Abstract
Translocator protein (18 kDa) (TSPO) is a marker of inflammation in the brain. Positron emission tomography (PET) scans with ligands for this receptor show increased expression of TSPO in many neuropathologic conditions. However, expression of TSPO in the periphery and its possible correlation to central nervous system (CNS) inflammation has been largely unstudied. In this paper PBR28, a recently synthesized ligand for TSPO that is shown to have 80-fold higher specific binding than its predecessor PK11195, is used to quantify peripheral TSPO. Data presented in this study show that monocytes account for the majority of TSPO measured in peripheral blood mononuclear cells (PBMC), and that TSPO expression is stable over time in healthy individuals. Previous studies show that areas of increased PBR28 binding in the brains of multiple sclerosis (MS) patients correlate with active demylinating lesions found during magnetic resonance imaging (MRI). To measure peripheral TSPO expression in an inflammatory disease of the CNS, PBR28 is used in an in vitro radioligand binding assay to measure the amount of TSPO in the PBMC of MS and healthy donor cohorts. Surprisingly, MS patients are found to have a significantly lower amount of peripheral TSPO than healthy donors. We suggest that TSPO protein expression is a potential peripheral biomarker of MS, more research is needed to determine if peripheral TSPO expression may also be altered in other neuroinflammatory conditions.
Keywords: TSPO, PBR28, Multiple sclerosis, Neuroinflammation
Introduction
Translocator protein 18 kDa (TSPO) is an outer mitochondrial membrane protein that is expressed in many varied cell and tissues types throughout the body, for example hematopoietic and lymphatic cells, and, heart, liver, adrenal and testes tissues (McEnery et al. 1992; Woods and Williams 1996). TSPO is thought to have many functions, among them cholesterol transport, steriodogenesis, apoptosis, and stress adaptation (Papadopoulos et al. 1997; Papadopoulos et al. 2006; Biggio et al. 2007; Veenman et al. 2007; Veenman et al. 2008). As evidenced by the embryonic lethal phenotype observed during efforts to create a knockout mouse, function of this protein is critical (Papadopoulos et al. 1997). TSPO expression is found to be upregulated in microglia and macrophages in sites of acute brain injury, which has made it a useful marker of neuroinflammation in the study of CNS inflammatory diseases (Banati 2002; Chen and Guilarte 2008; Kannan et al. 2009).
TSPO imaging through in vivo positron emission tomography (PET) scans and in vitro autoradiography has proven to be a useful tool in investigating chronic neuroinflammatory disease and inflammation following acute brain injury (Wilms et al. 2003; Papadopoulos and Lecanu 2009). The TSPO selective radioligand, PK11195, has been used extensively to study neuroinflammation in patients with diseases such as Alzheimer disease, multiple sclerosis (MS), traumatic brain injury (TBI), Parkinson disease, and brain cancer (Le Fur et al. 1983; Leschiner et al. 2000; Dolle et al. 2009). Recently, new TSPO ligands have been synthesized and confirmed to have higher specific binding than PK11195, and may yield a more sensitive detection of changes in inflammation levels (Briard et al. 2008). One of these new ligands, PBR28, is found to have an 80 fold higher specific binding in brain than PK11195 (Kreisl et al. 2010). The development of novel ligands, such as PBR28, represent an advance in the in vivo imaging of neuroinflammatory brain disease.
MS is a disease characterized by inflammatory demyelination in the central nervous system (CNS), activation of microglia and breakdown of the blood brain barrier (Sospedra and Martin 2005). MS patients routinely undergo MRI’s, and gadolinium enhancing lesions found in those scans are the standard measure of active disease and a primary measure of therapy efficacy (Sahraian and Eshaghi 2010). PETscans using [11C]PK11195 reveal areas of increased binding that correlate with active disease in MS patients seen on brain MRI’s, showing that upregulation of TSPO is a biomarker of MS (Banati et al. 2000). PET scans with [11C]PBR28 in MS patients have shown an increase in binding preceding a gadolinium enhancing lesion (Oh et al. 2010). These findings suggest that PBR28, in addition to PK11195, is a useful ligand for investigating disease in MS patients. In this study, using [3H]PBR28, we explore peripheral TSPO specific binding as an in vitro measure of receptor expression. We then investigate the possibility that peripheral expression of TSPO may be used as a biomarker of disease in MS patients.
