Abstract
Intestinal ischemia-reperfusion (I/R) causes severe destruction in remote organs. Lung damage is a frequently seen complication after intestinal I/R. Ukrain (NSC 631570) is a synthetic thiophosphate derivative of alkaloids from the extract of the celandine (Chelidonium majus L.) plant. We investigated the effect of Ukrain in animals with lung injury induced by intestinal I/R. Adult male Spraque-Dawley rats were randomly divided into four groups: control, Ukrain, I/R, I/R with Ukrain. Before intestinal I/R was induced, Ukrain was administered intraperitoneally at a dose of 7.0 mg/body weight. After 1 h ischemia and 2 h reperfusion period, lung tissues were excised. Tissue levels of total oxidative status (TOS), total antioxidant status (TAS) were measured and oxidative stress indices (OSI) were calculated. Lung tissues were also examined histopathologically. TOS and OSI levels markedly increased and TAS levels decreased in the I/R group compared to the control group (P < 0.05). TOS and OSI levels markedly decreased and TAS levels increased in the I/R with Ukrain group compared with the group subjected to IR only (P < 0.05). Severe hemorrhage, alveolar septal thickening, and leukocyte infiltration were observed in the I/R group. In the I/R with Ukrain group, morphologic changes occurring as a result of lung damage attenuated and histopathological scores reduced compared to the I/R group (P < 0.05). Our results suggest that Ukrain pretreatment could reduce lung injury induced by intestinal I/R induced via anti-inflammatory and antioxidant effects.
KEY WORDS: Ischemia-reperfusion injury, lung injury, oxidative stress, ukrain
INTRODUCTION
Intestinal ischemia and reperfusion (I/R) injury is commonly seen in some clinical situations, such as serious burns, hemorrhagic or traumatic shock, acute mesenteric ischemia, cardiopulmonary bypass, abdominal aortic aneurysm repair, organ transplantations [1]. Intestinal I/R most commonly leads to acute vascular deficiency and the multiple organ failure which are associated with high rates of morbidity and mortality [2]. Intestinal I/R also leads to the severe destruction of remote organs like the heart, lungs, liver, and kidneys. Lung damage is particularly a frequent complication after intestinal I/R [3]. Activation of immune cells within the endothelial cells of intestinal microcirculation may initiate a systemic inflammatory response with secondary injury to remote organs. Leakage of bacteria and toxins from intestinal mucosa may induce inflammation, causing remote organ dysfunction. Intestinal ischemia causes production of inflammatory mediators and adhesion molecules. During reperfusion, intestinal injury is increased by overproduction of reactive oxygen species (ROS) and reduction of endogenous antioxidant defence mechanisms. ROS activate neutrophils and activated neutrophils infiltrate intestinal epithelium and endothelial cells, leading to mucosal damage. Mucosal injury leads to increase in vascular permeability, causing translocation of enteric bacterial products [4,5].
Ukrain (NSC 631570) is a synthetic thiophosphate derivative of alkaloids from the extract of the celandine (Chelidonium majus L.) plant [6]. This plant contains various alkaloids, most particularly chelidonine, also known as benzophenanthridine alkaloid. Ukrain comprises one molecule thiophosphoric acid conjugated to three molecules of chelidonine [6,7]. Chelidonine has antispasmodic, analgesic, antiviral, antitumour, antibacterial, and anti-inflammatory properties. In animal studies, an extract of celandine increased bile flow, stimulated immune system by inducing the production of T-lymphocytes, and acted as a hepatoprotectant [7]. Ukrain has antineoplastic and immunomodulatory effects. Ukrain treatment can inhibit or reduce the growth of tumor mass, induce apoptosis, and also modulate the function of the immune system by increasing T-cells. The drug can interfere with the metabolism of cancer cells by reducing DNA, RNA, protein synthesis, and cellular oxygen depletion in malignant cells [8]. In addition to its antineoplastic potential, it has been reported a radioprotective effect of Ukrain against high-dose radiation exposure [9].
Respiratory failure is a frequent cause of mortality and morbidity after intestinal I/R. Thus, it is important to develop effective therapeutic strategies, however, specific therapeutic strategies are currently undefined. Our previous studies have demonstrated that Ukrain prevents intestinal tissue damage against intestinal I/R injury by antioxidant activity [10]. Therefore, we investigated the effect of Ukrain in animals with lung injury induced by intestinal I/R, using biochemical and histopathological analyses. To the best of our knowledge, the effect of Ukrain has not been previously investigated in the lung injury induced by intestinal I/R rat model.
