Abstract
Background and Purpose
Silent brain infarction (SBI) on magnetic resonance imaging (MRI) has been proposed as a subclinical risk marker for future symptomatic stroke. We performed a systematic review and meta-analysis to summarize the association between MRI-defined SBI and future stroke risk.
Methods
We searched the medical literature to identify cohort studies involving adults with MRI detection of SBI who were subsequently followed for incident clinically-defined stroke. Study data and quality assessment were recorded in duplicate with disagreements in data extraction resolved by a third reader. Strength association between MRI detected SBI and future symptomatic stroke measured by a hazard ratio (HR).
Results
The meta-analysis included 13 studies (14,764 subjects) with a mean follow-up ranging from 25.7 to 174 months. SBI predicted the occurrence of stroke with a random effects crude relative risk of 2.94 (95% CI 2.24–3.86, P<0.001; Q=39.65, P<0.001). In the eight studies of 10,427 subjects providing HR adjusted for cardiovascular risk factors, SBI was an independent predictor of incident stroke (HR 2.08 [95% CI 1.69–2.56, P<0.001]; Q=8.99, P=0.25). In a subgroup analysis pooling 9,483 stroke-free individuals from large population-based studies, SBI was present in ~18% of participants and remained a strong predictor of future stroke (HR 2.06 [95% CI 1.64–2.59], p<0.01).
Conclusions
SBI is present in approximately one in five stroke-free older adults and is associated with a 2-fold increased risk of future stroke. Future studies of in-depth stroke risk evaluations and intensive prevention measures are warranted in patients with clinically unrecognized radiologically evident brain infarctions.
Keywords: imaging, infarct or infarction, brain infarction, risk factor, asymptomatic
Introduction
Stroke is the second-leading cause of death worldwide and a leading cause of disability 1. Identifying subclinical stroke risk factors may allow for early and potentially more effective stroke prevention measures. One such potential stroke risk factor, silent brain infarction (SBI), is an increasingly detected abnormality with modern MRI techniques. Initially described by Fisher 2, mounting epidemiologic evidence has shown that SBI can contribute to cognitive dysfunction 3, dementia 4, and increased overall mortality 5. However, the most direct potential sequela of SBI is symptomatic stroke. Despite similar pathophysiologic pathways, it is unknown if SBI and stroke have identical mechanisms given the heterogeneity of SBI in terms of location, mechanism, and underlying risk factors. Furthermore, directly studying the mechanisms of SBI is challenging given that these lesions are incidentally detected and almost always of unknown age. The small size of many SBI, their non-eloquent location, and chronic ischemic preconditioning are attractive theories to explain why certain cerebrovascular events do not manifest clinically 6.
Understanding the extent to which MRI-defined SBI predicts the occurrence of stroke is important because if these lesions strongly predict stroke, then their detection might warrant the initiation of a thorough stroke evaluation and more aggressive medical management of stroke risk factors. Also, a more precise quantification of the relative risk of stroke in the presence of an SBI may allow the adoption of SBI as a surrogate endpoint in clinical trials and thereby potentially reduce the length and expense of trials that would otherwise have relied on stroke as a primary outcome measure 7. Although there have been many individual studies describing the predictive value of SBI, relying on single study samples results in wide confidence intervals for risk estimates. For these reasons, we performed a systematic review and meta-analysis evaluating whether MRI detection of SBI is a predictor of subsequent stroke.
Methods
We performed this study following the guidelines recommended by the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) group 8 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement 9. We also prospectively registered our study protocol on PROSPERO (CRD42014007016).
Data Sources and Searches
A research librarian performed comprehensive searches from database inception to April 3, 2015 in Ovid MEDLINE, Ovid Embase, and the Cochrane Library. An English language filter was not applied. The first search was conducted in Ovid MEDLINE. Subject headings and keywords were adapted for the other databases. Additional records were identified by employing the “Cited by” and “View references” features in Scopus on April 17, 2015 (see Supplemental Methods for search methodology details).
