Abstract
Human T-lymphotrophic virus type I (HTLV-I) is an oncogenic retrovirus and its infection is associated with a variety of human diseases including HTLV-I-associated myelopathy/tropic spastic paraparesis (HAM/TSP). Large numbers of epidemiological, virological, immunological, and clinical studies on HTLV-I- and HTLV-I-associated diseases have been published, although the pathogenesis of HAM/TSP remains to be fully understood. In the last several years, researchers have shown that several key factors are important in HTLV-I-associated neurologic disease including high HTLV-I proviral load and a strong immune response to HTLV-I. Here, we review pathophysiological findings on HAM/TSP and focus on viral-host immune responses to the virus in HTLV-I infected individuals. In particular, the role of HTLV-I-specific CD8+ T cell response is highlighted.
Keywords: HTLV, HTLV-1, HAM/TSP, myelopathy, uveitis, myositis, alveolitis, neuropathy, cytotoxic T lymphocyte, CTL, proviral load, pathology, immunology
Introduction
HTLV-I-associated myelopathy/tropic spastic paraparesis
Human T-lymphotrophic virus type I (HTLV-I) is the first human oncogenic retrovirus to be identified and is thought to infect approximately 10–20 million people worldwide (Poiesz et al. 1980; de The and Bomford 1993). Several endemic areas for HTLV-I are known in the world such as southern Japan, the Caribbean, Central and South America, Middle East, Central and West Africa, Melanesia, and there are smaller foci in the aboriginal populations of Australia, Papua New Guinea, and northern Japan (Gessain 1996). HTLV-I has been demonstrated to be the etiological agent of both adult T cell leukemia(ATL) and a progressive neurological disease termed HTLV-I-associated myelopathy/tropic spastic paraparesis (HAM/TSP) (Hinuma et al. 1981; Gessain et al. 1985; Osame et al. 1986) In 1985, Gessain first reported the high prevalence of anti-HTLV-I antibodies in the sera of the patients with tropic spastic paraparesis in Martinique of French West Indies. Subsequently, Rogers-Johnson reported similar findings in Jamaica and Colombia. In 1986, Osame reported the association between HTLV-I infection and spastic paraparesis in southern Japan, Kagoshima prefecture and proposed the diagnostic term HTLV-I-associated myelopathy. The official name of the disease, HAM/TSP, and the clinical and laboratory guidelines for the diagnosis of HAM/TSP have been made based on the World Health Organization guidelines in 1987 (WHO 1989; Osame 1990). HAM/TSP is characterized by unremitting myelopathic symptoms such as spastic paraparesis, persistent lower back pain, bladder dysfunction, and mild limb sensory disturbance. Of interest, these features are typically symmetrical in most patients with HAM/TSP, while there are some patients with a variation in progression and symptoms and some patients presenting with atypical neurological feature like mononeuropathy (Leite et al. 2004), amyotrophic lateral sclerosis (Matsuzaki et al. 2000), or spinocerebellar degeneration (Kira et al. 1993). The reason why most HAM/TSP patients show thoracic to lumbar myelopathy and a clinically symmetrical presentation remains unclear. Interestingly, the majority of infected individuals remain lifelong asymptomatic carriers. In the endemic area, the seroprevalence varies from 1% to 20%. Approximately 0.25% (Kaplan et al. 1990) to 5% of the infected individuals develops HAM/TSP while 2% to 5% develop ATL. Why only a subset of HTLV-I infected individuals develop clinical disease while the vast majority remain as asymptomatic carriers (AC) is not known, but it is believed to include genetic susceptibility associated with a dysregulated immune response that results in neuropathology.
Human T-lymphotrophic virus type I
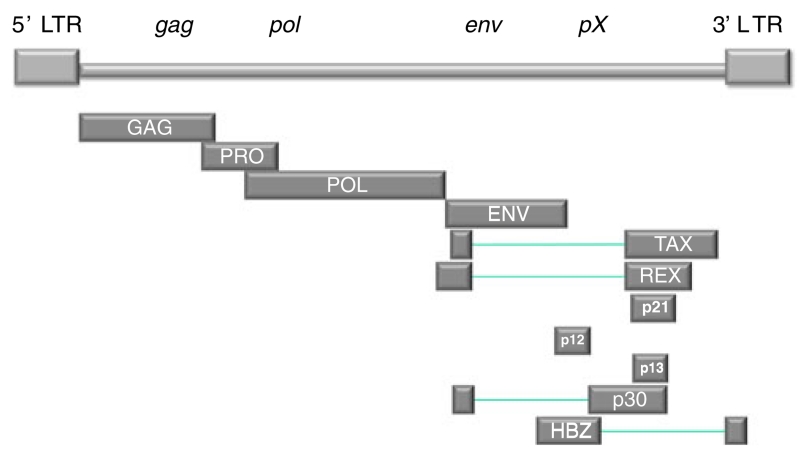
HTLV-I is an exogenous, human retrovirus, which varies little in sequence compared with human immunodeficiency virus type-1 (HIV-1). It is classified in the genus delta-retrovirus of the subfamily Orthoretrovirinae. HTLV-I genome contains three open reading frames that encode for proteins that can be found in the mature virus. The three typical structural and enzymatic genes (gag, pol, and env) are flanked by two long terminal repeats as seen in other retrovirus. In addition, a region called pX contains at least four partially overlapping leading flame (ORFs) encoding accessory proteins (p12, p13, p30), the post-transcriptional regulator REX (ORF3), and the TAX transactivator (ORF4; Fig. 1).
Fig. 1.
The HTLV-I proviral genome
Transmission of HTLV-I occurs through three main routes, breast-feeding, sexual contact, and blood transfusion. For all these routes, infected cells must pass from the infected individuals because HTLV-I transmits by cell–cell contact. HTLV-1 infected cells produce and transiently store virions in extracellular adhesive structures rich in extracellular matrix components and linker proteins that are crucial for HTLV-1 cell-to-cell transmission (Pais-Correia et al. 2009). When an infected cell contacts an uninfected cell, a microtubule-organizing center is polarized at the cell–cell junction, and a virological synapse forms at the interface (Igakura et al. 2003). HTLV-1 integrates randomly into the host genome (Doi et al. 2005). Sequential analyses of integration sites verify that the proliferation of HTLV-1-infected cells is clonal and persistent (Furukawa et al. 1992; Wattel et al. 1995; Etoh et al. 1997; Cavrois et al. 1998). Unlike the amplification of HIV-1 proviral genomes that is associated with de novo infection of new cells (Miyazato et al. 2006; Taylor et al. 2006; Feuer and Chen 1992), clonal proliferation of HTLV-I infected cells contribute to the increased number of infected cells in PBMC.
Location of HTLV-I infected cells and HTLV-I proviral expression in situ
HTLV-I in the CNS of HAM/TSP patients
There is a strong antibody response to HTLV-I with high titers of HTLV-I-specific IgM, IgA, and IgG, which are correlated with proviral load (PVL; Kira et al. 1992; Manns et al. 1999). Increased numbers of HTLV-I-specific HLA class I-restricted CD8+ CTLs is also one of the striking features of HAM/TSP (Jacobson et al. 1990; Pique et al. 2000). These results suggest that there must be persistent HTLV-I proviral expression in vivo. Although HTLV-I can infect a wide range of human and nonhuman cells in vitro, HTLV-I has been thought preferentially to infect CD4+ cells (Richardson et al. 1990; Trejo and Ratner 2000). High HTLV-I proviral loads were demonstrated in PBMC of patients with HAM/TSP and are higher than in asymptomatic carriers (Kubota et al. 1993; Hashimoto et al. 1998; Nagai et al. 1998), although HTLV-I tax expressing cells are difficult to detect. HTLV-I tax encodes a transactivator protein for virus expression and plays important roles in activating cellular genes including inflammatory cytokines. Tax also has a dominant epitope recognized by HTLV-I-specific CD8+ cytotoxic T lymphocytes (Niewiesk et al. 1995). Therefore, it has been suggested that strong HTLV-I tax expression, somewhere, is needed to drive these elevated CTL. HTLV-I proviral load and HTLV-I tax expression were found more frequently in the CSF than in the PBMC of the patients with HAM/TSP (Nagai et al. 2001a; Moritoyo et al. 1999). The ratio of HTLV-I PVL in the CSF cells to that in the PBMCs was significantly associated with clinically progressive disease and with recent onset of HAM/TSP (Takenouchi et al. 2003). In addition, HTLV-I infected lymphocytes shared the same HTLV-I integration site in cellular DNA in both CSF and PBMC of the HAM/TSP patients (Cavrois et al. 2000). These findings suggest that HTLV-I-infected lymphocytes migrate from the periphery into the CNS and that expression of HTLV-I tax in the CNS may induce a strong immune response and subsequent development of HAM/TSP.
Histological analysis of cellular localization for HTLV-I and tax expression in the CNS
Does HTLV-I exist in the CNS? Pathological studies revealed HTLV-I DNA was localized to inflammatory infiltrating T lymphocytes (UCHL-1 positive cells) but not in CD68+ cells in the affected spinal cord of HAM/TSP patients by in situ PCR techniques (Matsuoka et al. 1998). Another study showed that HTLV-I tax mRNA could be demonstrated in infiltrating CD4+ T lymphocytes in active lesions of the affected spinal cord using in situ hybridization (Moritoyo et al. 1996). Kubota et al. reported that quantitative PCR analysis showed the amount of HTLV-I DNA decreased concomitantly with the number of infiltrating CD4+ cells but not CD8+ cells nor macrophages in spinal cord lesions (Kubota et al. 1994). These findings suggest a preferential viral reservoir in infiltrating CD4+ T lymphocytes that express HTLV-I tax in the CNS. However, other cells may also harbor HTLV in the CNS. For example, HTLV-I RNA has been shown to localize to astrocytes (Lehky et al. 1995; Ozden et al. 2002) Recently, Afonso et al. reported the alteration of BBB function caused by HTLV-I infected endothelial cells that may be associated with increase migration of inflammatory cells into the CNS (Afonso et al. 2008).
General histology of the CNS of HAM/TSP patients
The brain is grossly unremarkable except for diffuse thickening of the leptomeninges (Izumo et al. 1992), while all patients with HAM/TSP have symptoms of a myelopathy but not meningitis. The spinal cord shows mild to severe atrophy with thickening of the leptomeninges. Atrophy of the spinal cord is often detected at the thoracic region with MRI/CT. Histochemically, chronic inflammation is mainly seen in mid- to lower spinal cord, especially at the thoracic region and is consistent with clinical features of the patients with HAM/TSP. A number of lymphocytic infiltration and foamy macrophages are seen in the thickened meninges, parenchyma, and perivascular area of the mid- to lower spinal cord of patients. Not only T cells but B cells are sparse in parenchyma and thickened meninges (Akizuki et al. 1989). Natural killer (NK) cells are rare in the lesion (Umehara et al. 1994a). Smaller numbers of infiltrating cells are also found in the midbrain, pons, medulla oblongata, cerebellum, and cerebral white matter (Akizuki et al. 1988). The abnormal signals of white matter in the cerebrum are also detected by MRI and have been associated with inflammation (Kira et al. 1991). These regions detected by MRI are usually small, less than 5 mm in the white matter, though almost all the patients with HAM/TSP do not show such symptoms. The reasons why lesions are scattered in the CNS, as well as why the thoracic level of the spinal cord is mainly affected, remain unclear. Of importance, the neuropathology of the spinal cord of HAM/TSP appears to change gradually during the progression of the disease (Umehara et al. 1993).
In cases with clinical history up to 3 years, parenchymal lesions with marked inflammation and those with degeneration rather than inflammatory changes coexist in the spinal cord. Lymphocyte exudation occurs randomly in both gray and white matter but is more frequently seen in the deeper portion of the cord than in the surface areas. Inflammation involves both white and gray matter, but the white matter is preferentially degenerated at the lesion. Interestingly, the lateral column is commonly, symmetrically, and most extensively involved with inflammation, while the anterior and posterior columns are usually less affected. These lesions are associated with the presence of a number of CD4+ and CD8+ cells, foamy macrophages, and fibrillary gliosis. It is commonly seen along the course of the small vessels running from the gray matter to the white matter. B cells are also found in the lesion but mainly located in perivascular spaces of larger vessels (Iwasaki 1993). A number of infiltrating macrophages and microglia are activated with the expression of MRP14 and MRP8 (Abe et al. 1999). Neurons in the anterior horn are relatively well preserved despite the presence of inflammatory cells. Immunoreactivity for HLA class I is present on endothelial cells, microglial cells, astrocytes, and infiltrating mononuclear cells (Wu et al. 1993). Upregulation of HLA class II expression is also found on endothelial cells, microglia, and infiltrating mononuclear cells in the lesion (Umehara et al. 1993). High expression of vascular cell adhesion molecule-1 on endothelium has been demonstrated (Umehara et al. 1996). Expression of vary late antigen-4 and monocyte chemoattractant protein-1 is also upregulated in the infiltrating cells in active-chronic lesions of affected spinal cord. Intracellular adhesion molecules-1 and its counterpart molecule lymphocytes function-associated antigen-1 are also suggested to be involved in the lymphocyte infiltration in the spinal cord (Cabre et al. 1999). Proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-1β were detected in perivascular infiltrating cells (Umehara et al. 1994a), while IL-1β were predominantly expressed on the infiltrating macrophage and parenchymal astrocytes. IFN-γ is also expressed on glial cells.
