Abstract
The resistance of cancer cells to chemotherapeutic agents represents the main problem in cancer treatment. Despite intensive research, mechanisms of resistance have not yet been fully elucidated. Six1 signaling has an important role in the expansion of progenitor cell populations during early embryogenesis. Six1 gene overexpression has been strongly associated with aggressiveness, invasiveness, and poor prognosis of different cancers. In this study, we investigated the role of Six1 signaling in resistance of MCF-7 breast cancer cells to taxanes. We first established in vitro paclitaxel-resistant MCF-7 breast cancer cells. Morphological modifications in paclitaxel-resistant cells were examined via light microscopic images and fluorescence-activated cell sorting analysis. Applying quantitative real-time polymerase chain reaction, we measured Six1, B-cell lymphoma/leukemia(BCL-2), BAX, and P53 mRNA expression levels in both non-resistant and resistant cells. Resistant cells were developed from the parent MCF-7 cells by applying increasing concentrations of paclitaxel up to 64 nM. The inhibitory concentration 50% value in resistant cells increased from 3.5 ± 0.03 to 511 ± 10.22 nM (p = 0.015). In paclitaxel-resistant cells, there was a significant increase in Six1 and BCL-2 mRNA levels (p = 0.0007) with a marked decrease in pro-apoptotic Bax mRNA expression level (p = 0.03); however, there was no significant change in P53 expression (p = 0.025). Our results suggest that identifying cancer patients with high Six1 expression and then inhibition of Six1 signaling can improve the efficiency of chemotherapeutic agents in the induction of apoptosis.
KEY WORDS: Six1, P53, apoptosis, MCF-7, paclitaxel-resistant cells
INTRODUCTION
Breast cancer is the most frequent cancer among women aged between 50 and 70. It affects one in eight women on average. About one million new cases of breast cancer are diagnosed each year worldwide. With the improvement of treatments, the survival rate of women diagnosed with breast cancer has increased; however, still the mortality among breast cancer patients remains significantly high [1-3]. Although, paclitaxel is one of the first-line treatments for breast cancer [4], the efficacy of this agent has been restricted by the acquired resistance in cancer cells [5]. The resistance of cancer cells to chemotherapeutic agents represents a major problem in cancer treatment. Despite all the efforts, mechanisms of resistance have not yet been fully understood. Paclitaxel is a common anticancer agent in a broad range of epithelial cancers, including carcinomas of the ovary, breast with high possibility of developing chemoresistance. One of the acquired tumor cell resistance mechanisms to paclitaxel is β-tubulin gene point mutations. P53 status is the singe of mitosis checkpoints to determine the sensitivity of cells to paclitaxel [6-8]. Investigation on the mechanisms underlying paclitaxel resistance in cancer cells can lead to novel strategies to improve the efficacy of the chemotherapeutic agents. B-cell lymphoma/leukemia (BCL-2) is known as a first proto-oncogene with an anti-apoptotic function which belongs to BCL-2 family of proteins acting as key regulators of apoptosis [9]. Some of these proteins including Bcl-2 and Bcl-XL play the anti-apoptotic role while others such as Bad, Bax, and Bid are pro-apoptotic. The balance of pro- and anti-apoptotic Bcl-2 proteins can regulate the sensitivity of the cells to cell death by altering the permeability of the outer mitochondrial membrane. The anti-apoptotic protein groups such as Bcl-2 and Bcl-XL, which mostly inhibit apoptosis lead to inactivation of the Bax/Bak proteins interaction [10]. Overexpression of bcl-2 can also block p53-induced apoptosis [11,12]. P53 (encoded by TP53) is a tumor suppressor that acts as a major control for the cellular response to chemotherapy. More than 50% of human cancer cells are associated with missense mutations or deletions of p53 which results in chemoresistance [13]. Mechanism of P53 action is based on protein-protein interactions and binding to specific promoter sequences to activate cell-cycle arrest, senescence, and apoptosis-related genes [14]. DNA damage induced by chemotherapy drugs is another factor for p53 activation. P53 expression level is decreased by Sineoculishomeobox homolog 1 (Six1) in response to DNA damage in a proteasome independent manner [15]. Six1 is one of the SIX family members, which was first identified and characterized as a mammalian homolog of the Drosophila sineoculis (so) gene [16]. This homeodomain transcription factor has been implicated in tumor progression and embryogenesis [17,18]. Six1 stimulates proliferation and survival of progenitor cells during normal development [19,20] which loss of its function leads to a reduction in size or the absence of various organs, because of a decrease in cell proliferation and increase in apoptosis [16,18,21]. Recent studies showed that Six1 overexpression is associated with a poor prognosis in numerous cancers including ovarian cancer, hepatocellular carcinoma, and cervical cancers [8,22]. The overexpression of Six1 protein likely contributes epithelial carcinogenesis by increasing of proliferation and decreasing apoptosis [23], or genomic instability [24]. In this study, after the establishment of paclitaxel-resistant MCF-7 cells, we first determined the inhibitory concentration 50% (IC50) values of both resistant and non-resistant cells. We also investigated morphological changes in the cells via diaminophenylindole (DAPI) staining. The mRNA expression levels of Six1, Bax, Bcl-2, and P53 were assessed by real-time (RT)-polymerase chain reaction (PCR) in both cell lines. Our findings add new insights into the mechanisms of resistance to paclitaxel in breast cancer cells.
