Abstract
Calprotectin is one of the major antimicrobial S100 leucocyte proteins. Serum calprotectin levels are associated with certain inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus and inflammatory bowel disease. The aim of this study was to investigate serum and fecal calprotectin levels in patients with ankylosing spondylitis (AS) and show their potential relations to the clinical findings of the disease. Fifty-one patients fulfilling the New York criteria of AS and 43 healthy age- and gender-matched volunteers were included in the study. Physical and locomotor system examinations were performed and history data were obtained for all patients. Disease activity parameters were assessed together with anthropometric parameters. Routine laboratory examinations and genetic testing (HLA-B27) were performed. Serum calprotectin levels and fecal calprotectin levels were measured by an enzyme-linked immunosorbent assay. The mean age of the patients was 41.5 years, the mean duration of the disease was 8.6 years, and the delay in diagnosis was 4.2 years. Serum calprotectin levels were similar in both AS patients and in the control group (p=0.233). Serum calprotectin level was correlated with Bath AS disease activity index (BASDAI) and Bath AS functional index (BASFI) (p=0.001, p=0.002, respectively). A higher level of fecal calprotectin was detected in AS patients when compared with the control group. A statistically significant correlation between fecal calprotectin level and BASDAI, BASFI, C-reactive protein and Erythrocyte sedimentation rate were detected (p=0.002, p=0.005, p=0.001, p=0.002, respectively). The results indicated that fecal calprotectin levels were associated with AS disease findings and activity parameters. Calprotectin is a vital disease activity biomarker for AS and may have an important role in the pathogenesis of the disease. Multi-centered prospective studies are needed in order to provide further insight.
KEY WORDS: Ankylosing spondylitis, calprotectin, disease activity
INTRODUCTION
Members of the S100 protein family are acidic proteins of low molecular mass characterized by cell-type-specific production and the presence of 2 EF-hand calcium-binding domains. Of these proteins, calprotectin has prompted particular interest among rheumatologists because it is a valuable marker of phagocyte activation [1]. Calprotectin is a calcium- and zinc-binding protein which is a potential antimicrobial marker of inflammation. It is also known as myeloid-related protein 8/14 (MRP8/14) and S100A8/A9. The secretion of calprotectin by neutrophils and macrophages is stimulated during these cells’ interaction with activated endothelial cells. Elevated concentrations of calprotectin have been measured in serum, synovial fluid, urine, and feces of patients with inflammatory diseases and chronic bacterial infections [2]. Ankylosing spondylitis (AS) is a chronic, progressive, rheumatic disease that affects sacroiliac joints, the spine, and peripheral joints. This inflammation may also affect extra articular parts like the intestines, heart, urinary system and eyes [3]. The pathogenesis of AS has remained unclear to date although there are genetic components, as well as environmental and infectious agents to be blamed. It is already known that intestinal inflammation is seen in some AS patients [4, 5]. Nearly 10% of AS cases are diagnosed with inflammatory bowel disease (IBD) and 70% of patients with AS have subclinical intestinal inflammation as well [6]. High concentrations of fecal calprotectin were detected as an inflammatory marker in patients with active IBD due to transmigration of neutrophils into the intestinal system [7]. The serum concentration of calprotectin has been correlated with many variables associated with disease activity in rheumatoid arthritis (RA), psoriatic arthritis (PsA) and AS, including such clinical variables as the number of swollen joints, the Ritchie index, the Disease Activity Score (DAS28), and such biological variables as C-reactive protein (CRP) concentration and erythrocyte sedimentation rate [8]. Fecal calprotectin is an acute phase protein which is very stable in the intestinal tract and can be measured in the stool. To a great extent, the quantity of fecal calprotectin helps to distinguish inflammatory bowel disease from functional bowel disorders. It is also used to track patients’ responses to treatment and to anticipate the relapse of the disease. Calprotectin is not only of potential use in the diagnosis of these diseases, but also may be a valuable biomarker of monitoring treatment and evaluating prognosis. Because AS is associated with chronic inflammatory bowel diseases, the serum and fecal calprotectin levels of AS patients are likely to be correlated with disease symptoms and activity parameters.