Study Selection
We included only studies with MRI characterization of SBI in subjects subsequently followed for development of future clinically overt stroke. Specific inclusion criteria were: (1) studies of adult subjects (>18 years of age); (2) at least 100 subjects; (3) MRI determination of SBI as lesions measuring 3 mm or greater with differentiation of SBI from leukoaraiosis; (4) mean follow-up >12 months after brain MRI; (5) clinical ascertainment of stroke during follow-up. In cases where the methods for detecting SBI or outcome data were not clear in the manuscript, we attempted to contact the corresponding author for additional details. Furthermore, if test data from a cohort was published more than once, only the paper with the largest person-years of follow up was included to minimize analysis of duplicate or overlapping samples.
Data Extraction and Quality Assessment
A single investigator read the title and abstract of all references produced by our database search. After preliminary articles were shortlisted as potentially eligible, the papers were read in their entirety by two readers to determine eligibility, with disagreements resolved by consensus. Data was extracted from articles meeting the inclusion criteria by a team of 2 readers using a prespecified data collection template, with disagreements in data extraction resolved by a third tie-breaking reader. Study characteristics that were extracted included study first author; study design (prospective or not); major study inclusion criteria; country of the study; total number of subjects; mean follow-up; SBI status at baseline; and the prevalence of stroke risk factors in the studied populations, including age, sex, hypertension, diabetes, chronic kidney disease, atrial fibrillation, coronary artery disease, hyperlipidemia, and smoking history. Additional study extraction focused on SBI status and stroke outcomes during follow up, including the number of subjects with and without SBI at baseline; the number of strokes (all strokes, ischemic strokes, and hemorrhagic strokes) that occurred during follow up in both SBI-positive and SBI-negative groups; the covariate-adjusted relative risk measure (hazard ratio [HR] or odds ratio [OR]) relating SBI and future stroke; and the specific vascular risk factors for which the relative risk measures were adjusted in multivariate analyses. Data were also extracted about the imaging definitions of SBI in each study, including MRI magnet field strength; MRI slice section thickness; MRI section gap; SBI size classification; SBI MRI signal characteristics; and means of differentiating SBI from perivascular spaces.
Since no standardized tool exists to assess the risk of bias in observational studies, we adapted the bias assessment criteria adapted from recently published meta-analyses focused on SBI in patients with atrial fibrillation 10 and imaging biomarkers of stroke risk 11, 12. A total of 11 questions were generated to evaluate potential selection, detection, misclassification, reporting, attrition, and confounding bias 13 (see Supplemental Table I). Risks of bias questions were assessed by two readers, with disagreements in assessment resolved by a third tie-breaking evaluator.
Data Synthesis and Analysis
We estimated the prevalence of SBI in the included studies as well as the total person-years of follow up in each study. Meta-analyses of the individual study crude relative risks (i.e., relative risk of stroke in the presence of an SBI) and covariate-adjusted hazard ratios were conducted with the use of StatsDirect statistical software (Version 2.7.9; 7/9/2012 StatsDirect Ltd, Cheshire, England). Each pooled risk ratio was calculated using a random effects (DerSimonian-Laird) model 14 and forest plots were generated to display the individual study risk ratios and the pooled risk ratio. We performed all analyses using a random effects model based on the conservative assumption that included studies did not have exactly the same effect size given the potential for heterogeneity between studies in terms of sample size, subject characteristics, and testing methods. To assess the combinability of the risk ratios, we calculated the p-value from the Cochrane Q statistical heterogeneity test. The results of each study were expressed as a risk ratio with a 95% confidence interval. For each meta-analysis, the presence of publication bias was evaluated through a Begg-Mazumdar rank-correlation test. All p-values <0.05 were considered statistically significant.