MATERIALS AND METHODS
Materials
Paclitaxel was purchased from Ebetaxel®, EBEWE Pharma (Unterach- Austuria); RPMI-1640 medium; 3-(4, 5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and penicillin–streptomycin were obtained from Sigma-Aldrich (St. Louis, MO, USA); fetal bovine serum (FBS) was from Gibco; Primers were purchased from (Ebersberg, Germany); RNX™-Plus Kit was obtained from CinnaGen (Tehran, Iran); REVERTA-L RT reagent kit was from Central Institute of Epidemiology (Moscow, Russia); SYBR green PCR Master Mix was purchased from Applied Biosystems (Warrington, UK).
Cell culture
The human MCF-7 breast cancer cells were purchased from National Cell Bank of Iran (Pasteur Institute, Iran). The MCF-7 cells were grown in RPMI 1640 medium containing 10% FBS along with 100 mg/mL streptomycin and 100 units/mL penicillin G in humidified 5% CO2 at 37°C incubator.
Development of paclitaxel-resistant MCF-7 cells
Cells with about 20-30% confluency were treated with increasing concentrations of paclitaxel. Initial treatment concentration of paclitaxel was one-tenth of IC50 value (0.5 nM) which was determined via MTT assay. Paclitaxel-resistant MCF-7 cells were established by treating cells with continues and stepwise increase in paclitaxel concentration (0.5-64 nM). Culture medium for growth of paclitaxel-resistant MCF-7 cells was enriched with 20% FBS and 10% conditioned medium. Conditioned medium was the supernatant medium of cultured non-resistant MCF-7 cells with about 80% confluency. Cells subcultured when became confluent, and then the concentration of paclitaxel was increased to 1.5 times higher than previous concentration. This process continued to the maximum concentration of 64 nM paclitaxel. MTT assay was applied to confirm the resistance of the cells to each concentration of paclitaxel [5,12].
Assessment of cell viability using MTT assay
MTT assay was applied for the assessment of cell growth inhibition. Cell proliferation and viability of sensitive MCF-7 cells and paclitaxel-resistant cells were determined using MTT, (5 mg/mL, Sigma) which evaluates the percentage of viable cells. The cells with about 70% confluency were collected from culture flask with 0.05% trypsin/ethylenediaminetetraacetic solution. Cells were seeded into a 96-well plate (200 µL/well) with concentration of 4 × 104 cells/cm2. MCF-7 and MCF-7/Pac 64 nM cells with about 50% confluency were treated with increasing concentrations of the paclitaxel 0.1-50 nM and 50-1000 nM, respectively. Four wells were remained untreated as controls. The cells were incubated with different concentrations of paclitaxel for 24, 48, and 72 hours. Then, 20 µL of MTT were added to each well, and the absorbance was measured at 570 nm on an ELISA plate reader (Biotek, EL x800, USA). The cell viability of each well was calculated compared to non-treated cells. Three independent experiments were performed for each paclitaxel concentration [25].
DAPI staining assay
MCF-7 and MCF-7/Pac 64 nM cells were seeded within chamber dishes and treated with and without different concentrations of paclitaxel for 48 hours. Then, the cells were washed with phosphate-buffered saline (PBS) twice and then fixed with 4% paraformaldehyde for 30 minutes. The fixed cells were then washed with PBS and then permeabilized with 0.1% Triton-X-100 for 10 minutes. Cells then were stained with DAPI (1:500 dilutions in PBS) for 10 minutes. Cells were evaluated as normal or apoptotic depending on morphological characteristics. Normal nuclei (smooth nuclear) and apoptotic nuclei (condensed or fragmented chromatin) were easily distinguished. Triplicate samples were prepared for each treatment, and at least 300 cells were counted in random fields for each sample and apoptotic nuclei were identified [26].
Assessment of cell death by flow cytometric assay
Apoptotic cells were detected by fluorescence-activated cell sorting (FACS) with the FITC Annexin V Apoptosis Detection Kit I (eBiosciences, San Diego, CA, USA). Briefly, MCF-7 and MCF-7/Pac 64 nM were harvested after the incubation with 64 nM concentrations of paclitaxel, washed twice with PBS, and re-suspended in the annexin-binding buffer at a concentration of 1 × 106 cells per mL. 100 µL of the cell solution was stained with 5 µL Annexin V and 5 µL PI at room temperature for 20 minutes in the dark. The data were collected and analyzed with a FACS calibur flow cytometer using the Flowjo Software Tree Star Software, San Carlos, California, USA [27].