Methods
PBMC preparation and separation
Whole blood or lymphocyte apheresis were obtained from the NIH Blood Bank, samples from healthy donors (HD) (n=25) and MS patients (n=32) of similar demographics are used (Table 1). All subjects provided informed written consent under a National Institutes of Health Clinical Center Institutional Review Board-approved research protocol. The peripheral blood mononuclear cells (PBMC) were collected after separation on a Ficol gradient using lymphocyte separation medium (BioWhittaker), then cryofrozen and stored in liquid nitrogen. For some HD, cryofrozen cells were thawed and CD14+, CD8+, and CD4+, cells were obtained using the MACS negative selection monocyte isolation kit (MiltenyiBiotec). A purity of >85 % was achieved, as determined by FACS analysis then, a binding assay or Taqman was run on the resulting populations.
Table 1.
Demographics of healthy donors (HD) and Multiple Sclerosis patients (MS)
| Subjects | Agea | Sex | Raceb | Treatmentc | Diagnosisd | |
|---|---|---|---|---|---|---|
| HD | 25 | 48.0±13.5 | 5F/20M | 14C/8B/3O | - | - |
| MS | 32 | 42.6±9.9 | 21F/11M | 23C/6B/3O | 7 B/6 Z/7 O/12 U | 25 RRMS/3 SPMS/4 O |
Ages shown as mean years ± SD, no significant difference is found
Caucasian (C), Black (B), and Other (O)
Betaseron (B), Zenapax (Z), other (O), and untreated (U)
Relapsing remitting MS (RRMS), secondary progressive MS (SPMS), and other (O)
Real-time PCR quantitation of TSPO
RNA was extracted from PBMC cell subsets using RNesay RNA extraction kit (Qiagen), the kit was used according to manufacturer’s instructions. cDNA was then synthesized using a MultiScribe Reverse Transcriptase kit (Applied Biosystems) according to manufacturer’s instructions. Taqman was run using the resulting cDNA and primers for TSPO (Applied Biosystems), and the housekeeping gene HPRT (Applied Biosystems), the reaction conditions and concentrations described by the manufacturer were used.
Radioligand binding assay
Cryofrozen PBMC were thawed, resuspended in 50 mM HEPES buffer, and homogenized using a dounce homogenizer. The homogenates were then put through one freeze thaw and normalized for protein concentration. The normalized homogenates were incubated with [3H]PBR28, in triplicate, for 90 min at 4 °C to obtain total binding. They were also incubated with a combination of [3H]PBR28 and PBR28, in triplicate, to obtain non-specific binding. Homogenate incubations were harvested using a TomTec Harvester 96 Mach III M over a glassfibre filterpaper (PerkinElmer) which was then placed in scintillation fluid (PerkinElmer) and radioactivity per well was counted using 1450 MicroBeta TriLuxplatereader (PerkinElmer). The mean specific binding was calculated by taking the mean total binding less the mean non-specific binding. Bmax was calculated for each sample by dividing the mean specific binding (pmol of radioactivity) by the protein (mg) used in the assay.
Mitochondrial membrane potential assay
Cryofrozen PBMC were thawed, making sure that a viability of >90 % was maintained. Cells were plated in cRPMI,10 % FCS, at a concentration of 2 million cells/mL. They were then cultured with or without 0.25 mM H2O2 for 1 h and then washed with PBS. After culture, 1, 2, and 5 h time-points were collected, the cells were then homogenized for use in a binding assay or stained as follows. Cultured cells were stained with CD14 and CD3 monoclonal antibodies for characterization, simultaneously they were stained with MitoProbe JC-1 following manufacturer’s instructions (Invitrogen) to determine mitochondrial membrane potential. Cells were then run on a FACS Calibur and analyzed using FlowJo.
Statistical analysis
Data are expressed as a mean±SD of (n) independent experiments. Two-tailed students t-test were used to assess significance. A p-value of less than or equal to 0.05 was considered significant.