In cases with duration of 4 to 6 years, smaller numbers of inflammatory cells are located in the meninges and perivascular spaces. CD8+ cell are predominantly seen in the parenchyma relative to CD4+ cells. Activated macrophages and microglia expressing MRP-8 are also present. The white matter is uniformly degenerated.
In HAM/TSP cases of longer duration of disease, myelin and axon are equally degenerated and lost, and tissue is largely replaced by glial scar with foamy cells, microglial cells, and a small number of lymphocytes, mostly CD8+ cells with concomitant downregulation of proinflammatory cytokine expression (with the exception of IFN-γ; Umehara et al. 1993; Iwasaki 1990). Although a number of macrophages are detectable in the lesions, the markers of activated marophages and microglia such as MRP-14 or 8 were downregulated (Abe et al. 1999). Fibrous thickening of aventitia and hyalinazed changes of the small vessels (hyalinous thickening of their wall) are conspicuous in both gray and white matter in the lesion of the patients with long history. Myelin staining revealed symmetrical myelin pallor of the lateral column that is most conspicuous in the entire length of the lateral pyramidal tract even in the cases with mild changes of the lateral column. Myelin pallor of the anterior or posterior funiculi is less involved and mild. Wallerian degeneration of ascending tracts in the posterior funiculi is less conspicuous than that of the pyramidal tract in the lumbar and sacral funiculi (Iwasaki 1990). In some cases with long history of disease of more than 20 years, a number of infiltrating CD8+ cells are reported in the spinal cord (Iwasaki et al. 2004). The rate of progression of disease depends on the individual.
Aye et al. reported that perivascular inflammatory infiltration in HAM/TSP cerebrum was seen in deep white matter and in the marginal area of cortex and white matter and that the types of the infiltrating cells were similar both in the spinal cord and cerebrum. In addition to infiltrating cells, demyelination and axonal damage were also seen in the lesion. Therefore, they suggested that HAM/TSP inflammatory process progressed and diminished in the entire area of the CNS simultaneously and that the lesions had a correlation with the site of slow blood flow in the spinal cord and the brain (Aye et al. 2000).
Distribution of HTLV-I other than the CNS
It remains unclear as to the extent that HTLV-I-infected cells proliferate and how much HTLV-I virus protein is expressed to stimulate the observed strong HTLV-I specific immune response seen in HAM/TSP patients. The proliferation and HTLV-I tax expression of HTLV-I infected cells has not been determined in either the CNS parenchyma or other tissue/organs of patients with HAM/TSP. In contrast, semi-quantitative studies on the distribution of HTLV-I proviral DNA in situ suggested that strong HTLV-I provirus signals could be detected by PCR in the spinal cord, peripheral nerve, muscle, lungs, and liver and that weak HTLV-I provirus signals were detected in the medulla oblongata, optic nerve, and lymph node (Sueyoshi et al. 1994). Documented in vivo infection under noncultured condition is rare but has been reported in salivary epithelium of the patient with Sjogren’s syndrome (Mariette et al. 1993), sweat gland epithelia of the patient with squamous cell carcinoma (Setoyama et al. 1999), and salivary glands of healthy carrier (Tangy et al. 1999). The distribution of HTLV-I infected cells in those studies were found to be scattered in few sections. Clearly, additional information is needed with regards to the extent and distribution of HTLV-I proviral DNA, sites of proliferation, and HTLV-I protein/tax expression in situ.
HTLV-I in PBMC
CD8+ cells including HTLV-I specific CD8+ CTLs have been shown to be infected with HTLV-I in PBMC (Hanon et al. 2000; Nagai et al. 2001b). Interestingly, HTLV-I tax expression in these infected CD8+ T cells rendered them susceptible to cytolysis mediated by autologous HTLV-I specific CD8+ CTLs (Hanon et al. 2000). HTLV-I specific CTLs may serve a dual function as both target and effector cells while HTLV-I infected CD8+ cells have not been demonstrated in the CNS parenchyma of HAM/TSP patients.
Previous studies have shown that mononuclear phagocytes (MPs), including monocytes, macrophages, and dendritic cells (DCs), are susceptible to HTLV in vivo, while the frequency of these infected MPs was determined to be 0.5–5% of the total MP population (Macatonia et al. 1992; Koyanagi et al. 1993; Makino et al. 1999). Although HTLV-I PVL in DCs is positively correlated with that of PBMC (Azakami et al. 2009), HTLV-I tax expression of the MPs including CD14+ cells and macrophages was barely detectable compared with CD4+ or CD8+ cells in the PBMC even after culture. More recent studies demonstrate that DCs can be efficiently infected by cell-free HTLV-I virions in vitro (Jones et al. 2008) although this seems unlikely to contribute to maintaining the high HTLV-I PVL in infected individuals since HTLV-I cell-free virions have not been reported in the periphery and are known to be poorly infectious to CD4+ cells.
Yamano et al. has reported that CD4+ CD25+ cells are the predominant viral reservoir in HAM/TSP PBMC and that the decreased Foxp3 expression in this subset was associated with a loss of suppressor function (Yamano et al. 2004; Oh et al. 2006; Michaelsson et al. 2008; Hayashi et al. 2008). It was suggested that this decrease of Foxp3 expression (loss of T regulatory cell function) may be involved in persistent T cell activation in lesions of HAM/TSP patients. This is in contrast with Toulza et al. who have reported that FoxP3+ cells in CD4+ T cell subset was not increased in HAM/TSP patients (Toulza et al. 2008). In the report, the frequency of tax negative Foxp3+ CD4+ cells was negatively correlated with the HTLV-I specific CTL frequency, while the frequency of tax positive Foxp3+ CD4+ cells was not. Recently, Yamano et al. reported the CD4+ CD25+ CCR4+ T cell subset was increased in HAM/TSP patients and also highly infected with HTLV-I compared with CD4+ CD25+ CCR4− cells (Yamano et al. 2009). Especially, Foxp3-negative population in CD4+ CD25+ CCR4+ T cells was significantly increased in HAM/TSP patients. In this study, purified CD4+ CD25+ CCR4+ T cells subset proliferated independently, while CD4+ CD25− CCR4− subset did. Asquith et al. has also shown that CD4+ CD45RO+ T cell proliferation, which are also susceptible to HTLV-I infection, was elevated in the PBMCs of HTLV-I-infected individuals using nonradioactive isotopes to dividing cells. In this study, the increased proliferation in vivo was correlated with ex vivo HTLV-I tax expression (Asquith et al. 2007). Collectively, these observations suggest that the strong immune response to HTLV-I seen in HTLV-I infected individuals may be due to increased proliferation of HTLV-I-infected CD4+ cells and their tax expression. HTLV-I tax expressing cells are barely detectable in PBMC ex vivo because of the strong HTLV-I specific CTL response that recognize and lyse these HTLV-I tax expressing cells.
Risk factors for HAM/TSP
A small percentage (less than 5%) of HTLV-I-infected individuals develops HAM/TSP while the majority remains lifelong asymptomatic carriers. It is therefore obvious that infection with HTLV-I is necessary but not sufficient to cause HAM/TSP. The crucial risk factors that determine the outcome of an HTLV-I infection is still unclear but include HTLV-I proviral load, host genetic factors, variants of HTLV-I, age, and gender.
HTLV-I PVL is an important risk factor for HAM/TSP
Higher HTLV-I PVLs have been demonstrated in PBMC of patients with HAM/TSP compared to asymptomatic carriers. Previous studies have shown that HTLV-I PVL of HAM/TPS patients were about 5- to 16-fold higher than those of asymptomatic carriers (Kubota et al. 1993; Hashimoto et al. 1998; Nagai et al. 1998). The study in Kagoshima, southern Japan which is one of the largest endemic areas in the worlds for HTLV-I showed that the prevalence of HAM/TSP rose steeply as the proviral load exceeded 1% of PBMCs (Nagai et al. 1998). Moreover, HTLV-I PVL was also increased in the CSF of the patients with HAM/TSP compared with that in the PBMC (Nagai et al. 2001a). Importantly, the ratio of PVL in the CSF cells to that in the PBMCs was significantly associated with clinically progressive disease and with recent onset of HAM/TSP (Takenouchi et al. 2003). In a small prospective cohort study performed in the UK, Taylor et al. have validated these observations and suggest that high PVL predispose to a high risk of onset of HAM/TSP (Taylor et al. 1999). Collectively, these results demonstrate that an increased HTLV-I PVL is a strong risk factor for HAM/TSP and is associated with clinical progression in this disorder.
Genomic integration and HTLV-I proviral load
HTLV-I tax expression in cultured PBMCs without CD8+ cells is more frequently detectable in HAM/TSP patients than in asymptomatic carriers at a given proviral load (Yamano et al. 2002; Asquith et al. 2005a). Asquith et al. reported that HTLV-I tax expressing rate in CD4+ cells after 18 h culture without CD8+ cells was positively correlated with HTLV-I PVL in PBMC (Asquith et al. 2005a). In this study, it was the rate of HTLV-I tax expression ex vivo in CD4+ cells that was thought to be a strong predictor for HAM/TSP independent of HTLV-I PVL. The authors suggested that the positive correlation between the HTLV-I tax expressing rate and HTLV-I PVL was attributed to the infected cell proliferation by tax-induced upregulation of cellular genes involved in proliferation and deregulating cell cycle checkpoint (Bex and Gaynor 1998; Hollsberg 1999; Mesnard and Devaux 1999). They went on to demonstrate that CD4+ CD45RO+ T lymphocyte proliferation was increased in HTLV-I-infected individuals in vivo and that the proliferation rate correlated with ex vivo HTLV-I viral expression, suggesting that persistent viral gene expression in vivo was necessary for the maintenance of HTLV-I PVL (Asquith et al. 2007). Recently, Meekings et al. reported that genomic integration in transcriptionally active genomic regions determines the rate of HTLV-I proviral expression (Meekings et al. 2008). The genomic integration of HTLV-I to certain regions may induce high HTLV-I tax expression and subsequent tax-induced proliferation that define HTLV-I PVL and that may drive the expansion of HTLV-I specific CTL (Bangham et al. 2009).
