Abstract
This review addresses fundamental mechanisms underlying how capillaries form in three-dimensional extracellular matrices and how endothelial cells (ECs) and pericytes co-assemble to form capillary networks. In addition to playing a critical role in supplying oxygen and nutrients to tissues, recent work suggests that blood vessels supply important signals to facilitate tissue development. Here, we hypothesize that another major function of capillaries is to supply signals to suppress major disease mechanisms including inflammation, infection, thrombosis, hemorrhage, edema, ischemic injury, fibrosis, autoimmune disease, and tumor growth/ progression. Capillary dysfunction plays a key pathogenic role in many human diseases, and thus, this suppressing function may be attenuated and central toward the initiation and progression of disease. We describe how capillaries form through creation of EC-lined tube networks and vascular guidance tunnels in 3D extracellular matrices. Pericytes recruit to the abluminal EC tube surface within these tunnel spaces, and work together to assemble the vascular basement membrane matrix. These processes occur under serum-free conditions in 3D collagen or fibrin matrices and in response to five key growth factors which are stem cell factor, interleukin-3, stromal-derived factor-1α, fibroblast growth factor-2, and insulin. In addition, we identified a key role for EC-derived platelet-derived growth factor-BB and heparin-binding epidermal growth factor in pericyte recruitment and proliferation to promote EC-pericyte tube co-assembly and vascular basement membrane matrix deposition. A molecular understanding of capillary morphogenesis and maturation should lead to novel therapeutic strategies to repair capillary dysfunction in major human disease contexts including cancer and diabetes.
Keywords: Endothelial cells, pericytes, extracellular matrix, basement membrane assembly, capillary morphogenesis
Introduction
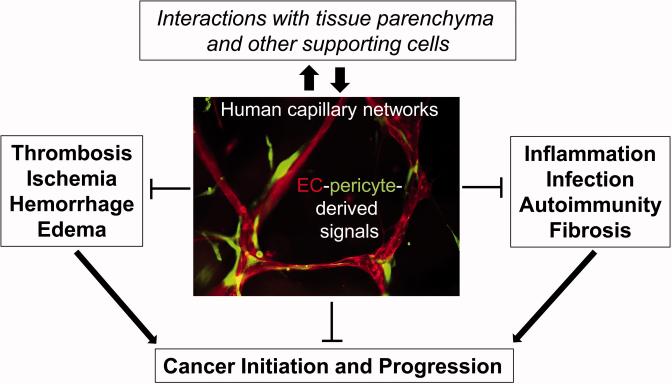
The predominant blood vessels within perfused tissues are capillaries which are critical conduits for the exchange of fluids, nutrients, and oxygen [1-10]. The health of our capillary networks is essential for proper tissue homeostasis [11, 12] and many disease states appear to have dysfunctional capillary structures [13-24]. An interesting new concept that we propose here is that healthy capillaries within tissues may play a fundamental role in suppressing disease by promoting the development and maintenance of healthy, non-diseased tissues (Figure 1). Major diseases where capillary dysfunction is known to play a key pathogenic component are cancer, diabetes, obesity, and Alzheimer's disease [13-16, 19, 21-29].
Figure 1. Hypothesis: Capillary tube networks directly suppress major pathogenic mechanisms of disease.
We propose the hypothesis that capillary tube networks (primarily composed of EC tubes and associated pericytes) provide signals to adjacent parenchymal and supporting cells within tissue stromal spaces to suppress major mechanisms of disease including cancer initiation and progression. These basic disease mechanisms are also direct contributors to cancer development (arrows). Many human diseases show evidence of capillary dysfunction and we propose that the disease processes directly result as a consequence of this microvascular dysfunction. Capillary dysfunction is a major pathogenic feature of key diseases including diabetes, cancer, obesity and Alzheimer's disease.
Capillaries consist of primarily two cell types which are endothelial cells (ECs) and pericytes which co-assemble to form this critical vascular conduit [1, 4, 30-32] (Figure 1). ECs are known to form cell-lined tubes (Figures 2, 3) and pericytes are known to recruit to the abluminal surface of these tubes (Figures 1, 4-7). Interestingly, capillaries within different tissues have distinct numbers of pericytes relative to ECs. Most tissues have about 20-25% of pericytes relative to ECs, while in the central nervous system; pericytes are much more abundant and are at a 1:1 ratio with ECs [4, 30, 31]. The molecular control of control of EC-pericyte tube co-assembly has been a major subject of investigation from our laboratory. Of great interest is that capillary tube assembly in large part functionally defines the role of both ECs and pericytes in that ECs form a perfusable network of tubes, while pericytes recruit to this network to facilitate maturation and stabilization of this capillary circuit [4, 31, 33]. Our laboratory first demonstrated that pericyte recruitment to EC tubes leads to another key feature of capillaries, the deposition of the vascular basement membrane which underlies the abluminal surface of the EC-lined tube and which is assembled between the two cell types [4, 31, 32] (Figures 6, 7). Pericyte recruitment occurs along this abluminal EC surface and dynamic imaging experiments have shown marked motility of ECs and pericytes along each other during tube co-assembly which is responsible for deposition of a continuous capillary basement membrane [3, 4, 31, 32] (Figures 6, 7).
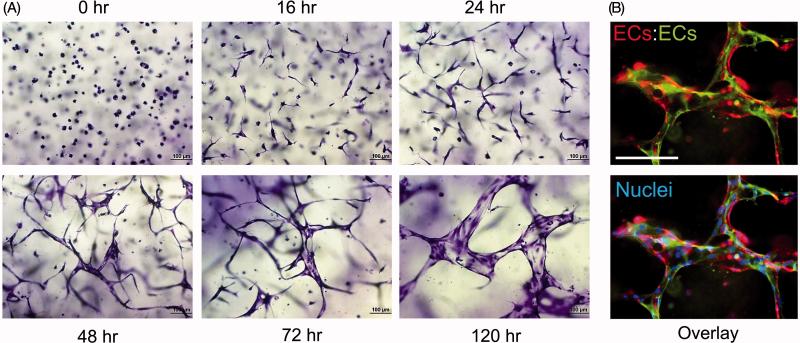
Figure 2. Time course of multicellular EC tube assembly in 3D collagen matrices.
(A) ECs were seeded as single cells within 3D collagen matrices (mimicking the developmental process of vasculogenesis) and form tubes in response to the “Factors”. They were fixed, stained, and photographed at the indicated time points. Marked EC tube morphogenesis is observed over time. Bar equals 100 μm. (B) GFP- and mCherry-labeled ECs were mixed together at a 1:1 ratio to reveal multicellular EC tube assembly after 72 hr of culture. Cultures were photographed under fluorescence, images were overlaid and the lower panel also shows nuclear staining after the addition of Hoechst dye. Bar equals 100 μm.