Quantitative RT-PCR
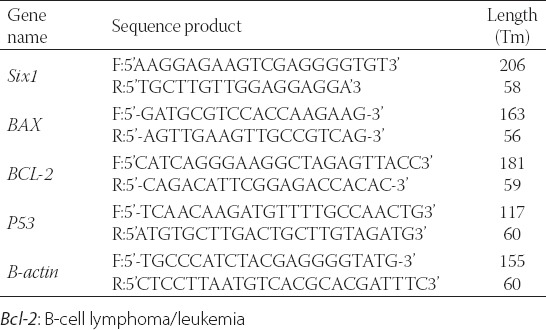
The mRNA expression of the genes involved in the apoptotic pathway was investigated in non-resistant MCF-7 cells and MCF-7/Pac 64 nM cells with different concentrations of paclitaxel. RNA extraction of the cells was done by RNA extraction kit according to the protocol. The quality of RNA was qualified by agarose gel electrophoresis. The concentration of extracted RNA was evaluated by optical density measurement (A260/A280 ratio) with NanoDrop 1000 Spectrophotometer (Wilmington, DE, USA). REVERTA-L RT reagents kit was applied for conversion of RNA to cDNA. Then the reaction tubes were incubated at 42°C for 60 minutes. RT-PCR was carried out using the SYBR Green-based PCR Master Mix and analyzed on a Corbett 6000 Rotor-Gene thermocycler (Corbett Research). Beacon Designer™ 5.01 software was applied to design Primers for cDNA amplification. The sequences for primers are shown in Table 1. The total volume of amplification reactions was 25 µL, and each well was included 12.5 µL of SYBR Green PCR Master Mix, 1 µL of cDNA, 70-100 nM of both forward and reverse special primers. The PCR thermal cycling steps were included 10 minutes at 95°C, 40 cycles of 25 seconds at 95°C for denaturation step, 25 seconds at annealing temperature, and 25 seconds at 72°C for the extension, respectively. Final 10 minutes incubation at 72°C was carried out to completion of amplicons. The β-actin expression was provided as an internal reference gene (housekeeping gene) to normalize the expression of the apoptotic genes. Melting-curve analysis was carried out after amplification to verification the validity of the amplicon. The Pfaffl method was applied for reporting gene expression level [28,29].
TABLE 1.
Corresponding primers which were used for gene expressions analysis
Statistical analysis
All data were represented as mean ± standard deviation of three independent experiments. A paired t-test was performed to determine the significance of differences between control and treatment groups, p< 0.05 was considered as statistically significant.
RESULTS
Anti-proliferation effects of paclitaxel on sensitive and resistant MCF-7 cells
To evaluate anti-proliferative and cytotoxic effects of paclitaxel, cells were exposed to various concentrations of paclitaxel and cell viability was evaluated via MTT assay. Then, paclitaxel-resistant MCF-7 cells were developed and MCF-7 resistant to 64 nM paclitaxel (MCF-7/Pac 64 nM) was selected for further investigation. IC50 of MCF-7/Pac 64 nM cells were increased from 0.2 to 1000 nM (p = 0.015) (Figure 1a and b).
FIGURE 1.
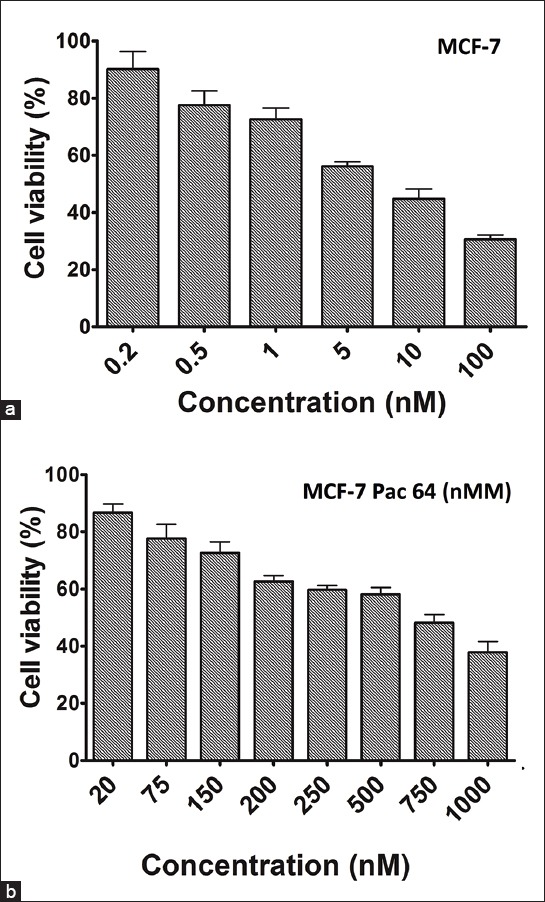
Determination of inhibitory concentration 50% of paclitaxel against MCF-7 cell. 3-(4, 5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide results of parent MCF-7 cell line (a), MCF-7/Pac 64 nM (b).