Results
CD14+ monocytes are a major TSPO expressing subset within PBMC
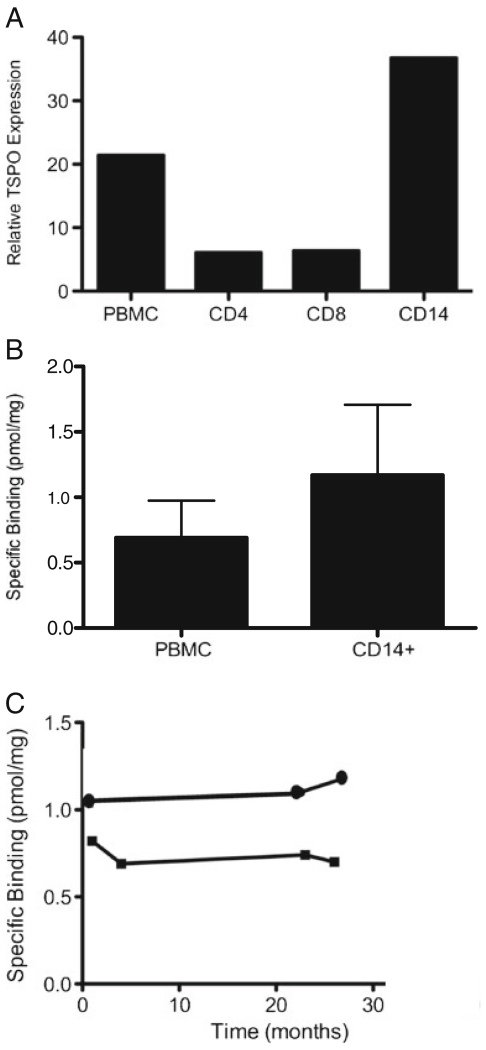
TSPO expression was examined in HD PBMC. Quantitative reverse transcriptase polymerase chain reaction (RT-PCR) showed that within PBMC, the CD14+ monocytes express six-fold higher TSPO than either the CD4+ or the CD8+ cells (Fig. 1a). The observation that TSPO is primarily expressed on CD14+ monocytes was further confirmed using a [3H] PBR28 radioligand binding assay. Purified CD14+ cells showed higher concentration of TSPO protein than total PBMC as indicated by higher Bmax (1.17 +/−0.54 vs 0.69 +/−0.28 pmol/mg; Fig. 1b). Thus, within the PBMC, CD14+ monocytes represent a major subset of TSPO expressing cells.
Fig. 1.
TSPO is expressed mainly on CD14+ monocytes and does not significantly vary over time within a donor. a TSPO expression of PBMC subsets is determined by Taqman, normalized to housekeeping gene HPRT, and expression relative to SupT1 cell line TSPO expression is graphed. Average relative expression from a representative experiment run in triplicate is shown. Total PBMC show a relative expression of 22, CD4+ and CD8+ populations show relative expression of approximately 7, CD14+ cells show a relative expression of 38. b TSPO expression as determined by a radioligand binding assay using [3H]PBR28, Bmax is plotted as the pmol of specific binding normalized to protein concentration. Mean Bmax of total PBMC from 3 HD is 0.69±0.28 pmol/mg, while mean Bmax of CD14+ cells from those HD is 1.17±0.54 pmol/mg. c [3H]PBR28 radioligand binding assays are used to determine the variability of peripheral TSPO expression over time. Bmax is plotted as the pmol of specific binding normalized to protein concentration. One HD has a Bmax of 1.05 pmol/mg at month 1, 1.1 pmol/mg at month 23, and 1.17 pmol/mg at month 27; the second HD has a Bmax of 0.8 pmol/mg at month 1, 0.7 pmol/mg at month 4, 0.72 pmol/mg at month 23, and 0.7 pmol/mg at month 27. No significant difference is found over time in these two donors
To further characterize TSPO expression in the peripheral blood, we measured within-subject changes in TSPO expression over time. The change in TSPO expression was less than 15 % over a 27 month period in the two individuals tested, suggesting that TSPO may be a stable marker over time in healthy donors (Fig. 1c).