Genetic factors and subtypes HTLV-I tax
While HTLV-I PVL is an important risk factor for HAM/TSP, the range of HTLV-I PVL seen in both groups of HAM/TSP patients and asymptomatic carriers is large with extensive overlap (Nagai et al. 1998). Of importance, HTLV-I PVL in asymptomatic carriers of families with HAM/TSP patients was higher than those of unrelated asymptomatic carriers. In addition, since HTLV-1 varies little in sequence other than tax gene either within or between hosts (Daenke et al. 1990), the variation in proviral load among HTLV-I infected individuals is thought to be caused by differences in the host rather than in the virus. There are wide variations (over 1,000-fold) in HTLV-I PVL among infected individuals, although within an individual, the HTLV-I PVLs is stable over time (Takenouchi et al. 2003; Matsuzaki et al. 2001). These findings suggest that the genetic factors as well as HTLV-I PVL and rates of HTLV-I tax expression in CD4+ cells play an important role in the development of HAM/TSP. Some HLA genotypes were found to influence HTLV-I PVL and the risk for HAM/TSP. In a case-control study, candidate gene association study in the HTLV-I endemic area of Kagoshima in Kyusyu Island, Japan (Jeffery et al. 1999, 2000; Vine et al. 2002), it was reported that HLA-A*02 or Cw*08 was independently associated with a lower risk (absence or presence) for HAM/TSP. The association between these two class I alleles and low HTLV-I PVL was found in only the group of asymptomatic carriers (Vine et al. 2002). HLA-B*5401 was also associated with higher HTLV-I PVL and high risk for HAM/TSP (Jeffery et al. 2000). Furthermore, individuals who were heterozygous at all three HLA class I loci have a lower PVL than those who were homozygous at one or more loci (Jeffery et al. 2000). It was suggested that HLA class I-restricted immune responses influence the HTLV-I PVL. HLA class II alleles such as HLA-DRB1*0101 was associated with high risk for HAM/TSP (Jeffery et al. 1999; Usuku et al. 1988). In another study, Borducchi et al. also reported that HLA-A*02 is associated with a low prevalence of HAM/TSP in white subjects but not Mestizo subjects in Brazil. The association of HLA-DR11 with HAM/TSP, which has previously described in Japanese patients (Usuku et al. 1988), was observed only in the Mestizo patients (Borducchi et al. 2003). These results suggest that a strong HLA class I-restricted T cell response is beneficial. Contrary, Takenouchi et al. demonstrated that HLA-A*2402 was significantly associated with a lower PVL and a higher risk for HAM/TSP in Kagoshima, Japan (unpublished data). In addition, Furukawa et al. reported an association between HTLV-I tax gene sequence variation and the risk of HAM/TSP. The HTLV-I tax subgroup A was more frequently observed in HAM/TSP patients than in ACs, and this effect was independent of HLA-A*02 (Furukawa et al. 2000). Similarly, Sabouri et al. also reported that HLA-A*02 did not give protection against HTLV-I with the HTLV-I cosmopolitan subtype A strain in Iran (Sabouri et al. 2005). Other genetic factors not associated with HLA have also been reported to be associated with HAM/TSP. Vine et al. reported that a promoter polymorphism in the cytokine gene TNF-α-863A, stromal cell-derived factor-1, and IL-15 also influenced the outcome of the HTLV-I infection (Vine et al. 2002). Clearly, a wide range of genes both within and outside the HLA region will be shown to influence susceptibility to HTLV-I and the clinical outcome of an HTLV-I infection.
Tax-specific CD8+ CTL
Increased tax-specific CD8+ CTLs have a strong association with the pathogenesis of HAM/TSP (Fig. 2)
Fig. 2.
Higher Tax expression and HTLV-I PVL drive HTLV-I specific CTL expansion in HAM/TSP
One of the most striking features of the cellular immune response in HTLV-I-infected individuals is the increased numbers of HTLV-I-specific HLA class I-restricted CD8+ CTLs in PBL and CSF cells (Jacobson et al. 1990; Elovaara et al. 1993; Parker et al. 1994; Greten et al. 1998; Nagai et al. 2001c; Kubota et al. 2002). Cytotoxic T cells (CTL) are an important component of the adaptive mammalian immune response to viruses and act by killing autologous cells that express viral antigen in association with MHC class I and by suppressing viral replication by secretion of IFN-γ. While HTLV-I specific CTLs are also detectable in PBMC of asymptomatic carriers (Parker et al. 1992), the magnitude and frequency of these responses are clearly higher in patients with HAM/TSP, particularly in the CSF (Nagai et al. 2001a; Elovaara et al. 1993). In HAM/TSP, most of these cells express IFN-γ and TNF-α, were CD8+ cells, and were HTLV-I-specific CTL. The frequency of IFN-γ+CD8+ cells (CTL) was significantly higher in the PBMC of HAM/TSP patients than in that of asymptomatic carriers or healthy controls and was correlated with HTLV-I PVL in PBMC (Kubota et al. 1998, 2000), although positive correlations between CTL frequency and HTLV-I PVL was not always seen in other studies. HTLV-I PVLs were also increased in CSF cells compared with that in the PBMC of HAM/TSP patients, and those were proportional to the frequency of HTLV-I specific CTLs in the CSF (Nagai et al. 2001a). These observations coupled with pathological findings that infiltrating TIA-1+ cells (CTL) appear to correlate with the presence of apoptotic CD4+ T cells in inflammatory lesion in HAM/TSP (Umehara et al. 1994b) suggests that the increased HTLV-I specific CTLs in the CNS are strongly associated with the pathogenesis of HAM/TSP.
Tax-specific CD8+ CTL clones have also been demonstrated to secrete various inflammatory cytokines, chemokines, and matrix metalloproteinase (MMP) such as IFN-g, TNF-α, monocyte inflammatory protein (MIP)-1α, MIP-1β, IL-16, and MMP-9 (Biddison et al. 1997). TNF-α induces cytotoxic damage to endothelial cells, thus decreasing the integrity of the BBB. It can also directly injure oligodendrocytes. MIP-1α and 1β can enhance transendothelial migration of lymphocytes into the CNS. IL-16 is a chemoattractant for CD4+ cells, and CD4+ T cells are the major source of IL-2 that is required by IL-2 nonproducer CD8+ cells for proliferation. Therefore, HTLV-I-specific CD8+ CTLs are the important source of proinflammatory soluble mediators that may contribute significantly to the pathogenesis of HAM/TSP.
Why does the high frequency of HTLV-I specific CTL not decrease HTLV-I PVL?
HTLV-I PVL and tax mRNA positively correlated with the frequency of HTLV-I-specific CTLs in PBMCs (Yamano et al. 2002; Nagai et al. 2001c; Kubota et al. 2000). Why does the high frequency of HTLV-I specific CTL therefore not contribute to a decrease in HTLV-I-infected cells and PVL in the periphery of infected individuals? One possibility is that HTLV-I-specific CTLs are functionally dysregulated. Sabouri et al. reported that the frequency of intracellular perforin-positive CD8+ T cells was significantly lower in both HAM/TSP and ACs than in healthy controls (HCs; Sabouri et al. 2008). In this paper, an inverse correlation between HTLV-1 PVL and the percent perforin-positive CD8+ T cells were observed only in HLA-A*02+ HCs but not in HAM/TSP patients. In this context, there may be differences in CTL function rather than CTL frequency. The CTL-mediated lysis effect (killing ability per CTL) of a CTL could be evaluated by measuring the effect of varying the frequency of CD8+ cells (not HTLV-I tax-specific CTLs) on the rate of disappearance of HTLV-I tax expressing cells ex vivo (Asquith et al. 2005b). Asquith et al. reported that the CTL lysis effect of HAM/TSP patients was not different from that of ACs. In addition, the variation in HTLV-I PVL among HTLV-I infected people was explained by differences in CTL lysis effect. The CTL lysis effect was negatively correlated with HTLV-I PVL in both HAM/TSP patients and ACs groups. Interestingly, HTLV-I PVL of HAM/TSP patients were significantly higher than AC at any given lytic effect of a CTL. These results suggest that CTLs do contribute to decrease HTLV-I PVL, and high HTLV-I PVL in HAM/TSP patients is not a consequence of weak CTL lysis. Again, additional factors must also be associated with high HTLV-I specific CTL that may be associated with the pathogenesis of HAM/TSP.
CTL activity is associated with CD244-SAP signaling in CD8+ cells
The ability of CD8+ T cells to degranulate (CD107α) has been directly correlated with cytolytic activity of effector CD8+ T cell and has been used as a useful tool to characterize total CD8+ T cell lytic activity in various diseases (Rubio et al. 2003; Betts et al. 2003; Kozako et al. 2006; Betts et al. 2006; Gehring et al. 2007). Enose-Akahata et al. recently reported an increased spontaneous degranulation with IFN-g expression (CD107α/IFN-γ) in CD8+ cells of HAM/TSP patients but not in asymptomatic carriers (Enose-Akahata et al. 2008). These authors have extended these observations and have shown that the CD244/SAP pathway in CD8+ cell is involved in the active regulation of CTL of patients with HAM/TSP (Plos Pathogens, in press). In this study, while the expression of CD244, a signaling lymphocyte activation molecule (SLAM) family receptor, was significantly higher on CD8+ T cells in both HAM/TSP patients and ACs than those on healthy normal donors (NDs), SLAM-related adapter protein (SAP) was overexpressed only in HAM/TSP patients compared to ACs and NDs. Blockade of CD244 inhibited the degranulation and IFN-γ production (CD107α/IFN-γ) and SAP knockdown by siRNA also inhibited IFN-γ production in CD8+ T cells of HAM/TSP patients. The results suggested that differential expression of SAP in the CD8+ cells results in a higher frequency of degranulation in HTLV-I infected individuals, thereby contributing to the higher CTL activity observed in patients with HAM/TSP.
Interaction between HTLV-I specific CD8+ CTL and mononuclear phagocytes
While high SAP expression of CD8+ cell in HAM/TSP patients was shown to influence CTL activity, additional factors are also important. Recently, there has been a greater appreciation for the role of MPs including CD14+ cells, macrophages, and dendritic cells. These populations have been shown to play a role in the spontaneous cell proliferation that is seen in cultured PBMC of HTLV-I infected individuals and is inhibited by blocking with antibodies against IL-2, IL2Rα, IL-15, and IL15R (Macatonia et al. 1992; Makino et al. 1999; Tendler et al. 1990; Ali et al. 1993; Azimi et al. 1999). Azimi et al. reported the role of MPs in proliferation of CTL in HAM/TSP patients (Azimi et al. 2001). Recently, Enose-Akahata et al. reported CTL degranulation to be mediated by HTLV-I infection of MPs with the concomitant expression of IL-15 (Enose-Akahata et al. 2008). In this study, the frequency of CD107α/IFN-γ cells in the CD8+ cells was reduced in purified CD8+ cells compared with total PBMC in HAM/TSP patients and then restored by the addition of autologous CD14+ cells but not CD4+ cells of HAM/TSP patients. They showed that cell-to-cell interaction with CD14+ cells was necessary for degranulation and that the frequency of CD107α/IFN-γ cells in CD8+ cells was increased by the addition of rhIL-15 or CD14+ cells and decreased by addition of both CD14+ cells and anti-IL-15 antibody. Lastly, IL-15 expression on CD14+ cells was increased in PBMC of HAM/TSP patients compared with ACs. These findings suggested that MPs play an important role in exacerbating CTLs degranulation in HAM/TSP patients. More recently, it has been demonstrated that CTL more frequently interact with CD14+ cells than with CD4+ cells in PBMC of HAM/TSP patients but not in that of normal donors (Matsuura et al., manuscript in preparation).
Although it has been reported that HTLV-I infection of MPs induce activation and secretion of MIP-1 and other chemokines (Sharma and Lorey 2001; Mori et al. 2004), the frequency of these infected MPs has been reported to be low (0.5–5% of the MPs; Macatonia et al. 1992; Makino et al. 1999). Although HTLV-I tax is barely detectable in CD14+ cells by flow cytometry even after culture, we have demonstrated that CD14+ cells in contact with CTLs were highly positive for HTLV-I tax (up to 12.5% of CD14+ cells). A recent study reported cell-free extracellular HTLV-I tax could induce maturation and functional alterations of DC without HTLV-I infection of DC (Jain et al. 2007). Together with our observations on the frequent interaction between CD14+ cells and CTLs, we have also detected that HTLV-I tax was often transferred from HTLV-I tax positive, CD4+ cells to CD14+ cells by phagocytosis. Collectively, these results suggest that not only HTLV-I-infected MPs but also activated MPs that uptake HTLV-I tax via phagocytosis may frequently interact with CTL and affect CTL degranulation. The lytic effect of a CTL in the CNS may therefore be influenced by a number of factors including MPs as well as the over-expression of SAP in CD8+ T cells of HAM/TSP patients.
Tax-specific CD8+ CTL in the brain
The phenotype of inflammatory cells in the CNS of HAM/TSP patients has been extensively studied. Significantly, later in disease, there is a predominance of CD8+ T cells in the regions of affected spinal cord in areas that express HLA class I antigens and from which functional HTLV-I-specific CD8+ CTL have been demonstrated (Levin et al. 1997). In addition, the infiltration of CD8+ CTLs in the affected spinal cord was characterized as TIA-1 positive (Umehara et al. 1994b; Anderson et al. 1990). TIA-1 is a monoclonal antibody that recognizes a 15-kDa granule-associated protein contained in CTLs and NK cells. Double staining for anti-CD8 and TIA revealed that 80% of TIA-1+ infiltrating cells throughout the parenchyma and perivascular area were positive for CD8. TIA-1+ cells were scarcely observed in inactive-chronic lesions, though CD8+ cells dominated in the parenchyma and perivascular area. The number of TIA-1+ cells was clearly related to the amount of the proviral DNA in situ and the number of infiltrating CD8+ cells appeared to correlate with the presence of apoptotic cells. More recently, HTLV-I tax-specific CD8+ CTLs in parenchyma and meninges were directly detected in the spinal cord of the patients with HAM/TSP by using HLA-A2 HTLV-I tax peptide pentamers (Fig. 3c). Of importance, about 30% of the infiltrating CD8+ cells were found to be HTLV-I specific CTLs (Matsuura et al., in preparation; Matsuura et al. 2006). These observations continue to support a role for HTLV-I specific CTL as a major contributing factor in HTLV-I-associated neurologic disease.