MATERIALS AND METHODS
The present study was executed in the Experimental Animals Application and Research Center of Dumlupinar University. The experimental design of study was confirmed by the the Dumlupinar University Local Ethics Committee for Animal Experiments (No: 51264181-604.01.02). Experimental studies were executed according to the Guide for the Care and Use of Laboratory Animals published by the Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council [11].
Animals
Male Sprague-Dawley rats (250-300 g, 10-12 weeks, N = 32) were used. Rats were purchased from Experimental Animals Application and Research Center of Dumlupinar University. Rats were sheltered in polycarbonate cages with a 12-:12-h light-dark cycle in a temperature-controlled (22 ± 1°C) room. All rats were fed with standard rat chow and allowed to fresh water ad libitum until the experiment started.
Chemicals
Ukrain ampoules were donated by Nowicky Pharmaceuticals (Margaretenstrasse 7, 1040 Vienna, Austria). The ampoules were protected from light and stored at 7°C until use.
Experimental study design
Rats were randomly divided into four groups (N = 8). Control group was subjected to abdominal median laparotomy and not to I/R conditions. Rats received 1 mL of sterile, pyrogen-free saline by intraperitoneal (i.p.) injection 1 h before laparotomy. Ukrain group was exposed to only abdominal median laparotomy and not to I/R. Rats received 1 mL of 7.0 mg/body weight (b.w.) Ukrain via i.p. injection 1 h before laparotomy. I/R was induced by 1 h of ischemia followed by 2 h of reperfusion. The rats received 1 mL of sterile, pyrogen-free saline via i.p. injection 1 h before laparotomy. I/R with Ukrain group was exposed to 1 h of ischemia followed by 2 h of reperfusion and 1 h before laparotomy, the rats received 1 mL of 7.0 mg/b.w. Ukrain via i.p. injection.
Surgical procedures
All rats were fasted for 12 h before the studies, but were allowed free access to water until 30 min before the experiment. The rats were weighed and anesthetized with ketamine (Ketalar, Pfizer, Istanbul, Turkey)-xylazine (Rompun, Bayer, Istanbul, Turkey) (70 and 10 mg/kg, respectively) by i.p. injection. Then the abdomen of the rats were shaved, cleaned with povidone–iodine 10% antiseptic solution, and opened via a midline incision under anesthesia. Intestinal Ischemia was initiated via ligation of the the superior mesenteric artery with a vascular clamp (Vascu Stop Bulldog Clamp, Istanbul, Turkey) for 1 h. Incised abdomen sites were subsequently closed and animals were observed. After 1 h of ischemia, the clamps were displaced to permit reperfusion for 2 h [12]. At the end of the reperfusion period, the animals were euthanized by exsanguination under anesthesia, and the lungs were bilaterally removed for biochemical and histopathological analyses. One part of lung samples were fixed with 10% formaldehyde and the other part of lung samples were put into an Eppendorf tube and stored at -80ºC.
Biochemical analyses
Preparation of lung tissue homogenates
The lung tissue samples were mixed with cold phosphate-buffered saline solution and homogenized with a mechanic homogenizer (Analytik Jena speedmill plus, Jena, Germany). The homogenates were centrifuged at 10000 × g for 10 min at 4 ºC and the supernatants were preserved for biochemical analysis by storing on ice. Tissue total antioxidant status (TAS) and total oxidant status (TOS) levels were measured on Beckman Coulter AU680 Chemistry System (Beckman Coulter, Miami, FL, USA) using commercial kits (Rel Assay Diagnostic, Gaziantep, Turkey). Tissue protein concentrations were measured based on the principal of the Bradford method on a Beckman Coulter AU680 Chemistry System (Beckman Coulter, Miami, FL, USA) [13].
Measurement of total antioxidant status (TAS)
Tissue TAS concentrations were measured with an automated assay method developed by Erel [14]. Free radical reactions were started with the production of hydroxyl radical by Fenton reaction and the rate of the reaction was monitored by following the absorbance of colored dianisidyl radicals. Antioxidants in the sample suppressed the color formation to a degree that is proportional to their concentrations [14]. The results were stated in term of micromolar Trolox equivalent per miligram tissue protein (µmol Trolox Eq/mg protein).
