Abstract
Background
FGF23 levels are elevated in cardiopulmonary bypass (CPB)-associated acute kidney injury (AKI); however, it is unknown how much of the circulating FGF23 is intact and bioactive. Hypoxia may induce FGF23 production, yet its impact in humans is unknown. Pediatric cardiac surgery patients have both a high incidence of CPB-associated AKI and a high prevalence of chronic hypoxemia.
Methods
We assessed the effects of hypoxemia and CPB-associated AKI on C-terminal FGF23 (cFGF23) and intact FGF23 (iFGF23) levels in 32 pediatric cardiac surgery patients with normal eGFR. Plasma cFGF23 and iFGF23 were measured pre-operatively and serially post-operatively.
Results
Despite normal renal and ventricular function, pre-operative cFGF23 levels were high and elevated out of proportion to iFGF23 levels. Pre-operative oxygen saturation measurements correlated inversely with FGF23 levels. Pre-operative cFGF23 and oxygen saturation both predicted post-operative AKI. Post-operatively, cFGF23 and iFGF23 increased by 2 hours post-reperfusion; iFGF23 then returned to baseline, but cFGF23 remained elevated through 24 hours post-reperfusion. Group status (AKI vs. non-AKI) modified the effect of time on changes in iFGF23 levels, but not cFGF23 levels.
Conclusions
Pre-operative cFGF23 may predict CPB-associated kidney dysfunction. Changes over time in cFGF23 and iFGF23 levels post-CPB differ. Chronic hypoxemia may affect FGF23 production in humans.
Keywords: Fibroblast Growth Factor 23, Acute Kidney Injury, Chronic Hypoxemia, Pediatrics, Cardiac Surgery
INTRODUCTION
Fibroblast growth factor 23 (FGF23) is secreted by osteocytes; it induces phosphaturia and decreases renal 1α-hydroxylase expression (1), physiologically functioning as a homeostatic regulator of phosphate and as a calcitriol counterregulatory hormone. In the setting of chronic kidney disease (CKD), circulating FGF23 levels increase as renal function declines (2,3) and are associated with progressive kidney disease (4,5) and increased cardiovascular morbidity and mortality (5,6).
Although FGF23 has been studied extensively in CKD, much less is known about FGF23 in the context of acute kidney injury (AKI). In a murine model of AKI, circulating FGF23 levels increased as early as one hour after AKI induction, and did so independently of phosphate, calcitriol, and PTH (7). Studies in humans with AKI have demonstrated that FGF23 concentrations, determined by the C-terminal assay, are increased in both adult (7–9) and pediatric (10) patients, and that these elevated levels are associated with poor clinical outcomes (8,9). However, it is unknown whether the high FGF23 levels in AKI reflect the intact, bioactive molecule or C-terminal fragments.
Not only are FGF23 levels increased in the setting of AKI, but in a small case-control study of pediatric cardiac surgery patients, Ali et al demonstrated that those patients who developed post-operative AKI had higher pre-operative FGF23 levels (10). The etiology of these elevated pre-operative levels is unclear, but subclinical kidney dysfunction (10) or poor cardiac function (11) may have been contributory. Yet, other factors may also promote FGF23 production. Hypoxia has been shown to induce FGF23 expression in osteoblast-like cell lines (12,13) and increase circulating FGF23 levels in rats (13); however, its impact in humans is unknown.
The current study aims to: (i) determine whether or not chronic hypoxemia is associated with FGF23 levels in humans; (ii) assess the ability of pre-operative FGF23 levels to predict post-operative AKI; and (iii) to determine how measurements of C-terminal and intact FGF23 are affected by AKI. The study population is comprised of pediatric patients undergoing cardiopulmonary bypass (CPB), which is a population well-suited for our study, as cyanotic (hypoxemic) congenital heart disease is not uncommon in the pediatric cardiology population (14), and there is a high incidence of CPB-associated AKI after surgical correction (15).
METHODS
Study participants
Study participants were recruited from the UCLA Pediatric Cardiothoracic Surgery program. Patients aged 0 to 18 years scheduled to undergo cardiac surgery requiring CPB were eligible. An additional inclusion criterion was an estimated glomerular filtration rate (eGFR) of ≥60 ml/min/1.73m2 at the time of surgery. Outpatient subjects were enrolled at their pre-operative clinic visits, which for almost all subjects occurred within six days of their operative dates, and inpatient subjects were enrolled from the wards within two days prior to surgery.