MS patients have reduced peripheral blood TSPO expression
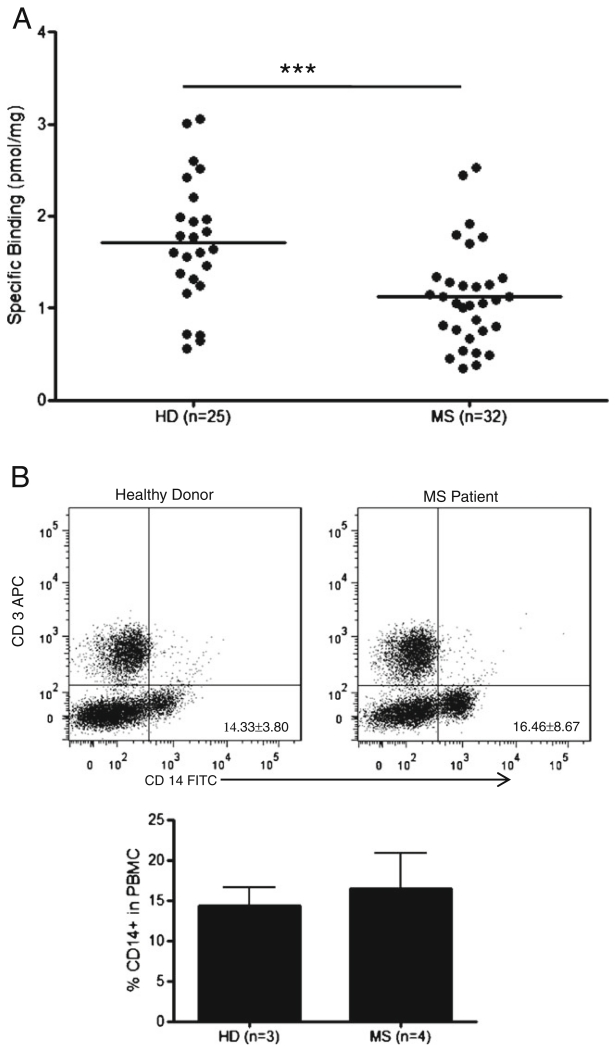
To examine TSPO expression in subjects with MS, TSPO protein expression was measured in the PBMC of HD (n=25) and MS (n=32) patients. The mean TSPO expression in the PBMC of MS patients was significantly lower than that of HD (1.7±0.69 vs. 1.1±0.55; p=0.0007; Fig. 2a).
Fig. 2.
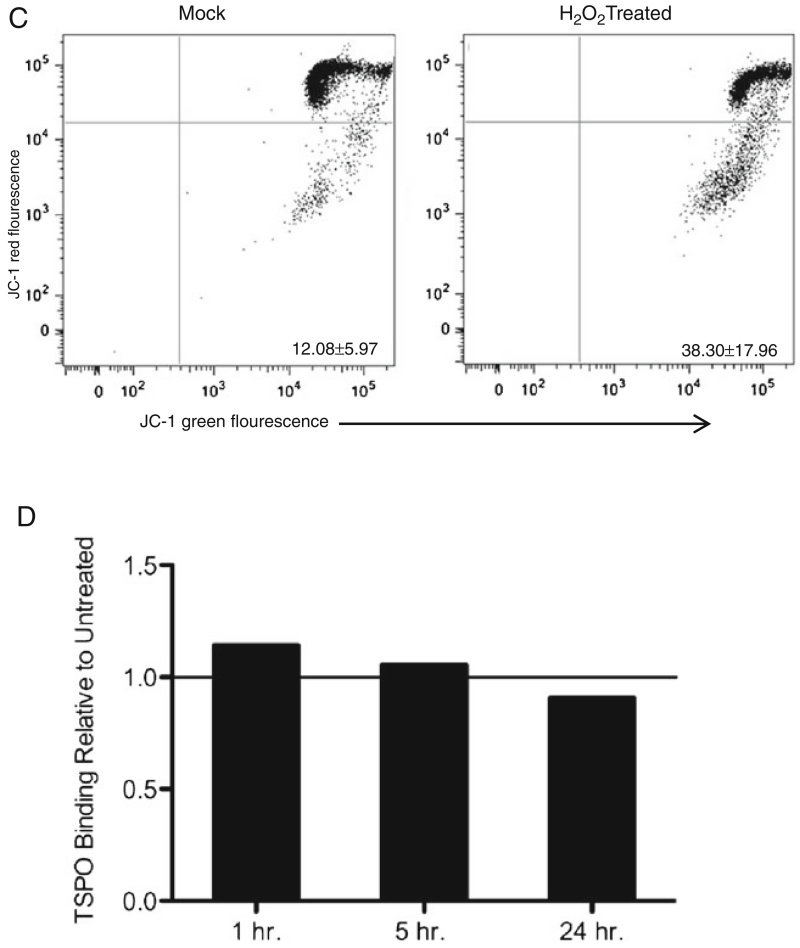
MS patients show significantly lower peripheral TSPO levels than HD, which is not due to acute mitochondrial injury. a Using a [3H]PBR28 radioligand binding assay, TSPO was measured in both HD (n=25) and MS patients (n=32), and Bmax is plotted as the pmol of specific binding normalized to protein concentration. The HD mean Bmax is found to be 1.7±0.69 pmol/mg, while the MS patient mean Bmax is found to be 1.1±0.55 pmol/mg. There is significantly lower TSPO expression in the MS cohort, students t-test p=0.0007. b Total PBMC from 3 HD and 4 MS patients were stained with CD14-FITC and CD3-APC antibodies and analyzed by flow cytometry. Representative plots of MS patient and HD cell profiles are shown along with a graph showing the mean ± SD percentage of CD14+ cells in PBMC for each cohort (HD= 14.33±3.80 %, MS=16.46±8.67 %), there are no significant differences seen in the percentages of each cell population. C. Representative plot, n=3, of CD14+ cells untreated and treated with H2O2 for 1 h. The lower right quadrant indicates mitochondrial membrane potential dysfunction, which was 12.08±5.97 % for mock, and 38.30±17.96 % for H2O2 treated. D. A radioligandbinding assay was used to measure specific binding of [3H]PBR28 to H2O2 treated PBMC relative to untreated. The relative amounts are 1.14 after 1 h, 1.06 after 5 h, and 0.91 after 24 h of treatment
To exclude the possibility that the differences in TSPO expression between subjects with MS and HD are due to differences in the frequencies of CD14+ cells, we analyzed the CD14+ populations in both cohorts. No significant difference was observed between the frequencies of CD14+ monocytes in PBMC of MS (16.46 %±8.67) and HD (14.33 %±3.80; Fig. 2b). These data suggest an alteration in peripheral TSPO expression in subjects with MS.
Acute mitochondrial injury does not alter TSPO expression
Given that TSPO is a mitochondrial membrane protein, we tested the possibility that mitochondrial dysfunction could explain changes in TSPO expression. CD14+ monocytes treated with H2O2 showed loss of mitochondrial membrane potential as indicated by the fluorescent cationic dye JC-1, indicating mitochondrial dysfunction resulting from oxidative stress (Fig. 2c). These monocytes showed an average of 38.3 % decrease mitochondrial membrane potential, after 1 h of treatment with H2O2, 25.6 % after 5 h, and 