Fig. 3.
A CD8 (Alexia-488, green) positive cells were found scattered in parenchyma of the spinal cord from HAM/TSP patient. DAPI (blue in A and B) was used for nuclear counter stain. B, C HTLV-I Tax11-19 specific CTLs were visualized by immunohistochemistry with fluorescent microscopy (B) and light microscopy (C) using HLA-A2 tax11-19-peptide complex tetramer which were visualized with Alexa-594 (red in B) or AEC (red in C; Matsuura et al., in preparation). Scale bar is 30 μm
HTLV-I infected CD4+ cell and its migration to extracellular matrix
HTLV-I-infected lymphocytes have been shown to adhere to endothelial monolayer cells and to migrate to a greater extent in the PBMC from HAM/TSP patients than ACs or NDs (Ichinose et al. 1996; Furuya et al. 1997; Al-Fahim et al. 1999; Romero et al. 2000). It has been reported that CD4+ cells in HAM/TSP patients deviate to Th1-like phenotype as characterized by upregulated secretion of proinflammatory cytokines such as IFN-g, TNF-a, and IL-2 and downregulation of Th 2 cytokines such as IL-4 (Watanabe et al. 1995; Nakamura et al. 2000; Horiuchi et al. 2000; Hanon et al. 2001; Goon et al. 2003). Th1 cells but not Th2 cells are reported to bind to P- and E-selectin expressed on endothelial cells (Austrup et al. 1997). HTLV-I-infected Th1 cells may therefore play a role in the triggering of the development of pathological process associated with HAM/TSP (Nakamura 2009). IL-1α and TNF-α secreted from HTLV-1-infected lymphocytes can induce a disruption of the tight junction between endothelial cells, a compartment of the blood-brain barrier (BBB), resulting in the increase of paracellular endothelial permeability and transcellular migration (Afonso et al. 2007). The alteration of the BBB function may enhance the migration of inflammatory cells including those infected with HTLV-I (Afonso et al. 2008). Migrating lymphocytes/macrophages encounter the extracellular matrix (ECM), passing through the basement membrane. Proteolytic disruption of ECM by MMPs is a key process for the damage of the BBB. Activated HTLV-I-infected CD4 T cells induced the production of MMP-2,3,9 and TIMP-1,2,3 in human astrocytes in vitro (Giraudon et al. 1996, 2000). MMP-9 and TIMP-3 were also shown to be elevated in the CSF of HAM/TSP patients compared with AC (Lezin et al. 2000). Moreover, the expression of MMP-2 and MMP-9 was detected on the perivascular infiltrating cells of the affected spinal cord in HAM/TSP (Umehara et al. 1998).
Natural killer cell, NKT cell, and gdT cell in patients with HAM/TSP
NK cell activity and frequency of this subset was reported to be significantly decreased in HAM/TSP compared with healthy controls (Azakami et al. 2009; Fujihara et al. 1991; Yu et al. 1991; Wu et al. 2000). Cytotoxic activity and antibody-dependent cell-mediated cytotoxicity were also lower in NK cells from HAM/TSP patients than those of controls (Yu et al. 1991). The study revealed NK cell activity was significantly increased after 4 weeks of oral administration of LcS preparation with improvements in spasticity (Modified Ashworth Scale Scores) and urinary symptoms, while HTLV-I PVL was not changed (Matsuzaki et al. 2005). These results suggested that low NK activity may be an additional risk factor for HAM/TSP.
Human natural killer cell receptors are expressed by NK cells and some T cells, primarily TCR+ CD8+ cytotoxic T lymphocytes. Inhibitory NK cell receptors (iNKRs) can downregulate antigen-mediated T cell effector functions including cytotoxic activity and cytokine release (Mingari et al. 1998; Biassoni et al. 2001). It is reported that CD8+ T cells that express the HLA-E ligand including an iNKR were significantly decreased in association with HAM/TSP but not in asymptomatic carriers (Saito et al. 2003). It was therefore suggested that the decrease in these iNKR cells in HAM/TSP patients contributes to the excessive antiviral immune response.
NKT subset was also significantly decreased in HAM/TSP compared with healthy controls (Azakami et al. 2009; Wu et al. 2000). iNKT cells are unique T cells that regulate the immune response to microbes, cancers, and autoimmunity. Frequency of iNKT, NK, and dendritic cells has been shown decreased in the peripheral blood of HAM/TSP and ATL patients (Azakami et al. 2009). In the study, an inverse correlation between the iNKT cell frequency and the HTLV-I proviral load was found in the peripheral blood of infected individuals. In vitro stimulation of PBMCs with alpha-galactosylceramide increased iNKT cells and subsequently decreased HTLV-I-infected T cells in samples from asymptomatic carriers but not HAM/TSP or ATL patients. The authors suggested that iNKT cells contribute to the immune defense against HTLV-1 and iNKT cell depletion plays an important role in the pathogenesis of HAM/TSP and ATL.
Other disorders associated with HTLV-I infection
HTLV-I has been known to be associated with not only HAM/TSP and ATL but also uveitis, alveolitis, myositis, arthritis, dermatitis, mononeuropathy, inclusion body myositis, Sjogren syndrome, Behcet disease, pseudohypoparathyroidism, and SLE (Leite et al. 2004; Morgan et al. 1989; Higuchi et al. 1992; Sugimoto et al. 1987; Vernant et al. 1988; Nakao et al. 1991; Nishioka et al. 1989; LaGrenade et al. 1990; Vernant et al. 1990; Cupler et al. 1996; Engel et al. 1997; Ozden et al. 2001; Matsuura et al. 2008; Yoshida et al. 2002). Although HAM/TSP patients with HCV hepatitis are sometimes seen, there is no significant epidemiological link (Taylor et al. 1999; Ijichi et al. 1993; Maruyama et al. 1995). Retrospective statistical studies show stronger associations of HTLV-I with HAM/TSP, uveitis, myositis, and peripheral neuropathy (Gessain et al. 1985; Morgan et al. 1989; Higuchi et al. 1992; Nakao et al. 1991; Gilbert et al. 2001; Leite et al. 2003), but less clear associations are for alveolitis, arthritis, and the other suggested diseases. While there is no epidemiological study for pulmonary diseases associated HTLV-I, some radiological studies suggest that there may be significantly higher radiological abnormalities in HTLV-I-infected subjects (Kohno et al. 1992; Okada et al. 2006). HTLV-I-associated myopathy, dermatitis, and peripheral neuropathy may be less frequent in southern regions of Japan than in the Caribbean and South America. Again, host genetic factors must clearly influence these conditions. Because monoclonal T cell expansion of HTLV-I is not seen in these HTLV-I-related disorders except ATL, the immune responses in these conditions are thought to be similar to that of HAM/TSP. However, unlike HAM/TSP, there are much less reports on the pathogenesis of these other HTLV-I-associated diseases. In this context, HTLV-I-associated myositis has been studied. Pathological studies have demonstrated HTLV-I proviral DNA and HTLV-I tax expression in the infiltrating cells in perimysium but not muscle fibers by in situ hybridization and in situ PCR, respectively (Higuchi et al. 1992, 1995). HTLV-I DNA and tax mRNA were also detected in the infiltrating cells in perimysium in inclusion body myositis (Ozden et al. 2001). Similar to HAM/TSP, HTLV-I tax-specific CTL were also detected in perimysium (Matsuura et al. 2008; Ozden et al. 2004). In this study, HTLV-I specific CTLs were found surrounding a single muscle fiber at the center of a patchy inflammatory lesion in affected muscle (Matsuura et al. 2008). These reports suggest that immunopathogenic CTL may also be playing a similar role in these other conditions associated with HTLV-I infection as has been suggested in HAM/TSP. Therefore, a better understanding of the pathophysiology of HAM/TSP may lead to therapies that may ameliorate a number of disorders in which HTLV-I is thought to play a role.
Concluding remarks
In HAM/TSP, localization of HTLV in CD4+ cells in the CNS may lead to the production of cytokines/chemokines that are thought to adversely effect resident cells in the CNS (bystander hypothesis) resulting in neurodegeneration of the long tract. However, neurons in the anterior horn are relatively preserved and neurodegeneration of the long tracts is commonly seen symmetrically. Histological studies in HAM/TSP patients have demonstrated that although CD4+ cells are predominantly infected with HTLV-I, other cells may also harbor HTLV-I including astrocytes and glia. All of these cells could potentially express HTLV-I antigens (processed HTLV-I peptides in association with HLA class I molecules) and are seen as targets by inflammatory, HTLV-I specific CTL. Recognition by CTL could lead to a cascade of events that produce toxic products within the CNS associated with death or dysfunction of important neuronal components leading to clinical symptoms of HAM/TSP. Although it has been known for a quarter of century that HTLV-I induces a chronic central nervous system dysfunction, the mechanisms associated with HTLV-I infection remains unclear. New factors for the development of HAM/TSP have recently been demonstrated. Differences between HAM/TSP patients and ACs include host HLA, HTLV-I genomic integration site, HTLV-I tax subtype, HTLV-I PVL, HTLV-I gene expression rate, adhesion or migration ability of infected CD4+ cells, and CTL activating factor (SAP in CD8+ cells and IL-15 in MPs). While high levels of HTLV-I tax expression has not been demonstrated to explain the strong virus-specific immune response seen in affected tissue, elevated CD4 and CD8 proliferation in vivo may explain the continuous tax expression that may serve to drive strong T cell responses. While HTLV-I specific CTL has been thought to play a role in decreasing HTLV-I PVL, these same cells may be activated by MPs and cause immunopathology in the CNS. It is clear that further information regarding the frequency, distribution, activity, and function of CTLs in the CNS of HAM/TSP as well as that of other HTLV-I-associated disorders will be needed to better understand the role of this virus played in these diseases.