Measurement of total oxidant status (TOS)
Tissue TOS concentrations were measured with an automated assay method developed by Erel [15]. The method is based on the principal of the oxidation of ferrous ion to ferric ion in the presence of various oxidants in the sample and the measurement of the ferric ion by xylenol orange. The assay was calibrated with hydrogen peroxide [15]. The results were stated in term of micromolar hydrogen peroxide equivalent per miligram tissue protein (mmol H2O2 Eq/mg protein).
Calculation of oxidative stress index (OSI)
The percent ratio of TOS to TAS was accepted as the OSI, an marker of the severity of oxidative stress. OSI was calculated as follows: OSI = [(TOS, μmol H2O2 Eq/mg protein)/(TAS, μmol Trolox Eq/mg protein) × 100] [16].
Histopathologic examinations
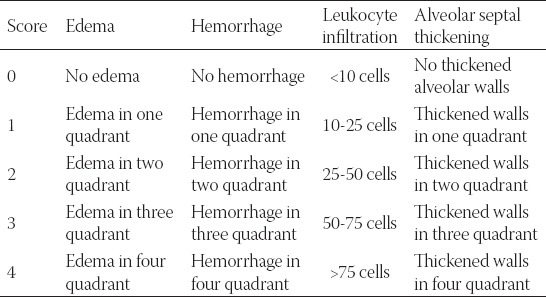
The lung samples were fixed in 10 % formaldehyde and embedded in paraffin. Thick paraffin sections (4 µm) were cut from each specimen. After deparaffinization and rehydration, all sections were stained with hematoxylin and eosin (H&E). Slides were examined and images were captured using a light microscope (Olympus BX51, Tokyo, Japan) by a pathologist who was blinded to the treatment each animal had received. The pathologic slides were graded according to a scoring system previously described by Yamanel et al. (Table 1) [17]. In this scoring system, four lung damage indices; edema (score: 0-4), hemorrhage (score: 0-4), leukocyte infiltration (score: 0-4), and alveolar septal thickening (score: 0-4) are graded for a total score of 0-16.
TABLE 1.
Histopathologic lung damage criteria according to according to Yamanel et al. [17]
Statistical analysis
Data were stated as means ± standard error of mean (SEM). Because of the small size of groups, nonparametric statistical tests were used. The differences were analyzed with the Kruskal-Wallis test among the multiple groups. The differences were analyzed with the Mann-Whitney U-test between two groups (GraphPad Prism version 6.05, GraphPad Software, Inc., La Jolla, CA, USA). A P value less than 0.05 was considered statistically significant.
RESULTS
Tissue TAS, TOS, and OSI levels are represented in Figure 1. Significant differences were observed between TAS, TOS, and OSI levels in the experimental study groups (P < 0.001, P = 0.001, P = 0.003, respectively). TOS and OSI levels increased and TAS levels decreased in the intestinal IR group (P < 0.05) compared with the control group (P < 0.05). TOS and OSI levels decreased and TAS levels increased in the I/R with Ukrain group compared with the group subjected to IR only (P < 0.05, Figure 1).
FIGURE 1.
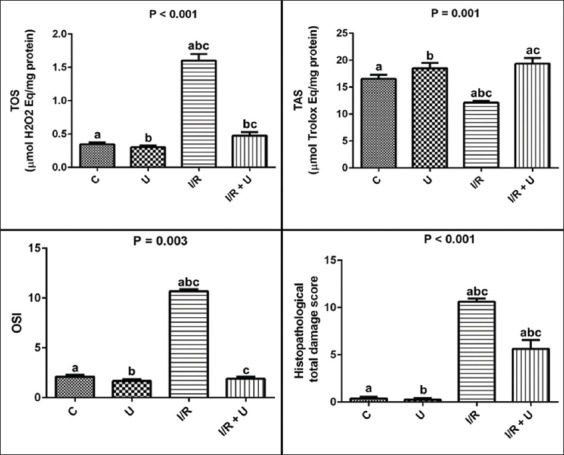
Pulmoner tissue TAS, TOS, OSI levels, and histopathological total lung damage scores in the study groups. Data are stated as the mean ± standard error of mean (SEM); N = 8. P: Demonstrates the differences among the groups (Kruskal-Wallis test). a,b,c: In each line, the differences between the two groups with same letters are significant, P < 0.05 (Mann-Whitney-U test). TAS: Total antioxidant status, TOS: Total oxidant status, OSI: Oxidative stress index, C: Control, U: Ukrain, I/R: Ischemia-reperfusion.