Following inclusion, demographic data were obtained from the medical records. Preoperative oxygen saturation measurements via pulse oximetry were recorded from the pre-operative clinic visit note, the pre-operative anesthesia note, or the pre-operative inpatient note. In order to verify stable oxygen saturations over time, we also recorded oxygen saturations documented at least one week, and preferably one month, preoperatively, and compared these remote measurements to the pre-operative values.
Study procedures
Plasma and serum were collected pre-operatively and at 2, 6, 12, 24, and 48 hours post-reperfusion. For outpatients, pre-operative blood was collected on the day of the pre-operative clinic visit, which occurred at a median of 3 (1, 4) days prior to surgery. For inpatients, pre-operative blood was collected 1–2 days prior to the operative date.
Samples were placed on ice, centrifuged, aliquoted, and stored at −80°C. Pre-operative and post-operative plasma C-terminal and intact FGF23 were measured in duplicate using standard ELISA kits (Immutopics, San Clemente, CA). Pre-operative and postoperative serum parathyroid hormone (PTH) was assayed using a second-generation assay (Immutopics). Pre-operative plasma B-type natriuretic peptide (BNP) was measured using the Triage® Kit (Alere, Waltham, MA).
As part of routine pre-operative care, serum creatinine, phosphate, and calcium were measured prior to surgery. Post-operatively, in the Cardiothoracic Intensive Care Unit, serum creatinine, phosphate, and calcium were measured daily. AKI was defined as an increase in serum creatinine of at least 50% from pre-operative levels, as established by the Acute Kidney Injury Network (16), occurring within 48 hours of surgery. A large study of pediatric cardiac surgery patients demonstrated that most patients who develop AKI, defined as a 50% increase in creatinine, did so within 24 hours of surgery, and almost all did so within 48 hours of surgery (15). As our serum creatinine measurements were determined by the alkaline picrate method (Jaffe method), eGFR was calculated by the original Schwartz equation (17,18). All pre-operative echocardiograms were interpreted by the same pediatric cardiologist.
Statistical analysis
Data are reported as means and standard errors of the means (SEM), or medians and interquartile ranges (IQR), as was suitable for the data distributions. Continuous or ordinal pre-operative variables were compared between the AKI and non-AKI groups using the Student t-test or the Mann-Whitney U-test, according to the data distributions. Categorical variables between groups were compared using the Fisher exact test. Comparisons were two-tailed, with p<0.05 considered significant. Correlations between pre-operative oxygen saturations and FGF23 levels were analyzed using Pearson product-moment correlation coefficients.
C-terminal FGF23 (cFGF23) levels vary physiologically with age in the pediatric population, decreasing from a median value of 105 (75, 153) RU/ml in infants to median values in the 60–80 RU/ml range for children and adolescents (19). To adjust for age, we calculated pre-operative age-related cFGF23 standard deviation scores (SDS), utilizing cFGF23 data obtained from a large, age-specific cohort of healthy children, determined by the Immutopics assay, as described by Fischer et al (19). The SDS, also known as a z-score, is defined as the number of standard deviations an observation is above the mean. Phosphate levels also vary with age, and published data was used to calculate pre-operative age-adjusted phosphate SDS (20). As age-adjusted normal values do not exist for intact FGF23 (iFGF23), the absolute value of this parameter was used in all analyses. For comparison, we also measured iFGF23 and cFGF23 levels, using the same Immutopics assays, in an institutional cohort of 47 healthy children.
Potential pre-operative predictors of post-operative kidney dysfunction were assessed for two outcomes: AKI vs. no AKI (dichotomous outcome), and maximum percent decrease in eGFR (continuous outcome). Receiver operating characteristic (ROC) curve analysis and multiple logistic regression modeling were used to assess the ability of pre-operative factors to predict post-operative CPB-associated AKI. Multiple linear regression modeling was used to assess the ability of pre-operative factors to predict post-operative percent decrease in eGFR. Regression models included FGF23 values or oxygen saturation, age, and CPB time; age and CPB time are known risk factors for CPB-associated AKI (15).
Linear mixed effects modeling was utilized to examine changes in FGF23 levels over time and the effects of group (AKI vs. non-AKI) on FGF23 levels. Both cFGF23 and iFGF23 values were log-transformed prior to modeling. The models included terms for time, group, and group by time interaction. To assess the effects on FGF23 levels through different time points, different models were created, sequentially truncating the data set by removing the last time point. As such, the full model included all data through the 48 hour time point, the first truncated model included all data through the 24 hour time point, the second truncated model included all data through the 12 hour time point, etc. Linear mixed effects modeling was also used to assess changes in phosphate, calcium, and PTH over time.
