Abstract
Objectives
Testing for human immunodeficiency virus (HIV) is the key first step in HIV treatment and prevention. In 2006, the Centers for Disease Control and Prevention (CDC) recommended annual HIV testing for people at high risk for HIV infection. We evaluated HIV testing among men with high-risk heterosexual (HRH) contact and sexually active men who have sex with men (MSM) before and after the CDC recommendations.
Methods
We used data from the National Survey of Family Growth, 2002 and 2006–2010, to assess proportions of HRH respondents and MSM reporting HIV testing in the prior 12 months, compare rates of testing before and after release of the 2006 CDC HIV testing guidelines, and examine demographic variables and receipt of health-care services as correlates of HIV testing.
Results
Among MSM, the proportion tested was 37.2% (95% confidence interval [CI] 28.2, 47.2) in 2002, 38.2% (95% CI 25.9, 52.2) in 2006–2008, and 41.7% (95% CI 29.2, 55.3) in 2008–2010; among HRH respondents, the proportion was 23.7% (95% CI 20.5, 27.3) in 2002, 24.5% (95% CI 20.9, 28.7) in 2006–2008, and 23.9% (95% CI 20.2, 28.1) in 2008–2010. HIV testing was more likely among MSM and HRH respondents who received testing or treatment for sexually transmitted disease in the prior 12 months, received a physical examination in the prior 12 months (MSM only), or were incarcerated in the prior 12 months.
Conclusions
The rate of annual HIV testing was low for men with sexual risk for HIV infection, and little improvement took place from 2002 to 2006–2010. Interventions aimed at men at risk, especially MSM, in both nonmedical and health-care settings, likely could increase HIV testing.
New infections of human immunodeficiency virus (HIV) occur in the United States at a rate of approximately 50,000 per year, driven mostly by sexual transmission, particularly among men who have sex with men (MSM).1 In 2010, male-to-male sexual contact accounted for 63% of new HIV infections (78% among males), and heterosexual contact accounted for 25% of new HIV infections (11% among males).1,2 Although overall incidence has been relatively stable since 2006, among young MSM, particularly young black MSM, new infections continue to increase.1,3
An estimated 14% of adults and adolescents living with HIV infection in the United States are undiagnosed, of whom 11% are males with high-risk heterosexual (HRH) contact and 62% are MSM.4 To increase the proportion of HIV-infected people who are aware of their status and link them to treatment and prevention services, the Centers for Disease Control and Prevention (CDC) recommended in 2006 that all people aged 13–64 years be tested at least once for HIV infection and that people at high risk for HIV infection, including men with HRH contact and sexually active MSM,5 be tested annually.
Using data from multiple waves of a nationally representative survey, we examined the percentage of HRH and MSM respondents who reported having been tested for HIV in the prior 12 months. We compared rates of testing before and after the revised HIV testing guidelines5 were released by CDC in 2006. Additionally, because the 2006 CDC guidelines recommended that HIV screening be conducted as part of routine clinical care in all health-care settings,5 we examined HIV testing among men stratified by their reported use of health-care services in the prior 12 months and by several sociodemographic variables.
METHODS
We used data from the National Survey of Family Growth (NSFG) for our analysis.6 NSFG, established in 1971, is a periodic cross-sectional household-based survey, conducted by the National Center for Health Statistics, employing multistage sampling methods to produce a nationally representative probability sample of males and females aged 15–44 years living in the United States.7,8 NSFG collects data on sexual behavior and reproductive health through in-person, voluntary, and confidential interviews conducted by trained female interviewers. The most sensitive items of the survey are administered with an audio computer-assisted self-interview to ensure privacy. In 2002, NSFG included men for the first time in its survey history. In 2006, to control costs and increase data quality, NSFG switched to a continuous survey design, in which interviewing was done every year by a smaller number of interviewers working consistently over survey years. We used data from male respondents from two NSFG cycles, Cycle 6 (2002) and continuous Cycle 7 (2006–2010). The response rate of male respondents was 78% in 2002 and 75% in 2006–2010. We used NSFG's sampling weights in our analysis to account for the complex sample design to ensure nationally representative estimates.7,8 Sample weights for data from 2006–2010 were available only for the full four-year period from June 2006 through June 2010 or in two-year increments from June 2006 through June 2008 and July 2008 through June 2010. Sample weights were not available for the separate years of 2006, 2007, 2008, 2009, or 2010.
Our analysis focused on men at risk for contracting HIV through sex as adapted from risk groups defined in CDC's revised HIV testing guidelines:5 sexually active MSM, defined as men who reported at least one male partner in the prior 12 months, regardless of whether they self-identified as gay, bisexual, or other; and HRH, defined as men who did not have sex with other men in the prior 12 months and who reported sex in the prior 12 months with multiple female partners; with a female partner who was non-monogamous, injected drugs, or was HIV-infected; or with a female partner in exchange for drugs or money. Men who did not have any sexual risk were defined as men who did not meet the criteria for MSM or HRH. The NSFG surveys did not include a question about current HIV status; thus, we were not able to exclude men who had been diagnosed with HIV infection prior to the survey.