We performed subgroup analyses limited to the following patient samples: (1) stroke-free participants recruited from population-based or community-dwelling samples; (2) stroke-free participants in studies with inclusion criteria including at least 1 known stroke risk factor (such as hypertension, diabetes, atrial fibrillation, coronary artery disease, hyperlipidemia, or chronic kidney disease); (3) patients with a history of documented prior stroke followed for recurrent stroke.
Role of Funding Source
This study received no external funding.
Results
Study Selection
We screened a total of 1654 titles and abstracts from which we identified 13 manuscripts that met all inclusion criteria for the systematic review 15–27. One study 27 that met our inclusion criteria was published after our search was completed during the data extraction phase but was included as it provided follow-up cohort data for a manuscript initially included in our original literature search 28. Study selection steps are summarized in Supplemental Figure I. A crude relative risk expressing the association between SBI and incident stroke was calculable in all 13 studies from raw data. The most commonly provided adjusted-risk metric was the HR, with 8 studies 15, 17, 19, 20, 22, 23, 25, 27 providing covariate-adjusted HRs of SBI as a predictor of incident stroke. One study provided a covariate-adjusted RR of SBI as a predictor of stroke 16 and 2 studies 18, 24 provided covariate-adjusted or age- and sex-matched ORs.
Qualitative Study Characteristics
Of the 13 articles meeting inclusion criteria (Supplemental Table II), all except one 22 were prospective studies. One study was conducted as an international multicenter study 24, five were in Japan 16–18, 21, 26, four in the United States 15, 19, 25, 27, and one each in Finland 20, Canada 22, and the Netherlands 23. Eleven studies 15–19, 21, 23–27 included cohorts with mean or median ages above 50 years (range 56.0 to 75.2), while 2 studies were focused on evaluation of stroke in younger patients with mean ages of 39.4 22 and 40.0 years 20. Subjects in all studies were followed for at least 25.7 months (range 25.7–174 months) for ascertainment of clinically-defined stroke.
Definitions of SBI and Stroke Outcomes
All studies defined SBI as lesions ≥3 mm that were hyperintense on T2-weighted images (see Supplemental Table III for details). One study provided additional separate analysis of putative vascular lesions <3 mm that were too small to definitely characterize as SBI 27. All studies described the use of additional MRI pulse sequences to differentiate SBI from adjacent white matter leukoaraiosis, with most studies relying on T1-weighted hypointensity as a feature suggestive of SBI rather than nonspecific white matter leukoaraiosis. There were variable methods used to distinguish SBI from dilated perivascular spaces including the presence of hyperintensity on T2-weighted fluid attenuated inversion recovery images 18, 20–22, 24, 26, proton density hyperintensity 15, 18, 27, and lesion morphology/location 19, 25. MRI magnet field strengths varied from 0.2 Tesla to 3.0 Tesla, with most studies using a 1.5 Tesla scanner. Most studies used clinically-based definitions of ischemic stroke based on a focal neurologic deficit lasting longer than 24 hours and without evidence of hemorrhage on brain imaging (see Supplemental Table IV for details). A majority of studies used a combination of hospital and outpatient medical records as well as telephone interviews to ascertain stroke outcome events.
Association between SBI and Future Stroke: Crude Relative Risks
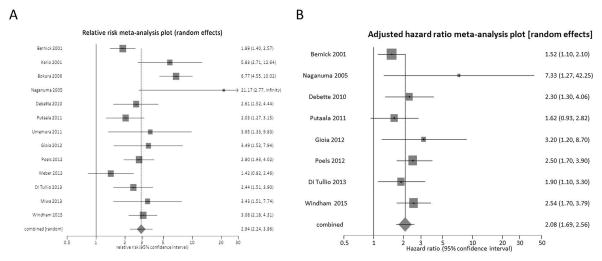
We were able to obtain sufficient raw data to calculate a crude relative risk for future stroke in the presence of SBI for each of the included 13 studies (Supplemental Table V). In this analysis of 13 studies (14,764 subjects), there was a mean follow-up ranging from 25.7 to 174 months (mean ~76.0), yielding a total of 108,360 person-years of follow-up. There was statistically significant heterogeneity (Q=39.65, P<0.001) but no significant publication bias (Kendall’s tau score=0.26, P=0.25) present in this analysis. We found a significant positive relationship between the presence of SBI and the risk of stroke with a random effects crude RR of 2.94 (95% CI 2.24–3.86, P<0.001, Figure 1A). Of the total study sample, 3007 subjects (20.4%) had a positive MRI test for SBI, whereas 11,757 (79.6%) had a negative test for SBI.