References
- Abe M, Umehara F, Kubota R, Moritoyo T, Izumo S, Osame M. Activation of macrophages/microglia with the calcium-binding proteins MRP14 and MRP8 is related to the lesional activities in the spinal cord of HTLV-I associated myelopathy. J Neurol. 1999;246:358–364. doi: 10.1007/s004150050363. [DOI] [PubMed] [Google Scholar]
- Afonso PV, Ozden S, Prevost MC, Schmitt C, Seilhean D, Weksler B, Couraud PO, Gessain A, Romero IA, Ceccaldi PE. Human blood-brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganization. J Immunol. 2007;179:2576–2583. doi: 10.4049/jimmunol.179.4.2576. [DOI] [PubMed] [Google Scholar]
- Afonso PV, Ozden S, Cumont MC, Seilhean D, Cartier L, Rezaie P, Mason S, Lambert S, Huerre M, Gessain A, Couraud PO, Pique C, Ceccaldi PE, Romero IA. Alteration of blood-brain barrier integrity by retroviral infection. PLoS Pathog. 2008;4:e1000205. doi: 10.1371/journal.ppat.1000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akizuki S, Setoguchi M, Nakazato O, Yoshida S, Higuchi Y, Yamamoto S, Okajima T. An autopsy case of human T-lymphotropic virus type I-associated myelopathy. Hum Pathol. 1988;19:988–990. doi: 10.1016/s0046-8177(88)80017-0. [DOI] [PubMed] [Google Scholar]
- Akizuki S, Yoshida S, Setoguchi M, Higuchi Y, Yamamoto S, Nakazato O, Okajima T. The neuropathology of human T cell lymphotropic virus type I-associated myelopathy. In: Roman GC, Vernant JC, Osame M, editors. HTLV-I and the nervous system. Alan R. Liss; New York: 1989. pp. 253–260. [Google Scholar]
- Al-Fahim A, Cabre P, Kastrukoff L, Dorovini-Zis K, Oger J. Blood mononuclear cells in patients with HTLV-I-associated myelopathy: lymphocytes are highly activated and adhesion to endothelial cells is increased. Cell Immunol. 1999;198:1–10. doi: 10.1006/cimm.1999.1580. [DOI] [PubMed] [Google Scholar]
- Ali A, Patterson S, Cruickshank K, Rudge P, Dalgleish AG, Knight SC. Dendritic cells infected in vitro with human T cell leukaemia/lymphoma virus type-1 (HTLV-1); enhanced lymphocytic proliferation and tropical spastic paraparesis. Clin Exp Immunol. 1993;94:32–37. doi: 10.1111/j.1365-2249.1993.tb05973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Nagler-Anderson C, O’Brien C, Levine H, Watkins S, Slayter HS, Blue ML, Schlossman SF. A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol. 1990;144:574–582. [PubMed] [Google Scholar]
- Asquith B, Mosley AJ, Heaps A, Tanaka Y, Taylor GP, McLean AR, Bangham CR. Quantification of the virus-host interaction in human T lymphotropic virus I infection. Retrovirology. 2005a;2:75. doi: 10.1186/1742-4690-2-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asquith B, Mosley AJ, Barfield A, Marshall SE, Heaps A, Goon P, Hanon E, Tanaka Y, Taylor GP, Bangham CR. A functional CD8+ cell assay reveals individual variation in CD8+ cell antiviral efficacy and explains differences in human T-lymphotropic virus type 1 proviral load. J Gen Virol. 2005b;86:1515–1523. doi: 10.1099/vir.0.80766-0. [DOI] [PubMed] [Google Scholar]
- Asquith B, Zhang Y, Mosley AJ, de Lara CM, Wallace DL, Worth A, Kaftantzi L, Meekings K, Griffin GE, Tanaka Y, Tough DF, Beverley PC, Taylor GP, Macallan DC, Bangham CR. In vivo T lymphocyte dynamics in humans and the impact of human T-lymphotropic virus 1 infection. Proc Natl Acad Sci USA. 2007;104:8035–8040. doi: 10.1073/pnas.0608832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- Aye MM, Matsuoka E, Moritoyo T, Umehara F, Suehara M, Hokezu Y, Yamanaka H, Isashiki Y, Osame M, Izumo S. Histopathological analysis of four autopsy cases of HTLV-I-associated myelopathy/tropical spastic paraparesis: inflammatory changes occur simultaneously in the entire central nervous system. Acta Neuropathol. 2000;100:245–252. doi: 10.1007/s004019900170. [DOI] [PubMed] [Google Scholar]
- Azakami K, Sato T, Araya N, Utsunomiya A, Kubota R, Suzuki K, Hasegawa D, Izumi T, Fujita H, Aratani S, Fujii R, Yagishita N, Kamijuku H, Kanekura T, Seino KI, Nishioka K, Nakajima T, Yamano Y. Severe loss of invariant NKT cells exhibiting anti-HTLV-1 activity in patients with HTLV-1-associated disorders. Blood. 2009;114:3208–3215. doi: 10.1182/blood-2009-02-203042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi N, Jacobson S, Leist T, Waldmann TA. Involvement of IL-15 in the pathogenesis of human T lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis: implications for therapy with a monoclonal antibody directed to the IL-2/15R beta receptor. J Immunol. 1999;163:4064–4072. [PubMed] [Google Scholar]
- Azimi N, Nagai M, Jacobson S, Waldmann TA. IL-15 plays a major role in the persistence of Tax-specific CD8 cells in HAM/TSP patients. Proc Natl Acad Sci USA. 2001;98:14559–14564. doi: 10.1073/pnas.251540598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham CR, Meekings K, Toulza F, Nejmeddine M, Majorovits E, Asquith B, Taylor GP. The immune control of HTLV-1 infection: selection forces and dynamics. Front Biosci. 2009;14:2889–2903. doi: 10.2741/3420. [DOI] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Meth. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bex F, Gaynor RB. Regulation of gene expression by HTLV-I Tax protein. Methods. 1998;16:83–94. doi: 10.1006/meth.1998.0646. [DOI] [PubMed] [Google Scholar]
- Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, Bottino C, Moretta A. Human natural killer cell receptors and co-receptors. Immunol Rev. 2001;181:203–214. doi: 10.1034/j.1600-065x.2001.1810117.x. [DOI] [PubMed] [Google Scholar]
- Biddison WE, Kubota R, Kawanishi T, Taub DD, Cruikshank WW, Center DM, Connor EW, Utz U, Jacobson S. Human T cell leukemia virus type I (HTLV-I)-specific CD8+ CTL clones from patients with HTLV-I-associated neurologic disease secrete proinflammatory cytokines, chemokines, and matrix metalloproteinase. J Immunol. 1997;159:2018–2025. [PubMed] [Google Scholar]
- Borducchi DM, Gerbase-DeLima M, Morgun A, Shulzhenko N, Pombo-de-Oliveira MS, Kerbauy J, Rodrigues de Oliveira JS. Human leucocyte antigen and human T-cell lymphotropic virus type 1 associated diseases in Brazil. Br J Haematol. 2003;123:954–955. doi: 10.1046/j.1365-2141.2003.04711.x. [DOI] [PubMed] [Google Scholar]
- Cabre P, al-Fahim A, Oger J. Enhanced adherence of endothelial cells to blood mononuclear cells in HAM/TSP. Rev Neurol (Paris) 1999;155:273–279. [PubMed] [Google Scholar]
- Cavrois M, Leclercq I, Gout O, Gessain A, Wain-Hobson S, Wattel E. Persistent oligoclonal expansion of human T-cell leukemia virus type 1-infected circulating cells in patients with Tropical spastic paraparesis/HTLV-1 associated myelopathy. Oncogene. 1998;17:77–82. doi: 10.1038/sj.onc.1201906. [DOI] [PubMed] [Google Scholar]
- Cavrois M, Gessain A, Gout O, Wain-Hobson S, Wattel E. Common human T cell leukemia virus type 1 (HTLV-1) integration sites in cerebrospinal fluid and blood lymphocytes of patients with HTLV-1-associated myelopathy/tropical spastic paraparesis indicate that HTLV-1 crosses the blood-brain barrier via clonal HTLV-1-infected cells. J Infect Dis. 2000;182:1044–1050. doi: 10.1086/315844. [DOI] [PubMed] [Google Scholar]
- Cupler EJ, Leon-Monzon M, Miller J, Semino-Mora C, Anderson TL, Dalakas MC. Inclusion body myositis in HIV-1 and HTLV-1 infected patients. Brain. 1996;119(Pt 6):1887–1893. doi: 10.1093/brain/119.6.1887. [DOI] [PubMed] [Google Scholar]
- Daenke S, Nightingale S, Cruickshank JK, Bangham CR. Sequence variants of human T-cell lymphotropic virus type I from patients with tropical spastic paraparesis and adult T-cell leukemia do not distinguish neurological from leukemic isolates. J Virol. 1990;64:1278–1282. doi: 10.1128/jvi.64.3.1278-1282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de The G, Bomford R. An HTLV-I vaccine: why, how, for whom? AIDS Res Hum Retroviruses. 1993;9:381–386. doi: 10.1089/aid.1993.9.381. [DOI] [PubMed] [Google Scholar]
- Doi K, Wu X, Taniguchi Y, Yasunaga J, Satou Y, Okayama A, Nosaka K, Matsuoka M. Preferential selection of human T-cell leukemia virus type I provirus integration sites in leukemic versus carrier states. Blood. 2005;106:1048–1053. doi: 10.1182/blood-2004-11-4350. [DOI] [PubMed] [Google Scholar]
- Elovaara I, Koenig S, Brewah AY, Woods RM, Lehky T, Jacobson S. High human T cell lymphotropic virus type 1 (HTLV-1)-specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-1-associated neurological disease. J Exp Med. 1993;177:1567–1573. doi: 10.1084/jem.177.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel WK, Haginoya K, Alvarez RB, Sabetian K, Bajoghli M, Askanas V. S-IBM in an HTLV-1-positive Iranian Muslim. Neurology. 1997;48:124. [Google Scholar]
- Enose-Akahata Y, Oh U, Grant C, Jacobson S. Retrovirally induced CTL degranulation mediated by IL-15 expression and infection of mononuclear phagocytes in patients with HTLV-I-associated neurologic disease. Blood. 2008;112:2400–2410. doi: 10.1182/blood-2008-02-138529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etoh K, Tamiya S, Yamaguchi K, Okayama A, Tsubouchi H, Ideta T, Mueller N, Takatsuki K, Matsuoka M. Persistent clonal proliferation of human T-lymphotropic virus type I-infected cells in vivo. Cancer Res. 1997;57:4862–4867. [PubMed] [Google Scholar]
- Feuer G, Chen IS. Mechanisms of human T-cell leukemia virus-induced leukemogenesis. Biochim Biophys Acta. 1992;1114:223–233. doi: 10.1016/0304-419x(92)90017-s. [DOI] [PubMed] [Google Scholar]
- Fujihara K, Itoyama Y, Yu F, Kubo C, Goto I. Cellular immune surveillance against HTLV-I infected T lymphocytes in HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/TSP) J Neurol Sci. 1991;105:99–107. doi: 10.1016/0022-510x(91)90125-q. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Fujisawa J, Osame M, Toita M, Sonoda S, Kubota R, Ijichi S, Yoshida M. Frequent clonal proliferation of human T-cell leukemia virus type 1 (HTLV-1)-infected T cells in HTLV-1-associated myelopathy (HAM-TSP) Blood. 1992;80:1012–1016. [PubMed] [Google Scholar]
- Furukawa Y, Yamashita M, Usuku K, Izumo S, Nakagawa M, Osame M. Phylogenetic subgroups of human T cell lymphotropic virus (HTLV) type I in the tax gene and their association with different risks for HTLV-I-associated myelopathy/tropical spastic paraparesis. J Infect Dis. 2000;182:1343–1349. doi: 10.1086/315897. [DOI] [PubMed] [Google Scholar]
- Furuya T, Nakamura T, Shirabe S, Nishiura Y, Tsujino A, Goto H, Nakane S, Eguchi K, Nakamura H, Nagataki S. Height-ened transmigrating activity of CD4-positive T cells through reconstituted basement membrane in patients with human T-lymphotropic virus type I-associated myelopathy. Proc Assoc Am Physicians. 1997;109:228–236. [PubMed] [Google Scholar]
- Gehring AJ, Sun D, Kennedy PTF, Nolte-’T Hoen E, Lim SG, Wasser S, Selden C, Maini MK, Davis DM, Nassal M, Bertoletti A. The level of viral antigen presented by hepatocytes influences CD8 T-cell function. J Virol. 2007;81:2940–2949. doi: 10.1128/JVI.02415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A. Epidemiology of HTLV-I and associated diseases. In: Höllsberg P, Hafler DA, editors. Human T-cell Lymphotropic Virus Type I. Wiley; Chichester: 1996. pp. 33–64. [Google Scholar]
- Gessain A, Barin F, Vernant JC. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Gilbert DT, Morgan O, Smikle MF, Simeon D, Barton EN. HTLV-1 associated polymyositis in Jamaica. Acta Neurol Scand. 2001;104:101–104. doi: 10.1034/j.1600-0404.2001.104002101.x. [DOI] [PubMed] [Google Scholar]