Our primary outcome measure, self-reported HIV testing in the prior 12 months, did not include testing performed as part of blood donation. Stratification variables included receipt of physical examination in the prior 12 months, receipt of testing or treatment for sexually transmitted infections (STIs) other than HIV in the prior 12 months, current health insurance status, and other sociodemographic variables: age, race/ethnicity, income, and sexual identity; and in the prior 12 months, use of injected drugs, a shelter stay, and incarceration. Data on income were dichotomized (#annual poverty threshold or >annual poverty threshold defined by the U.S. Census Bureau9). For 2006–2010 (but not for 2002), respondents were asked to choose a reason for their most recent HIV test from 13 options.
We used SUDAAN® version 1010 to estimate weighted proportions of male respondents who reported receiving HIV testing in the prior 12 months. We conducted the same analysis separately for HRH respondents and for MSM respondents, in which we compared HIV testing between 2002 and three other periods (2002 vs. 2006–2010, 2002 vs. 2006–2008, and 2002 vs. 2008–2010) and assessed correlates of HIV testing using univariate logistic regression. We included variables with a p-value <0.10 in univariate analysis in multivariable logistic regression models. We estimated strength of association with HIV testing using relative risk (RR) based on average marginal predictions. For each of the two NSFG cycles, we weighted estimates for unequal selection probabilities and nonresponse to adjust for the complex NSFG sampling design. We applied weights separately to each cycle before pooling the data from the two NSFG cycles.
In our overall analysis, we combined data from 15,331 male respondents interviewed in 2002 (n=4,928) and 2006–2010 (n=10,403) to obtain a nationally representative sample of men aged 15–44 years. Combined data from NSFG 2002 and 2006–2010 yielded a weighted estimate of 24,407,000 HRH respondents and 2,924,000 MSM. The number of HRH respondents was 1,334 in 2002 and 2,453 in 2006–2010 (combined n=3,787); the number of MSM respondents was 197 in 2002 and 272 in 2006–2010 (combined n=469).
RESULTS
In the NSFG sample of 15,331 male respondents for all years combined, 19.5% (95% confidence interval [CI] 18.5, 20.8) reported HRH contact and 2.4% (95% CI 2.0, 2.8) reported sex with a male partner in the prior 12 months. Sociodemographic characteristics of male respondents in 2002 did not differ significantly from those of male respondents in 2006–2010.
Most HRH respondents (56%, 95% CI 53, 60) and MSM respondents (61%, 95% CI 54, 68)) were non-Hispanic white, followed by Hispanic and non-Hispanic black, and most had annual incomes above the annual poverty threshold (Table 1). Among HRH respondents, 70% (95% CI 67, 72) had health insurance (private, Medicaid, Medicare, military, or Veterans Administration), 48% (95% CI 46, 51) had received a physical examination in the prior 12 months, and 28% (95% CI 26, 30) had received testing or treatment for an STI other than HIV in the prior 12 months. Among MSM, 58% (95% CI 52, 64) identified as homosexual or gay, 21% (95% CI 17, 25) as bisexual, and 16% (95% CI 11, 22) as heterosexual or straight. Among MSM, 76% had health insurance (private, Medicaid, Medicare, military, or Veterans Administration), 52% (95% CI 46, 59) had received a physical examination in the prior 12 months, and 40% (95% CI 33, 47) had received testing or treatment for STIs in the prior 12 months.