Evaluation of the formation of apoptotic nuclei in resistant cells
To investigate, the effects of paclitaxel on the apoptotic response, DAPI nuclear staining was applied. We selected resistant concentration of paclitaxel in MCF-7 cells. Once apoptosis was triggered, morphological changes including apoptotic bodies were evaluated. After treatment of MCF-7 and MCF-7/Pac 64 nM with IC50 concentration of paclitaxel, the morphology of the MCF-7 cells changed with the development of apoptotic bodies. Our results showed that the percentage of apoptotic cells decreased from 35% in MCF-7 cells (Figure 2a) to 10% in MCF-7/Pac 64 nM cells (p = 0.032) (Figure 2b) when we incubated the cells with 64 nM of paclitaxel for 48 hours.
FIGURE 2.
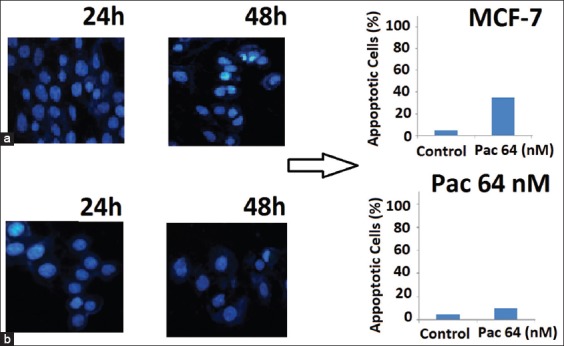
Effect of 64 nM concentration of paclitaxel on morphology of cells. Apoptotic cells were characterized by condense nuclei and non-apoptotic dead cells: Diaminophenylindole images of cells treated with 64 nM concentration of paclitaxel in MCF-7 (a) and MCF-7/Pac 64 nM (b).
Paclitaxel-induced apoptosis in non-resistant and resistant MCF-7 cells
The effects of various concentrations of paclitaxel in the induction of apoptosis and necrosis were measured at 48 hours after treatment. To study, the effect of paclitaxel on resistant cells, after determination of IC50 value (64 nM), various concentrations of paclitaxel (0-1000 nM) were incubated with the cells for 48 hours. The percentage of apoptosis at 64 nM was similar to control(Figure 3a) which verified the resistance of MCF-7 in our research (Figure 3b). The percentage of apoptosis at 200 and 500 nM concentrations showed no significant differences (80% cell death) (Figure 3c and d). Incubation of the cells with 700 nM for 48 hours showed a maximum 87.3% apoptosis (Figure 3e). The relative frequencies of apoptotic and necrotic cells in the presence of different concentrations of paclitaxel using flow cytometry with Annexin-V staining showed that the apoptosis was the main type of cell death involved in the inhibition of cell survival compared to necrosis.
FIGURE 3.
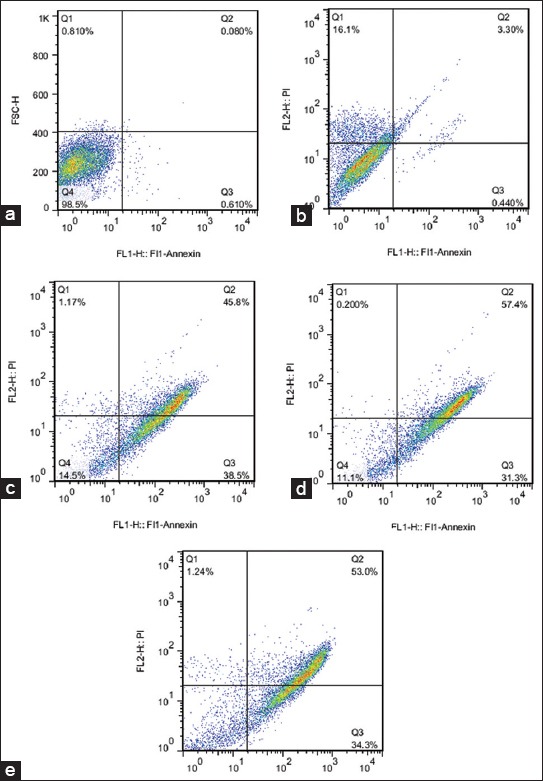
The effects of different paclitaxel concentrations (nM) on the rates of apoptosis of MCF-7/Pac 64 nM cells. The values were shown in percentage (%) from the most appropriate individual test. Flow cytometric measurement was performed by staining with annexin-V and the corresponding scatter plots were depicted for different treated concentrations of paclitaxel as control, 0 µM (a), 64 (b), 200 (c), 500 (d), and 700 (e). Apoptosis, the percentage of necrosis and viability of these effects were also underlined in each scatter plot.