- Giraudon P, Buart S, Bernard A, Thomasset N, Belin MF. Extracellular matrix-remodeling metalloproteinases and infection of the central nervous system with retrovirus human T-lymphotropic virus type I (HTLV-I) Prog Neurobiol. 1996;49:169–184. doi: 10.1016/0301-0082(96)00017-2. [DOI] [PubMed] [Google Scholar]
- Giraudon P, Szymocha R, Buart S, Bernard A, Cartier L, Belin MF, Akaoka H. T lymphocytes activated by persistent viral infection differentially modify the expression of metalloproteinases and their endogenous inhibitors, TIMPs, in human astrocytes: relevance to HTLV-I-induced neurological disease. J Immunol. 2000;164:2718–2727. doi: 10.4049/jimmunol.164.5.2718. [DOI] [PubMed] [Google Scholar]
- Goon PK, Igakura T, Hanon E, Mosley AJ, Asquith B, Gould KG, Taylor GP, Weber JN, Bangham CR. High circulating frequencies of tumor necrosis factor alpha- and interleukin-2-secreting human T-lymphotropic virus type 1 (HTLV-1)-specific CD4+ T cells in patients with HTLV-1-associated neurological disease. J Virol. 2003;77:9716–9722. doi: 10.1128/JVI.77.17.9716-9722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten TF, Slansky JE, Kubota R, Soldan SS, Jaffee EM, Leist TP, Pardoll DM, Jacobson S, Schneck JP. Direct visualization of antigen-specific T cells: HTLV-1 Tax11-19-specific CD8(+) T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc Natl Acad Sci USA. 1998;95:7568–7573. doi: 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanon E, Stinchcombe JC, Saito M, Asquith BE, Taylor GP, Tanaka Y, Weber JN, Griffiths GM, Bangham CR. Fratricide among CD8(+) T lymphocytes naturally infected with human T cell lymphotropic virus type I. Immunity. 2000;13:657–664. doi: 10.1016/s1074-7613(00)00065-0. [DOI] [PubMed] [Google Scholar]
- Hanon E, Goon P, Taylor GP, Hasegawa H, Tanaka Y, Weber JN, Bangham CR. High production of interferon gamma but not interleukin-2 by human T-lymphotropic virus type I-infected peripheral blood mononuclear cells. Blood. 2001;98:721–726. doi: 10.1182/blood.v98.3.721. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Higuchi I, Osame M, Izumo S. Quantitative in situ PCR assay of HTLV-1 infected cells in peripheral blood lymphocytes of patients with ATL, HAM/TSP and asymptomatic carriers. J Neurol Sci. 1998;159:67–72. doi: 10.1016/s0022-510x(98)00138-5. [DOI] [PubMed] [Google Scholar]
- Hayashi D, Kubota R, Takenouchi N, Tanaka Y, Hirano R, Takashima H, Osame M, Izumo S, Arimura K. Reduced Foxp3 expression with increased cytomegalovirus-specific CTL in HTLV-I-associated myelopathy. J Neuroimmunol. 2008;200:115–124. doi: 10.1016/j.jneuroim.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Higuchi I, Nerenberg M, Yoshimine K, Yoshida M, Fukunaga H, Tajima K, Osame M. Failure to detect HTLV-I by in situ hybridization in the biopsied muscles of viral carriers with polymyositis. Muscle Nerve. 1992;15:43–47. doi: 10.1002/mus.880150108. [DOI] [PubMed] [Google Scholar]
- Higuchi I, Hashimoto K, Kashio N, Izumo S, Inose M, Izumi K, Ohkubo R, Nakagawa M, Arimura K, Osame M. Detection of HTLV-I provirus by in situ polymerase chain reaction in mononuclear inflammatory cells in skeletal muscle of viral carriers with polymyositis. Muscle Nerve. 1995;18:854–858. doi: 10.1002/mus.880180809. [DOI] [PubMed] [Google Scholar]
- Hinuma Y, Nagata K, Hanaoka M. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollsberg P. Mechanisms of T-cell activation by human T-cell lymphotropic virus type I. Microbiol Mol Biol Rev. 1999;63:308–333. doi: 10.1128/mmbr.63.2.308-333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi I, Kawano Y, Yamasaki K, Minohara M, Furue M, Taniwaki T, Miyazaki T, Kira J. Th1 dominance in HAM/TSP and the optico-spinal form of multiple sclerosis versus Th2 dominance in mite antigen-specific IgE myelitis. J Neurol Sci. 2000;172:17–24. doi: 10.1016/s0022-510x(99)00232-4. [DOI] [PubMed] [Google Scholar]
- Ichinose K, Nakamura T, Nishiura Y, Tsujino A, Goto H, Shirabe S, Furuya T, Nagataki S. Characterization of T cells transmigrating through human endothelial cells in patients with HTLV-I-associated myelopathy. Immunobiology. 1996;196:485–490. doi: 10.1016/s0171-2985(97)80065-4. [DOI] [PubMed] [Google Scholar]
- Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, Tanaka Y, Osame M, Bangham CR. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- Ijichi T, Miyata K, Mori S, Nakajima K, Okanoue T, Tsuchihashi Y. Asymptomatic primary biliary cirrhosis in HTLV-I-associated myelopathy. Am J Gastroenterol. 1993;88:2107–2109. [PubMed] [Google Scholar]
- Iwasaki Y. Pathology of chronic myelopathy associated with HTLV-I infection (HAM/TSP) J Neurol Sci. 1990;96:103–123. doi: 10.1016/0022-510x(90)90060-z. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y. Human T cell leukemia virus type I infection and chronic myelopathy. Brain Pathol. 1993;3:1–10. doi: 10.1111/j.1750-3639.1993.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Sawada K, Aiba I, Mukai E, Yoshida M, Hashizume Y, Sobue G. Widespread active inflammatory lesions in a case of HTLV-I-associated myelopathy lasting 29 years. Acta Neuropathol. 2004;108:546–551. doi: 10.1007/s00401-004-0924-1. [DOI] [PubMed] [Google Scholar]
- Izumo S, Ijichi T, Higuchi I, Tashiro A, Takahashi K, Osame M. Neuropathology of HTLV-I-associated myelopathy—a report of two autopsy cases. Acta Paediatr Jpn. 1992;34:358–364. [PubMed] [Google Scholar]
- Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- Jain P, Ahuja J, Khan ZK, Shimizu S, Meucci O, Jennings SR, Wigdahl B. Modulation of dendritic cell maturation and function by the Tax protein of human T cell leukemia virus type 1. J Leukoc Biol. 2007;82:44–56. doi: 10.1189/jlb.1006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery KJ, Usuku K, Hall SE, Matsumoto W, Taylor GP, Procter J, Bunce M, Ogg GS, Welsh KI, Weber JN, Lloyd AL, Nowak MA, Nagai M, Kodama D, Izumo S, Osame M, Bangham CR. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci USA. 1999;96:3848–3853. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery KJ, Siddiqui AA, Bunce M, Lloyd AL, Vine AM, Witkover AD, Izumo S, Usuku K, Welsh KI, Osame M, Bangham CR. The influence of HLA class I alleles and heterozygosity on the outcome of human T cell lymphotropic virus type I infection. J Immunol. 2000;165:7278–7284. doi: 10.4049/jimmunol.165.12.7278. [DOI] [PubMed] [Google Scholar]
- Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med. 2008;14:429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- Kaplan JE, Osame M, Kubota H, Igata A, Nishitani H, Maeda Y, Khabbaz RF, Janssen RS. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J Acquir Immune Defic Syndr. 1990;3:1096–1101. [PubMed] [Google Scholar]
- Kira J, Fujihara K, Itoyama Y, Goto I, Hasuo K. Leukoence-phalopathy in HTLV-I-associated myelopathy/tropical spastic paraparesis: MRI analysis and a two year follow-up study after corticosteroid therapy. J Neurol Sci. 1991;106:41–49. doi: 10.1016/0022-510x(91)90192-a. [DOI] [PubMed] [Google Scholar]
- Kira J, Nakamura M, Sawada T, Koyanagi Y, Ohori N, Itoyama Y, Yamamoto N, Sakaki Y, Goto I. Antibody titers to HTLV-I-p40tax protein and gag-env hybrid protein in HTLV-I-associated myelopathy/tropical spastic paraparesis: correlation with increased HTLV-I proviral DNA load. J Neurol Sci. 1992;107:98–104. doi: 10.1016/0022-510x(92)90215-7. [DOI] [PubMed] [Google Scholar]
- Kira J, Goto I, Otsuka M, Ichiya Y. Chronic progressive spinocerebellar syndrome associated with antibodies to human T-lymphotropic virus type I: clinico-virological and magnetic resonance imaging studies. J Neurol Sci. 1993;115:111–116. doi: 10.1016/0022-510x(93)90075-a. [DOI] [PubMed] [Google Scholar]
- Kohno S, Higashiyama Y, Mukae H, Morikawa N, Kadota J, Koga H, Hara K, Ikeda S, Tomonaga M, Katamine S, et al. Epidemiology of HTLV-I carriers in Hirado Island and virological and immunological investigation of HTLV-I associated pulmonary disease. Nihon Kyobu Shikkan Gakkai Zasshi. 1992;30:763–769. [PubMed] [Google Scholar]
- Koyanagi Y, Itoyama Y, Nakamura N, Takamatsu K, Kira J, Iwamasa T, Goto I, Yamamoto N. In vivo infection of human T-cell leukemia virus type I in non-T cells. Virology. 1993;196:25–33. doi: 10.1006/viro.1993.1451. [DOI] [PubMed] [Google Scholar]
- Kozako T, Arima N, Toji S, Masamoto I, Akimoto M, Hamada H, Che XF, Fujiwara H, Matsushita K, Tokunaga M, Haraguchi K, Uozumi K, Suzuki S, Takezaki T, Sonoda S. Reduced frequency, diversity, and function of human T cell leukemia virus type 1-specific CD8+ T cell in adult T cell leukemia patients. J Immunol. 2006;177:5718–5726. doi: 10.4049/jimmunol.177.8.5718. [DOI] [PubMed] [Google Scholar]
- Kubota R, Fujiyoshi T, Izumo S, Yashiki S, Maruyama I, Osame M, Sonoda S. Fluctuation of HTLV-I proviral DNA in peripheral blood mononuclear cells of HTLV-I-associated myelopathy. J Neuroimmunol. 1993;42:147–154. doi: 10.1016/0165-5728(93)90004-i. [DOI] [PubMed] [Google Scholar]
- Kubota R, Umehara F, Izumo S, Ijichi S, Matsumuro K, Yashiki S, Fujiyoshi T, Sonoda S, Osame M. HTLV-I proviral DNA amount correlates with infiltrating CD4+ lymphocytes in the spinal cord from patients with HTLV-I-associated myelopathy. J Neuroimmunol. 1994;53:23–29. doi: 10.1016/0165-5728(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Kubota R, Kawanishi T, Matsubara H, Manns A, Jacobson S. Demonstration of human T lymphotropic virus type I (HTLV-I) tax-specific CD8+ lymphocytes directly in peripheral blood of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by intracellular cytokine detection. J Immunol. 1998;161:482–488. [PubMed] [Google Scholar]
- Kubota R, Kawanishi T, Matsubara H, Manns A, Jacobson S. HTLV-I specific IFN-gamma+CD8+ lymphocytes correlate with the proviral load in peripheral blood of infected individuals. J Neuroimmunol. 2000;102:208–215. doi: 10.1016/s0165-5728(99)00175-7. [DOI] [PubMed] [Google Scholar]
- Kubota R, Soldan SS, Martin R, Jacobson S. Selected cytotoxic T lymphocytes with high specificity for HTLV-I in cerebrospinal fluid from a HAM/TSP patient. J Neurovirol. 2002;8:53–57. doi: 10.1080/135502802317247811. [DOI] [PubMed] [Google Scholar]
- LaGrenade L, Hanchard B, Fletcher V, Cranston B, Blattner W. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. Lancet. 1990;336:1345–1347. doi: 10.1016/0140-6736(90)92896-p. [DOI] [PubMed] [Google Scholar]
- Lehky TJ, Fox CH, Koenig S, Levin MC, Flerlage N, Izumo S, Sato E, Raine CS, Osame M, Jacobson S. Detection of human T-lymphotropic virus type I (HTLV-I) tax RNA in the central nervous system of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by in situ hybridization. Ann Neurol. 1995;37:167–175. doi: 10.1002/ana.410370206. [DOI] [PubMed] [Google Scholar]
- Leite AC, Mendonca GA, Serpa MJ, Nascimento OJ, Araujo AQ. Neurological manifestations in HTLV-I-infected blood donors. J Neurol Sci. 2003;214:49–56. doi: 10.1016/s0022-510x(03)00179-5. [DOI] [PubMed] [Google Scholar]
- Leite AC, Silva MT, Alamy AH, Afonso CR, Lima MA, Andrada-Serpa MJ, Nascimento OJ, Araujo AQ. Peripheral neuropathy in HTLV-I infected individuals without tropical spastic paraparesis/HTLV-I-associated myelopathy. J Neurol. 2004;251:877–881. doi: 10.1007/s00415-004-0455-7. [DOI] [PubMed] [Google Scholar]