Table 1.
Sociodemographic characteristics and health-care utilization among men at risk for acquiring HIV infection, National Survey of Family Growth, 2002 and 2006–2010 combineda
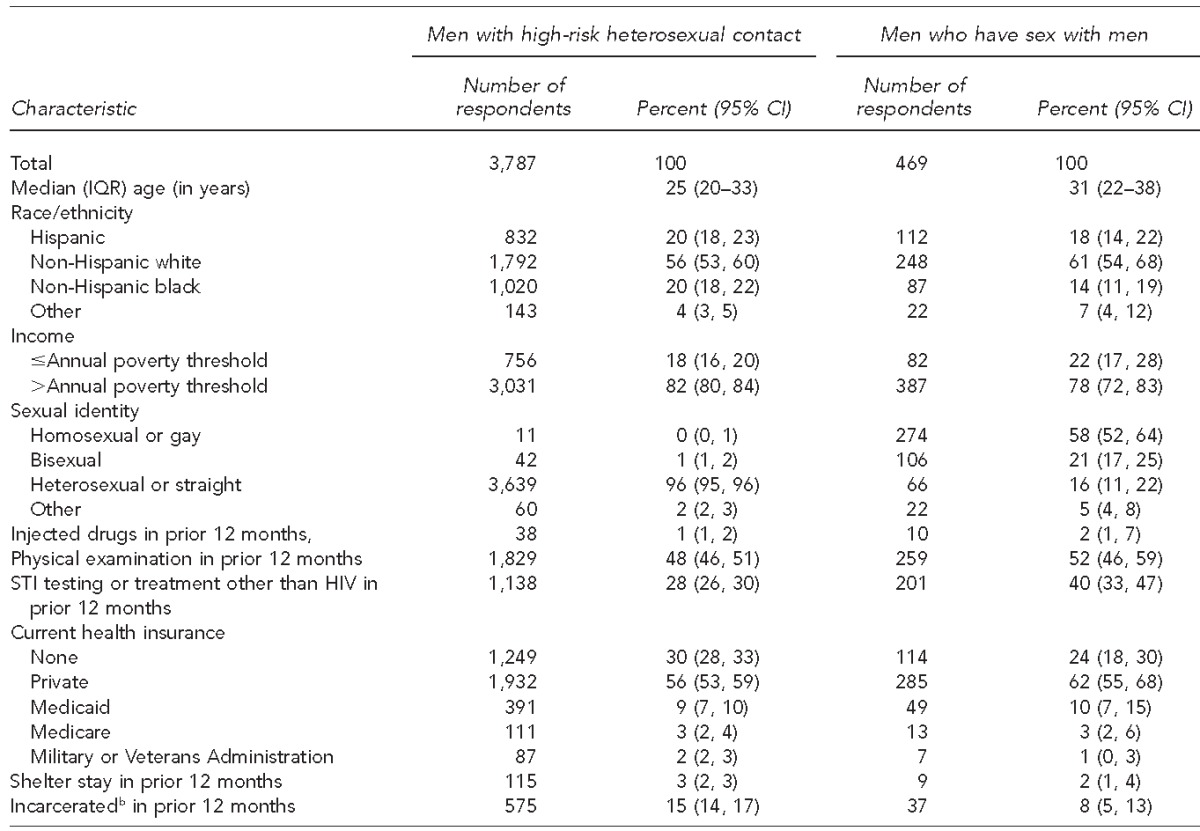
Combined data from National Survey of Family Growth 2002 (Cycle 6) and 2006–2010 (Cycle 7) yielding a weighted estimate of 24,407,000 high-risk heterosexual (HRH) respondents and 2,924,000 men who have sex with men (MSM). The number of HRH respondents was 1,334 in 2002 and 2,453 in 2006–2010; the number of MSM respondents was 197 in 2002 and 272 in 2006–2010. Percentages reported were derived from weighted numerators and denominators (rather than actual numbers of respondents) to account for the complex sampling design and to adjust for unequal selection probabilities and non-response.
bSpent any time in a jail, prison, or juvenile detention facility in prior 12 months
HIV = human immunodeficiency virus
IQR = interquartile range
CI = confidence interval
STI = sexually transmitted infection
For all years combined, HIV testing occurred in the prior 12 months among 11% (95% confidence interval [CI] 10, 12) of the male respondents who did not have any sexual risk, 24% (95% CI 22, 26) of HRH respondents, and 38% (95% CI 31, 45) of MSM (-Figure 1). Compared with men with no sexual risk, HRH respondents were more than twice as likely (unadjusted RR=2.29, 95% CI 2.01, 2.61) and MSM were more than three times as likely (unadjusted RR=3.59, 95% CI 2.93, 4.40) to report having received HIV testing in the prior 12 months.
Figure 1.
Percentage and unadjusted relative risk of men who received HIV testing in the previous 12 months, by sexual risk group, National Survey of Family Growth, 2002 and 2006–2010 combineda
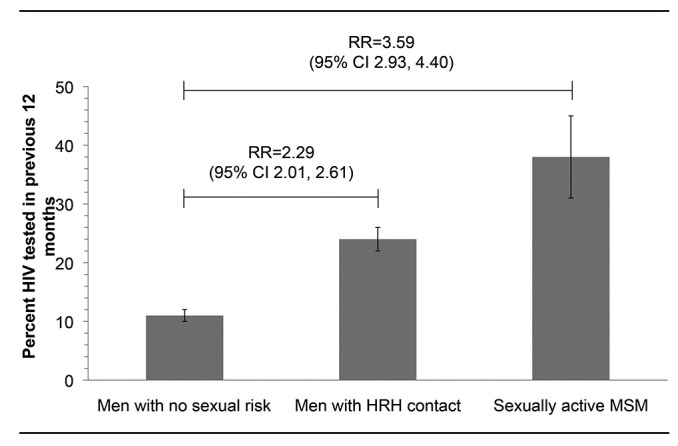
aCombined data from National Survey of Family Growth 2002 (Cycle 6) and 2006–2010 (Cycle 7) yielding a weighted estimate of 24,407,000 HRH and 2,924,000 MSM. The number of HRH respondents was 1,334 in 2002 and 2,453 in 2006–2010 (combined n=3,787); the number of MSM respondents was 197 in 2002 and 272 in 2006–2010 (combined n=469). Error bars indicate 95% confidence intervals.
RR = relative risk
CI = confidence interval
HIV = human immunodeficiency virus
HRH = high-risk heterosexual
MSM = men who have sex with men
HIV testing proportions before and after the revised 2006 CDC HIV testing guidelines did not differ significantly. Among HRH respondents, HIV testing in the prior 12 months was stable, with 23.7% in 2002 (95% CI 20.5, 27.3) and 24.4% in 2006–2010 (95% CI 21.7, 27.4). Similarly, among MSM, HIV testing in the prior 12 months was stable, with 37.2% in 2002 (95% CI 28.2, 47.2) and 38.5% in 2006–2010 (95% CI 29.3, 48.4). Further comparison of data for 2002 with those for 2006–2008 and 2008–2010 showed stable HIV testing proportions among both groups of men over time (Figure 2): 24.5% (95% CI 20.9, 28.7) in 2006–2008 and 23.9% (95% CI 20.2, 28.1) in 2008–2010 among HRH respondents, and 38.2% (95% CI 25.9, 52.2) in 2006–2008 and 41.7% (95% CI 29.2, 55.3) in 2008–2010 among MSM.