Expression levels of pro/anti-apoptotic genes in response to paclitaxel
Our results from RT-PCR showed that in MCF-7/Pac 64 nM cells, the mRNA expression level of the pro-apoptotic gene, P53, did not changed significantly (Figure 4a) while the mRNA expression level of anti- apoptotic Six1 gene was elevated 1.5-fold compared to non-resistant cells (p< 0.001) (Figure 4b).
FIGURE 4.
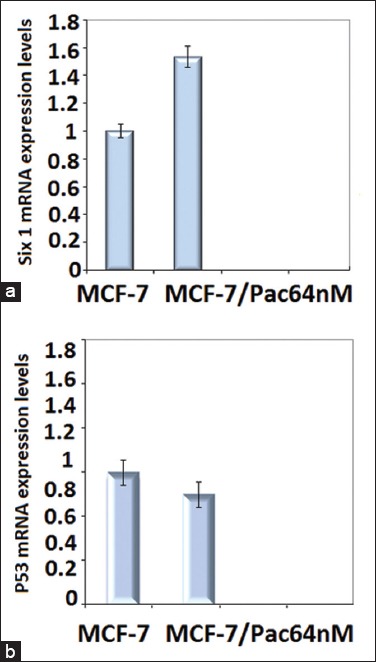
Gene expression patterns of Six1 and P53 gene in MCF-7 and MCF-7/Pac 64 nM cells. Ribosomal β-actin was used as housekeeping gene. Effects of paclitaxel on pro/anti-apoptotic genes: (a) Level of mRNA of Six1 was increased significantly in MCF-7/Pac 64 nM (p = 0.0007) compared with MCF-7 cell line, (b) Level of p53 did not change significantly in paclitaxel-resistant cell line compare with parent MCF-7 cell line MCF-7/Pac 64 nM.
DISCUSSION AND CONCLUSIONS
Paclitaxel is an important anticancer drug that is clinically used for patients with various types of cancers; however, tumor cell resistance to this agent remains the main obstacle in successful cancer therapy. Investigation of molecular mechanisms involved in the chemo-resistance can be helpful in designing novel strategies in cancer treatment. Paclitaxel-resistant MCF-7 cell line was previously developed to study the mechanisms of resistance and key modulator molecules including paclitaxel binding proteins, extracellular matrix proteins, drug transporters, and β-tubulin isoforms [30]. Six1 expression is associated with poor prognosis in luminal breast cancers, especially in the aggressive luminal B subtype [31]. Six1 mRNA is over expressed in pancreatic cancer which is associated with the advanced tumor stage. Six1induces up-regulation of cyclinD1 in promoting cell cycle progression and proliferation [32]. However, the role of Six1 signaling in survival of resistant cancer cells is still un-known. We first established paclitaxel-resistant breast cancer cells from MCF-7 cell line. After determination of cytotoxicity in resistant and non-resistant cells, the effects of high concentrations of paclitaxel were examined on resistant-MCF-7 cells to evaluate mRNA expression levels of Six1 and P53 [12]. Our findings demonstrate that Six1 enhances cellular proliferation in MCF-7 cells in response to lower concentrations of paclitaxel. The induction of cellular proliferation by Six1 signaling was dependent on the regulation of pro/anti-apoptotic genes. Our results showed that increasing anti-apoptotic gene expression including Six1 decreased the percentage of apoptotic cells as determined by Annexin V staining and FACS analysis. To examine the mechanism by which Six1 enhanced breast cancer cell proliferation, we considered the anti/pro-apoptotic genes as potential targets of Six1-induced cell proliferation. Paclitaxel changed Six1 gene expression which caused changing the expression levels of Bcl-2 and Bax. Bcl-2 gene was elevated 1.13-fold and Bax mRNA level decreased 0.4-fold relative to MCF-7 cells [12]; however, it did not have a significant effect on changing P53 gene expression. Microtubule-stabilizing paclitaxel is considered to have p53-independent effects. TP53 shows fidelity within tumor types, but it is promiscuous from one cancer to the next [33]. Response to paclitaxcel has been observed in the cells with mutations or lack of p53 expression [34,35]. In this study, the level of p53 gene expression did not change significantly in MCF-7/Pac 64 nM cells compared which are consistent with the previous study. This finding can be explained by p53-independent mechanisms of paclitaxel resistance. The ratio of Bcl-2/Bax determines the susceptibility of a cell to apoptosis. The higher expression of Bcl-2 was associated with longer overall survival [36]. In addition, many of the components in this pathway are currently being explored as markers for tumorigenesis and potential targets for treatment. Our results