Figure 1.
Forest plots of the association between MRI-determined silent brain infarction and future stroke in all included studies15–27. Meta-analysis calculated using a random-effects mode, with crude relative risks pooled in panel A and adjusted-hazard ratios pooled in panel B. Squares represent point estimates for the effect sizes. The size of the squares is proportional to the inverse of the variance of the estimate. Diamond represents the pooled estimate and the horizontal lines represent the 95% confidence interval.
Association between SBI and Future Stroke: Adjusted Hazard Ratios
Eight of the included studies provided adjusted hazard ratios describing the strength of association between SBI and future stroke. In the analysis of these 8 studies (10,427 subjects), there was a mean follow-up ranging from 25.7 to 174 months (mean ~88.8), yielding a total of 85,001 person-years of follow-up. There was neither significant heterogeneity (Q=8.99, P=0.25) nor significant publication bias (Kendall’s tau score=0.29, P=0.40) present in this analysis. We found a significant positive relationship between the presence of SBI and the risk of stroke with a random effects adjusted HR of 2.08 (95% CI 1.69–2.56, P<0.001 Figure 1B). Of the total study sample, 1962 subjects (18.8%) had a positive MRI test for SBI, whereas 8465 (81.2%) had a negative test for SBI.
Subgroup Meta-Analysis Results
A statistically significant random-effects crude RR (Table 1) and adjusted-HR (Table 2) was preserved in the following subgroup analyses: (1) stroke-free patients recruited from population-based or community-dwelling studies; (2) stroke-free participants in studies with inclusion criteria requiring at least one known stroke risk factor, including hypertension 16, chronic renal disease requiring hemodialysis 17, diabetes mellitus 21, or greater than one cardiovascular risk factor 26; (3) patients presenting with first time symptomatic acute stroke followed for recurrent stroke in whom clinically silent brain infarction was evident on baseline imaging performed for the initial acute stroke diagnostic evaluation. Of the studies providing an adjusted HR, the risk of stroke in the presence of SBI conferred an approximately 2-fold increased risk of future stroke in both stroke-free patients (HR 2.06, 95% CI 1.64–2.59) and in patients with prior stroke (HR 2.00, 95% CI 1.08–3.71). Based on the 5 studies 15, 19, 23, 25, 27 with adjusted HRs derived from population-based studies of 9,483 stroke-free individuals with mean or median study ages between 62 and 76, the prevalence of SBI was 18.7%.
Table 1.
Meta-Analysis Summary for Subgroups Analyses of Crude Relative Risk of Stroke in Patients with MRI defined Silent Brain Infarct
| Test of Heterogeneity | Publication bias | |||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | No. of Studies | Total person-years of follow-up | SBI Prevalence | Pooled RR (95% CI) | RR P-value | Cochran’s Q P-value | Kendall’s τ | P-value |
| Stroke-free individuals recruited from population-based or community-dwelling samples15, 18, 19, 23, 25, 28 | 6 | 95,449.2 | 17.7% | 2.81 (2.40–3.28) | <0.01 | <0.01 | 0.07 | 0.99 |
| Stroke-free individuals with at least 1 known stroke risk factor16, 17, 21, 26 | 4 | 5,813.4 | 38.1% | 4.54 (2.76–7.45) | <0.01 | 0.50 | 0.33 | 0.75 |
| Known history of stroke followed for stroke recurrence20, 22, 24 | 3 | 7097.6 | 27.5% | 1.99 (1.30–3.04) | <0.01 | 0.21 | ||
Publication bias test only performed on greater than 3 studies
CI indicates confidence interval; RR, relative risk
Table 2.