Figure 2.
Percentage of men with sexual risk who received HIV testing in the previous 12 months, by year interviewed, National Survey of Family Growth, 2002 and 2006–2010 combineda
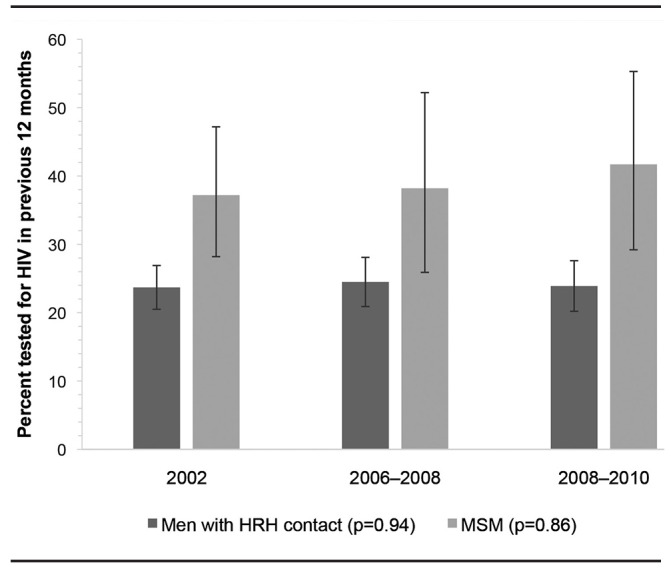
aCombined data from National Survey of Family Growth 2002 (Cycle 6) and 2006–2010 (Cycle 7) yielding a weighted estimate of 24,407,000 HRH and 2,924,000 MSM. The number of HRH respondents was 1,334 in 2002 and 2,453 in 2006–2010 (combined n=3,787); the number of MSM respondents was 197 in 2002 and 272 in 2006–2010 (combined n=469). Error bars indicate 95% confidence intervals. P-value reflects c2 test of any differences among the defined time intervals (2002, 2006–2008, and 2008–2010).
HIV = human immunodeficiency virus
HRH = high-risk heterosexual
MSM = men who have sex with men
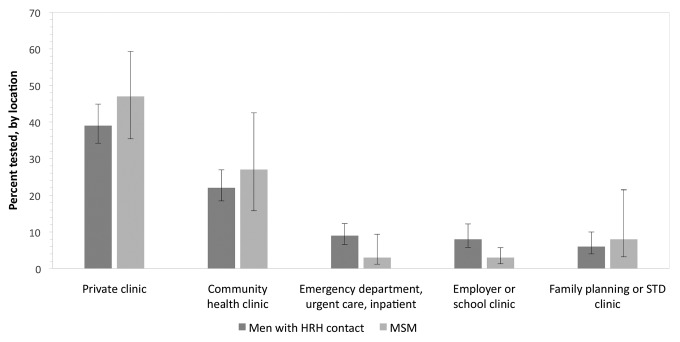
Among all HRH respondents, the multivariable model indicated that receipt of a physical examination, testing or treatment for STIs, having government health insurance other than Medicaid (Medicare, military, or Veterans Administration), and having spent time in jail, prison, or a juvenile detention facility in the prior 12 months were independently associated with a higher likelihood of having HIV testing in the prior 12 months (Table 2). Among HRH respondents who received HIV testing in the prior 12 months, 39% (95% CI 34, 45) received their most recent HIV test at a private clinic, and 22% (95% CI 19, 27) received their most recent test at a community health clinic (Figure 3). For 2006–2010, the most frequently cited reasons for obtaining the most recent HIV test were as follows: “wanted to find out if infected or not” (49%, 95% CI 43, 55), “testing was part of a medical checkup or surgical procedure” (24%, 95% CI 19, 30), “possible exposure through sex or drug use” (7%, 95% CI 4, 10), and “for military service or a job” (7%, 95% CI 3, 12).
Table 2.