showed 2.82-fold increase in Bcl-2/Bax ratios in MCF-7/Pac 64 nM cells compared to non-resistant cells (Bcl-2/Bax ratio = 1). The high Bcl-2/Bax ratio can cause inhibition of apoptosis and resistance to chemotherapeutic agents [12,37]. The current study extends our understanding about the requirement of Six1 in breast tumor proliferation and growth. The ability of Six1 to induce proliferation of breast cancer cells may vary in different cancer cells or tumors. The abundance of Six1 is regulated through distinct mechanisms, including post-translational modification by induction of mRNA and/or gene transcription. Six1 levels are elevated in paclitaxel-resistant MCF-7cells. Inhibition of ERK signaling in resistant MCF7 cells with MEK inhibitor, PD98059, restored the population of breast cancer cells similar to control cells (data not shown) [38]. We showed for the first time that Six1 and dependent pro/anti-apoptotic gene expressions increased in MCF-7 resistant cells in response to paclitaxel; furthermore, there is no significant association between paclitaxel resistance and gene expression in MCF-7/Pac 64 nM. This study can be expanded to other cancer cell lines and even the biopsies from the cancer patient with different biomarker distributions. Our data suggest that identifying cancer patients with high Six1 activity and then inhibition of this signaling can promote the efficacy of chemotherapeutic agents in cancer patients.
ACKNOWLEDGMENTS
This study was financially supported by grant from Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
DECLARATION OF INTERESTS
The authors report that there are no conflicts of interest.
REFERENCES
- [1].Alphandéry E. Perspectives of breast cancer thermotherapies. J Cancer. 2014;5(6):472–9. doi: 10.7150/jca.8693. http://dx.doi.org/10.7150/jca.8693 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tinoco G, Warsch S, Glück S, Avancha K, Montero AJ. Treating breast cancer in the 21st century:emerging biological therapies. J Cancer. 2013;4(2):117–32. doi: 10.7150/jca.4925. http://dx.doi.org/10.7150/jca.4925 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tabasinezhad M, Samadi N, Ghanbari P, Mohseni M, Saei AA, Sharifi S, et al. Sphingosin 1-phosphate contributes in tumor progression. J Cancer Res Ther. 2013;9(4):556–63. doi: 10.4103/0973-1482.126446. http://dx.doi.org/10.4103/0973-1482.126446 . [DOI] [PubMed] [Google Scholar]
- [4].Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–65. doi: 10.1038/nrc1317. http://dx.doi.org/10.1038/nrc1317 . [DOI] [PubMed] [Google Scholar]
- [5].Coley HM. Development of drug-resistant models. Methods Mol Med. 2004;88:267–73. doi: 10.1385/1-59259-406-9:267. [DOI] [PubMed] [Google Scholar]
- [6].Wahl AF, Donaldson KL, Fairchild C, Lee FY, Foster SA, Demers GW, et al. Loss of normal p53 function confers sensitization to Taxol by increasing G2/M arrest and apoptosis. Nat Med. 1996;2(1):72–9. doi: 10.1038/nm0196-72. http://dx.doi.org/10.1038/nm0196-72 . [DOI] [PubMed] [Google Scholar]
- [7].Li Y, Benezra R. Identification of a human mitotic checkpoint gene:hsMAD2. Science. 1996;274(5285):246–8. doi: 10.1126/science.274.5285.246. http://dx.doi.org/10.1126/science.274.5285.246 . [DOI] [PubMed] [Google Scholar]
- [8].Tan J, Zhang C, Qian J. Expression and significance of Six1 and Ezrin in cervical cancer tissue. TumourBiol. 2011;32(6):1241–7. doi: 10.1007/s13277-011-0228-8. http://dx.doi.org/10.1007/s13277-011-0228-8 . [DOI] [PubMed] [Google Scholar]
- [9].Reed JC. Bcl-2-family proteins and hematologic malignancies:history and future prospects. Blood. 2008;111(7):3322–30. doi: 10.1182/blood-2007-09-078162. http://dx.doi.org/10.1182/blood-2007-09-078162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sasi N, Hwang M, Jaboin J, Csiki I, Lu B. Regulated cell death pathways:new twists in modulation of BCL2 family function. Mol Cancer Ther. 2009;8(6):1421–9. doi: 10.1158/1535-7163.MCT-08-0895. http://dx.doi.org/10.1158/1535-7163.MCT-08-0895 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kurvinen K, Syrjänen K, Syrjänen S. p53 and bcl-2 proteins as prognostic markers in human papillomavirus-associated cervical lesions. J ClinOncol. 1996;14(7):2120–30. doi: 10.1200/JCO.1996.14.7.2120. [DOI] [PubMed] [Google Scholar]