Meta-Analysis Summary for Subgroups Analyses of Risk Factor Adjust-Hazard Ratios of Stroke in Patients with MRI defined Silent Brain Infarct CI indicates confidence interval; HR, hazard ratio
| Test of Heterogeneity | Publication bias | |||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | No. of Studies | Total person-years of follow-up | SBI Prevalence | Pooled HR (95% CI) | HR P-value | Cochran’s Q P-value | Kendall’s τ | P-value |
| Stroke-free individuals recruited from population-based or community-dwelling samples15, 19, 23, 25, 27 | 5 | 78,540 | 18.7% | 2.06 (1.64–2.59) | <0.01 | 0.24 | 0 | 0.82 |
| Known history of stroke followed for stroke recurrence20, 22 | 2 | 6062.6 | 16.2% | 2.00 (1.08,3.71) | 0.03 | 0.24 | ||
Publication bias test only performed on greater than 3 studies
Assessment of the Quality of the Included Studies
The results from the quality assessment questionnaire are shown in Supplemental Table VI. All included studies involved more than one investigator independently evaluating MRI studies for the presence of SBI. In all but two studies 16, 17, perivascular spaces were systematically differentiated from SBI. In seven of the 13 studies 15, 18, 19, 23–26, the risk of selection bias was minimized by either random selection of subjects or recruitment from a community-dwelling population. Six studies explicitly reported that investigators were blinded to SBI status when determining stroke outcomes 15, 19, 20, 23, 25, 27. All but two studies 21, 26 corrected for covariate risk factors in their analysis of the association between SBI and incident stroke and provided an adjusted disease association measure such as a HR or OR. Five studies 15, 19, 23, 25, 27 had the lowest overall risk of bias, with each of these studies demonstrating low potential for bias in all questions but one. All five of these studies were of stroke-free individuals recruited from large population-based studies including the Cardiovascular Health Study (CHS) 15, the Rotterdam Scan Study 23, the Framingham Offspring Cohort Study 19, the Northern Manhattan Study (NOMAS) 25, and the Atherosclerosis Risk in Communities Study (ARIC) 27.
Discussion
SBI is often incidentally detected in patients during MRI evaluation. In this systematic review and meta-analysis of studies involving over 14,000 subjects and over 100,000 person-years of follow-up, we found that the presence of SBI conferred an approximately 3-fold increased risk of subsequent stroke. After adjustment for potentially confounding vascular risk factors, the presence of an SBI was associated with an approximately 2-fold higher risk of future stroke. This approximate doubling of the independent risk of incident stroke was seen in both stroke-free patients and patients with prior stroke. Approximately one in five stroke-free individuals in these studies had SBI.
Our results are particularly applicable to stroke-free, community-dwelling individuals over 60 years of age who are incidentally found to have an SBI on brain MRI. In our subgroup analysis of five large, diverse population-based studies (CHS, ARIC, NOMAS, Rotterdam Scan Study, and the Framingham Offspring Cohort Study), the presence of an SBI conferred a ~2-fold increase in incident stroke risk after adjustment for vascular risk factors. These five studies had the lowest risk of bias in our assessment and pooled together over 9,400 subjects totaling ~78,000 person-years of follow-up, thereby increasing our confidence in the conclusion from this analysis.