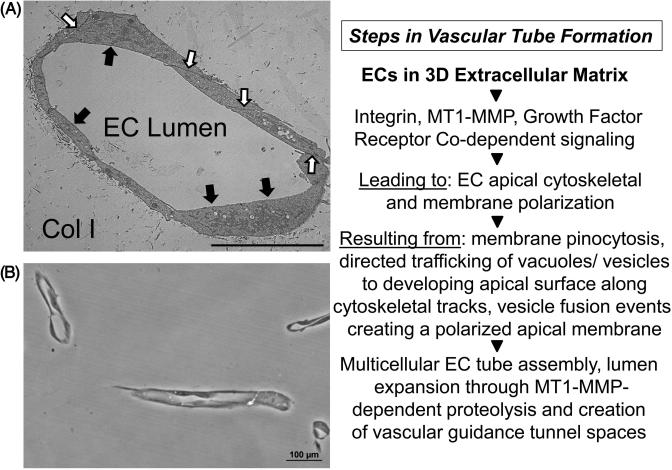
Figure 3. Molecular events controlling EC lumen formation during “Factor”-induced EC tube assembly in 3D collagen matrices.
ECs were seeded as single cells and allowed to form tubes over 120 hr prior to fixation and processing for transmission electron microscopy (A) or plastic thin sectioning (B) to demonstrate EC lumen formation following cross-sectioning of 3D collagen gels. Black arrows indicate the EC apical surface; white arrows indicate EC junctional contacts, Col I indicates the 3D collagen type I matrix. Key regulatory steps that control the EC lumen formation process are highlighted in the right panel. Bar equals 10 μm (A); Bar equals 100 μm (B).
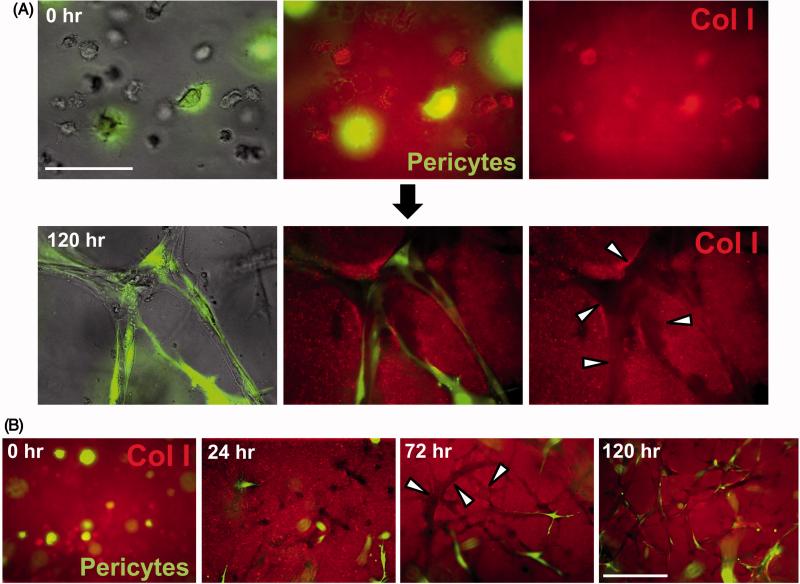
Figure 4. Capillary tube assembly in 3D collagen matrices and role of vascular guidance tunnels in EC-pericyte tube co-assembly.
(A) ECs were co-cultured with GFP-pericytes and at the indicated times, cultures were fixed with paraformaldehyde and stained using anti-collagen type I (Col I) antibodies, and were photographed using light or fluorescent microscopy. EC tubes form, vascular guidance tunnels appear and pericytes are observed to recruit to these tubes on the EC tube abluminal surface within vascular guidance tunnels. Arrowheads indicate the borders of vascular guidance tunnels. Bar equals 50 μm. (B) ECs were co-cultured with GFP-pericytes and at the indicated times, cultures were fixed, immunostained with anti-collagen type I antibodies and photographed using fluorescence microscopy. Marked recruitment of pericytes is observed to EC-lined tubes which are present within vascular guidance tunnels over time. Arrowheads indicate the borders of vascular guidance tunnels. Bar equals 100 μm.
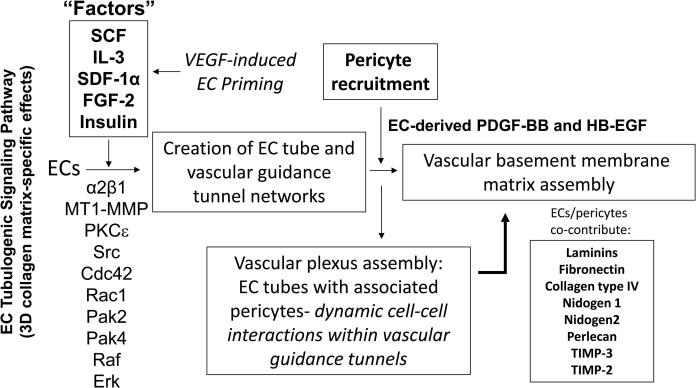
Figure 7. Molecular control of human capillary tube morphogenesis and maturation in 3D matrices.
Schematic diagram showing key molecular regulators of EC tubulogenesis and pericyte recruitment to EC tubes which control capillary network formation and maturation. Dynamic and polarized EC-pericyte interactions within vascular guidance tunnels results in abluminal vascular basement membrane matrix assembly. EC tubulogenesis is driven by growth factor-dependent signals secondary to a special combination of five growth factors (“Factors”) which are SCF, IL-3, SDF-1α, FGF-2 and Insulin. VEGF can prime ECs in an upstream step to facilitate their responsiveness to the “Factors”. These “Factors” stimulate an integrin-, MT1-MMP-, and Rho GTPase-dependent signaling cascade which controls the development of EC tube networks and vascular guidance tunnels in 3D matrices. These networks produce PDGF-BB and HB-EGF to induce recruitment and proliferation of pericytes and together ECs and pericytes co-assemble within tunnel spaces to co-contribute and deposit the vascular basement membrane along the abluminal EC tube surface in between the two cell types.
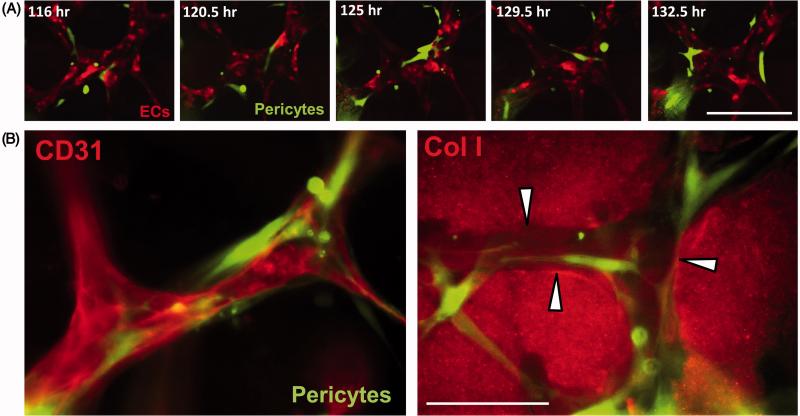
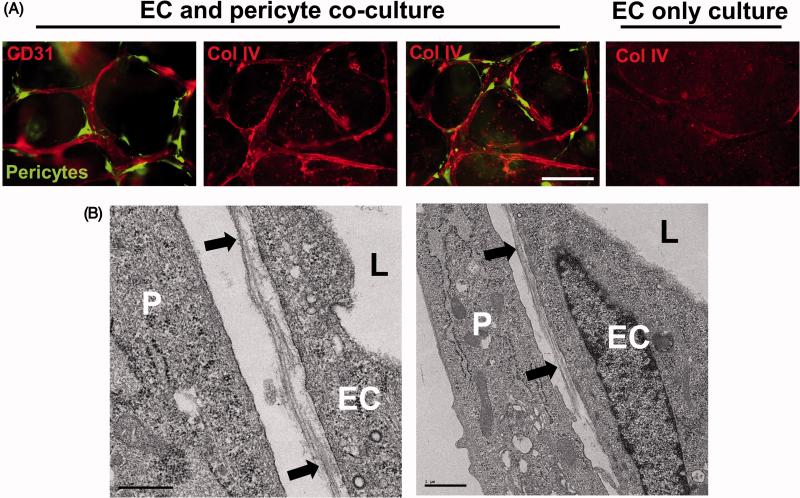
Figure 6. EC-pericyte tube co-assembly leads to vascular basement membrane matrix deposition.