16.6 % after 24 h, showing that they are able to respond to and recover from the acute injury. Mock cultured CD14+ cells gave a consistent average of 12.08 % decreased mitochondrial membrane potential. Shortly after oxidative stress, nonsignificant increases in TSPO were seen which returned to baseline by 24 h (Fig. 2d), this does not correlate with the changes seen in JC-1 staining. TSPO expression was not significantly altered in this in vitro model of acute mitochondrial injury.
Discussion
While TSPO expression in the CNS has been studied as a marker of neuroinflammation, less is known about the TSPO expression in the periphery and how it may relate to disease. Understanding the peripheral expression patterns and fluctuations in TSPO expression may allow a broader utilization of this protein as a marker of inflammation. Using PBR28 we found that within PBMCs the CD14+ monocyte population is a major TSPO expressing subset, corroborating data using PK11195 from Canat et al. (Canat et al. 1993). TSPO expression appeared to be stable over time in healthy individuals, suggesting that larger changes in TSPO expression may reflect pathophysiologic changes.
In contrast to findings within the CNS that show upregulation of TSPO correlating with disease state (Banati et al. 2000), TSPO expression in the periphery, measured using PBR28, is significantly decreased in MS patients compared to healthy donors (Fig. 2). This is consistent with findings from Ferrero et al. that found a lower amount of peripheral TSPO in MS patients using PK11195 (Ferrero et al. 1992). The reduced TSPO expression in subjects with MS was not explained by alterations in the frequencies of total peripheral CD14+ monocytes. Subjects with MS in which cell counts were measured did not differ with respect to frequencies of peripheral CD14+ monocytes compared to healthy donors. It is of note that levels of activated CD14+ cells and other immune subsets are shown to vary with disease in many neuroinflammatory conditions such as Alzheimer’s disease and sporadic amyotrophic lateral sclerosis (Zhang et al. 2005). Also that peripheral monocyte levels in MS patients have been shown to be affected by drug treatment, such as Natalizumab (Skarica et al. 2011). We did not see significant variation in CD14+ levels of the patients tested however, it is expected that among the larger cohort there might be more variation as compared to CD14+ amounts in healthy donor PBMC. However, investigation of this variation among our patients’ subset is beyond the scope of this report. This variation may be contributing to our results and a larger study is needed to determine how monocyte frequency and activation status may be playing a role in the observed decrease in peripheral TSPO of MS patients.