Lung damage scores in H&E staining are represented in Table 2. In the histopathological examinations, total lung damage scores were higher in the I/R group compared to the control group (P < 0.05, Table 2). Ukrain treatment ameliorated morphological changes occurring in lung tissue as a result of I/R. Histopathological scores were lower in the I/R with Ukrain group compared to the I/R group (P < 0.05, Table 2). Severe hemorrhage, alveolar septal thickening, and leukocyte infiltration were observed in the I/R group (Figure 2A-C). However, the microscopic views of lung samples were approximately normal in the I/R with Ukrain group. Slight hemorrhage and leukocyte infiltration were observed in the I/R with Ukrain group (Figure 2D).
TABLE 2.
Comparison of scored histopathological values in the experimental study groups (mean±SEM, N=8)
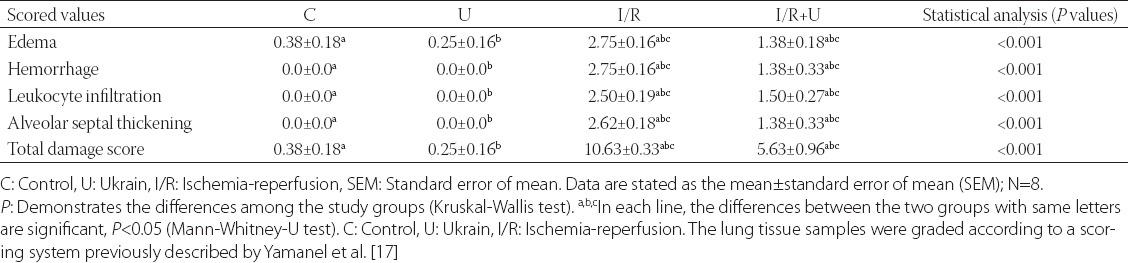
FIGURE 2.
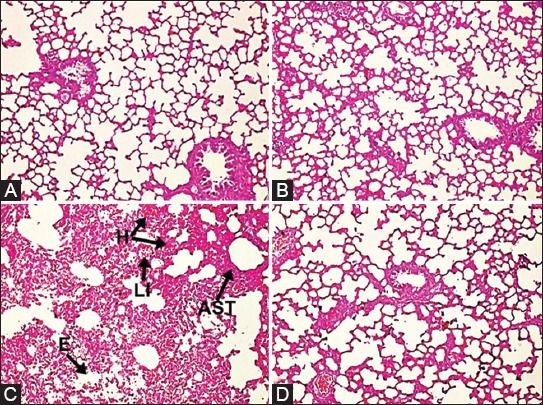
Microphotographs of lung tissues in the study groups. (A,B) Hematoxylin and eosin staining (H&E x 100) revealed that there was no any change in the control and Ukrain groups, (C) Severe edema, hemorrhage, leukocyte infiltration, and alveolar septal thickening were observed in the I/R group. The “H” denotes hemorrhage, “LI” denotes leukocyte infiltration, “AST” denotes alveolar septal thickening, and “E” denotes edema. (D) The microscopic views of lung tissues were near normal in the I/R with Ukrain group. Minimal hemorrhage, leukocyte infiltration, alveolar septal thickening, and a comparatively preserved pulmoner architectures were observed in the I/R with Ukrain group. The slides were graded according to a scoring system previously described by Yamanel et al. [17].
DISCUSSION
We investigated the effects of Ukrain in rats subjected to the experimental lung injury induced by intestinal I/R in this study. This is the first report of the effect of Ukrain in a model of lung damage induced by intestinal I/R. Our findings revealed that 1 h of superior mesenteric obstruction followed by 2 h of reperfusion led to serious lung injury. However, Ukrain treatment showed remarkable protective effects against intestinal I/R induced lung damage. This protective effect was evident as a decrease in TOS and OSI levels and an increase in TAS levels in lung tissue. Additionally, the histopathological examination of lung tissues supported the protective effects of Ukrain.
Intestinal ischemia generally occurs due to arterial obstruction by thrombi or embolisms and also by non-obstructive processes such as cardiac failure, sepsis, the usage of alpha-adrenergic agents or digitalis. Mesenteric venous occlusion may lead to infarction with acute mesenteric ischemia and irreversible tissue lesions [18,19]. Intestinal I/R may also cause remote organ injury through several mechanisms such as inflammatory cells, ROS, and cytokines. Increased production of ROS, reduced endogenous antioxidative defense mechanisms may cause I/R injury. The lung is very prone to ROS induced by I/R, which cause oxidative damage to lipids, proteins, and DNA, leading to organ failure and cell death [20]. Lung injury as a result of I/R induced remote organ injury increases mortality and morbidity [21].