(A) ECs alone or ECs and GFP-pericytes were cultured for 120 hr and then fixed and stained for CD31 or collagen type IV (Col IV), a key basement membrane component. Fixed cultures were not permeabilized with detergent, so that only collagen type IV that was deposited extracellularly is observed. Note that marked basement membrane deposition, as indicated by collagen type IV deposition, is observed only when ECs and pericytes are cultured together. Bar equals 50 μm. (B) EC-pericyte co-cultures were examined by transmission electron microscopy and vascular basement membrane deposition is observed between the two cell types (black arrows). P indicates pericytes, EC indicates endothelial cells, and L indicates the luminal space. Left image- Bar equals 0.5 μm; Right image- Bar equals 1 μm.
Molecular control of capillary tube morphogenesis
Considerable progress has occurred in recent years regarding how ECs form multicellular capillary tube structures (Figures 2, 3). The identified mechanisms show similarities to how epithelial cells form tubes, although there are also clear distinctions between them [34, 35]. Recent reviews have covered these issues quite thoroughly [2, 34-37]. Key regulators of capillary tube formation are integrins, the surface matrix metalloproteinase MT1-MMP, Src family isoforms, protein kinase C epsilon (PKCε), the small GTPases, Cdc42, Rac1 and RhoJ, and a series of downstream effectors and regulators including Pak2, Pak4, Par6b, Par3, Rasip1, Raf and Erk kinases [2, 37-44] (Figure 7). Interestingly, one of the functions of this set of molecules appears to suppress RhoA and Rho kinase activities which probably play a key role in stabilizing EC-EC junctional contacts and maintaining the flat shape of ECs within a capillary tube [2, 5, 37]. Other key molecular regulators of capillary formation are the junctional adhesion molecules, VE-cadherin, JamB and JamC, and they all share the ability to interact with a major polarity molecule, Par3, which has been implicated in vessel formation in vitro and in vivo [37, 38, 45-48].
In all of the cases that we have observed, capillary tube assembly is accompanied by the creation of physical spaces in the extracellular matrix which we have termed vascular guidance tunnels [44] (Figures 4, 5). Interference with the tube formation process also results in complete blockade of tunnel formation[44]. These matrix-free spaces are created through matrix proteolysis derived from cell surface matrix metalloproteinases. In collagen matrices, the primary enzyme responsible for the creation of these tunnels is MT1-MMP [44]. Vascular guidance tunnels are observed in either collagen type I or fibrin matrices, the two major matrix substrates which facilitate vascular morphogenesis and, thus, these tunnels are observed in conjunction with the process of capillary formation [31, 44, 49]. Another key point is that the EC-lined tubes that are formed in vitro in collagen or fibrin matrices resemble capillaries in vivo in that they possess a fluid-filled apical compartment and a basal surface in contact with the ECM. The ECs within the tubes are embedded in the 3D matrices within vascular guidance tunnels, and they are free to move within these spaces and remodel the tube structure. Interestingly, initial EC motility in 3D collagen matrices is completely dependent on MT1-MMP and this leads to generation of the vascular guidance tunnels in conjunction with other tube regulatory signals such as the α2β1 integrin (a collagen receptor) which is directly associated with MT1-MMP during this process [43, 44, 46, 50]. Once vascular guidance tunnels have been established in 3D matrices, ECs can move in an MMP-independent manner within these spaces [44]. The establishment of this initial polarized tube structure is critical for EC-lined tubes to receive an additional polarizing and maturation stimulus, which is the recruitment of pericytes to the abluminal tube surface [4, 31] (Figures 1, 4-7).
Figure 5. Pericyte motility is observed along EC tubes within vascular guidance tunnels in 3D matrices.
(A) mRFP-labeled ECs and GFP-pericytes were co-cultured and videos were made of EC tubulogenesis, pericyte recruitment and pericyte motility along EC tubes over time. Still images from these videos are shown at the indicated times of culture and reveal marked pericyte motility along the abluminal EC tube surface. Note that EC tube remodeling is also occurring while pericytes are moving along the tubes. Bar equals 50 μm. (B) EC and GFP-pericytes were co-cultured and after 120 hr, cultures were fixed and stained with anti-CD31 and anti-collagen type I (Col I) antibodies. Note that pericytes are observed on the EC tube abluminal surface and this recruitment is observed within vascular guidance tunnels (arrowheads indicate the borders of these tunnels), which are generated as a result of the EC tubulogenic process. Bar equals 50 μm.
Role of vascular guidance tunnels in capillary remodeling, dynamic EC-pericyte interactions and vascular basement membrane matrix assembly
ECs remodel the ECM in which they are embedded to create networks of vascular guidance tunnels that directly result from the EC tubulogenic signaling pathway [44]. Thus, matrix proteolysis and the creation of these tunnel spaces is a pre-requisite and essential step in the assembly of EC-lined tubes. During the dynamic process of EC tubulogenesis, more tunnels are created than are occupied at any given time, and this allows for motility of individual or groups of ECs to remodel the tube networks [44]. Pericytes recruit to EC-lined tubes that are embedded in tunnels, and, thus the pericytes enter vascular guidance tunnels allowing them to migrate along the abluminal surface of tubes [4, 31, 32, 49]. We have previously demonstrated marked motility of both ECs and pericytes together as they co-assemble into capillary tube structures within EC-generated tunnel spaces [31, 49] (Figure 4). An interesting issue concerns how pericytes move in 3D matrices and whether they create related tunnel spaces as they invade as single cells. Of great interest is that pericyte motility in 3D collagen matrices required the co-presence of ECs due to their secretion of PDGF-BB and HB-EGF [4, 32] (Figures 4, 7) (see below). Pericyte invasion of 3D collagen matrices is MMP-dependent and is completely inhibited in the presence of the MMP inhibitor, GM6001 [33]. In related work, we previously reported that tumor cell motility in 3D collagen matrices requires MT1-MMP-dependent proteolysis [51, 52]. In contrast, tumor cell motility on 2D collagen surfaces did not require MT1-MMP. In 3D matrices, we showed that tumor cells could invade as single cells via the creation of MT1-MMP-dependent tunnel structures (we termed these single cell invasion tunnels- SCITs) that were the identical in width to that of the nucleus of the cell that was invading [52]. This was also demonstrated with human fibroblasts and is also true for pericytes which invade as single cells as they approach the EC-lined tube networks [52]. Once pericytes enter the vascular guidance tunnels created by EC tubulogenesis (and which are much larger in diameter than SCITs), they are free to move in an MMP-independent manner, just like the ECs since they both are in a 2D-like vascular guidance tunnel space in a 3D matrix environment [31]. This dynamic and polarized motility of ECs and pericytes results in ECM remodeling events leading to tube maturation (see below). Another important finding that we observed is that EC-pericyte interactions lead to the upregulation of TIMP-2 (primarily EC-derived) and TIMP-3 (primarily pericyte-derived) [43]. These two MMP inhibitors interestingly restrict EC tube diameter by blocking MT1-MMP activity [43, 44], facilitate ECM remodeling by reducing MMP-dependent degradation of ECM components [31], and help maintain the EC-pericyte co-assembled tube by preventing either cell type from leaving the vascular guidance tunnel spaces via inhibition of MT1-MMP-dependent invasion. TIMP-2 and TIMP-3 also have been reported to inhibit growth factor-dependent signaling responses [53, 54] that would be necessary to initiate angiogenic sprouting responses, and in this manner, they contribute to capillary tube stabilization through this additional mechanism.