The aim of this study was to detect serum and fecal calprotectin levels in AS patients and discuss the potential relationship between calprotectin and the clinical symptoms of the disease.
MATERIALS AND METHODS
Fifty-one patients who consecutively presented to Sifa University rheumatology clinic, and who satisfied the modified New York criteria of AS at the time of enrollment, were included in the study. Physical and locomotor system examinations were performed for all patients, and their medical history was taken. Anthropometric parameters (hand-ground distance, occiput-wall distance, Schober test, chest expansion) and disease activity indices Bath AS disease activity index (BASDAI), Bath AS functional index (BASFI) were evaluated. Routine laboratory tests were done and acute phase reactants the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and HLA-B27 were monitored. The control group included forty-three age- and gender-matched healthy hospital employees. Healthy controls were free from any history of rheumatic disease or IBD. AS patients and controls with a personal or family history of IBD were excluded from the study. Serum and stool samples of both patients and volunteers were collected after their consent was given. Serum samples were stored at -20°C prior to analysis. Serum calprotectin test (MRP8/14, calprotectin S100A8/A9) and ELISA (Bühlmann Laboratories AG, Switzerland) were used in the study. In accordance with the instructions of the manufacturing company, 2.9 µg/mL serum calprotectin (SC) and higher values were accepted as positive. Extracts of feces were prepared using a Roche Smart-Prep Faecal Sample Preparation Kit. The extracts were stored at -20°C prior to analysis. Quantum Blue Calprotectin lateral flow kit (Bühlmann Laboratories AG, Schönenbuch, Switzerland), having an immunochromatographic method and using a highly specific monoclonal antibody to calprotectin, was used to test fecal calprotectin. The results of the fecal calprotectin test, having a point-of-care testing (POCT) method, were read quantitatively using the Quantum Blue Reader. Values 50 mg/g and higher were considered as positive, per the manufacturer’s instructions [9]. The study was acknowledged by Sifa University Ethics Committee and funded by Sifa University Scientific Research Projects Unit.
Statistical analysis
Data was analyzed by the Statistical Package for the Social Sciences (SPSS) 20.0 software for Windows (SPSS, Chicago, Illinois, USA). Cross-tabulation was used for categorical data, and chi-square and the Fisher’s exact tests were performed. First, the Shapiro-Wilk test was used to assess the normality of the numeric data. Normally distributed data was assessed through t-tests. The Mann–Whitney U test was used to assess the data which was not normally distributed. Statistical significance was set as p < 0.05.
RESULTS
The average age of 51 patients (30 male) was 41.5, the average duration of the disease was 8.6 years, and the delay in diagnosis was 4.2 years. Presenting symptoms of the patients were as follows: 37 patients with inflammatory back pain, 2 with wrist arthritis, 2 with arthritis in the knee joint, 5 with hip pain, 2 with uveitis, and 3 with ankle pain. HLA-B27 was positive in 28 (54.9%) patients. The presentation of the clinical phenotype of AS patients was as follows: 51 (100%) patients were diagnosed with sacroiliitis and enthesitis, 17 (33.3%) with hip involvement, 14 (27.5%) with knee joint involvement, 27 (52.9%) with cervical involvement, 12 (23.5%) with uveitis, and 5 (9.8%) with bamboo spine deformity. Twenty-five (49%) of the patients were smokers. As for the medication, 37 (72.5%) of the patients used sulphasalazine, 4 (7.8%) used methotrexate, 48 (94.1%) used NSAIDs and 4 (7.8%) used anti-TNF alpha drugs. High levels of serum calprotectin were detected in 27 (52.9%) AS patients; this number was 26 (60%) in the healthy control group (Table 1). Serum calprotectin levels were similar in both AS patients and the control group, and it was statistically non-significant (p=0.233). A correlation between serum calprotectin level and AS activity indices (BASDAI, BASFI) was found (p=0.002, p=0.005, respectively) (Table 2). High levels of fecal calprotectin were found in 38 (74.5%) of the AS patients and 13 (30.2%) subjects in the control group. A higher level of fecal calprotectin was detected in AS patients when compared with the control group (p=0.001). Statistically significant correlation was detected between fecal calprotectin level and some clinical and laboratory findings (BASDAI, BASFI, CRP, ESR) (p=0.002, p=0.005, p=0.001, p=0.002, respectively). Fecal calprotectin level also correlated with some anthropometric parameters (chest expansion, hand-ground distance, Schober test) (p=0.002, p=0.001, p=0.002, respectively) (Table 3).