Correlates of HIV testing in the 12 months prior to interview among men at risk for acquiring HIV infection—National Survey of Family Growth, 2002 and 2006–2010 combineda
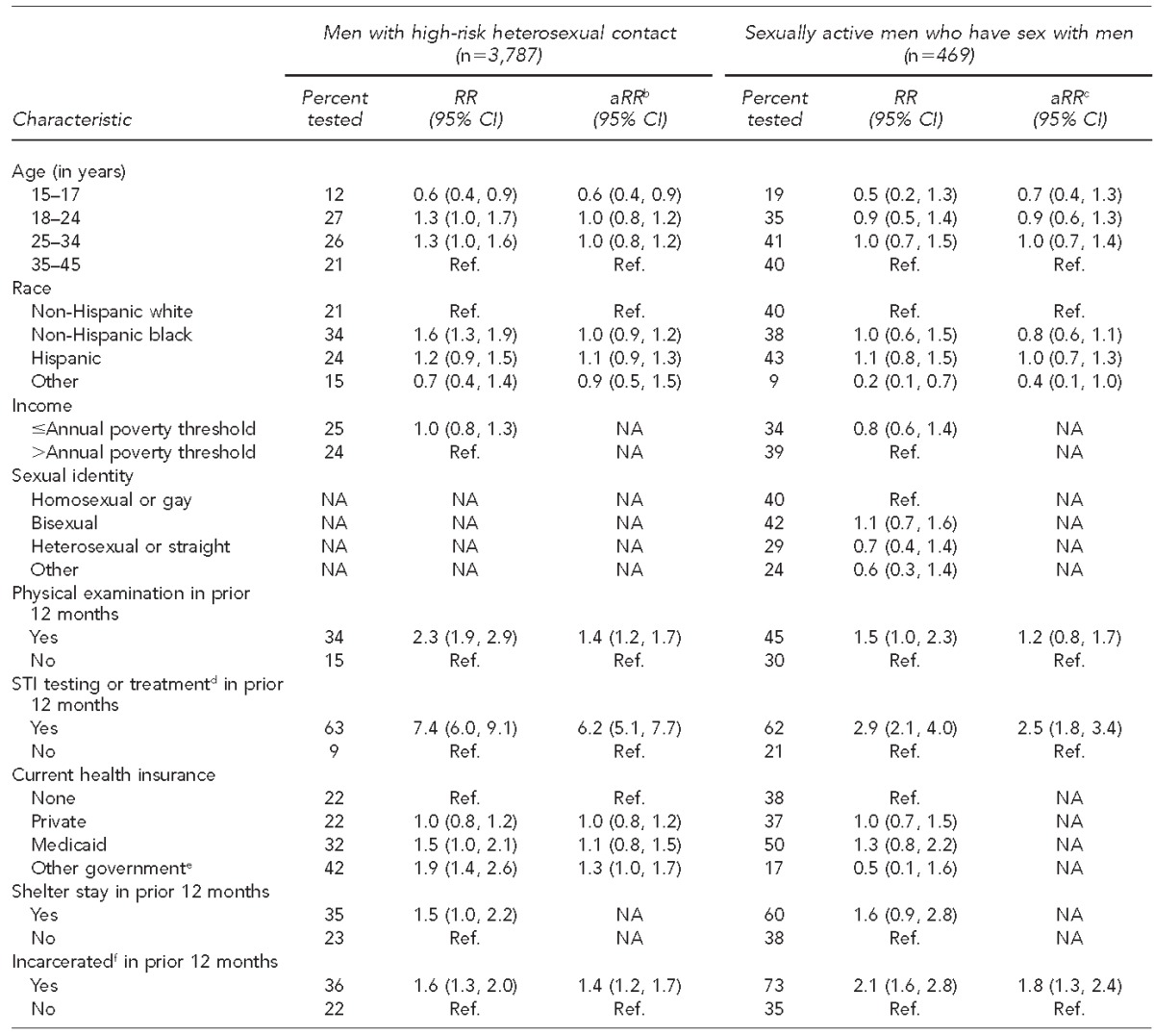
Combined data from National Survey of Family Growth 2002 (Cycle 6) and 2006–2010 (Cycle 7) yielding a weighted estimate of 24,407,000 high-risk heterosexual (HRH) respondents and 2,924,000 men who have sex with men (MSM). The number of HRH respondents was 1,334 in 2002 and 2,453 in 2006–10; the number of MSM respondents was 197 in 2002 and 272 in 2006–2010.
bThe multivariable model for HRH men included age, race, physical examination, testing or treatment for STI, health insurance, and jail.
cThe multivariable model for sexually active MSM included age, race, physical examination, testing or treatment for STI, and jail.
dOther than HIV
eMedicare, or military or Veterans Administration insurance
fSpent any time in a jail, prison, or a juvenile detention facility in prior 12 months
HIV = human immunodeficiency virus
RR = rate ratio
CI = confidence interval
aRR = adjusted rate ratio
Ref. = reference group
NA = not applicable or not assessed
STI = sexually transmitted infection
Figure 3.
Primary location of most recenta HIV test among men at risk who received HIV test in previous 12 months, National Survey of Family Growth, 2002 and 2006–2010 combinedb
aMost recent test prior to interview for National Survey of Family Growth.
bCombined data from National Survey of Family Growth 2002 (Cycle 6) and 2006–2010 (Cycle 7) yielding a weighted estimate of 24,407,000 HRH and 2,924,000 MSM. The number of HRH respondents was 1,334 in 2002 and 2,453 in 2006–2010 (combined n=3,787); the number of MSM respondents was 197 in 2002 and 272 in 2006–2010 (combined n=469). Error bars indicate 95% confidence intervals.
HIV = human immunodeficiency virus
HRH = high-risk heterosexual
MSM = men who have sex with men
STD = sexually transmitted disease
Among MSM, HIV testing in the prior 12 months was independently associated with testing or treatment for an STI other than HIV and with having spent time in jail, prison, or a juvenile detention facility in the prior 12 months (Table 2). Among MSM who received HIV testing in the prior 12 months, 47% (95% CI 35, 59) received their most recent HIV test at a private clinic, and 27% (95% CI 16, 43) received their most recent HIV test at a community health clinic (Figure 3). For 2006–2010, the most frequently cited reasons for their most recent HIV test were as follows: “wanted to find out if infected or not” (46%, 95% CI 33, 60), “testing was part of a medical checkup or surgical procedure” (20%, 95% CI 11, 32), and “possible exposure through sex or drug use” (18%, 95% CI 8, 38).