EC-pericyte interactions are necessary for capillary basement membrane assembly
In previous work, we demonstrated that ECs and pericytes co-assemble in order to regulate the maturation of this vascular network, by inducing assembly of the vascular basement membrane which is deposited abluminally and in between ECs and pericytes [31, 49] (Figures 4-7). This abluminal deposition constitutes an additional polarity signal for ECs and pericytes during this tube maturation process. Deposition of the basement membrane during capillary assembly restricts vessel diameters and, thus, the EC-pericyte co-assembled tubes are much narrower compared to EC only tubes [31]. An important point is that EC only tubes fail to deposit a basement membrane [31] as indicated by both immunofluorescence microscopy (Figure 6A) or by examining the tubes by transmission electron microscopy. Thus far, we have observed that pericytes are highly efficient at recruiting to EC tubes and inducing vascular basement membrane matrix deposition (Figures 1, 4-7). Other mural cells such as vascular smooth muscle cells failed to recruit well to tubes in our models and also failed to induce basement membrane formation. We have observed the same results using different origins of human ECs (umbilical vein or artery cells) and when combined with either human or bovine pericytes [33]. Furthermore, EC-pericyte tube co-assembly leads to basement membrane formation using either collagen or fibrin matrices [31, 49]. Both cell types are necessary to contribute key basement membrane components to deposit this remodeled matrix [31] (Figures 6, 7). Thus, we conclude that ECs and pericytes co-assemble in a highly efficient manner to form polarized capillary networks with associated basement membranes in 3D matrices and therefore, ECs and pericytes can be functionally defined in this manner [31, 33]. ECs are defined by their ability to form multicellular tube networks (ECs do not proliferate during these morphogenic processes) and pericytes are defined by their ability to recruit to these tubes and proliferate in response to EC-derived factors.
In further support of these findings, we also demonstrated that integrin requirements change during EC-pericyte tube co-assembly compared to EC tubulogenesis with ECs alone. In the latter case, when ECs are embedded in 3D collagen matrices by themselves, they require the α2β1 integrin to form tubes and maintain them [50]. We have demonstrated this result using blocking antibodies, siRNAs and chemical inhibitors. Of great interest, integrin requirements change when ECs and pericytes are co-cultured compared to EC only cultures in 3D collagen matrices. ECs express many integrins including α1β1, α2β1, α3β1, α5β1, α6β1, and various αv integrins such as αvβ3 and αvβ5. Pericytes express all of these integrins and also express α4β1. Many of these integrins bind basement membrane components including laminin isoforms, fibronectin and collagen type IV. We demonstrated that α1β1, α3β1, α5β1, and α6β1 play a key role in EC-pericyte tube co-assembly [31]. However, they do not play a role when ECs are cultured alone in 3D collagen matrices [50]. All of these integrins recognize basement membrane matrix proteins which show little deposition around tubes in the absence of pericytes [31]. Thus, these basement membrane-binding integrins become critical once ECs and pericytes work together to deposit the vascular basement membrane during capillary tube assembly. In contrast, α2β1 integrin plays a dominant role during EC tubulogenesis when ECs are by themselves in 3D collagen matrices, but assumes a diminished role over time when ECs and pericytes co-assemble and then contact basement membrane matrices by engaging α1β1, α3β1, α5β1, and α6β1 [31].
Defined growth factors controls distinct steps in capillary morphogenesis and maturation
The systems that we have developed to investigate capillary tube assembly are serum-free models that depend on defined growth factors that allow for the above described events to occur [33, 49, 55, 56]. One of the critical questions that we have been addressing is to define the nature and number of growth factors that are necessary for EC-pericyte tube co-assembly (i.e. capillary morphogenesis and maturation). We have identified five defined growth factors that must be added in combination to allow for this process to occur using human ECs and pericytes. The five growth factors (“Factors”) are stem cell factor (SCF), interleukin-3 (IL-3), stromal-derived factor-1α (SDF-1α), fibroblast growth factor-2 (FGF-2) and insulin [33, 49, 55, 56] (Figure 7). We have yet to identify other growth factors that will substitute for this combination of factors to regulate human capillary morphogenesis under defined serum-free conditions in 3D collagen matrices. Interestingly, we identified that Flt3 ligand addition could enhance the effect of the five factors in 3D fibrin matrices under defined serum-free conditions [49]. Importantly, the addition of vascular endothelial cell growth factor (VEGF) by itself or in combination with FGF-2 fails to stimulate EC tubulogenesis or EC-pericyte tube co-assembly in either 3D collagen or fibrin matrices [49, 55]. However, we made the important observation that ECs can be activated in an upstream priming event by VEGF [55] (Figure 7). If ECs are pre-treated with VEGF for at least 8 hr, they are primed to respond much faster to the five factor combination of SCF, IL-3, SDF-1α, FGF-2 and insulin [55] (Figure 7). The latter factor combination is what is necessary to drive EC tubulogenesis [55]. VEGF does not directly stimulate EC tubulogenesis, because it acts upstream to activate the ability of ECs to respond to a distinct downstream set of “Factors” that directly control vascular morphogenesis in 3D matrices. We demonstrated that VEGF can upregulate the expression of the receptors for SCF, IL-3 and SDF-1α, which are c-Kit, IL-3Rα, and CXCR4, respectively [55]. This finding is one explanation for why VEGF primes downstream morphogenic responses to these “Factors”. Considerable effort is currently ongoing to elucidate the underlying signaling pathways that control the VEGF priming response which is distinct from the downstream EC tubulogenic signaling pathway stimulated by SCF, IL-3, SDF-1α, FGF-2 and insulin (Figure 7).