All statistical analysis was performed using SigmaPlot 11.0 (San Jose, CA) software, with the exception of linear mixed effects modeling, which was performed using SAS (Cary, NC) software.
RESULTS
Pre-operative patient characteristics
Table 1 shows pre-operative characteristics of the 32 study patients. The median age was 37 (10, 80) months, and mean eGFR was 141 ± 42 ml/min/1.73m2. cFGF23 levels were elevated compared to controls (295 [109, 547] vs. 59 [39, 76] RU/ml, p<0.001). The mean age-adjusted cFGF23 standard deviation score (SDS) was 2.28 ± 0.34. iFGF23 levels, however, were similar to controls (56 [38, 72] vs. 50 [35, 87] pg/ml, p=0.84).
Table 1.
Patient characteristics by group (AKI vs. non-AKI). All variables are preoperative except for CPB time and AXC time
| All | AKI Patients | Non-AKI Patients |
p value (AKI vs. Non-AKI) |
|
|---|---|---|---|---|
| Number | 32 | 20 | 12 | n/a |
| Sex (% male) | 56 | 60 | 50 | 0.72 |
| Age (months) | 37 (10, 80) | 15 (7, 49) | 91 (41, 146) | 0.005 |
| Weight SDS | −1.49 ± 0.33 | −1.36 ± 0.35 | −1.70 ± 0.67 | 0.63 |
| Height SDS | −1.22 ± 0.28 | −1.22 ± 0.36 | −1.22 ± 0.45 | 1.00 |
| Oxygen Saturation (%) | 96 (80, 99) | 87 (74, 98) | 99 (96, 100) | 0.01 |
| Hemoglobin (g/dl) | 13.1 (12.1, 16.3) | 15.0 (12.5, 17.3) | 12.7 (11.9, 13.2) | 0.07 |
| BNP (pg/ml) | <20 (<20<20) | <20 (<20, <20) | 22.5 (<20, 74) | n/a |
| RACHS-1 Score (21) | 2 (2, 3) | 2 (2, 3) | 2 (2, 2) | 0.07 |
| CPB Time (min) | 44 (34, 68) | 46 (35, 65) | 42 (33, 76) | 0.61 |
| AXC Time (min) | 21 (16, 45) | 22 (9, 41) | 21 (17, 51) | 0.98 |
| Diuretic Use (%) | 25 | 30 | 17 | 0.68 |
| ACEI Use (%) | 31 | 35 | 25 | 0.70 |
| Creatinine (mg/dl) | 0.38 ± 0.03 | 0.32 ± 0.03 | 0.48 ± 0.05 | 0.003 |
| eGFR (ml/min/1.73m2) | 141 ± 42 | 144 ± 47 | 136 ± 33 | 0.65 |
| Calcium (mg/dl) | 10.1 ± 0.1 | 10.1 ± 0.1 | 9.9 ± 0.2 | 0.19 |
| Phosphate (mg/dl) | 4.9 ± 0.1 | 5.1 ± 0.2 | 4.4 ± 0.2 | 0.01 |
| Phosphate SDS | −1.49 ± 0.27 | −1.58 ± 0.34 | −1.33 ± 0.46 | 0.66 |
| PTH (pg/ml) | 64 ± 5 | 60 ± 6 | 69 ± 10 | 0.39 |
| C-terminal FGF23 (RU/ml) | 295 (109, 547) | 437 (232, 705) | 119 (80, 153) | 0.006 |
| C-terminal FGF23 SDS | 2.28 ± 0.34 | 2.81 ± 0.44 | 1.38 ± 0.42 | 0.04 |
| Intact FGF23 (pg/ml) | 56 (38, 72) | 57 (48, 76) | 41 (36, 62) | 0.19 |
Data are presented as mean ± SEM or median (IQR). AKI: acute kidney injury; SDS: standard deviation score; BNP: B-type natriuretic peptide; RACHS-1: risk adjustment for congenital heart surgery; CPB: cardiopulmonary bypass; AXC: aortic cross-clamp; ACEI: angiotensin-converting-enzyme inhibitor; eGFR: estimated glomerular filtration rate; PTH: parathyroid hormone; FGF23: fibroblast growth factor 23; n/a: test not performed.