DISCUSSION
Our analysis of data from a nationally representative sample suggests low adherence to the 2006 recommendations for annual HIV testing among men with sexual risk for HIV infection in the years immediately following the recommendations. Fewer than half of HRH and MSM respondents had received HIV testing in the prior 12 months, with no significant change after the 2006 revised CDC HIV testing recommendations. Similarly, analysis of data from the 2003–2010 National Health and Nutrition Examination Survey (NHANES) showed that HIV testing did not change significantly among high-risk groups, including MSM, before and after the 2006 CDC guidelines, although testing among males increased modestly.11
Our findings differ from those reported in an analysis of 2008 and 2011 data from the National HIV Behavioral Surveillance System (NHBS), which found a significant increase in percentages of MSM tested in the prior 12 months, from 63% in 2008 to 67% in 2011.12,13 However, the differences in the percentage tested (3 percentage points between 2006–2008 and 2008–2010 in our analysis of NSFG data, and 4 -percentage points between 2008 and 2011 in the NHBS analysis) are not substantially different. Because of its larger sample size of MSM, the NHBS had stronger statistical power to detect modest differences in testing among MSM. One reason NHBS found higher rates of HIV testing among MSM is that, in contrast to NSFG, which was a household-based, nationally representative sample, NHBS used venue-based (e.g., bars, clubs, street locations) sampling methods in 21 U.S. cities to select for MSM participants.12,13 Compared with the exclusively urban, venue-visiting MSM surveyed in NHBS, NSFG respondents represent more of a general cross-section of MSM, including those residing in rural locations who may have been less likely to receive HIV testing because of less accepting social or sexual environments for MSM, concerns about loss of confidentiality, limited access to HIV testing, underestimation of their personal risk for HIV, or fear of testing positive.14–16 A few U.S. studies showed that people living in rural areas are less likely than those living in urban areas to report prior HIV testing and, if HIV infected, more likely to be diagnosed late and to delay entry to HIV care.17–19 Additionally, given that 16% of MSM surveyed in NSFG identified as heterosexual or straight, a significant minority may not test annually out of concern about loss of confidentiality.20–22 Because NSFG uses a nationally representative sample, our results provide useful additional data to existing surveillance data to better understand HIV testing behaviors of MSM in the United States.
When we examined the use of health-care services and HIV testing to assess whether men at risk who utilized health-care services were more or less likely to have received HIV testing, we found that most HRH respondents and MSM had health insurance and approximately half had received a physical examination in the prior 12 months. However, among men who received a physical examination, fewer than half reported HIV testing during that period. Furthermore, among MSM, neither having health insurance nor having received a physical examination was associated with receiving HIV testing in the prior 12 months. Although our analysis did not address knowledge and practices of health-care providers, providers may be uncomfortable discussing sexual histories or HIV testing, may underestimate the sexual risk behaviors of men (especially MSM under their care), may not be aware of the revised 2006 guidelines for annual HIV screening, or may lack the time, funding, or trained staff to conduct HIV testing.20,23–26 On the other hand, our analysis found that testing or treatment for STIs other than HIV was associated with HIV testing in the prior 12 months for both HRH and MSM respondents. Our findings suggest that additional community-based testing and heightened efforts to conduct routine testing in health-care settings might have increased annual HIV testing for MSM in the years following the 2006 recommendations. For example, strengthening and expanding community-based outreach and testing might have helped increase testing among the 48% of MSM who did not receive a physical examination in the prior 12 months. Additional programs to train providers to discuss sexual histories and to educate both providers and patients on the 2006 recommendations might have helped increase annual HIV testing for the 28% of MSM who utilized health-care services but did not receive HIV testing.
To help promote HIV testing after dissemination of the revised CDC recommendations, CDC's expanded HIV testing initiatives included the Expanded HIV Testing for Populations Disproportionately Affected program for 25 jurisdictions from 2007 to 2010 and for 30 jurisdictions from 2010 to 2012, and the Comprehensive HIV Prevention Programs for Health Departments for 36 jurisdictions from 2012 to 2016. Programs to increase annual HIV testing for men at high risk for acquisition of HIV infection, especially MSM, who account for more than half of all new HIV infections in the United States each year, are critically needed, because testing is the key first step in HIV treatment and prevention.27,28
Limitations
Limitations of this study included a self-reported history of HIV testing, which may have been subject to recall errors, particularly in the setting of opt-out testing, in which HIV testing is performed routinely (i.e., without pretest counseling and informed consent) unless a patient explicitly refuses. However, it is unlikely that opt-out testing was a prevalent practice during the years of the survey: CDC recommended opt-out testing in medical settings in 2006, and evidence suggests low uptake of opt-out HIV testing in the first few years after the revised recommendations.5,29,30
Additionally, because HIV status was not assessed by NSFG, the sample may have included respondents who had previously tested positive for HIV, some of whom may not have needed to be tested in the prior 12 months. Had we been able to remove these previously diagnosed people with no need for testing from the denominator, our estimate of the proportion of undiagnosed people tested for HIV in the prior 12 months might have been slightly higher. The impact of this limitation was likely minimal, because the prevalence of HIV infection in the general population is approximately 0.5% and approximately 9% among MSM.31–34
We did not define injection drug use as a risk behavior in this analysis because our objective was to evaluate HIV testing in relation to sexual risk behavior. Additionally, the percentage reporting injection drug use in the prior 12 months was minimal (1% among HRH respondents and 2% among MSM) and unlikely to be a substantial confounding factor. Our findings for MSM should be cautiously interpreted, because the number of MSM surveyed by NSFG was relatively small; the surveys did not oversample MSM, and the power to detect differences among MSM across years was low. Finally, the survey data in our analysis preceded legislative measures enacted since 2010 to promote opt-out testing (e.g., the Veterans Health Administration eliminated the requirement for written informed consent in August 2009)35 and preceded the enactment of the Affordable Care Act,36 which mandates that health insurance plans cover HIV screening. Thus, more recent surveys may find increased uptake of annual HIV testing among MSM and other people at risk for HIV infection.