TABLE 1.
Positivity rates of serum and fecal calprotectin in ankylosing spondylitis (AS) patients and healthy controls (HC)
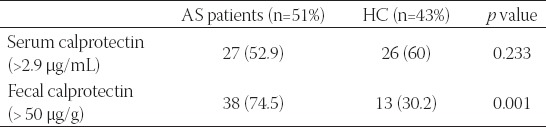
TABLE 2.
Demographic data for ankylosing spondylitis patients stratified by serum calprotectin (SC) positivity
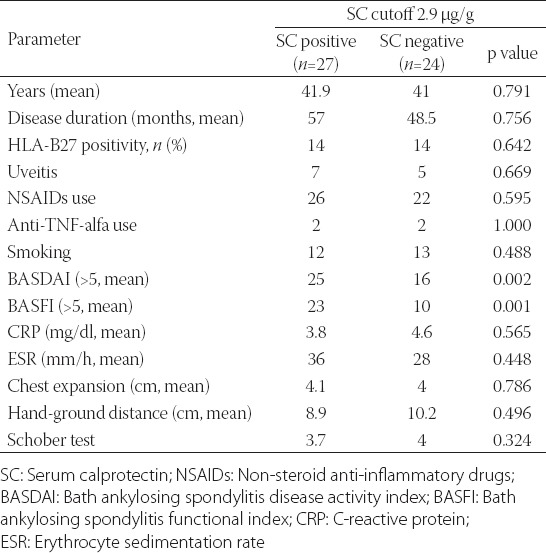
TABLE 3.
Demographic data for ankylosing spondylitis patients stratified by fecal calprotectin (FC) positivity
DISCUSSION
Our study showed that a higher level of fecal calprotectin was detected in AS patients when compared with the control group, and serum calprotectin levels were similar in both groups. A statistically significant correlation was also found between the fecal calprotectin level and some clinical and laboratory findings, and anthropometric parameters (BASDAI, BASFI, CRP, ESR, chest expansion, hand-ground distance, Schober test). In addition, none of the patients had gastrointestinal complaints. There is limited data concerning serum calprotectin levels in AS patients in the literature, with different and contradictory results. Oktayoğlu et al. found that serum calprotectin levels were significantly higher in AS patients compared with controls but this was not correlated with disease activity indices [10]. Klingberg et al. investigated serum and fecal calprotectin levels in AS patients and found that serum calprotectin levels were similar to those of the control group and fecal calprotectin levels were higher in AS patients. In addition, they reported a correlation between fecal calprotectin level and some disease parameters (advanced age, duration of disease, ESR, CRP) [11]. Matzkies et al. looked at calprotectin levels of 81 AS patients and reported a high level of serum calprotectin in 41% of the AS patients [12]. When compared with the control group, there was positivity in IBD-associated antibodies (pANCA, anti-Saccharomyces cerevisiae) in AS patients classified according to fecal calprotectin level. Turina et al. measured serum calprotectin levels of 76 patients with axial spondyloarthritis (axSpA). They found higher levels of serum calprotectin in axSpA patients when compared with the control group and showed that this could be an independent risk factor for radiographic progression [13]. Davida et al. tested fecal calprotectin levels of 17 AS patients and found 57% positivity. While 81.8% of the patients who had fecal calprotectin values higher than the cut-off point were diagnosed with inflammatory bowel disease, only 12.5% of the patients who had fecal calprotectin values lower than the cut-off point were diagnosed with the same disease. Researchers highlighted that high levels of fecal calprotectin in AS patients might be a biomarker for IBD diagnosis [14]. In another study, Turina et al. found high levels of serum calprotectin, hs-CRP, VEGF and MM-3 in SpA patients when compared with the control group [15]. They emphasized that serum calprotectin might be an important biomarker in response to the treatment of peripheral and axial SpA patients. The findings of our study are in parallel with the results in the literature. Unlike the other studies in the literature, our study focused solely on AS patients and the participants in our study were homogeneous. In addition, both serum and fecal calprotectin levels were studied separately. IBD and AS are similar