Ventricular function, assessed qualitatively by echocardiogram, was normal in 29 patients (91%); 3 patients had mildly depressed function. The surgical procedures performed are listed in Table 2. Post-operatively, 20 patients (62.5%) developed at least Stage 1 AKI by the Acute Kidney Injury Network (AKIN) criteria (16). The AKI group was younger than the non-AKI group, and thus had lower pre-operative creatinine; however, pre-operative eGFR did not differ between the groups. Preoperative cFGF23 levels, as well as age-adjusted cFGF23 SDS, were higher in the AKI group than in the non-AKI group; however, iFGF23 levels did not differ between the groups. Pre-operative oxygen saturation (SpO2) was lower in the AKI group (Table 1). Of the 15 patients with pre-operative SpO2 <95%, cardiac surgery improved saturations in all patients, and normalized saturations in 10 patients.
Table 2.
Surgical procedures performed.
| Surgical Procedure | n |
|---|---|
| Tetralogy of Fallot repair | 9 |
| Ventricular septal defect repair | 6 |
| Fontan procedure | 5 |
| Mitral valve replacement | 2 |
| Pulmonary valve replacement | 2 |
| Subaortic stenosis resection | 2 |
| Arterial switch | 1 |
| Complete atrioventricular canal repair | 1 |
| Ebstein anomaly repair | 1 |
| Glenn procedure | 1 |
| Partial anomalous pulmonary venous return repair | 1 |
| Septal myomectomy | 1 |
Post-operative renal function
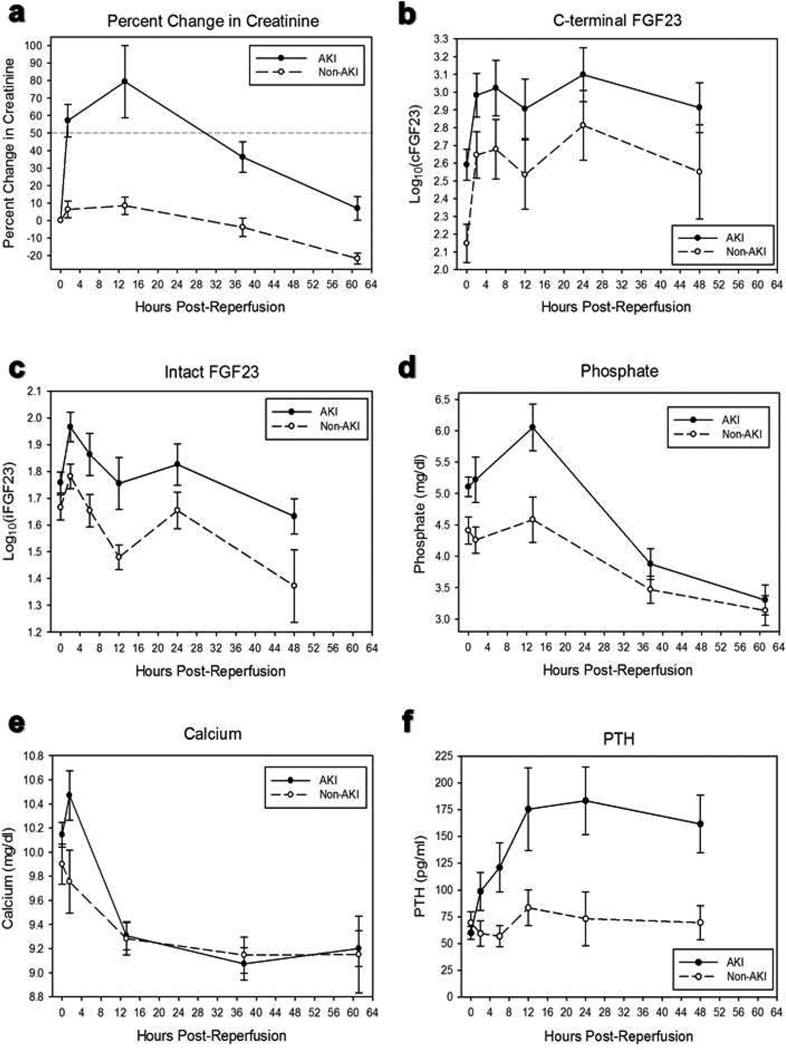
Peak post-operative percent change in serum creatinine was 74 (67, 100) % in the AKI group and 21 (0, 25) % in the non-AKI group (p<0.001). Of the AKI patients, all developed AKI within 24 hours of surgery, with the exceptions of two patients who were diagnosed with AKI at 26 hours and 39 hours post-reperfusion. A large prospective study of pediatric cardiac surgery patients observed a similar early development of CPB-associated AKI (15). Percent change in creatinine over time is shown in Figure 1a. Serum creatinine levels returned to baseline by post-operative day 5 in all patients. No patient became oliguric post-operatively or required renal replacement therapy.