Using these systems, we have also begun to define the role of growth factors in pericyte recruitment and proliferation [4, 32, 49]. Of great interest is our finding that pericyte motility in 3D collagen matrices under defined serum-free conditions is minimal in the absence of ECs [32]. In contrast, if ECs are present, pericyte motility can be readily observed and they migrate long distances as they are recruited to EC tubes and then migrate along the abluminal EC surface within vascular guidance tunnels as described above. We identified the dual role of EC-derived PDGF-BB and HB-EGF in these events [32] (Figure 7). Using either blocking antibodies or soluble receptor traps with specific affinity for these growth factors, we demonstrated that these two factors in combination marked control both pericyte recruitment to tubes, and pericyte proliferation [32]. Pericytes fail to proliferate in the absence of ECs under these culture conditions, but with ECs present, they proliferate [32]. Importantly, ECs do not proliferate under the same culture conditions, and, we hypothesize that this inability to proliferate plays a key role in their ability to form tubes. Many years ago using DNA microarray approaches, we first demonstrated marked downregulation of cell cycle genes that are necessary for EC proliferation while they are forming tubes in 3D matrices [57]. Also, we were able to show that PDGF-BB and HB-EGF controlled pericyte recruitment to EC-lined tubes in vivo in developing quail embryos [4, 32]. We utilized blocking antibodies as well as chemical inhibitors of PDGFRβ and EGFR, which are receptors for PDGF-BB and HBEGF, respectively. Current work in our laboratory is addressing whether additional endogenous growth factors (i.e. EC- or pericyte-derived) affect EC tubulogenesis and EC-pericyte tube co-assembly. The number of growth factors that control capillary morphogenesis will be many and we are focused on identifying these molecules and the downstream signaling pathways regulated by them using our defined serum-free model systems in 3D matrices.
Another important aspect of this process is how growth factor-dependent pathways overlap with ECM-dependent signaling pathways and how this works in concert with assembly of the vascular basement membrane to promote EC and pericyte differentiation and maturation [3, 4, 58, 59]. Growth factors (from ECs, pericytes or surrounding parenchymal cells/ supporting cell types such as macrophages and mast cells) will specifically anchor to particular components of the basement membrane matrix which will create very unique signaling events (e.g. co-clustering of growth factors with particular integrins) to control capillary maturation and stabilization. Examples are the binding of bone morphogenic proteins to collagen type IV [60], the interaction of VEGF with fibronectin [61], or angiopoietin-1 with insoluble ECM [62], thus, creating opportunities for co-signaling between receptor tyrosine kinases and integrins leading to unique signals to ECs [58, 63], pericytes and other cell types that come into contact with vascular basement membranes.
Capillary abnormalities underlie the pathogenesis of key human diseases: cancer and diabetes: Hypothesis- healthy capillaries directly suppress major human diseases
It is well known the capillaries are abnormal in the malignant tumor microenvironment or in diseases such as diabetes, obesity, and Alzheimer's disease [13-16, 19, 21-29]. A critical question that remains unresolved is whether capillary abnormalities are primary causative factors in the pathogenic manifestations of these diseases or do they become abnormal as a downstream consequence of the disease. We hypothesize here that healthy capillary networks within tissues suppress diseases via their production of growth factors and other molecules leading to signaling cross-talk with adjacent parenchymal cells resulting in proper tissue homeostasis (Figure 1). EC-pericyte tube networks may produce a wide variety of molecules to actively suppress major disease processes including inflammation, infection, thrombosis, hemorrhage, edema, ischemic injury, fibrosis, autoimmune disease, and tumor growth/ progression (Figure 1). Several very interesting examples that have been reported are that pericytes lose their ability to interact with capillaries and become pro-fibrotic cells leading to tissue fibrosis associated with chronic kidney, liver, or pancreatic injury [16, 64-71]. Similar mechanisms might occur within the tumor microenvironment where fibrosis is associated with tumors including pancreatic, hepatocellular, and breast carcinomas or in variants of Hodgkin's disease [64-69, 72-74]. In these instances, capillary dysfunction that predisposes pericytes to becoming pro-fibrotic cells may represent an important pathogenic step in the development of these cancers. In the cases of chronic liver fibrosis secondary to chronic hepatitis or cirrhotic liver disease, it is interesting to speculate that longstanding capillary dysfunction might also underlie the fibrotic response leading to an increased propensity to develop hepatocellular carcinoma. Furthermore, the EC apical domain is well known to be an anti-coagulant surface through the thrombomodulin/ activated protein C/ protein S signaling cascade [75-78] and EC-EC junctional controls through VE-cadherin-mediated cell-cell adhesion regulates vessel integrity to reduce disease processes such as edema (secondary to increased vascular permeability), thrombosis and hemorrhage [45, 79, 80]. EC-derived nitric oxide reduces inflammation, thrombosis and platelet activation, and has antimicrobial activity, along with EC-derived antimicrobial peptides [81-83]. Also, pericytes are known to have immunosuppressive functions [84] and, thus, capillaries might suppress autoimmune diseases directed at tissue parenchymal cells (e.g. beta cells in type I diabetes).
As mentioned earlier, EC-pericyte tube co-assembly leads to vascular basement membrane matrix deposition in 3D fibrin matrices [49]. In contrast, they do not deposit type I or type III collagens in these matrices despite the presence of abundant mRNA transcripts of these genes as well as other interstitial matrix proteins in pericytes (unpublished data). Does this indicate that EC-pericyte interactions lead to specific deposition of basement membrane matrices, and suppression of interstitial matrix protein deposition? If these interactions were disrupted due to chronic tissue injury with concomitant capillary dysfunction (or vice-versa), might this result in aberrant deposition of interstitial matrix collagens leading to fibrotic responses. This idea could be directly tested using our new model system.
In support of our hypothesis that healthy capillaries suppress disease (Figure 1) is that the incidence of inflammation, thrombosis, hemorrhage, cancer, etc. are all increased in diabetic patients who are known to have systemic capillary dysfunction [15, 19, 21, 22, 85]. Loss of pericytes around capillaries is a hallmark of diabetes, and is particularly apparent in capillaries of the central nervous system (e.g. retina) leading to diabetic retinopathy [21, 86]. Loss of pericytes leads to abnormalities in vascular basement membranes, and disruption of capillary vessel stabilization leads to focal hemorrhagic lesions, thrombosis and ischemic damage [21, 86]. Further work is necessary to elucidate the molecular details for how EC-pericyte interactions are altered under conditions of hyperglycemia or in conjunction with low insulin signaling. Our finding that insulin is a required growth factor for capillary tube assembly suggests one reason why diabetes results in capillary dysfunction. Our model systems are particularly well suited to investigate such questions.
Capillary dysfunction within the tumor microenvironment
One approach to evaluate tumor angiogenic responses is to consider tumor tissues as new tissues (i.e. similar to developmental processes or regenerative events) that must be vascularized like any developing tissue. A key point is that it is necessary to understand the specific growth factor requirements for different stages of vascular morphogenesis, such as priming, tube morphogenesis, EC-pericyte tube co-assembly, maintenance of these formed vessels, and also, regression of vessels in the context of tissue vascularization [2, 10, 33, 56] (Figure 7). We believe that parenchymal and other stromal support cells within different tissues such as macrophages, mast cells, fibroblasts, etc. will supply the necessary factors in combination for capillary assembly and maturation (e.g. “Factors” that we have described [33, 49, 55, 56]), and normal tissues have the appropriate cell types to supply these factors. In turn, capillaries will supply growth factors and other signaling molecules to facilitate tissue parenchymal cell development and maintenance. With regard to malignant tumor tissues (compared to normal tissues), we propose that major imbalances in the growth factor milieu will be presented to the newly formed tumor vasculature that will favor capillary instability over time (i.e. blood and/ or lymphatic vasculatures). It is known that EC-pericyte interactions are impaired within the tumor microenvironment and this will account for the abnormal vasculature that is observed [14, 23, 24, 87]. One important characteristic of highly aggressive cancers is that they appear to outgrow their blood supply and become hypoxic [13]. Another interpretation of these observations is that the vasculature becomes progressively abnormal over time leading to hypoperfusion of the tumor tissue and hypoxia.