- [12].Sharifi S, Barar J, Hejazi MS, Samadi N. Roles of the Bcl-2/Bax ratio, caspase-8 and 9 in resistance of breast cancer cells to paclitaxel. Asian Pac J Cancer Prev. 2014;15(20):8617–22. doi: 10.7314/apjcp.2014.15.20.8617. http://dx.doi.org/10.7314/APJCP.2014.15.20.8617 . [DOI] [PubMed] [Google Scholar]
- [13].Huang SM, Tsai CF, Chen DR, Wang MY, Yeh WL. p53 is a key regulator for osthole-triggered cancer pathogenesis. Biomed Res Int 2014. 2014. p. 175247. http://dx.doi.org/10.1155/2014/175247 . [DOI] [PMC free article] [PubMed]
- [14].Jackson JG, Pant V, Li Q, Chang LL, Quintás-Cardama A, Garza D, et al. p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell. 2012;21(6):793–806. doi: 10.1016/j.ccr.2012.04.027. http://dx.doi.org/10.1016/j.ccr.2012.04.027 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jin A, Xu Y, Liu S, Jin T, Li Z, Jin H, et al. Sineoculishomeobox homolog 1 protein overexpression as an independent biomarker for pancreatic ductal adenocarcinoma. ExpMolPathol. 2014;96(1):54–60. doi: 10.1016/j.yexmp.2013.11.003. http://dx.doi.org/10.1016/j.yexmp.2013.11.003 . [DOI] [PubMed] [Google Scholar]
- [16].Ozaki H, Nakamura K, Funahashi J, Ikeda K, Yamada G, Tokano H, et al. Six1 controls patterning of the mouse otic vesicle. Development. 2004;131(3):551–62. doi: 10.1242/dev.00943. http://dx.doi.org/10.1242/dev.00943 . [DOI] [PubMed] [Google Scholar]
- [17].Hendry CE, Vanslambrouck JM, Ineson J, Suhaimi N, Takasato M, Rae F, et al. Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J Am SocNephrol. 2013;24(9):1424–34. doi: 10.1681/ASN.2012121143. http://dx.doi.org/10.1681/ASN.2012121143 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426(6964):247–54. doi: 10.1038/nature02083. http://dx.doi.org/10.1038/natur.e02083 . [DOI] [PubMed] [Google Scholar]
- [19].Ikeda K, Kageyama R, Suzuki Y, Kawakami K. Six1 is indispensable for production of functional progenitor cells during olfactory epithelial development. Int J DevBiol. 2010;54(10):1453–64. doi: 10.1387/ijdb.093041ki. http://dx.doi.org/10.1387/ijdb.093041ki . [DOI] [PubMed] [Google Scholar]
- [20].McCoy EL, Iwanaga R, Jedlicka P, Abbey NS, Chodosh LA, Heichman KA, et al. Six1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transition. J Clin Invest. 2009;119(9):2663–77. doi: 10.1172/JCI37691. http://dx.doi.org/10.1172/JCI37691 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130(17):3989–4000. doi: 10.1242/dev.00628. http://dx.doi.org/10.1242/dev.00628 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ng KT, Man K, Sun CK, Lee TK, Poon RT, Lo CM, et al. Clinicopathological significance of homeoprotein Six1 in hepatocellular carcinoma. Br J Cancer. 2006;95(8):1050–5. doi: 10.1038/sj.bjc.6603399. http://dx.doi.org/10.1038/sj.bjc.6603399 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Behbakht K, Qamar L, Aldridge CS, Coletta RD, Davidson SA, Thorburn A, et al. Six1 overexpression in ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and is associated with poor survival. Cancer Res. 2007;67(7):3036–42. doi: 10.1158/0008-5472.CAN-06-3755. http://dx.doi.org/10.1158/0008-5472.CAN-06-3755 . [DOI] [PubMed] [Google Scholar]
- [24].Coletta RD, Christensen KL, Micalizzi DS, Jedlicka P, Varella-Garcia M, Ford HL. Six1 overexpression in mammary cells induces genomic instability and is sufficient for malignant transformation. Cancer Res. 2008;68(7):2204–13. doi: 10.1158/0008-5472.CAN-07-3141. http://dx.doi.org/10.1158/0008-5472.CAN-07-3141 . [DOI] [PubMed] [Google Scholar]