Few guidelines exist on how to manage patients with incidentally found SBI. In contrast, professional societies publish detailed guidelines on secondary stroke prevention in patients with clinically overt stroke 29. Given that the likelihood of a brain lesion presenting clinically depends more on the eloquence of the brain region affected rather than the underlying pathogenesis of the lesion 30, 31, the distinction between silent and overt brain ischemia may need re-examination. In this meta-analysis, we found that SBI confers a similar relative degree of risk of future stroke as a prior history of clinically overt stroke in certain settings. In patients with atrial fibrillation, for example, a prior history of stroke is associated with a HR of 2.3 for recurrent stroke 32, comparable to the magnitude of risk we determined in our meta-analysis for first-time symptomatic stroke after SBI. Such considerations raise the question of whether SBI should be considered as a manifestation of cerebrovascular disease that requires intensive evaluation and secondary prevention, as in clinically overt stroke, rather than simply being considered an incidental finding.
Our study has revealed some limitations about the MRI techniques used to detect SBI in the existing body of literature summarizing stroke risk after SBI. First, though there was general agreement on the MRI features of SBI, including size (≥3 mm, T2 hyperintensity distinguishable from leukoaraiosis), we found variability in the methods used to determine the presence of SBI, including MRI magnet field strength (ranging from 0.2T to 3.0T) and specific MRI sequences obtained for evaluation. Most of the included studies (12 out of 13) did not perform MRI on higher field strength 3T magnets, which are becoming increasingly common and are more likely to allow detection of small lesions. The prevalence and predictive value of SBI detected on these higher field MRI magnets requires additional study. Furthermore, the methods used to distinguish SBI from perivascular spaces, a potential mimic on imaging studies, was variably reported and raises the possibility that some perivascular spaces were misclassified as SBI. Such misclassification, however, may not significantly affect our study’s overall conclusions given recent data suggesting that perivascular spaces are a manifestation of cerebral small vessel disease 33 and that very small brain lesions <3 mm that are too small to definitely distinguish from small perivascular spaces are still predictive of incident stroke 27. Nonetheless, our study suggests that further standardization of SBI definitions would allow for the more widespread use of SBI as an imaging risk biomarker 6, 34.
Several additional limitations of our study are important to consider. First, we performed one of our meta-analyses using a crude RR because the covariate risk factors varied across studies. However, the relatively higher quality studies included in our analysis provided adjusted HRs, and though there were some differences in the specific risk factors for which each study performed statistical correction, it appears unlikely that these relatively minor inter-study differences impacted our overall conclusions. Second, given the variability in length of follow-up between studies, the existing data are not amenable to the calculation of absolute stroke rates. Third, there was variable reporting of stroke subtypes (ischemic versus hemorrhagic) in studies. It would seem likely that SBI would more strongly predict ischemic rather than hemorrhage stroke risk, though further work is warranted to investigate this issue. Fourth, it is unclear in a majority of studies, especially the large population-based studies, to what extent imaging confirmation of stroke outcomes occurred. A more standardized definition of stroke outcomes using modern imaging-based criteria would be helpful in future studies of SBI and stroke risk. Fifth, stroke prevention measures have substantially decreased the incidence of stroke 1 since many of the cohorts in this study were recruited (some more than 20 years ago), possibly limiting the validity of using these data to extrapolate stroke risk in present-day populations. Despite this reduction in absolute stroke risk in the general population, we believe that the longer-term trends in stroke incidence are unlikely to substantially affect our study’s main conclusions given that our focus was on relative risk. Furthermore, to our knowledge, there are no data to suggest that the recent reductions in stroke incidence would be different in patients with or without SBI. Finally, the RR and HR we determined in our meta-analyses are probably most applicable to older adults given that the majority of participants in these studies were middle-aged or elderly. Further studies are warranted to assess whether the future stroke risk conferred by SBI in young adults is substantially different compared to relatively older individuals.
In summary, our systematic review and meta-analysis suggests that SBI is significantly associated with an increased risk of symptomatic stroke. Our study specifically suggests that approximately one in five older, stroke-free adults may be harboring a SBI, which may in turn confer more than double the risk of future first-time stroke. These data indicate a need for future studies of in-depth stroke risk evaluations and intensive prevention measures, including lifestyle modification and proven medical therapies for stroke reduction, in patients with these commonly discovered brain lesions.