chronical inflammatory diseases with unknown etiologies. Although their clinical manifestations are different, there are many clinical and genetic findings that support their pathogenic relationship [16]. There is a strong possibility of silent inflammatory bowel disease in AS patients. Fecal calprotectin levels may be significant in terms of early development of IBD or subclinical bowel inflammation in these patients [17]. It may also be important in making decisions about using NSAIDs, which may affect the course of the treatment. Looking at fecal calprotectin levels is a fast, simple and noninvasive method that helps patients and professionals in many ways without using invasive methods like colonoscopy. Since these tests have high sensitivity and specificity, they could be used for AS patients by clinicians. AS and IBD may accompany each other, which means a fecal calprotectin test in AS patients may be used in the early diagnosis of IBD and in patient monetarization [18].
Our study has some limitations. Some of these are: Having a limited number of participants both in the patients and the control group, and being unable to demonstrate the relation between radiological progression of ankylosing spondylitis and calprotectin. Another limitation is that we did not study the potential correlation between calprotectin and a cure for AS.
CONCLUSION
We found a correlation between fecal calprotectin levels and AS symptoms and its activity parameters. Calprotectin is a significant biomarker for AS and may have an important role in disease pathogenesis. Multicentered prospective studies are needed in order to provide further insight into this subject.
DECLARATION OF INTERESTS
The authors declare no conflict of interest.
REFERENCES
- [1].Montalto M, Gallo A, Santoro L, D’Onofrio F, Landolfi R, Gasbarrini A. Role of fecal calprotectin in gastrointestinal disorders. Eur Rev Med Pharmacol Sci. 2013;17(12):1569–82. [PubMed] [Google Scholar]
- [2].Hetland G, Talgö GJ, Fagerhol MK. Chemotaxins C5a and fMLP induce release of calprotectin (leucocyte L1 protein) from polymorphonuclear cells in vitro. Mol Pathol. 1998;51(3):143–8. doi: 10.1136/mp.51.3.143. http://dx.doi.org/10.1136/mp.51.3.143 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mielants H, Veys EM, Goemaere S, Goethals K, Cuvelier C, De Vos M. Gut inflammation in the spondyloarthropathies:clinical, radiologic, biologic and genetic features in relation to the type of histology. A prospective study. J Rheumatol. 2015 Apr 16;18(10):1542–51. [PubMed] [Google Scholar]
- [4].Rudwaleit M, Baeten D. Ankylosing spondylitis and bowel disease. Best Pract Res Clin Rheumatol. 2006;20(3):451–71. doi: 10.1016/j.berh.2006.03.010. http://dx.doi.org/10.1016/j.berh.2006.03.010 . [DOI] [PubMed] [Google Scholar]
- [5].Costello ME, Elewaut D, Kenna TJ, Brown M. Microbes, the gut and ankylosing spondylitis. Arthritis Res Ther. 2013;15(3):214. doi: 10.1186/ar4228. http://dx.doi.org/10.1186/ar4228 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD:useful, magic, or unnecessary toys? Gut. 2006;55(3):426–31. doi: 10.1136/gut.2005.069476. http://dx.doi.org/10.1136/gut.2005.069476 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Osipenko MF, Livzan MA, Skalinskaia MI, Lialiukova EA. Fecal calprotectin concentration in the differential diagnosis of bowel diseases. Ter Arkh. 2015;87(2):30–3. doi: 10.17116/terarkh201587230-33. [DOI] [PubMed] [Google Scholar]