Capillary instability, in contrast to capillary stabilization, will lead to continuous attempts at vessel formation, as well as vessel breakdown and regression events [2, 10, 88]. Thus, the tissue is unable to properly mature both with respect to the tumor cells and supporting cellular elements including unstable capillaries with impaired EC-pericyte interactions, creating a form of chronic tissue injury. In general terms, normal tissues in embryonic and adult life appear to have a balance of factors that favor capillary formation and maintenance, while in disease states such as cancer, these factors may be absent or alternatively, are present but are overwhelmed by factors that cause capillary dysfunction. Thus, does capillary dysfunction primarily result from a lack of appropriate pro-morphogenic factors or the presence of inhibitory factors (i.e. pro-regressive factors) which cause vessel regression (or both)? Not enough emphasis has yet been placed on this latter aspect, which could underlie the basis for capillary abnormalities in many diseases. The identification of inhibitory factors that disrupt capillary function and cause capillary regression is a very critical area for future investigation, since blockade of such factors or their downstream signaling pathways might restore capillary function and thereby reduce key disease mechanisms (Figure 1).
Major efforts have been ongoing to correct the vascular defects within malignant tumor tissues [89-91]. Can these abnormal vessels be repaired and if so, does this enhance our ability to treat cancers? Considerable effort has been undertaken to normalize the tumor vasculature (such as blockade of VEGF and VEGF-dependent signaling) with the hope that better delivery of anti-tumor agents could be delivered to the malignant tumor through the repaired vascular networks [89, 90, 92-95]. Manipulation of angiopoietin-2 signaling (which appears to destabilize the vasculature) vs. angiopoietin-1 (which promotes normalization) is another approach to promote vessel normalization [96]. Angiopoietin-1 activates Rac1 which facilitates EC junction assembly, and another molecule, intermedin, has similar effects and also promotes tumor vessel normalization [95, 96]. Other interesting molecules that promote tumor vessel normalization include a prolyl hydroxylase, PHD2, and R-Ras [97, 98]. One study identified an adhesion molecule, L1, which is typically expressed in neural tissues, to be elevated in expression on tumor vessel ECs and which contributed to vessel instability [99]. Blockade of L1 function led to tumor vessel normalization. Overall, there is considerable evidence to suggest that tumor vessel normalization can facilitate the treatment of malignant cancers by facilitating anti-tumor drug delivery. Here, we hypothesize that capillary normalization within malignant tumors might enhance therapeutic efficacy for other reasons. For example, repair of capillaries might reduce key tissue injury responses and injury stimuli (e.g. inflammation, edema, thrombosis and ischemia) (Figure 1) present within the tumor microenvironment, leading to normalization of tumor cells themselves to a less malignant phenotype by enhancing cellular differentiation and reducing tumor cell proliferation.
Major questions remain concerning the nature of the problem in the tumor microenvironment, which directly impacts our approach to therapeutics. Are the particular characteristics of malignant tumor cells the key problem or is it their interaction with non-malignant host cells such as the vasculature or perivascular cell types (e.g. macrophages, mast cells, fibroblasts) that are central to defining the nature of the malignant tumor phenotype? The majority of studies have directly focused on the tumor cells and their altered genetic, signaling and metabolic profiles. However, their interactions with the host (e.g. leading to capillary abnormalities, vascular disruption, and stromal reactions such as fibrosis) may be just as crucial toward determining the malignant behavior of tumor cells over time in a clinical setting.
Critical issues and questions such as these remain and for certain, the molecular basis for capillary morphogenesis and maintenance is considerably more complex than previously realized. The growth factor requirements and signaling pathways alone are highly complex under normal conditions and it is necessary for these fundamental processes to be elucidated as soon as possible so that more sophisticated treatment approaches can be devised to address different disease states. This information is also essential for efforts in regenerative medicine and tissue engineering where many problems have arisen with regard to integrating a stable microvasculature within tissue constructs. Our belief is that a detailed understanding of the fundamental cell biology and cell-cell interactions in these contexts will ultimately reveal the solutions to these complex clinical issues. For example, many of our central assumptions regarding tumors and blood vessels should be questioned and re-considered based on our current knowledge for how normal capillary networks are assembled, maintained and stabilized, and then how malignant tumors interact to impact these processes. Similar considerations should be made for key diseases such as diabetes, obesity, and neurodegenerative conditions (e.g. Alzheimer's disease) where capillary dysfunction plays a key pathogenic role.
Conclusions
In this review, we have discussed how ECs and pericytes co-assemble to form networks of capillary tubes, a fundamental requirement for tissue development and homeostasis. We hypothesize that normal capillaries through ECs and pericytes deliver key signals to parenchymal cells within tissues (and vice-versa) that facilitate tissue health, and in so doing, actually suppress primary pathologic events including inflammation, infection, thrombosis, hemorrhage, edema, ischemic injury, fibrosis, autoimmune disease, and tumor growth/ progression. In major human diseases such as cancer and diabetes, capillary dysfunction represents a primary problem that directly contributes to the initiation and progression of the disease process. Although great progress has been made toward our understanding of the assembly and function of capillaries, considerably more effort needs to be placed at understanding the fundamental cell biology of ECs and pericytes and their ability to interact with parenchymal cells and other supporting cell types within tissues (in health and disease). It is our contention that capillary dysfunction underlies the pathogenesis of many disease states and repair of this dysfunction, although a complex problem, may represent a key way to improve the health of vascularized tissues and the overall patient. Developing specific therapeutics to repair systemic or localized capillary dysfunction might represent a major advance to combating or reducing the negative impact of many human diseases via promotion of capillary-tissue parenchyma cross-talk leading to suppression of major underlying mechanisms of disease.