In this study, intestinal I/R caused increase in pulmoner tissue TOS and OSI levels consistent with previous studies [22,23]. The TOS/TAS ratio, in other words the OSI, is a marker of the severity of oxidative stress and demonstrates the redox balance between antioxidant and oxidant status [24]. TOS level is a sensitive index of oxidative stress [25]. TAS, as an antioxidant, can protect tissue from the oxidative damage by scavenging ROS. Therefore, the measurement of TAS level provides a sensitive index of antioxidant capacity [10]. In addition, intestinal I/R caused severe histopathological pulmoner damage including edema, hemorrhage, increased thickness of the alveolar wall and the number of neutrophils that infiltrated the interstitium and alveoli, consistent with previous studies [22,23,26]. Therefore, our findings suggest that intestinal ischemia-reperfusion may induce oxidative stress, leading to severe damage in pulmoner tissue.
The Ukrain is a synthetic derivative of thiotepa and the chelidonine, a main component of the greater celandine (Chelidonium majus L.) plant [27]. It has been reported that Ukrain has spesific properties such as a selective cytotoxic effect on malignant human tumour cells, but not on normal cells in a concentration not cytotoxic in normal cells. Authors suggest that this may be explained by the difference in metabolic activities of drug between normal and malignant cells. Normal cells are able to metabolize Ukrain, whereas Ukrain is cytotoxic for malignant cells [28]. Hohenwarter et al. [29] observed Ukrain in much higher concentration in malignant cells than in normal cell cultures. Thus, the authors suggest that Ukrain exerts selective cytotoxic effects on tumour cells without adverse side effects on normal cells. The cytotoxic effect of Ukrain may be due to breakdown in the metabolism of cancer cells, the reduction of DNA, RNA, protein synthesis, and cellular oxygen depletion [8,30,31]. In a previous study, it has been shown that Ukrain inhibits the protein synthesis by preventing amino acid transport into tumor cells. Ukrain not only inhibits protein synthesis, but also inhibits gluconeogenesis and energy production [32]. It has been reported that Ukrain induces mitochondrial damage and apoptosis in malignant cells by activating caspases [33,34], inhibiting proapoptotic Bcl-2 [35,36], upregulating cyclin-dependent kinase inhibitor p27 [37,38]. In a study by Skivka et al. [39], it has been demonstrated that Ukrain restores proinflammatory functions of macrophages and recovers ROS production. Koriem et al. [40] reported that Chelidonium majus leaves methanol extract has natriuretic, antidiuretic, and nephroprotective effects against cadmium-induced nephrotoxicity in rats. In a previous study, Chelidonium majus exhibited anti-inflammatory activity by inhibiting inducible nitric oxide synthase and cyclooxygenase-2 against lipopolysaccharide-induced inflammation in macrophage RAW264.7 cells [41]. The findings of this study revealed that Ukrain treatment decreased the pulmonary inflammation by decreasing inflammatory cell infiltration, alveolar septal thickening, and alveolar edema. In addition, Ukrain treatment reduced oxidative stress by decreasing TOS levels and OSI values, increasing TAS levels, consistent with our previous study [10]. Taken together, the findings of this study suggest that treatment with Ukrain may protect against remote organ damage induced by intestinal I/R via antioxidant and antiinflammatory effects.
CONCLUSION
In conclusion, this is the first study that reports the protective effects of Ukrain in lung injury after experimental intestinal I/R model in rats. We demonstrated the intestinal I/R caused the oxidative stress in lung tissue. The Ukrain treatment prevented lung injury via antioxidant and anti-inflammatory effects. These results suggest that oxidative stress plays an important role in the lung injury induced by intestinal I/R and Ukrain provides protective action against remote organ injury induced by intestinal I/R. It can be commented that the protective effect of Ukrain can be attributed to its antioxidant and antiinflammatory properties. Consequently, Ukrain may be an effective therapeutic alternate in protecting lung tissue against I/R induced oxidative injury.
DECLARATION OF INTERESTS
The authors declare that they have no conflicts of interest to disclose.
ACKNOWLEDGMENTS
The authors are thankful to Nowicky Pharmaceuticals for donating Ukrain.