- [8].Mariani A, Marsili M, Nozzi M, Faricelli R, Chiarelli F, Breda L. Serum calprotectin:review of its usefulness and validity in paediatric rheumatic diseases. Clin Exp Rheumatol. 2014;33(1):109–14. [PubMed] [Google Scholar]
- [9].Wassell J, Wallage M, Brewer E. Evaluation of the Quantum Blue(R) rapid test for faecal calprotectin. Ann Clin Biochem. 2012;49(1):55–8. doi: 10.1258/acb.2011.011106. http://dx.doi.org/10.1258/acb.2011.011106 . [DOI] [PubMed] [Google Scholar]
- [10].Oktayoglu P, Bozkurt M, Mete N, Caglayan M, Em S, Nas K. Elevated serum levels of calprotectin (myeloid-related protein 8/14) in patients with ankylosing spondylitis and its association with disease activity and quality of life. J Investig Med. 2014;62(6):880–4. doi: 10.1097/JIM.0000000000000095. http://dx.doi.org/10.1097/JIM.0000000000000095 . [DOI] [PubMed] [Google Scholar]
- [11].Klingberg E, Carlsten H, Hilme E, Hedberg M, Forsblad-d’Elia H. Calprotectin in ankylosing spondylitis - frequently elevated in feces, but normal in serum. Scand J Gastroenterol. 2012;47(4):435–44. doi: 10.3109/00365521.2011.648953. http://dx.doi.org/10.3109/00365521.2011.648953 . [DOI] [PubMed] [Google Scholar]
- [12].Matzkies FG, Targan SR, Berel D, Landers CJ, Reveille JD, McGovern DP, et al. Markers of intestinal inflammation in patients with ankylosing spondylitis:a pilot study. Arthritis Res Ther. 2012;14(6):R261. doi: 10.1186/ar4106. http://dx.doi.org/10.1186/ar4106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Turina M, Sieper J, Yeremenko N, Conrad K, Haibel H, Rudwaleit M, et al. Calprotectin serum level is an independent marker for radiographic spinal progression in axial spondyloarthritis. Ann Rheum Dis. 2014;73:1746–1748. doi: 10.1136/annrheumdis-2014-205506. http://dx.doi.org/10.1136/annrheumdis-2014-205506 . [DOI] [PubMed] [Google Scholar]
- [14].Dávida L, Szántó S, Kacska S, Haraszti B, Brúgós B, Altorjay I, et al. The role of fecal calprotectin in the prediction of ankylosing spondylitis associated with early IBD. J Crohns Colitis. 2015;9(Suppl 1):S108–9. http://dx.doi.org/10.1093/ecco-jcc/jju027.182 . [Google Scholar]
- [15].Turina MC, Yeremenko N, Paramarta JE, De Rycke L, Baeten D. Calprotectin (S100A8/9) as serum biomarker for clinical response in proof-of-concept trials in axial and peripheral spondyloarthritis. Arthritis Res Ther. 2014;16(4):413. doi: 10.1186/s13075-014-0413-4. http://dx.doi.org/10.1186/s13075-014-0413-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Manz M, Burri E, Rothen C, Tchanguizi N, Niederberger C, Rossi L, et al. Value of fecal calprotectin in the evaluation of patients with abdominal discomfort:an observational study. BMC Gastroenterol. 2012;12(1):5. doi: 10.1186/1471-230X-12-5. http://dx.doi.org/10.1186/1471-230X-12-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Smith J. Update on Ankylosing Spondylitis:Current Concepts in Pathogenesis. Curr Allergy Asthma Rep. 2014;15(1) doi: 10.1007/s11882-014-0489-6. [DOI] [PubMed] [Google Scholar]
- [18].Rashid T, Wilson C, Ebringer A. The link between ankylosing spondylitis, Crohn’s disease, Klebsiella, and starch consumption. Clin Dev Immunol. 2013 Jan;2013:8726–32. doi: 10.1155/2013/872632. [DOI] [PMC free article] [PubMed] [Google Scholar]