Acknowledgments
The authors would like to thank Dr. Amber Stratman for her outstanding work and contributions to our understanding of human capillary tube assembly. This work was supported by NIH grants HL105606 and HL126518.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- 1.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 2.Davis GE, Stratman AN, Sacharidou A, Koh W. Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. Int Rev Cell Mol Biol. 2011;288:101–165. doi: 10.1016/B978-0-12-386041-5.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol. 2011;3:a005090. doi: 10.1101/cshperspect.a005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stratman AN, Davis GE. Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization. Microsc Microanal. 2012;18:68–80. doi: 10.1017/S1431927611012402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu K, Cleaver O. Tubulogenesis during blood vessel formation. Semin Cell Dev Biol. 2011;22:993–1004. doi: 10.1016/j.semcdb.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 7.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kume T. Specification of arterial, venous, and lymphatic endothelial cells during embryonic development. Histol Histopathol. 2010;25:637–646. doi: 10.14670/hh-25.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocha SF, Adams RH. Molecular differentiation and specialization of vascular beds. Angiogenesis. 2009;12:139–147. doi: 10.1007/s10456-009-9132-x. [DOI] [PubMed] [Google Scholar]
- 10.Davis GE. Molecular regulation of vasculogenesis and angiogenesis: Recent advances and future directions. In: Homeister JW, Willis MS, editors. Molecular and Translational Vascular Medicine. Springer; New York: 2012. pp. 169–206. [Google Scholar]
- 11.Ramasamy SK, Kusumbe AP, Adams RH. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 2015;25:148–157. doi: 10.1016/j.tcb.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleaver O, Dor Y. Vascular instruction of pancreas development. Development. 2012;139:2833–2843. doi: 10.1242/dev.065953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Nagy JA, Dvorak AM, Dvorak HF. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med. 2012;2:a006544. doi: 10.1101/cshperspect.a006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prieto D, Contreras C, Sanchez A. Endothelial dysfunction, obesity and insulin resistance. Curr Vasc Pharmacol. 2014;12:412–426. doi: 10.2174/1570161112666140423221008. [DOI] [PubMed] [Google Scholar]
- 16.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2011;20:297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 17.van Hinsbergh VW. Endothelium--role in regulation of coagulation and inflammation. Semin Immunopathol. 2012;34:93–106. doi: 10.1007/s00281-011-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trojanowska M. Cellular and molecular aspects of vascular dysfunction in systemic sclerosis. Nat Rev Rheumatol. 2010;6:453–460. doi: 10.1038/nrrheum.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2009;335:165–189. doi: 10.1007/s00441-008-0685-6. [DOI] [PubMed] [Google Scholar]
- 20.Fligny C, Duffield JS. Activation of pericytes: recent insights into kidney fibrosis and microvascular rarefaction. Curr Opin Rheumatol. 2013;25:78–86. doi: 10.1097/BOR.0b013e32835b656b. [DOI] [PubMed] [Google Scholar]
- 21.Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res 37 Suppl. 2005;1:39–43. doi: 10.1055/s-2005-861361. [DOI] [PubMed] [Google Scholar]
- 22.Karaca U, Schram MT, Houben AJ, Muris DM, Stehouwer CD. Microvascular dysfunction as a link between obesity, insulin resistance and hypertension. Diabetes Res Clin Pract. 2014;103:382–387. doi: 10.1016/j.diabres.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163:1801–1815. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 26.Ostergaard L, Aamand R, Gutierrez-Jimenez E, Ho YC, Blicher JU, Madsen SM, Nagenthiraja K, Dalby RB, Drasbek KR, Moller A, et al. The capillary dysfunction hypothesis of Alzheimer's disease. Neurobiol Aging. 2013;34:1018–1031. doi: 10.1016/j.neurobiolaging.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Perlmutter LS, Chui HC. Microangiopathy, the vascular basement membrane and Alzheimer's disease: a review. Brain Res Bull. 1990;24:677–686. doi: 10.1016/0361-9230(90)90007-m. [DOI] [PubMed] [Google Scholar]
- 28.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 29.Weis SM. Vascular permeability in cardiovascular disease and cancer. Curr Opin Hematol. 2008;15:243–249. doi: 10.1097/MOH.0b013e3282f97d86. [DOI] [PubMed] [Google Scholar]
- 30.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–4730. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowers SL, Meng CX, Davis MT, Davis GE. Investigating human vascular tube morphogenesis and maturation using endothelial cell-pericyte co-cultures and a doxycycline-inducible genetic system in 3D extracellular matrices. Methods Mol Biol. 2015;1189:171–189. doi: 10.1007/978-1-4939-1164-6_12. [DOI] [PubMed] [Google Scholar]
- 34.Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr Biol. 2011;21:R126–136. doi: 10.1016/j.cub.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16:222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacharidou A, Stratman AN, Davis GE. Molecular mechanisms controlling vascular lumen formation in three-dimensional extracellular matrices. Cells Tissues Organs. 2012;195:122–143. doi: 10.1159/000331410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci. 2008;121:989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- 39.Koh W, Sachidanandam K, Stratman AN, Sacharidou A, Mayo AM, Murphy EA, Cheresh DA, Davis GE. Formation of endothelial lumens requires a coordinated PKC{epsilon}-, Src-, Pak- and Raf-kinase-dependent signaling cascade downstream of Cdc42 activation. J Cell Sci. 2009;122:1812–1822. doi: 10.1242/jcs.045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu K, Sacharidou A, Fu S, Chong DC, Skaug B, Chen ZJ, Davis GE, Cleaver O. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell. 2011;20:1–14. doi: 10.1016/j.devcel.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan L, Sacharidou A, Stratman AN, Le Bras A, Zwiers PJ, Spokes K, Bhasin M, Shih SC, Nagy JA, Molema G, et al. RhoJ is an endothelial cell-restricted Rho GTPase that mediates vascular morphogenesis and is regulated by the transcription factor ERG. Blood. 2011;118:1145–1153. doi: 10.1182/blood-2010-10-315275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci. 2002;115:1123–1136. doi: 10.1242/jcs.115.6.1123. [DOI] [PubMed] [Google Scholar]
- 43.Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, Davis MJ, Davis GE. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood. 2009;114:237–247. doi: 10.1182/blood-2008-12-196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dejana E, Vestweber D. The role of VE-cadherin in vascular morphogenesis and permeability control. Prog Mol Biol Transl Sci. 2013;116:119–144. doi: 10.1016/B978-0-12-394311-8.00006-6. [DOI] [PubMed] [Google Scholar]
- 46.Sacharidou A, Koh W, Stratman AN, Mayo AM, Fisher KE, Davis GE. Endothelial lumen signaling complexes control 3D matrix-specific tubulogenesis through interdependent Cdc42- and MT1-MMP-mediated events. Blood. 2010 doi: 10.1182/blood-2009-11-252692. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zovein AC, Luque A, Turlo KA, Hofmann JJ, Yee KM, Becker MS, Fassler R, Mellman I, Lane TF, Iruela-Arispe ML. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell. 2010;18:39–51. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, Dejana E. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci. 2010;123:1073–1080. doi: 10.1242/jcs.059329. [DOI] [PubMed] [Google Scholar]