- Levin MC, Lehky TJ, Flerlage AN, Katz D, Kingma DW, Jaffe ES, Heiss JD, Patronas N, McFarland HF, Jacobson S. Immunologic analysis of a spinal cord-biopsy specimen from a patient with human T-cell lymphotropic virus type I-associated neurologic disease. N Engl J Med. 1997;336:839–845. doi: 10.1056/NEJM199703203361205. [DOI] [PubMed] [Google Scholar]
- Lezin A, Buart S, Smadja D, Akaoka H, Bourdonne O, Perret-Liaudet A, Cesaire R, Belin MF, Giraudon P. Tissue inhibitor of metalloproteinase 3, matrix metalloproteinase 9, and neopterin in the cerebrospinal fluid: preferential presence in HTLV type I-infected neurologic patients versus healthy virus carriers. AIDS Res Hum Retroviruses. 2000;16:965–972. doi: 10.1089/08892220050058380. [DOI] [PubMed] [Google Scholar]
- Macatonia SE, Cruickshank JK, Rudge P, Knight SC. Dendritic cells from patients with tropical spastic paraparesis are infectedwith HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses. 1992;8:1699–1706. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- Makino M, Shimokubo S, Wakamatsu SI, Izumo S, Baba M. The role of human T-lymphotropic virus type 1 (HTLV-1)-infected dendritic cells in the development of HTLV-1-associated myelopathy/tropical spastic paraparesis. J Virol. 1999;73:4575–4581. doi: 10.1128/jvi.73.6.4575-4581.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns A, Miley WJ, Wilks RJ, Morgan OS, Hanchard B, Wharfe G, Cranston B, Maloney E, Welles SL, Blattner WA, Waters D. Quantitative proviral DNA and antibody levels in the natural history of HTLV-I infection. J Infect Dis. 1999;180:1487–1493. doi: 10.1086/315088. [DOI] [PubMed] [Google Scholar]
- Mariette X, Agbalika F, Daniel MT, Bisson M, Lagrange P, Brouet JC, Morinet F. Detection of human T lymphotropic virus type I tax gene in salivary gland epithelium from two patients with Sjogren’s syndrome. Arthritis Rheum. 1993;36:1423–1428. doi: 10.1002/art.1780361015. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Okayama A, Kawano T. Human T-cell lymphotropic virus-I coinfection in patients with chronic hepatitis and hepatocellular carcinoma associated with hepatitis C virus. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:262. [Google Scholar]
- Matsuoka E, Takenouchi N, Hashimoto K, Kashio N, Moritoyo T, Higuchi I, Isashiki Y, Sato E, Osame M, Izumo S. Perivascular T cells are infected with HTLV-I in the spinal cord lesions with HTLV-I-associated myelopathy/tropical spastic paraparesis: double staining of immunohistochemistry and polymerase chain reaction in situ hybridization. Acta Neuropathol. 1998;96:340–346. doi: 10.1007/s004010050903. [DOI] [PubMed] [Google Scholar]
- Matsuura E, Kubota R, Saito M, Suehara M, Matsuzaki T, Arimura K, Osame M, Izumo S. Visualization of HTLV-I Tax-specific cytotoxic T lymphocytes in the central nervous system of HTLV-I-associated myelopathy. J Neuroimmunol. 2006;178:257. [Google Scholar]
- Matsuura E, Umehara F, Nose H, Higuchi I, Matsuoka E, Izumi K, Kubota R, Saito M, Izumo S, Arimura K, Osame M. Inclusion body myositis associated with human T-lymphotropic virus-type I infection: eleven patients from an endemic area in Japan. J Neuropathol Exp Neurol. 2008;67:41–49. doi: 10.1097/nen.0b013e31815f38b7. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Nakagawa M, Nagai M, Nobuhara Y, Usuku K, Higuchi I, Takahashi K, Moritoyo T, Arimura K, Izumo S, Akiba S, Osame M. HTLV-I-associated myelopathy (HAM)/tropical spastic paraparesis (TSP) with amyotrophic lateral sclerosis-like manifestations. J Neurovirol. 2000;6:544–548. doi: 10.3109/13550280009091955. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Nakagawa M, Nagai M, Usuku K, Higuchi I, Arimura K, Kubota H, Izumo S, Akiba S, Osame M. HTLV-I proviral load correlates with progression of motor disability in HAM/TSP: analysis of 239 HAM/TSP patients including 64 patients followed up for 10 years. J Neurovirol. 2001;7:228–234. doi: 10.1080/13550280152403272. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Saito M, Usuku K, Nose H, Izumo S, Arimura K, Osame M. A prospective uncontrolled trial of fermented milk drink containing viable Lactobacillus casei strain Shirota in the treatment of HTLV-1 associated myelopathy/tropical spastic paraparesis. J Neurol Sci. 2005;237:75–81. doi: 10.1016/j.jns.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Meekings KN, Leipzig J, Bushman FD, Taylor GP, Bangham CR. HTLV-1 integration into transcriptionally active genomic regions is associated with proviral expression and with HAM/TSP. PLoS Pathog. 2008;4:e1000027. doi: 10.1371/journal.ppat.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnard JM, Devaux C. Multiple control levels of cell proliferation by human T-cell leukemia virus type 1 Tax protein. Virology. 1999;257:277–284. doi: 10.1006/viro.1999.9685. [DOI] [PubMed] [Google Scholar]
- Michaelsson J, Barbosa HM, Jordan KA, Chapman JM, Brunialti MK, Neto WK, Nukui Y, Sabino EC, Chieia MA, Oliveira AS, Nixon DF, Kallas EG. The frequency of CD127low expressing CD4+CD25high T regulatory cells is inversely correlated with human T lymphotrophic virus type-1 (HTLV-1) proviral load in HTLV-1-infection and HTLV-1-associated myelopathy/tropical spastic paraparesis. BMC Immunol. 2008;9:41. doi: 10.1186/1471-2172-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingari MC, Ponte M, Bertone S, Schiavetti F, Vitale C, Bellomo R, Moretta A, Moretta L. HLA class I-specific inhibitory receptors in human T lymphocytes: interleukin 15-induced expression of CD94/NKG2A in superantigen- or alloantigen-activated CD8+ T cells. Proc Natl Acad Sci USA. 1998;95:1172–1177. doi: 10.1073/pnas.95.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazato P, Yasunaga J, Taniguchi Y, Koyanagi Y, Mitsuya H, Matsuoka M. De novo human T-cell leukemia virus type 1 infection of human lymphocytes in NOD-SCID, common gamma-chain knockout mice. J Virol. 2006;80:10683–10691. doi: 10.1128/JVI.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan OS, Rodgers-Johnson P, Mora C, Char G. HTLV-1 and polymyositis in Jamaica. Lancet. 1989;2:1184–1187. doi: 10.1016/s0140-6736(89)91793-5. [DOI] [PubMed] [Google Scholar]
- Mori N, Krensky AM, Ohshima K, Tomita M, Matsuda T, Ohta T, Yamada Y, Tomonaga M, Ikeda S, Yamamoto N. Elevated expression of CCL5/RANTES in adult T-cell leukemia cells: possible transactivation of the CCL5 gene by human T-cell leukemia virus type I tax. Int J Cancer. 2004;111:548–557. doi: 10.1002/ijc.20266. [DOI] [PubMed] [Google Scholar]
- Moritoyo T, Reinhart TA, Moritoyo H, Sato E, Izumo S, Osame M, Haase AT. Human T-lymphotropic virus type I-associated myelopathy and tax gene expression in CD4+ T lymphocytes. Ann Neurol. 1996;40:84–90. doi: 10.1002/ana.410400114. [DOI] [PubMed] [Google Scholar]
- Moritoyo T, Izumo S, Moritoyo H, Tanaka Y, Kiyomatsu Y, Nagai M, Usuku K, Sorimachi M, Osame M. Detection of human T-lymphotropic virus type I p40tax protein in cerebrospinal fluid cells from patients with human T-lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis. J Neurovirol. 1999;5:241–248. doi: 10.3109/13550289909015810. [DOI] [PubMed] [Google Scholar]
- Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, Hashiguchi S, Ichinose M, Bangham CR, Izumo S, Osame M. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–593. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- Nagai M, Yamano Y, Brennan MB, Mora CA, Jacobson S. Increased HTLV-I proviral load and preferential expansion of HTLV-I Tax-specific CD8+ T cells in cerebrospinal fluid from patients with HAM/TSP. Ann Neurol. 2001a;50:807–812. doi: 10.1002/ana.10065. [DOI] [PubMed] [Google Scholar]
- Nagai M, Brennan MB, Sakai JA, Mora CA, Jacobson S. CD8 (+) T cells are an in vivo reservoir for human T-cell lymphotropic virus type I. Blood. 2001b;98:1858–1861. doi: 10.1182/blood.v98.6.1858. [DOI] [PubMed] [Google Scholar]
- Nagai M, Kubota R, Greten TF, Schneck JP, Leist TP, Jacobson S. Increased activated human T cell lymphotropic virus type I (HTLV-I) Tax11-19-specific memory and effector CD8+ cells in patients with HTLV-I-associated myelopathy/tropical spastic paraparesis: correlation with HTLV-I provirus load. J Infect Dis. 2001c;183:197–205. doi: 10.1086/317932. [DOI] [PubMed] [Google Scholar]
- Nakamura T. HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP): the role of HTLV-I-infected Th1 cells in the pathogenesis, and therapeutic strategy. Folia Neuropathol. 2009;47:182–194. [PubMed] [Google Scholar]
- Nakamura T, Furuya T, Nishiura Y, Ichinose K, Shirabe S, Eguchi K. Importance of immune deviation toward Th1 in the early immunopathogenesis of human T-lymphotropic virus type I-associated myelopathy. Med Hypotheses. 2000;54:777–782. doi: 10.1054/mehy.1999.0949. [DOI] [PubMed] [Google Scholar]
- Nakao K, Matsumoto M, Ohba N. Seroprevalence of antibodies to HTLV-I in patients with ocular disorders. Br J Ophthalmol. 1991;75:76–78. doi: 10.1136/bjo.75.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiesk S, Daenke S, Parker CE, Taylor G, Weber J, Nightingale S, Bangham CR. Naturally occurring variants of human T-cell leukemia virus type I Tax protein impair its recognition by cytotoxic T lymphocytes and the transactivation function of Tax. J Virol. 1995;69:2649–2653. doi: 10.1128/jvi.69.4.2649-2653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K, Maruyama I, Sato K, Kitajima I, Nakajima Y, Osame M. Chronic inflammatory arthropathy associated with HTLV-I. Lancet. 1989;1:441. doi: 10.1016/s0140-6736(89)90038-x. [DOI] [PubMed] [Google Scholar]
- Oh U, Grant C, Griffith C, Fugo K, Takenouchi N, Jacobson S. Reduced Foxp3 protein expression is associated with inflammatory disease during human t lymphotropic virus type 1 Infection. J Infect Dis. 2006;193:1557–1566. doi: 10.1086/503874. [DOI] [PubMed] [Google Scholar]
- Okada F, Ando Y, Yoshitake S, Yotsumoto S, Matsumoto S, Wakisaka M, Maeda T, Mori H. Pulmonary CT findings in 320carriers of human T-lymphotropic virus type 1. Radiology. 2006;240:559–564. doi: 10.1148/radiol.2402050886. [DOI] [PubMed] [Google Scholar]
- Osame M. Review of WHO Kagoshima meeting and diagnostic guidelines for HAM/TSP. In: HTLVWA B, editor. Human retrovirology. Raven Press; New York: 1990. p. 191. [Google Scholar]
- Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Ozden S, Gessain A, Gout O, Mikol J. Sporadic inclusion body myositis in a patient with human T cell leukemia virus type 1-associated myelopathy. Clin Infect Dis. 2001;32:510–514. doi: 10.1086/318506. [DOI] [PubMed] [Google Scholar]
- Ozden S, Seilhean D, Gessain A, Hauw JJ, Gout O. Severe demyelinating myelopathy with low human T cell lymphotropic virus type 1 expression after transfusion in an immunosuppressed patient. Clin Infect Dis. 2002;34:855–860. doi: 10.1086/338868. [DOI] [PubMed] [Google Scholar]
- Ozden S, Cochet M, Mikol J, Teixeira A, Gessain A, Pique C. Direct evidence for a chronic CD8+-T-cell-mediated immune reaction to tax within the muscle of a human T-cell leukemia/lymphoma virus type 1-infected patient with sporadic inclusion body myositis. J Virol. 2004;78:10320–10327. doi: 10.1128/JVI.78.19.10320-10327.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CE, Daenke S, Nightingale S, Bangham CR. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology. 1992;188:628–636. doi: 10.1016/0042-6822(92)90517-s. [DOI] [PubMed] [Google Scholar]
- Pais-Correia AM, Sachse M, Guadagnini S, Robbiati V, Lasserre R, Gessain A, Gout O, Alcover A, Thoulouze MI. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat Med. 2009;16:83–89. doi: 10.1038/nm.2065. [DOI] [PubMed] [Google Scholar]