REFERENCES
- [1].Teke Z, Sacar M, Yenisey C, Atalay AO, Kavak T, Erdem E. Activated protein C attenuates intestinal mucosal injury after mesenteric ischemia/reperfusion. J Surg Res. 2008;149:219–30. doi: 10.1016/j.jss.2007.10.011. http://dx.doi.org/10.1016/j.jss.2007.10.011 . [DOI] [PubMed] [Google Scholar]
- [2].Berlanga J, Prats P, Remirez D, Gonzalez R, Lopez-Saura P, Aguiar J, et al. Prophylactic use of epidermal growth factor reduces ischemia/reperfusion intestinal damage. Am J Pathol. 2002;161:373–9. doi: 10.1016/S0002-9440(10)64192-2. http://dx.doi.org/10.1016/S0002-9440(10)64192-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhao W, Gan X, Su G, Wanling G, Li S, Hei Z, et al. The interaction between oxidative stress and mast cell activation plays a role in acute lung injuries induced by intestinal ischemia-reperfusion. J Surg Res. 2014;187:542–52. doi: 10.1016/j.jss.2013.10.033. http://dx.doi.org/10.1016/j.jss.2013.10.033 . [DOI] [PubMed] [Google Scholar]
- [4].Khanna A, Rossman JE, Fung HL, Caty MG. Intestinal and hemodynamic impairment following mesenteric ischemia/reperfusion. J Surg Res. 2001;99:114–9. doi: 10.1006/jsre.2001.6103. http://dx.doi.org/10.1006/jsre.2001.6103 . [DOI] [PubMed] [Google Scholar]
- [5].Swank GM, Deitch EA. Role of the gut in multiple organ failure:bacterial translocation and permeability changes. World J Surg. 1996;20:411–7. doi: 10.1007/s002689900065. http://dx.doi.org/10.1007/s002689900065 . [DOI] [PubMed] [Google Scholar]
- [6].Uglyanitsa KN, Nefyodov LI, Doroshenko YM, Nowicky JW, Volchek IV, Brzosko WJ, Hodysh YJ. Ukrain:a novel antitumor drug. Drugs Exp Clin Res. 2000;26:341–56. [PubMed] [Google Scholar]
- [7].Ernst E, Schmidt K. Ukrain - a new cancer cure? A systematic review of randomised clinical trials. BMC Cancer. 2005;5:69. doi: 10.1186/1471-2407-5-69. http://dx.doi.org/10.1186/1471-2407-5-69 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jagiello-Wójtowicz E, Kleinrok Z, Urbanska EM. Ukrain (NSC-631570) in experimental and clinical studies:a review. Drugs Exp Clin Res. 1998;24:213–9. [PubMed] [Google Scholar]
- [9].Cordes N, Plasswilm L, Bamberg M, Rodemann HP. Ukrain, an alkaloid thiophosphoric acid derivative of Chelidonium majus L. protects human fibroblasts but not human tumour cells in vitro against ionizing radiation. Int J Radiat Biol. 2002;78:17–27. doi: 10.1080/09553000110089991. http://dx.doi.org/10.1080/09553000110089991 . [DOI] [PubMed] [Google Scholar]
- [10].Akcilar R, Akcilar A, Savran B, Ayada C, Kocak C, Kocak FE, Genc O. Effects of ukrain in rats with intestinal ischemia and reperfusion. J Surg Res. 2015;195:67–73. doi: 10.1016/j.jss.2014.12.040. http://dx.doi.org/10.1016/j.jss.2014.12.040 . [DOI] [PubMed] [Google Scholar]
- [11].Institute for Laboratory Animal Research, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Academy Press; 2010. p. 248. [Google Scholar]
- [12].Wasserberg N, Pileggi A, Salgar SK, Ruiz P, Ricordi C, Inverardi L, et al. Heme oxygenase-1 upregulation protects against intestinal ischemia/reperfusion injury:a laboratory based study. Int J Surg. 2007;5:216–24. doi: 10.1016/j.ijsu.2006.06.001. http://dx.doi.org/10.1016/j.ijsu.2006.06.001 . [DOI] [PubMed] [Google Scholar]
- [13].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. http://dx.doi.org/10.1016/0003-2697(76)90527-3 . [DOI] [PubMed] [Google Scholar]
- [14].Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–9. doi: 10.1016/j.clinbiochem.2003.10.014. http://dx.doi.org/10.1016/j.clinbiochem.2003.10.014 . [DOI] [PubMed] [Google Scholar]