Supplementary Material
Acknowledgments
The authors wish to thank Drs. Jukka Putaala, Toshitaka Umemura, M. Arfan Ikram, Marileen L. Portegies, Beverly G. Windham, Michael E. Griswold, and Wanmei Wang for providing both unpublished data and clarification of published data used in this meta-analysis.
Sources of Funding
Dr. Wright reports grants support (NIH NS 29993) for the Northern Manhattan Study. Drs. Seshadri and Beiser report grant support (NIH NS017950 and AG0008122) for the Framingham Cohort Study.
Footnotes
Disclosures
None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Fisher CM. Lacunes: Small, deep cerebral infarcts. Neurology. 1965;15:774–784. doi: 10.1212/wnl.15.8.774. [DOI] [PubMed] [Google Scholar]
- 3.Longstreth WT, Jr, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr, O’Leary D, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: The cardiovascular health study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 4.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 5.Bokura H, Kobayashi S, Yamaguchi S, Iijima K, Nagai A, Toyoda G, et al. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: A prospective cohort study. J Stroke Cerebrovasc Dis. 2006;15:57–63. doi: 10.1016/j.jstrokecerebrovasdis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Fanning JP, Wesley AJ, Wong AA, Fraser JF. Emerging spectra of silent brain infarction. Stroke. 2014;45:3461–3471. doi: 10.1161/STROKEAHA.114.005919. [DOI] [PubMed] [Google Scholar]
- 7.Vickrey BG, Brott TG, Koroshetz WJ, et al. Stroke Research Priorities Meeting Steering C, the National Advisory Neurological D Stroke C. Research priority setting: A summary of the 2012 ninds stroke planning meeting report. Stroke. 2013;44:2338–2342. doi: 10.1161/STROKEAHA.113.001196. [DOI] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 10.Kalantarian S, Ay H, Gollub RL, Lee H, Retzepi K, Mansour M, et al. Association between atrial fibrillation and silent cerebral infarctions: A systematic review and meta-analysis. Ann Intern Med. 2014;161:650–658. doi: 10.7326/M14-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A, Kesavabhotla K, Baradaran H, Kamel H, Pandya A, Giambrone AE, et al. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: A systematic review and meta-analysis. Stroke. 2015;46:91–97. doi: 10.1161/STROKEAHA.114.006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, et al. Carotid plaque mri and stroke risk: A systematic review and meta-analysis. Stroke. 2013;44:3071–3077. doi: 10.1161/STROKEAHA.113.002551. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Se G. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated march 2011] The Cochrane Collaboration. 2011 [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Bernick C, Kuller L, Dulberg C, Longstreth WT, Jr, Manolio T, Beauchamp N, et al. Silent mri infarcts and the risk of future stroke: The cardiovascular health study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 16.Kario K, Shimada K, Schwartz JE, Matsuo T, Hoshide S, Pickering TG. Silent and clinically overt stroke in older japanese subjects with white-coat and sustained hypertension. J Am Coll Cardiol. 2001;38:238–245. doi: 10.1016/s0735-1097(01)01325-0. [DOI] [PubMed] [Google Scholar]
- 17.Naganuma T, Uchida J, Tsuchida K, Takemoto Y, Tatsumi S, Sugimura K, et al. Silent cerebral infarction predicts vascular events in hemodialysis patients. Kidney international. 2005;67:2434–2439. doi: 10.1111/j.1523-1755.2005.00351.x. [DOI] [PubMed] [Google Scholar]