MS lesions are characterized in part by the infiltration of monocytes and macrophages and increases in TSPO correlate with lesion localization in MS (Banati et al. 2000). Increased expression of TSPO is also seen in other inflammatory brain diseases and is attributed mostly to microglia and infiltrating macrophage, suggesting that a population of macrophage expressing high levels of TSPO infiltrate the CNS and account for the increased TSPO seen in the brains of MS patients (Papadopoulos and Lecanu 2009). Interestingly, when using PBR28 in a PET scan an increase in TSPO expression is seen in the brain of MS patients preceding the appearance of a gadolinium enhancing lesion (Oh et al. 2010). This increase is also attributed mostly to an increase in activated microglia and macrophages, even before lesion relapse. Thus, while the total number of peripheral monocytes is unchanged, the sequestration of macrophage expressing high levels of TSPO in tissue may in part account for the reduction seen in TPSO expression in the periphery and this reduction may serve as a useful biomarker or warning for an increase in MS disease activity. Following this observation of reduction in peripheral TSPO expression of MS patients it is now important to investigate expression levels in PBMC from patients with other neuroinflammatory conditions. This will help determine if TSPO has potential to be a specific biomarker of MS or if it is a marker of generalized inflammatory CNS disease.
To determine if peripheral decrease in TSPO is specific to inflammatory CNS disease, peripheral TSPO from a small cohort (n=4) of traumatic brain injury (TBI) patients was measured using a [3H]PBR28 binding assay. No difference in peripheral TSPO level is seen between TBI patients and HD (data not shown). This finding suggests that the decrease in peripheral TSPO seen in MS patients may be specific to inflammatory conditions as opposed to simply neurologic injury. Investigation into TSPO expression in other inflammatory disease is necessary to further describe this observation but is beyond the scope of this study. The aforementioned studies are critical to further this discovery and should be carried out in a larger multi-group study.
TSPO is partially responsible for regulating mitochondrial membrane potential by interacting with the mitochondrial permeability pore (Papadopoulos and Lecanu 2009). Mitochondrial dysfunction has been implicated in the progression of MS, and enhanced density and activity of mitochondria is observed in areas of demylination in MS patient brains (Mao and Reddy 2010). This increase in active mitochondria is thought to create free radicals leading to oxidative stress, which has been associated with axonal degeneration (Su et al. 2009; Witte et al. 2009; Higgins et al. 2010). Given that TSPO is a mitochondrial membrane protein, alterations in TSPO expression could reflect changes in mitochondrial function. In our study, we induced acute mitochondrial injury in cultured monocytes to test the hypothesis that mitochondrial dysfunction could be associated with changes in TSPO expression. We did not find significant changes in TSPO expression associated with acute mitochondrial dysfunction (Fig. 2). While no differences were seen in our in vitro model of acute mitochondrial injury, we have not ruled out the possibility that chronic mitochondrial dysfunction could be associated with alterations in TSPO expression.
The existence of mixed and low affinity binders in brain tissue to TSPO ligands, excluding PK11195, has recently been reported (Owen et al. 2011). These mixed affinity levels do not correlate with disease state, and are shown to be less dramatic in platelets than brain tissue. During our study we found about 10 % of both the healthy donors and MS patients tested to be low affinity binders, this percentage is consistent with previously reported levels (Owen et al. 2011). These PBR28 low affinity binders do not give a detectable signal in our binding assay and were excluded from this study. The range of binding seen within each cohort may in part be due to mixed affinity binding. However, because affinity for the ligand does not correlate with disease, we expect the observation that MS patients have a significantly decreased average peripheral TSPO expression to also be seen using high affinity ligands without mixed affinity binding. The development of TSPO ligands without mixed affinity binding, and with even higher specificity then PBR28 is an active area of research and many newly synthesized ligands are currently being evaluated. These ligands will allow us to further elucidate the range of TSPO levels within patients that may be indicative of different disease states, therefore aiding in diagnosis and treatment.