- 49.Smith AO, Bowers SL, Stratman AN, Davis GE. Hematopoietic stem cell cytokines and fibroblast growth factor-2 stimulate human endothelial cell-pericyte tube co-assembly in 3D fibrin matrices under serum-free defined conditions. PLoS One. 2013;8:e85147. doi: 10.1371/journal.pone.0085147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis GE, Camarillo CW. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 51.Fisher KE, Pop A, Koh W, Anthis NJ, Saunders WB, Davis GE. Tumor cell invasion of collagen matrices requires coordinate lipid agonist-induced G-protein and membrane-type matrix metalloproteinase-1-dependent signaling. Mol Cancer. 2006;5:69. doi: 10.1186/1476-4598-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisher KE, Sacharidou A, Stratman AN, Mayo AM, Fisher SB, Mahan RD, Davis MJ, Davis GE. MT1-MMP- and Cdc42-dependent signaling co-regulate cell invasion and tunnel formation in 3D collagen matrices. J Cell Sci. 2009;122:4558–4569. doi: 10.1242/jcs.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 54.Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9:407–415. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- 55.Stratman AN, Davis MJ, Davis GE. VEGF and FGF prime vascular tube morphogenesis and sprouting directed by hematopoietic stem cell cytokines. Blood. 2011;117:3709–3719. doi: 10.1182/blood-2010-11-316752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis GE, Kim DJ, Meng CX, Norden PR, Speichinger KR, Davis MT, Smith AO, Bowers SL, Stratman AN. Control of vascular tube morphogenesis and maturation in 3D extracellular matrices by endothelial cells and pericytes. Methods Mol Biol. 2013;1066:17–28. doi: 10.1007/978-1-62703-604-7_2. [DOI] [PubMed] [Google Scholar]
- 57.Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114:2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- 58.Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost 5 Suppl. 2007;1:32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- 59.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 61.Wijelath ES, Murray J, Rahman S, Patel Y, Ishida A, Strand K, Aziz S, Cardona C, Hammond WP, Savidge GF, et al. Novel vascular endothelial growth factor binding domains of fibronectin enhance vascular endothelial growth factor biological activity. Circ Res. 2002;91:25–31. doi: 10.1161/01.res.0000026420.22406.79. [DOI] [PubMed] [Google Scholar]
- 62.Xu Y, Yu Q. Angiopoietin-1, unlike angiopoietin-2, is incorporated into the extracellular matrix via its linker peptide region. J Biol Chem. 2001;276:34990–34998. doi: 10.1074/jbc.M103661200. [DOI] [PubMed] [Google Scholar]
- 63.Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol. 2005;170:993–1004. doi: 10.1083/jcb.200507082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124:2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Apte MV, Pirola RC, Wilson JS. Pancreatic stellate cells: a starring role in normal and diseased pancreas. Front Physiol. 2012;3:344. doi: 10.3389/fphys.2012.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carloni V, Luong TV, Rombouts K. Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: more complicated than ever. Liver Int. 2014;34:834–843. doi: 10.1111/liv.12465. [DOI] [PubMed] [Google Scholar]
- 67.Coulouarn C, Clement B. Stellate cells and the development of liver cancer: therapeutic potential of targeting the stroma. J Hepatol. 2014;60:1306–1309. doi: 10.1016/j.jhep.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Hellerbrand C. Hepatic stellate cells--the pericytes in the liver. Pflugers Arch. 2013;465:775–778. doi: 10.1007/s00424-012-1209-5. [DOI] [PubMed] [Google Scholar]
- 69.Kolodecik T, Shugrue C, Ashat M, Thrower EC. Risk factors for pancreatic cancer: underlying mechanisms and potential targets. Front Physiol. 2013;4:415. doi: 10.3389/fphys.2013.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902–1910. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith SW, Schrimpf C, Parekh DJ, Venkatachalam M, Duffield JS. Kidney pericytes: a novel therapeutic target in interstitial fibrosis. Histol Histopathol. 2012;27:1503–1514. doi: 10.14670/HH-27.1503. [DOI] [PubMed] [Google Scholar]
- 72.Kadin ME. Pathology of Hodgkin's disease. Curr Opin Oncol. 1994;6:456–463. doi: 10.1097/00001622-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dahlback B, Villoutreix BO. Molecular recognition in the protein C anticoagulant pathway. J Thromb Haemost. 2003;1:1525–1534. doi: 10.1046/j.1538-7836.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 76.Esmon CT. The endothelial protein C receptor. Curr Opin Hematol. 2006;13:382–385. doi: 10.1097/01.moh.0000239712.93662.35. [DOI] [PubMed] [Google Scholar]
- 77.Mohan Rao LV, Esmon CT, Pendurthi UR. Endothelial cell protein C receptor: a multiliganded and multifunctional receptor. Blood. 2014;124:1553–1562. doi: 10.1182/blood-2014-05-578328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xue M, Minhas N, Chow SO, Dervish S, Sambrook PN, March L, Jackson CJ. Endogenous protein C is essential for the functional integrity of human endothelial cells. Cell Mol Life Sci. 2010;67:1537–1546. doi: 10.1007/s00018-010-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 80.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26:441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 81.Burgey C, Kern WV, Romer W, Sakinc T, Rieg S. The innate defense antimicrobial peptides hBD3 and RNase7 are induced in human umbilical vein endothelial cells by classical inflammatory cytokines but not Th17 cytokines. Microbes Infect. 2015 doi: 10.1016/j.micinf.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 82.Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olas B. Gasomediators (NO, CO, and HS) and their role in hemostasis and thrombosis. Clin Chim Acta. 2015;445:115–121. doi: 10.1016/j.cca.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 84.Maier CL, Pober JS. Human placental pericytes poorly stimulate and actively regulate allogeneic CD4 T cell responses. Arterioscler Thromb Vasc Biol. 2011;31:183–189. doi: 10.1161/ATVBAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Potenza MA, Gagliardi S, Nacci C, Carratu MR, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem. 2009;16:94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 86.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nagy JA, Chang SH, Dvorak AM, Dvorak HF. Why are tumour blood vessels abnormal and why is it important to know? Br J Cancer. 2009;100:865–869. doi: 10.1038/sj.bjc.6604929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davis GE, Senger DR. Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr Opin Hematol. 2008;15:197–203. doi: 10.1097/MOH.0b013e3282fcc321. [DOI] [PubMed] [Google Scholar]
- 89.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 90.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 91.Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect Med. 2012;2:a006486. doi: 10.1101/cshperspect.a006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mancuso MR, Davis R, Norberg SM, O'Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoang MV, Smith LE, Senger DR. Moderate GSK-3beta inhibition improves neovascular architecture, reduces vascular leakage, and reduces retinal hypoxia in a model of ischemic retinopathy. Angiogenesis. 2010;13:269–277. doi: 10.1007/s10456-010-9184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoang MV, Nagy JA, Senger DR. Cdc42-mediated inhibition of GSK-3beta improves angio-architecture and lumen formation during VEGF-driven pathological angiogenesis. Microvasc Res. 2011;81:34–43. doi: 10.1016/j.mvr.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoang MV, Nagy JA, Senger DR. Active Rac1 improves pathologic VEGF neovessel architecture and reduces vascular leak: mechanistic similarities with angiopoietin-1. Blood. 2011;117:1751–1760. doi: 10.1182/blood-2010-05-286831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Falcon BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A, Oliner JD, McDonald DM. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol. 2009;175:2159–2170. doi: 10.2353/ajpath.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sawada J, Urakami T, Li F, Urakami A, Zhu W, Fukuda M, Li DY, Ruoslahti E, Komatsu M. Small GTPase R-Ras regulates integrity and functionality of tumor blood vessels. Cancer Cell. 2012;22:235–249. doi: 10.1016/j.ccr.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Magrini E, Villa A, Angiolini F, Doni A, Mazzarol G, Rudini N, Maddaluno L, Komuta M, Topal B, Prenen H, et al. Endothelial deficiency of L1 reduces tumor angiogenesis and promotes vessel normalization. J Clin Invest. 2014;124:4335–4350. doi: 10.1172/JCI70683. [DOI] [PMC free article] [PubMed] [Google Scholar]