- 18.Bokura H, Kobayashi S, Yamaguchi S, Iijima K, Nagai A, Toyoda G, et al. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: A prospective cohort study. J Stroke Cerebrovasc Dis. 2006;15:57–63. doi: 10.1016/j.jstrokecerebrovasdis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of mri markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The framingham offspring study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Putaala J, Haapaniemi E, Kurkinen M, Salonen O, Kaste M, Tatlisumak T. Silent brain infarcts, leukoaraiosis, and long-term prognosis in young ischemic stroke patients. Neurology. 2011;76:1742–1749. doi: 10.1212/WNL.0b013e31821a44ad. [DOI] [PubMed] [Google Scholar]
- 21.Umemura T, Kawamura T, Umegaki H, Mashita S, Kanai A, Sakakibara T, et al. Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small-vessel disease and cognitive impairment: A 6-year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry. 2011;82:1186–1194. doi: 10.1136/jnnp.2010.217380. [DOI] [PubMed] [Google Scholar]
- 22.Gioia LC, Tollard E, Dubuc V, Lanthier S, Deschaintre Y, Chagnon M, et al. Silent ischemic lesions in young adults with first stroke are associated with recurrent stroke. Neurology. 2012;79:1208–1214. doi: 10.1212/WNL.0b013e31826aacac. [DOI] [PubMed] [Google Scholar]
- 23.Poels MM, Steyerberg EW, Wieberdink RG, Hofman A, Koudstaal PJ, Ikram MA, et al. Assessment of cerebral small vessel disease predicts individual stroke risk. J Neurol Neurosurg Psychiatry. 2012;83:1174–1179. doi: 10.1136/jnnp-2012-302381. [DOI] [PubMed] [Google Scholar]
- 24.Weber R, Weimar C, Wanke I, Möller-Hartmann C, Gizewski ER, Blatchford J, et al. Risk of recurrent stroke in patients with silent brain infarction in the prevention regimen for effectively avoiding second strokes (profess) imaging substudy. Stroke. 2012;43:350–355. doi: 10.1161/STROKEAHA.111.631739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Tullio MR, Jin Z, Russo C, Elkind MS, Rundek T, Yoshita M, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol. 2013;62:35–41. doi: 10.1016/j.jacc.2013.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miwa K, Tanaka M, Okazaki S, Furukado S, Sakaguchi M, Mochizuki H, et al. Association between interleukin-6 levels and first-ever cerebrovascular events in patients with vascular risk factors. Arterioscler Thromb Vasc Biol. 2013;33:400–405. doi: 10.1161/ATVBAHA.112.300350. [DOI] [PubMed] [Google Scholar]
- 27.Windham BG, Deere B, Griswold ME, Wang W, Bezerra DC, Shibata D, et al. Small brain lesions and incident stroke and mortality: A cohort study. Ann Intern Med. 2015;163:22–31. doi: 10.7326/M14-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Windham BG, Griswold ME, Shibata D, Penman A, Catellier DJ, Mosley TH., Jr Covert neurological symptoms associated with silent infarcts from midlife to older age: The atherosclerosis risk in communities study. Stroke. 2012;43:1218–1223. doi: 10.1161/STROKEAHA.111.643379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 30.Leary MC, Saver JL. Annual incidence of first silent stroke in the united states: A preliminary estimate. Cerebrovasc Dis. 2003;16:280–285. doi: 10.1159/000071128. [DOI] [PubMed] [Google Scholar]
- 31.Brott T, Tomsick T, Feinberg W, Johnson C, Biller J, Broderick J, et al. Baseline silent cerebral infarction in the asymptomatic carotid atherosclerosis study. Stroke. 1994;25:1122–1129. doi: 10.1161/01.str.25.6.1122. [DOI] [PubMed] [Google Scholar]
- 32.Diener HC, Eikelboom J, Connolly SJ, Joyner CD, Hart RG, Lip GY, et al. Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: A predefined subgroup analysis from averroes, a randomised trial. Lancet Neurol. 2012;11:225–231. doi: 10.1016/S1474-4422(12)70017-0. [DOI] [PubMed] [Google Scholar]
- 33.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10:376–381. doi: 10.1111/ijs.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.