- [25].Sabzichi M, Hamishehkar H, Ramezani F, Sharifi S, Tabasinezhad M, Pirouzpanah M, et al. Luteolin-loaded phytosomes sensitize human breast carcinoma MDA-MB 231 cells to doxorubicin by suppressing Nrf2 mediated signalling. Asian Pac J Cancer Prev. 2014;15(13):5311–6. doi: 10.7314/apjcp.2014.15.13.5311. http://dx.doi.org/10.7314/APJCP.2014.15.13.5311 . [DOI] [PubMed] [Google Scholar]
- [26].Ghanbari P, Mohseni M, Tabasinezhad M, Yousefi B, Saei AA, Sharifi S, et al. Inhibition of survivin restores the sensitivity of breast cancer cells to docetaxel and vinblastine. ApplBiochemBiotechnol. 2014;174(2):667–81. doi: 10.1007/s12010-014-1125-6. http://dx.doi.org/10.1007/s12010-014-1125-6 . [DOI] [PubMed] [Google Scholar]
- [27].Pirouzpanah MB, Sabzichi M, Pirouzpanah S, Chavoshi H, Samadi N. Silibilin-induces apoptosis in breast cancer cells by modulating p53, p21, Bak and Bcl-XL pathways. Asian Pac J Cancer Prev. 2015;16(5):2087–92. doi: 10.7314/apjcp.2015.16.5.2087. http://dx.doi.org/10.7314/APJCP.2015.16.5.2087 . [DOI] [PubMed] [Google Scholar]
- [28].Samadi N, Bekele RT, Goping IS, Schang LM, Brindley DN. Lysophosphatidate induces chemo-resistance by releasing breast cancer cells from taxol-induced mitotic arrest. PLoS One. 2011;6(5):e20608. doi: 10.1371/journal.pone.0020608. http://dx.doi.org/10.1371/journal.pone.0020608 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Samadi N, Ghanbari P, Mohseni M, Tabasinezhad M, Sharifi S, Nazemieh H, et al. Combination therapy increases the efficacy of docetaxel, vinblastine and tamoxifen in cancer cells. J Cancer Res Ther. 2014;10(3):715–21. doi: 10.4103/0973-1482.139152. [DOI] [PubMed] [Google Scholar]
- [30].Wang H, Vo T, Hajar A, Li S, Chen X, Parissenti AM, et al. Multiple mechanisms underlying acquired resistance to taxanes in selected docetaxel-resistant MCF-7 breast cancer cells. BMC Cancer. 2014;14(1):37. doi: 10.1186/1471-2407-14-37. http://dx.doi.org/10.1186/1471-2407-14-37 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Iwanaga R, Wang CA, Micalizzi DS, Harrell JC, Jedlicka P, Sartorius CA, et al. Expression of Six1 in luminal breast cancers predicts poor prognosis and promotes increases in tumor initiating cells by activation of extracellular signal-regulated kinase and transforming growth factor-beta signaling pathways. Breast Cancer Res. 2012;14(4) doi: 10.1186/bcr3219. http://dx.doi.org/10.1186/bcr3219 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li Z, Tian T, Lv F, Chang Y, Wang X, Zhang L, et al. Six1 promotes proliferation of pancreatic cancer cells via upregulation of cyclin D1 expression. PLoS One. 2013;8(3):e59203. doi: 10.1371/journal.pone.0059203. http://dx.doi.org/10.1371/journal.pone.0059203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Turajlic S, McGranahan N, Swanton C. Inferring mutational timing and reconstructing tumour evolutionary histories. BiochimBiophysActa. 2015;1855(2):264–75. doi: 10.1016/j.bbcan.2015.03.005. http://dx.doi.org/10.1016/j.bbcan.2015.03.005 . [DOI] [PubMed] [Google Scholar]
- [34].Khan SH, Wahl GM. p53 and pRb prevent rereplication in response to microtubule inhibitors by mediating a reversible G1 arrest. Cancer Res. 1998;58(3):396–401. [PubMed] [Google Scholar]
- [35].Delia D, Mizutani S, Lamorte G, Goi K, Iwata S, Pierotti MA. p53 activity and chemotherapy. Nat Med. 1996;2(7):724–5. doi: 10.1038/nm0796-724. http://dx.doi.org/10.1038/nm0796-724 . [DOI] [PubMed] [Google Scholar]
- [36].Bilalovic N, Vranic S, Hasanagic S, Basic H, Tatarevic A, Beslija S, et al. The Bcl-2 protein:a prognostic indicator strongly related to ER and PR in breast cancer. Bosn J Basic Med Sci. 2004;4(4):5–12. doi: 10.17305/bjbms.2004.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62(16):4592–8. [PubMed] [Google Scholar]
- [38].Turajlic S, Ali Z, Yousaf N, Larkin J. Phase I/II RAF kinase inhibitors in cancer therapy. Expert OpinInvestig Drugs. 2013;22(6):739–49. doi: 10.1517/13543784.2013.797964. http://dx.doi.org/10.1517/13543784.2013.797964 . [DOI] [PubMed] [Google Scholar]