CONCLUSION
Our analysis demonstrated that a low proportion of men at high risk for HIV infection received HIV testing in the prior year, and testing did not increase appreciably from 2002 to 2006–2010, despite CDC's expanded HIV testing recommendations in 2006. More provider training and patient education might have helped increase uptake of annual HIV testing for MSM who utilized routine medical services, and might still help today. Interventions are critically needed to increase uptake of annual HIV testing for men at risk, especially for MSM, in both nonmedical and health-care settings.
Footnotes
The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The use of the public dataset from the National Survey of Family Growth did not require institutional review board review or an exemption determination.
REFERENCES
- 1.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. Estimated HIV incidence in the United States, 2006–2009. PloS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (US) Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report, 2012; vol. 17 [cited 2014 Jul 12] Available from: http://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf.
- 3.Biedrzycki P, Vergeront J, Gasiorowicz M, Bertolli J, Oster A, Spikes PS, et al. Increase in newly diagnosed HIV infections among young black men who have sex with men—Milwaukee County, Wisconsin, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(4):99–102. [PubMed] [Google Scholar]
- 4.Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV—United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Morb Mortal Wkly Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (US) National Survey of Family Growth [cited 2014 Jul 12] Available from: http://www.cdc.gov/nchs/nsfg.htm.
- 7.Lepkowski JM, Mosher WD, Davis KE, Groves RM, Van Hoewyk J. The 2006–2010 National Survey of Family Growth: sample design and analysis of a continuous survey. Vital Health Stat. 2010;2(150) [PubMed] [Google Scholar]
- 8.Lepkowski JM, Mosher WD, Davis KE, Groves RM, van Hoewyk J, Willem J. National Survey of Family Growth, cycle 6: sample design, weighting, imputation, and variance estimation. Vital Health Stat. 2006;2(142) [PubMed] [Google Scholar]
- 9.National Center for Health Statistics (US) National Survey of Family Growth 2006–2010. NSFG user's guide appendix ec: male file recode specifications [cited 2014 Jul 12] Available from: http://www.cdc.gov/nchs/data/nsfg/NSFG_2006-2010_UG_app3c_malerecodespecs.pdf.
- 10.RTI International. Research Triangle Park (NC): RTI International; 2012. SUDAAN®: Version 10. [Google Scholar]
- 11.Woodring JV, Kruszon-Moran D, Oster AM, McQuillan GM. Did CDC's 2006 revised HIV testing recommendations make a difference? Evaluation of HIV testing in the US household population, 2003–2010. J Acquir Immun Defic Syndr. 2014;67:331–40. doi: 10.1097/QAI.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oster AM, Miles IW, Le BC, DiNenno EA, Wiegand RE, Heffelfinger JD, et al. HIV testing among men who have sex with men—21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep. 2011;60(21):694–9. [PubMed] [Google Scholar]
- 13.Cooley LA, Oster AM, Rose CE, Wejnert C, Le BC, Paz-Bailey G. Increases in HIV testing among men who have sex with men—National HIV Behavioral Surveillance System, 20 U.S. metropolitan statistical areas, 2008 and 2011. PloS One. 2014;9:e104162. doi: 10.1371/journal.pone.0104162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams ML, Bowen AM, Horvath KJ. The social/sexual environment of gay men residing in a rural frontier state: implications for the development of HIV prevention programs. J Rural Health. 2005;21:48–55. doi: 10.1111/j.1748-0361.2005.tb00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hormes JM, Theall KP. Accessibility and effectiveness of sources of information about HIV/AIDS in a rural population. South Med J. 2013;106:599–604. doi: 10.1097/SMJ.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 16.Harmon JL, Collins-Ogle M, Bartlett JA, Thompson J, Barroso J. Integrating routine HIV screening into a primary care setting in rural North Carolina. J Assoc Nurses AIDS Care. 2014;25:70–82. doi: 10.1016/j.jana.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohl M, Tate J, Duggal M, Skanderson M, Scotch M, Kaboli P, et al. Rural residence is associated with delayed care entry and increased mortality among veterans with human immunodeficiency virus infection. Med Care. 2010;48:1064–70. doi: 10.1097/MLR.0b013e3181ef60c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohl ME, Perencevich E. Frequency of human immunodeficiency virus (HIV) testing in urban vs. rural areas of the United States: results from a nationally-representative sample. BMC Public Health. 