Figure 1.
(a) Percent change in serum creatinine over time. The dotted line represents the acute kidney injury (AKI) threshold (50% increase in creatinine from baseline). (b) C-terminal fibroblast growth factor-23 (FGF23) levels over time. Linear mixed effects modeling showed that cFGF23 levels were significantly higher than pre-operative values by 2 hours post-reperfusion and through 24 hours post-reperfusion. Group status (AKI vs. non-AKI) did not modify the effect of time on cFGF23 levels. (c) Intact FGF23 levels over time. Linear mixed effects modeling showed that iFGF23 levels were significantly higher than pre-operative values at 2 hours post-reperfusion but not at subsequent time points. Group status (AKI vs. non-AKI) modified the effect of time on iFGF23 levels at the 2 hour time point. (d) Serum phosphate levels over time. Serum phosphate levels peaked around 13 hours postreperfusion, then decreased to below pre-operative values. (e) Serum calcium levels over time. By 13 hours post-reperfusion, serum calcium levels decreased to below preoperative values. (f) Serum parathyroid hormone (PTH) levels over time. PTH increased over time to a greater extent in the AKI group. All data are presented as means and SEM.
Post-operative changes in cFGF23 levels
For all patients, the median peak cFGF23 level was 1629 (596, 5085) RU/ml, with a median 6 (2, 18) fold change. cFGF23 levels increased early and markedly post-reperfusion (Figure 1b). Linear mixed effects modeling revealed that cFGF23 levels were higher than pre-operative values by 2 hours post-reperfusion (p<0.001) and remained higher than pre-operative levels through 24 hours post-reperfusion (p<0.001). However, there was no evidence of an interaction between group and time, such that group status (AKI vs. non-AKI) did not modify the effect of time on cFGF23 levels.
Post-operative changes in iFGF23 levels
For all patients, the median peak iFGF23 level was 84 (62, 152) pg/ml, with a median 1.7 (1.2, 2.6) fold change. Like cFGF23 levels, iFGF23 levels increased in the early post-operative period; however, unlike cFGF23 levels, iFGF23 levels returned to baseline soon thereafter (Figure 1c). We observed that iFGF23 levels were higher than pre-operative values at 2 hours post-reperfusion (p<0.001), but not at subsequent time points. In fact, at the 48 hour time point, iFGF23 levels were lower than pre-operative values (p<0.001). At the 2 hour time point, there was an interaction between group and time, such that iFGF23 levels increased to a greater degree in the AKI group than in the non-AKI group (p=0.04). There was no interaction detected at the 48 hour time point.
Post-operative changes in phosphate, calcium, and PTH levels
Post-operative serum phosphate concentrations peaked at an average of 13 hours post-reperfusion, increasing by 21 ± 8 % in the AKI group and 2 ± 9 % in the non-AKI group (p=0.12). Serum phosphate then decreased to levels significantly lower than preoperative values (p<0.001, Figure 1d). By an average of 13 hours post-reperfusion, serum calcium levels decreased to below pre-operative levels, with a nadir decrease of 10 ± 2 % in the AKI group and 7 ± 2 % in the non-AKI group (p=0.21). Serum calcium levels then remained significantly lower than pre-operative values (p<0.001, Figure 1e). PTH levels increased over time to a greater degree in the AKI group than in the non-AKI group (p=0.04, Figure 1f).