- Parker CE, Nightingale S, Taylor GP, Weber J, Bangham CR. Circulating anti-Tax cytotoxic T lymphocytes from human T-cell leukemia virus type I-infected people, with and without tropical spastic paraparesis, recognize multiple epitopes simultaneously. J Virol. 1994;68:2860–2868. doi: 10.1128/jvi.68.5.2860-2868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique C, Ureta-Vidal A, Gessain A, Chancerel B, Gout O, Tamouza R, Agis F, Dokhelar MC. Evidence for the chronic in vivo production of human T cell leukemia virus type I Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J Exp Med. 2000;191:567–572. doi: 10.1084/jem.191.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz BJ, Ruscetti FW, Gazdar AF. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JH, Edwards AJ, Cruickshank JK, Rudge P, Dalgleish AG. In vivo cellular tropism of human T-cell leukemia virus type 1. J Virol. 1990;64:5682–5687. doi: 10.1128/jvi.64.11.5682-5687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero IA, Prevost MC, Perret E, Adamson P, Greenwood J, Couraud PO, Ozden S. Interactions between brain endothelial cells and human T-cell leukemia virus type 1-infected lymphocytes: mechanisms of viral entry into the central nervous system. J Virol. 2000;74:6021–6030. doi: 10.1128/jvi.74.13.6021-6030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- Sabouri AH, Saito M, Usuku K, Bajestan SN, Mahmoudi M, Forughipour M, Sabouri Z, Abbaspour Z, Goharjoo ME, Khayami E, Hasani A, Izumo S, Arimura K, Farid R, Osame M. Differences in viral and host genetic risk factors for development of human T-cell lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis between Iranian and Japanese HTLV-1-infected individuals. J Gen Virol. 2005;86:773–781. doi: 10.1099/vir.0.80509-0. [DOI] [PubMed] [Google Scholar]
- Sabouri AH, Usuku K, Hayashi D, Izumo S, Ohara Y, Osame M, Saito M. Impaired function of human T-lymphotropic virus type 1 (HTLV-1)-specific CD8+ T cells in HTLV-1-associated neurologic disease. Blood. 2008;112:2411–2420. doi: 10.1182/blood-2008-02-140335. [DOI] [PubMed] [Google Scholar]
- Saito M, Braud VM, Goon P, Hanon E, Taylor GP, Saito A, Eiraku N, Tanaka Y, Usuku K, Weber JN, Osame M, Bangham CR. Low frequency of CD94/NKG2A+T lymphocytes in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis, but not in asymptomatic carriers. Blood. 2003;102:577–584. doi: 10.1182/blood-2002-09-2855. [DOI] [PubMed] [Google Scholar]
- Setoyama M, Mizoguchi S, Eizuru Y. Human T-cell lymphotropic virus type I infects eccrine sweat gland epithelia. Int J Cancer. 1999;80:652–655. doi: 10.1002/(sici)1097-0215(19990301)80:5<652::aid-ijc3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Sharma V, Lorey SL. Autocrine role of macrophage inflammatory protein-1 beta in human T-cell lymphotropic virus type-I tax-transfected Jurkat T-cells. Biochem Biophys Res Commun. 2001;287:910–913. doi: 10.1006/bbrc.2001.5671. [DOI] [PubMed] [Google Scholar]
- Sueyoshi K, Goto M, Johnosono M, Sato E, Shibata D. Anatomical distribution of HTLV-I proviral sequence in an autopsy case of HTLV-I associated myelopathy: a polymerase chain reaction study. Pathol Int. 1994;44:27–33. doi: 10.1111/j.1440-1827.1994.tb02582.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Nakashima H, Watanabe S, Uyama E, Tanaka F, Ando M, Araki S, Kawasaki S. T-lymphocyte alveolitis in HTLV-I-associated myelopathy. Lancet. 1987;2:1220. doi: 10.1016/s0140-6736(87)91362-6. [DOI] [PubMed] [Google Scholar]
- Takenouchi N, Yamano Y, Usuku K, Osame M, Izumo S. Usefulness of proviral load measurement for monitoring of disease activity in individual patients with human T-lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis. J Neurovirol. 2003;9:29–35. doi: 10.1080/13550280390173418. [DOI] [PubMed] [Google Scholar]
- Tangy F, Ossondo M, Vernant JC, Smadja D, Bletry O, Baglin AC, Ozden S. Human T cell leukemia virus type I expression in salivary glands of infected patients. J Infect Dis. 1999;179:497–502. doi: 10.1086/314588. [DOI] [PubMed] [Google Scholar]
- Taylor GP, Tosswill JH, Matutes E, Daenke S, Hall S, Bain BJ, Davis R, Thomas D, Rossor M, Bangham CR, Weber JN. Prospective study of HTLV-I infection in an initially asymptomatic cohort. J Acquir Immune Defic Syndr. 1999;22:92–100. doi: 10.1097/00042560-199909010-00012. [DOI] [PubMed] [Google Scholar]
- Taylor GP, Goon P, Furukawa Y, Green H, Barfield A, Mosley A, Nose H, Babiker A, Rudge P, Usuku K, Osame M, Bangham CR, Weber JN. Zidovudine plus lamivudine in Human T-Lymphotropic Virus type-I-associated myelopathy: a randomised trial. Retrovirology. 2006;3:63. doi: 10.1186/1742-4690-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendler CL, Greenberg SJ, Blattner WA, Manns A, Murphy E, Fleisher T, Hanchard B, Morgan O, Burton JD, Nelson DL, et al. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: pathogenic implications and a rationale for immunotherapy. Proc Natl Acad Sci USA. 1990;87:5218–5222. doi: 10.1073/pnas.87.13.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulza F, Heaps A, Tanaka Y, Taylor GP, Bangham CR. High frequency of CD4+FoxP3+ cells in HTLV-1 infection: inverse correlation with HTLV-1-specific CTL response. Blood. 2008;111:5047–5053. doi: 10.1182/blood-2007-10-118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo SR, Ratner L. The HTLV receptor is a widely expressed protein. Virology. 2000;268:41–48. doi: 10.1006/viro.2000.0143. [DOI] [PubMed] [Google Scholar]
- Umehara F, Izumo S, Nakagawa M, Ronquillo AT, Takahashi K, Matsumuro K, Sato E, Osame M. Immunocytochemical analysis of the cellular infiltrate in the spinal cord lesions in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol. 1993;52:424–430. doi: 10.1097/00005072-199307000-00010. [DOI] [PubMed] [Google Scholar]
- Umehara F, Izumo S, Ronquillo AT, Matsumuro K, Sato E, Osame M. Cytokine expression in the spinal cord lesions in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol. 1994a;53:72–77. doi: 10.1097/00005072-199401000-00009. [DOI] [PubMed] [Google Scholar]
- Umehara F, Nakamura A, Izumo S, Kubota R, Ijichi S, Kashio N, Hashimoto K, Usuku K, Sato E, Osame M. Apoptosis of T lymphocytes in the spinal cord lesions in HTLV-I-associated myelopathy: a possible mechanism to control viral infection in the central nervous system. J Neuropathol Exp Neurol. 1994b;53:617–624. doi: 10.1097/00005072-199411000-00009. [DOI] [PubMed] [Google Scholar]
- Umehara F, Izumo S, Takeya M, Takahashi K, Sato E, Osame M. Expression of adhesion molecules and monocyte chemoattractant protein -1 (MCP-1) in the spinal cord lesions in HTLV-I-associated myelopathy. Acta Neuropathol. 1996;91:343–350. doi: 10.1007/s004010050435. [DOI] [PubMed] [Google Scholar]
- Umehara F, Okada Y, Fujimoto N, Abe M, Izumo S, Osame M. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol. 1998;57:839–849. doi: 10.1097/00005072-199809000-00005. [DOI] [PubMed] [Google Scholar]
- Usuku K, Sonoda S, Osame M, Yashiki S, Takahashi K, Matsumoto M, Sawada T, Tsuji K, Tara M, Igata A. HLA haplotype-linked high immune responsiveness against HTLV-I in HTLV-I-associated myelopathy: comparison with adult T-cell leukemia/lymphoma. Ann Neurol. 1988;23(Suppl):S143–150. doi: 10.1002/ana.410230733. [DOI] [PubMed] [Google Scholar]
- Vernant JC, Buisson G, Magdeleine J, De Thore J, Jouannelle A, Neisson-Vernant C, Monplaisir N. T-lymphocyte alveolitis, tropical spastic paresis, and Sjogren syndrome. Lancet. 1988;1:177. doi: 10.1016/s0140-6736(88)92744-4. [DOI] [PubMed] [Google Scholar]
- Vernant JC, Bellance R, Buisson GG, Havard S, Mikol J, Roman G. Human Retrovirology: HTLV-I. Raven Press; New York: 1990. Peripheral neuropathies and myositis associated to HTLV-I infection in Martinique; pp. 225–235. [Google Scholar]
- Vine AM, Witkover AD, Lloyd AL, Jeffery KJ, Siddiqui A, Marshall SE, Bunce M, Eiraku N, Izumo S, Usuku K, Osame M, Bangham CR. Polygenic control of human T lymphotropic virus type I (HTLV-I) provirus load and the risk of HTLV-I-associated myelopathy/tropical spastic paraparesis. J Infect Dis. 2002;186:932–939. doi: 10.1086/342953. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Nakamura T, Nagasato K, Shirabe S, Ohishi K, Ichinose K, Nishiura Y, Chiyoda S, Tsujihata M, Nagataki S. Exaggerated messenger RNA expression of inflammatory cytokines in human T-cell lymphotropic virus type I-associated myelopathy. Arch Neurol. 1995;52:276–280. doi: 10.1001/archneur.1995.00540270068021. [DOI] [PubMed] [Google Scholar]
- Wattel E, Vartanian JP, Pannetier C, Wain-Hobson S. Clonal expansion of human T-cell leukemia virus type I-infected cells in asymptomatic and symptomatic carriers without malignancy. J Virol. 1995;69:2863–2868. doi: 10.1128/jvi.69.5.2863-2868.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Virus diseases: human T-lymphotropic virus type I, HTLV-I. WHO Weekly Epidemiol Rec. 1989;49:382. [Google Scholar]
- Wu E, Dickson DW, Jacobson S, Raine CS. Neuroaxonal dystrophy in HTLV-1-associated myelopathy/tropical spastic paraparesis: neuropathologic and neuroimmunologic correlations. Acta Neuropathol. 1993;86:224–235. doi: 10.1007/BF00304136. [DOI] [PubMed] [Google Scholar]
- Wu XM, Osoegawa M, Yamasaki K, Kawano Y, Ochi H, Horiuchi I, Minohara M, Ohyagi Y, Yamada T, Kira JI. Flow cytometric differentiation of Asian and Western types of multiple sclerosis, HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) and hyperIgEaemic myelitis by analyses of memory CD4 positive T cell subsets and NK cell subsets. J Neurol Sci. 2000;177:24–31. doi: 10.1016/s0022-510x(00)00322-1. [DOI] [PubMed] [Google Scholar]
- Yamano Y, Nagai M, Brennan M, Mora CA, Soldan SS, Tomaru U, Takenouchi N, Izumo S, Osame M, Jacobson S. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8(+) T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP) Blood. 2002;99:88–94. doi: 10.1182/blood.v99.1.88. [DOI] [PubMed] [Google Scholar]
- Yamano Y, Cohen CJ, Takenouchi N, Yao K, Tomaru U, Li HC, Reiter Y, Jacobson S. Increased expression of human T lymphocyte virus type I (HTLV-I) Tax11-19 peptide-human histocompatibility leukocyte antigen A*201 complexes on CD4 + CD25+ T Cells detected by peptide-specific, major histocompatibility complex-restricted antibodies in patients with HTLV-I-associated neurologic disease. J Exp Med. 2004;199:1367–1377. doi: 10.1084/jem.20032042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano Y, Araya N, Sato T, Utsunomiya A, Azakami K, Hasegawa D, Izumi T, Fujita H, Aratani S, Yagishita N, Fujii R, Nishioka K, Jacobson S, Nakajima T. Abnormally high levels of virus-infected IFN-gamma+CCR4+ CD4+ CD25+ T cells in a retrovirus-associated neuroinflammatory disorder. PLoS ONE. 2009;4:e6517. doi: 10.1371/journal.pone.0006517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Machigashira N, Wang SY, Osame M. A patient with acute-onset HAM/TSP after blood transfusion complicated with pseudopseudohypoparathyroidism. Intern Med. 2002;41:899–900. doi: 10.2169/internalmedicine.41.899. [DOI] [PubMed] [Google Scholar]
- Yu F, Itoyama Y, Fujihara K, Goto I. Natural killer (NK) cells in HTLV-I-associated myelopathy/tropical spastic paraparesis-decrease in NK cell subset populations and activity in HTLV-I seropositive individuals. J Neuroimmunol. 1991;33:121–128. doi: 10.1016/0165-5728(91)90056-d. [DOI] [PubMed] [Google Scholar]