TSPO imaging and peripheral quantification are evolving into important tools for monitoring disease progression in neurodegenerative and traumatic brain disorders, particularly MS. Using PBR28, which has higher specific binding than previously used ligands, we observe a significant decrease in TSPO expression in MS patients as compared to a similar cohort of healthy donors. With the development and use of more advanced ligands an even greater disparity in peripheral TSPO expression between MS patients of varying stages of disease and healthy donors may be able to be observed. Measurement of peripheral TSPO levels may represent a biomarker indicative of MS disease state. Upon further development, this method may prove to be a rapid and in vitro way to provide quantitative information to aid in diagnosis of MS, and possibly other neuroinflammatory disease, patients.
Acknowledgements
The authors would like to thank Dr. Robert Innis for his kind donation of the [3H]PBR28, and his lab, especially Kimberly Jenko, for help in developing our radioligand binding assay methods.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- Banati RB. Visualising microglial activation in vivo. Glia. 2002;40:206–217. doi: 10.1002/glia.10144. [DOI] [PubMed] [Google Scholar]
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123(Pt 11):2321–2337. doi: 10.1093/brain/123.11.2321. [DOI] [PubMed] [Google Scholar]
- Biggio G, Concas A, Follesa P, Sanna E, Serra M. Stress, ethanol, and neuroactive steroids. Pharmacol Ther. 2007;116:140–171. doi: 10.1016/j.pharmthera.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briard E, Zoghbi SS, Imaizumi M, Gourley JP, Shetty HU, Hong J, Cropley V, Fujita M, Innis RB, Pike VW. Synthesis and evaluation in monkey of two sensitive 11C-labeled aryloxyanilide ligands for imaging brain peripheral benzodiazepine receptors in vivo. J Med Chem. 2008;51:17–30. doi: 10.1021/jm0707370. [DOI] [PubMed] [Google Scholar]
- Canat X, Carayon P, Bouaboula M, Cahard D, Shire D, Roque C, Le Fur G, Casellas P. Distribution profile and properties of peripheral-type benzodiazepine receptors on human hemopoietic cells. Life Sci. 1993;52:107–118. doi: 10.1016/0024-3205(93)90293-c. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle F, Luus C, Reynolds A, Kassiou M. Radiolabelled molecules for imaging the translocator protein (18 kDa) using positron emission tomography. Curr Med Chem. 2009;16:2899–2923. doi: 10.2174/092986709788803150. [DOI] [PubMed] [Google Scholar]
- Ferrero P, Rocca P, Benna P, De Leo C, Montalenti E, Ravizza L, Bergamasco B. An analysis of peripheral type benzodiaze-pine receptors on blood mononuclear cells during high dose steroid treatment of multiple sclerosis. Ital J Neurol Sci. 1992;13:685–691. doi: 10.1007/BF02334972. [DOI] [PubMed] [Google Scholar]
- Higgins GC, Beart PM, Shin YS, Chen MJ, Cheung NS, Nagley P. Oxidative stress: emerging mitochondrial and cellular themes and variations in neuronal injury. J Alzheimers Dis. 2010;20(Suppl 2):S453–473. doi: 10.3233/JAD-2010-100321. [DOI] [PubMed] [Google Scholar]
- Kannan S, Balakrishnan B, Muzik O, Romero R, Chugani D. Positron emission tomography imaging of neuroinflammation. J Child Neurol. 2009;24:1190–1199. doi: 10.1177/0883073809338063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Fujita M, Fujimura Y, Kimura N, Jenko KJ, Kannan P, Hong J, Morse CL, Zoghbi SS, Gladding RL, Jacobson S, Oh U, Pike VW, Innis RB. Comparison of [(11)C]-(R)-PK 11195 and [(11)C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: implications for positron emission tomographic imaging of this inflammation biomarker. Neuroimage. 2010;49:2924–2932. doi: 10.1016/j.neuroimage.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Fur G, Guilloux F, Rufat P, Benavides J, Uzan A, Renault C, Dubroeucq MC, Gueremy C. Peripheral benzodiazepine binding sites: effect of PK 11195, 1-(2-chlorophenyl)-N-methyl-(1-methylpropyl)-3 isoquinolinecarboxamide. II. In vivo studies. Life Sci. 1983;32:1849–1856. doi: 10.1016/0024-3205(83)90063-2. [DOI] [PubMed] [Google Scholar]