- [15].Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. http://dx.doi.org/10.1016/j.clinbiochem.2005.08.008 . [DOI] [PubMed] [Google Scholar]
- [16].Tufek A, Tokgoz O, Aliosmanoglu I, Alabalik U, Evliyaoglu O, Ciftci T, et al. The protective effects of dexmedetomidine on the liver and remote organs against hepatic ischemia reperfusion injury in rats. Int J Surg. 2013;11:96–100. doi: 10.1016/j.ijsu.2012.12.003. http://dx.doi.org/10.1016/j.ijsu.2012.12.003 . [DOI] [PubMed] [Google Scholar]
- [17].Yamanel L, Kaldirim U, Oztas Y, Coskun O, Poyrazoglu Y, Durusu M, et al. Ozone therapy and hyperbaric oxygen treatment in lung injury in septic rats. Int J Med Sci. 2011;8:48–55. doi: 10.7150/ijms.8.48. http://dx.doi.org/10.7150/ijms.8.48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stoney RJ, Cunninghan CG. Acute mesenteric ischemia. Surgery. 1993;114:489–90. [PubMed] [Google Scholar]
- [19].Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion:a review. Acta Cir Bras. 2005;20:336–43. doi: 10.1590/s0102-86502005000400013. http://dx.doi.org/10.1590/S0102-86502005000400013 . [DOI] [PubMed] [Google Scholar]
- [20].Shen L, Zhang J. Ginsenoside Rg1 increases ischemia-induced cell proliferation and survival in the dentate gyrus of adult gerbils. Neurosci Lett. 2003;344:1–4. doi: 10.1016/s0304-3940(03)00318-5. http://dx.doi.org/10.1016/S0304-3940(03)00318-5 . [DOI] [PubMed] [Google Scholar]
- [21].Teke Z, Sacar M, Yenisey C, Atalay AO, Bicakci T, Erdem E. Activated protein C attenuates intestinal reperfusion-induced acute lung injury:an experimental study in a rat model. Am J Surg. 2008;195:861–73. doi: 10.1016/j.amjsurg.2007.06.025. http://dx.doi.org/10.1016/j.amjsurg.2007.06.025 . [DOI] [PubMed] [Google Scholar]
- [22].Boyuk A, Onder A, Kapan M, Gumus M, Firat U, Basaralι MK, et al. Ellagic acid ameliorates lung injury after intestinal ischemia-reperfusion. Pharmacogn Mag. 2011;7:224–8. doi: 10.4103/0973-1296.84236. http://dx.doi.org/10.4103/0973-1296.84236 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Luo C, Yuan D, Zhao W, Chen H, Luo G, Su G, et al. Sevoflurane ameliorates intestinal ischemia-reperfusion-induced lung injury by inhibiting the synergistic action between mast cell activation and oxidative stress. Mol Med Rep. 2015;12:1082–90. doi: 10.3892/mmr.2015.3527. http://dx.doi.org/10.3892/mmr.2015.3527 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Torun E, Gedik AH, Cakir E, Umutoglu T, Gok O, Kilic U. Serum paraoxonase, TAS, TOS and ceruloplasmin in brucellosis. Int J Clin Exp Med. 2014;7:1592–97. [PMC free article] [PubMed] [Google Scholar]
- [25].Aycicek A, Ipek A. Maternal active or passive smoking causes oxidative stress in cord blood. Eur J Pediatr. 2008;167:81–5. doi: 10.1007/s00431-007-0433-z. http://dx.doi.org/10.1007/s00431-007-0433-z . [DOI] [PubMed] [Google Scholar]
- [26].Guzel A, Kanter M, Guzel A, Pergel A, Erboga M. Anti-inflammatory and antioxidant effects of infliximab on acute lung injury in a rat model of intestinal ischemia/reperfusion. J Mol Histol. 2012;43:361–9. doi: 10.1007/s10735-012-9396-0. http://dx.doi.org/10.1007/s10735-012-9396-0 . [DOI] [PubMed] [Google Scholar]
- [27].Habermehl D, Kammerer B, Handrick R, Eldh T, Gruber C, Cordes N, et al. Proapoptotic activity of Ukrain is based on Chelidonium majus L. alkaloids and mediated via a mitochondrial death pathway. BMC Cancer. 2006;6:14. doi: 10.1186/1471-2407-6-14. http://dx.doi.org/10.1186/1471-2407-6-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nowicky JW, Manolakis G, Meijer D, Vatanasapt V, Brzosko WJ. Ukrain both as an anticancer and immunoregulatory agent. Drugs Exp Clin Res. 1992;18(Suppl 5):1–4. [PubMed] [Google Scholar]
