exposure. Toxicol Sci. 2013;134(2):304–311. doi: 10.1093/toxsci/kft107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnebaum SM, Patterson C. The FoxO family in cardiac function and dysfunction. Annu Rev Physiol. 2010;72:81–94. doi: 10.1146/annurev-physiol-021909-135931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancini G, Farina F, Battaglia C, Cifola I, Mangano E, Mantecca P, Camatini M, Palestini P. Health risk assessment for air pollutants: alterations in lung and cardiac gene expression in mice exposed to Milano winter fine particulate matter (PM2.5). PLoS One. 2014;9(10):e109685. doi: 10.1371/journal.pone.0109685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisler JC, Willis MS, Patterson C. You spin me round: MaFBx/Atrogin-1 feeds forward on FOXO transcription factors (like a record). Cell Cycle. 2008;7(4):440–443. doi: 10.4161/cc.7.4.5451. [DOI] [PubMed] [Google Scholar]
- Schmid R, Baum P, Ittrich C, Fundel-Clemens K, Huber W, Brors B, Eils R, Weith A, Mennerich D, Quast K. Comparison of normalization methods for Illumina BeadChip HumanHT-12 v3. BMC Genomics. 2010;11:349. doi: 10.1186/1471-2164-11-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher A. Effect of ex vivo storage and Cyto-Chex on the expression of P-selectin glycoprotein ligand-1 (PSGL-1) on human peripheral leukocytes. J Immunol Methods. 2007;323(1):24–30. doi: 10.1016/j.jim.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Shi W, Chen F, Cardoso WV. Mechanisms of lung development: contribution to adult lung disease and relevance to chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2009;6(7):558–563. doi: 10.1513/pats.200905-031RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TS, Lai CH, Hung HF, Ku SY, Tsai PJ, Yang T, Liou SH, Loh CH, Jaakkola JJ. Elemental and organic carbon exposure in highway tollbooths: a study of Taiwanese toll station workers. Sci Total Environ. 2008;402(2-3):163–170. doi: 10.1016/j.scitotenv.2008.04.051. [DOI] [PubMed] [Google Scholar]
- Sikorski K, Czerwoniec A, Bujnicki JM, Wesoly J, Bluyssen HA. STAT1 as a novel therapeutical target in pro-atherogenic signal integration of IFNgamma, TLR4 and IL-6 in vascular disease. Cytokine Growth Factor Rev. 2011;22(4):211–219. doi: 10.1016/j.cytogfr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2(72) doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobus JR, Pleil JD, Madden MC, Funk WE, Hubbard HF, Rappaport SM. Identification of surrogate measures of diesel exhaust exposure in a controlled chamber study. Environ Sci Technol. 2008;42(23):8822–8828. doi: 10.1021/es800813v. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takyar S, Vasavada H, Zhang JG, Ahangari F, Niu N, Liu Q, Lee CG, Cohn L, Elias JA. VEGF controls lung Th2 inflammation via the miR-1-Mpl (myeloproliferative leukemia virus oncogene)-P-selectin axis. J Exp Med. 2013;210(10):1993–2010. doi: 10.1084/jem.20121200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauters A, Esmaeilzadeh F, Bladt S, Beukinga I, Wijns W, van de Borne P, Pradier O, Argacha JF. Pro-thrombotic effect of exercise in a polluted environment: a P-selectin- and CD63-related platelet activation effect. Thromb Haemost. 2015;113(1):118–124. doi: 10.1160/TH14-03-0251. [DOI] [PubMed] [Google Scholar]
- Wingender E, Chen X, Fricke E, Geffers R, Hehl R, Liebich I, Krull M, Matys V, Michael H, Ohnhauser R, Pruss M, Schacherer F, Thiele S, Urbach S. The TRANSFAC system on gene expression regulation. Nucleic Acids Res. 2001;29(1):281–283. doi: 10.1093/nar/29.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender E, Chen X, Hehl R, Karas H, Liebich I, Matys V, Meinhardt T, Pruss M, Reuter I, Schacherer F. TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res. 2000;28(1):316–319. doi: 10.1093/nar/28.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Goya I, Graf D, Craig S, Martin-Orozco N, Dong C. A butyrophilin family member critically inhibits T cell activation. J Immunol. 2010;185(10):5907–5914. doi: 10.4049/jimmunol.1000835. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117(6):898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Duan H, Gao F, Li Y, Huang C, Niu Y, Gao W, Yu S, Zheng Y. Increased Micronucleus, Nucleoplasmic Bridge, and Nuclear Bud Frequencies in the Peripheral Blood Lymphocytes of Diesel Engine Exhaust-Exposed Workers. Toxicol Sci. 2014 doi: 10.1093/toxsci/kfu239. [DOI] [PubMed] [Google Scholar]