- Leschiner S, Weizman R, Shoukrun R, Veenman L, Gavish M. Tissue-specific regulation of the peripheral benzodiazepine receptor by antidepressants and lithium. Neuropsychobiology. 2000;42:127–134. doi: 10.1159/000026682. [DOI] [PubMed] [Google Scholar]
- Mao P, Reddy PH. Is multiple sclerosis a mitochondrial disease? Biochim Biophys Acta. 2010;1802:66–79. doi: 10.1016/j.bbadis.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci U S A. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh U, Fujita M, Ikonomidou VN, Evangelou IE, Matsuura E, Harberts E, Ohayon J, Pike VW, Zhang Y, Zoghbi SS, Innis RB, Jacobson S. Translocator protein PET imaging for glial activation in multiple sclerosis. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2010 doi: 10.1007/s11481-010-9243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, Innis RB, Pike VW, Reynolds R, Matthews PM, Parker CA. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med. 2011;52:24–32. doi: 10.2967/jnumed.110.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Lecanu L. Translocator protein (18 kDa) TSPO: an emerging therapeutic target in neurotrauma. Exp Neurol. 2009;219:53–57. doi: 10.1016/j.expneurol.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, Hardwick M, Li H, Vidic B, Brown AS, Reversa JL, Bernassau JM, Drieu K. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62:21–28. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Sahraian MA, Eshaghi A. Role of MRI in diagnosis and treatment of multiple sclerosis. Clin Neurol Neurosurg. 2010;112:609–615. doi: 10.1016/j.clineuro.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Skarica M, Eckstein C, Whartenby KA, Calabresi PA. Novel mechanisms of immune modulation of natalizumab in multiple sclerosis patients. J Neuroimmunol. 2011;235:70–76. doi: 10.1016/j.jneuroim.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Su KG, Banker G, Bourdette D, Forte M. Axonal degeneration in multiple sclerosis: the mitochondrial hypothesis. Curr Neurol Neurosci Rep. 2009;9:411–417. doi: 10.1007/s11910-009-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenman L, Papadopoulos V, Gavish M. Channel-like functions of the 18-kDa translocator protein (TSPO): regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr Pharm Des. 2007;13:2385–2405. doi: 10.2174/138161207781368710. [DOI] [PubMed] [Google Scholar]
- Veenman L, Shandalov Y, Gavish M. VDAC activation by the 18 kDa translocator protein (TSPO), implications for apoptosis. J Bioenerg Biomembr. 2008;40:199–205. doi: 10.1007/s10863-008-9142-1. [DOI] [PubMed] [Google Scholar]
- Wilms H, Claasen J, Rohl C, Sievers J, Deuschl G, Lucius R. Involvement of benzodiazepine receptors in neuroinflammatory and neurodegenerative diseases: evidence from activated micro-glial cells in vitro. Neurobiol Dis. 2003;14:417–424. doi: 10.1016/j.nbd.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Witte ME, Bo L, Rodenburg RJ, Belien JA, Musters R, Hazes T, Wintjes LT, Smeitink JA, Geurts JJ, De Vries HE, van der Valk P, van Horssen J. Enhanced number and activity of mitochondria in multiple sclerosis lesions. J Pathol. 2009;219:193–204. doi: 10.1002/path.2582. [DOI] [PubMed] [Google Scholar]
- Woods MJ, Williams DC. Multiple forms and locations for the peripheral-type benzodiazepine receptor. Biochem Pharmacol. 1996;52:1805–1814. doi: 10.1016/s0006-2952(96)00558-8. [DOI] [PubMed] [Google Scholar]
- Zhang R, Gascon R, Miller RG, Gelinas DF, Mass J, Hadlock K, Jin X, Reis J, Narvaez A, McGrath MS. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS) J Neuroimmunol. 2005;159:215–224. doi: 10.1016/j.jneuroim.2004.10.009. [DOI] [PubMed] [Google Scholar]