2011;11:681. doi: 10.1186/1471-2458-11-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trepka MJ, Fennie KP, Sheehan DM, Lutfi K, Maddox L, Lieb S. Late HIV diagnosis: differences by rural/urban residence, Florida, 2007–2011. AIDS Patient Care STDS. 2014;28:188–97. doi: 10.1089/apc.2013.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstein KT, Liu KL, Begier EM, Koblin B, Karpati A, Murrill C. Same-sex attraction disclosure to health care providers among New York City men who have sex with men: implications for HIV testing approaches. Arch Intern Med. 2008;168:1458–64. doi: 10.1001/archinte.168.13.1458. [DOI] [PubMed] [Google Scholar]
- 21.Mackellar DA, Hou SI, Whalen CC, Samuelsen K, Sanchez T, Smith A, et al. Reasons for not HIV testing, testing intentions, and potential use of an over-the-counter rapid HIV test in an Internet sample of men who have sex with men who have never tested for HIV. Sex Transm Dis. 2011;38:419–28. doi: 10.1097/OLQ.0b013e31820369dd. [DOI] [PubMed] [Google Scholar]
- 22.White BL, Walsh J, Rayasam S, Pathman DE, Adimora AA, Golin CE. What makes me screen for HIV? Perceived barriers and facilitators to conducting recommended routine HIV testing among primary care physicians in the southeastern United States. J Int Assoc Provid AIDS Care. 2015;14:127–35. doi: 10.1177/2325957414524025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson CV, Mimiaga MJ, Reisner SL, VanDerwarker R, Mayer KH. Barriers and facilitators to routine HIV testing: perceptions from Massachusetts Community Health Center personnel. AIDS Patient Care STDS. 2011;25:647–55. doi: 10.1089/apc.2011.0180. [DOI] [PubMed] [Google Scholar]
- 24.Jain CL, Wyatt CM, Burke R, Sepkowitz K, Begier EM. Knowledge of the Centers for Disease Control and Prevention's 2006 routine HIV testing recommendations among New York City internal medicine residents. AIDS Patient Care STDS. 2009;23:167–76. doi: 10.1089/apc.2008.0130. [DOI] [PubMed] [Google Scholar]
- 25.Mohajer MA, Lyons M, King E, Pratt J, Fichtenbaum CJ. Internal medicine and emergency medicine physicians lack accurate knowledge of current CDC HIV testing recommendations and infrequently offer HIV testing. J Int Assoc Physicians AIDS Care (Chic) 2012;11:101–8. doi: 10.1177/1545109711430165. [DOI] [PubMed] [Google Scholar]
- 26.Shirreffs A, Lee DP, Henry J, Golden MR, Stekler JD. Understanding barriers to routine HIV screening: knowledge, attitudes, and practices of healthcare providers in King County, Washington. PloS One. 2012;7:e44417. doi: 10.1371/journal.pone.0044417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frieden TR, Das-Douglas M, Kellerman SE, Henning KJ. Applying public health principles to the HIV epidemic. N Engl J Med. 2005;353:2397–402. doi: 10.1056/NEJMsb053133. [DOI] [PubMed] [Google Scholar]
- 29.Berg LJ, Delgado MK, Ginde AA, Montoy JC, Bendavid E, Camargo CA., Jr Characteristics of U.S. emergency departments that offer routine human immunodeficiency virus screening. Acad Emerg Med. 2012;19:894–900. doi: 10.1111/j.1553-2712.2012.01401.x. [DOI] [PubMed] [Google Scholar]
- 30.Hoover JB, Tao G, Heffelfinger JD. Monitoring HIV testing at visits to emergency departments in the United States: very-low rate of HIV testing. J Acquir Immune Defic Syndr. 2013;62:90–4. doi: 10.1097/QAI.0b013e3182742933. [DOI] [PubMed] [Google Scholar]
- 31.McQuillan GM, Kruszon-Moran D. HIV infection in the United States household population aged 18–49 years: results from 1999–2006. NCHS Data Brief. 2008;(4):1–8. [PubMed] [Google Scholar]
- 32.McQuillan GM, Kruszon-Moran D, Granade T, Feldman JW. Seroprevalence of human immunodeficiency virus in the US household population aged 18–49 years: the National Health and Nutrition Examination Surveys, 1999–2006. J Acquir Immun Defic Syndr. 2010;53:117–23. doi: 10.1097/QAI.0b013e3181b3a8e3. [DOI] [PubMed] [Google Scholar]
- 33.Xu F, Sternberg MR, Markowitz LE. Men who have sex with men in the United States: demographic and behavioral characteristics and prevalence of HIV and HSV-2 infection: results from National Health and Nutrition Examination Survey 2001–2006. Sex Transm Dis. 2010;37:399–405. doi: 10.1097/OLQ.0b013e3181ce122b. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (US) HIV surveillance report, 2010 [cited 2014 Jul 13] Available from: http://www.cdc.gov/hiv/surveillance/resources/reports.
- 35.Department of Veterans Affairs (US) Informed consent for clinical treatments and procedures. VHA handbook 1004.01. 2009 [cited 2015 Dec 3] Available from: http://www1.va.gov/vhapublications/viewpublication.asp?pub_ID=2055.
- 36. 42 U.S.C. §18001 et seq. (2010)