- [29].Hohenwarter O, Strutzenberger K, Katinger H, Liepins A, Nowicky JW. Selective inhibition of in vitro cell growth by the anti-tumour drug Ukrain. Drugs Exp Clin Res. 1992;18(Suppl):1–4. [PubMed] [Google Scholar]
- [30].Nowicky JW, Hiesmayr W, Nowicky W, Liepins A. Influence of Ukrain on DNA, RNA and protein synthesis in malignant cells. Drugs Exp Clin Res. 1996;22:81–91. [PubMed] [Google Scholar]
- [31].Zhalilo LI, Susak YM, Zemskov SV, Susak IA. Influence of Ukrain on the redox processes of hepatocytes. Drugs Exp Clin Res. 1996;22:189–91. [PubMed] [Google Scholar]
- [32].Nefyodov LI, Uglyanitsa KN, Nechiporenko NA, Smirnov VY, Brzosko W, Karavay NL. New biochemical mechanisms of the anticancer effect of Ukrain in the treatment of cancer of the urinary bladder. Drugs Exp Clin Res. 2000;26:195–9. [PubMed] [Google Scholar]
- [33].Savran B, Yerlikaya A, Erdogan E, Genc O. Anticancer agent ukrain and bortezomib combination is synergistic in 4T1 breast cancer cells. Anticancer Agents Med Chem. 2014;14:466–72. doi: 10.2174/18715206113139990318. http://dx.doi.org/10.2174/18715206113139990318 . [DOI] [PubMed] [Google Scholar]
- [34].Habermehl D, Kammerer B, Handrick R, Eldh T, Gruber C, Cordes N, et al. Proapoptotic activity of Ukrain is based on Chelidonium majus L. alkaloids and mediated via a mitochondrial death pathway. BMC Cancer. 2006;6:14. doi: 10.1186/1471-2407-6-14. http://dx.doi.org/10.1186/1471-2407-6-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chan SL, Lee MC, Tan KO, Yang LK, Lee AS, Flotow H, et al. Identification of chelerythrine as an inhibitor of BclXL function. J Biol Chem. 2003;278:20453–6. doi: 10.1074/jbc.C300138200. http://dx.doi.org/10.1074/jbc.C300138200 . [DOI] [PubMed] [Google Scholar]
- [36].Roublevskaia IN, Haake AR, Polevoda BV. Bcl-2 overexpression protects human keratinocyte cells from Ukrain-induced apoptosis but not from G2/M arrest. Drugs Exp Clin Res. 2000;26:149–56. [PubMed] [Google Scholar]
- [37].Roublevskaia IN, Polevoda BV, Ludlow JW, Haake AR. Induced G2/M arrest and apoptosis in human epidermoid carcinoma cell lines by semisynthetic drug Ukrain. Anticancer Res. 2000;20:3163–7. [PubMed] [Google Scholar]
- [38].Roublevskaia IN, Haake AR, Ludlow JW, Polevoda BV. Induced apoptosis in human prostate cancer cell line LNCaP by Ukrain. Drugs Exp Clin Res. 2000;26:141–7. [PubMed] [Google Scholar]
- [39].Skivka LM, Fedorchuk OG, Rudyk MP, Pozur VV, Khranovska NM, Grom MY, et al. Antineoplastic drug NSC631570 modulates functions of hypoxic macrophages. Tsitol Genet. 2013;47:70–82. http://dx.doi.org/10.3103/s0095452713050095 . [PubMed] [Google Scholar]
- [40].Koriem KM, Arbid MS, Asaad GF. Chelidonium majus leaves methanol extract and its chelidonine alkaloid ingredient reduce cadmium-induced nephrotoxicity in rats. J Nat Med. 2013;67:159–67. doi: 10.1007/s11418-012-0667-6. http://dx.doi.org/10.1007/s11418-012-0667-6 . [DOI] [PubMed] [Google Scholar]
- [41].Park JE, Cuong TD, Hung TM, Lee I, Na M, Kim JC, et al. Alkaloids from Chelidonium majus and their inhibitory effects on LPS-induced NO production in RAW264.7 cells. Bioorg Med Chem Lett. 2011;21:6960–3. doi: 10.1016/j.bmcl.2011.09.128. http://dx.doi.org/10.1016/j.bmcl.2011.09.128 . [DOI] [PubMed] [Google Scholar]