Pre-operative FGF23 as a predictor of CPB-associated AKI
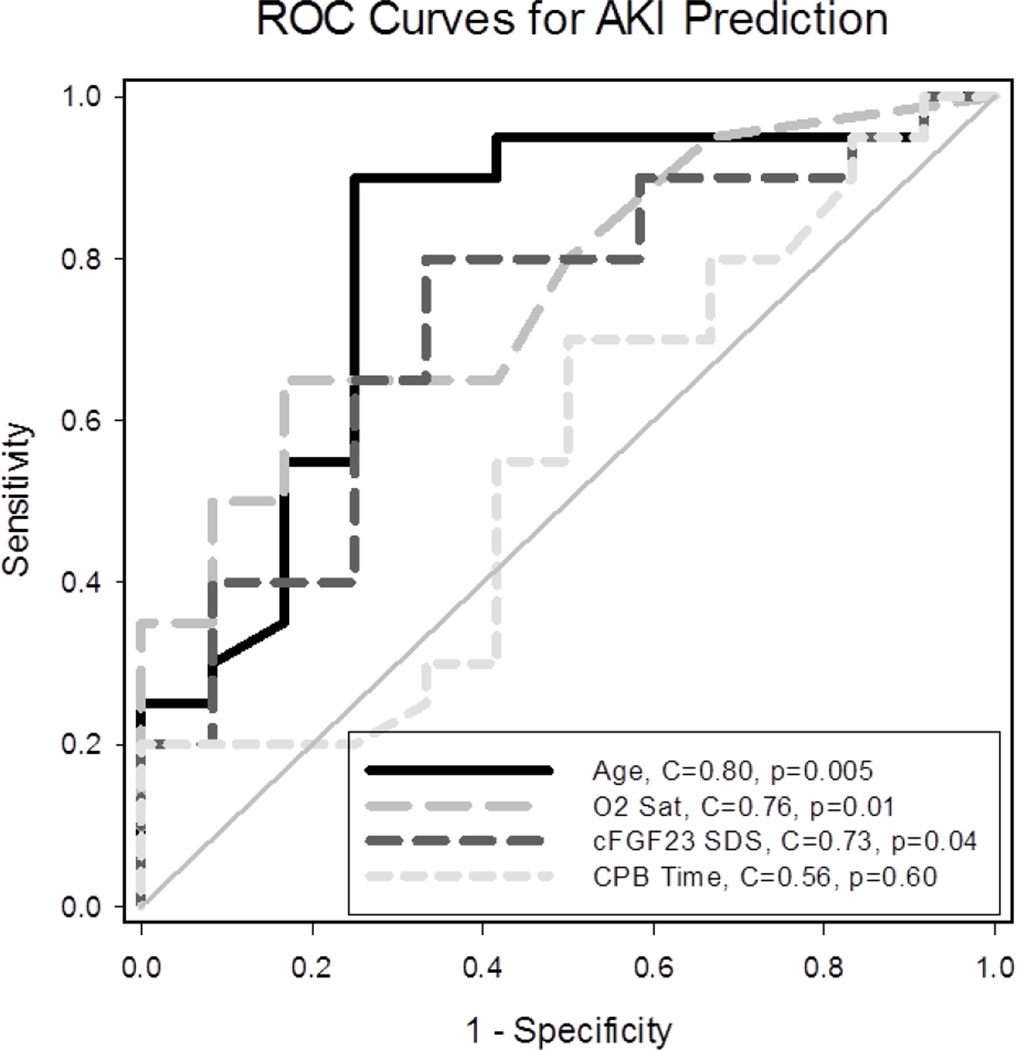
Pre-operative cFGF23 SDS predicted post-operative CPB-associated AKI (Figure 2). In multiple logistic regression modeling, after adjusting for age and CPB time, preoperative cFGF23 SDS was positively associated with AKI (OR = 2.07 (95% CI: 1.13, 3.79), p=0.02). Yet, given the loss of power inherent to outcome dichotomization (AKI vs. no AKI), and given that decrements in kidney function of less than 50% (not meeting the AKI threshold) may still be relevant, we also assessed a continuous outcome variable, the percent decrease in eGFR. Multiple linear regression modeling revealed that, after adjusting for age and CPB time, pre-operative cFGF23 SDS was positively associated with post-operative percent decrease in eGFR (B = 3.5 (95% CI: 1.0, 6.0), p=0.008). All three covariates were significantly associated with the outcome. The addition of cFGF23 SDS as a covariate to a model that already included age and CPB time increased the adjusted R2 from 0.46 to 0.57, indicating a relatively large increase in the degree to which the covariates explain the variability in the outcome measure. Using cFGF23 SDS as a covariate, as opposed to cFGF23 levels, allowed us to assess the age-independent effects of cFGF23 and avoid the collinearity that arose when including cFGF23 levels with age in the model. After adjustment for age and CPB time, iFGF23 levels were not associated with post-operative percent decrease in eGFR (p=0.33).
Figure 2.
Receiver operating characteristic (ROC) curves for acute kidney injury (AKI) prediction. The C statistic represents the area under the ROC curve. The C statistic ranges from 0.5, which is denoted by the diagonal line and corresponds to no predictive ability, to 1.0, which represents perfect predictive ability. Individually, age, pre-operative oxygen saturation, and pre-operative cFGF23 age-adjusted SDS predicted post-operative CPB- associated AKI.
Association of pre-operative oxygen saturation and FGF23 levels
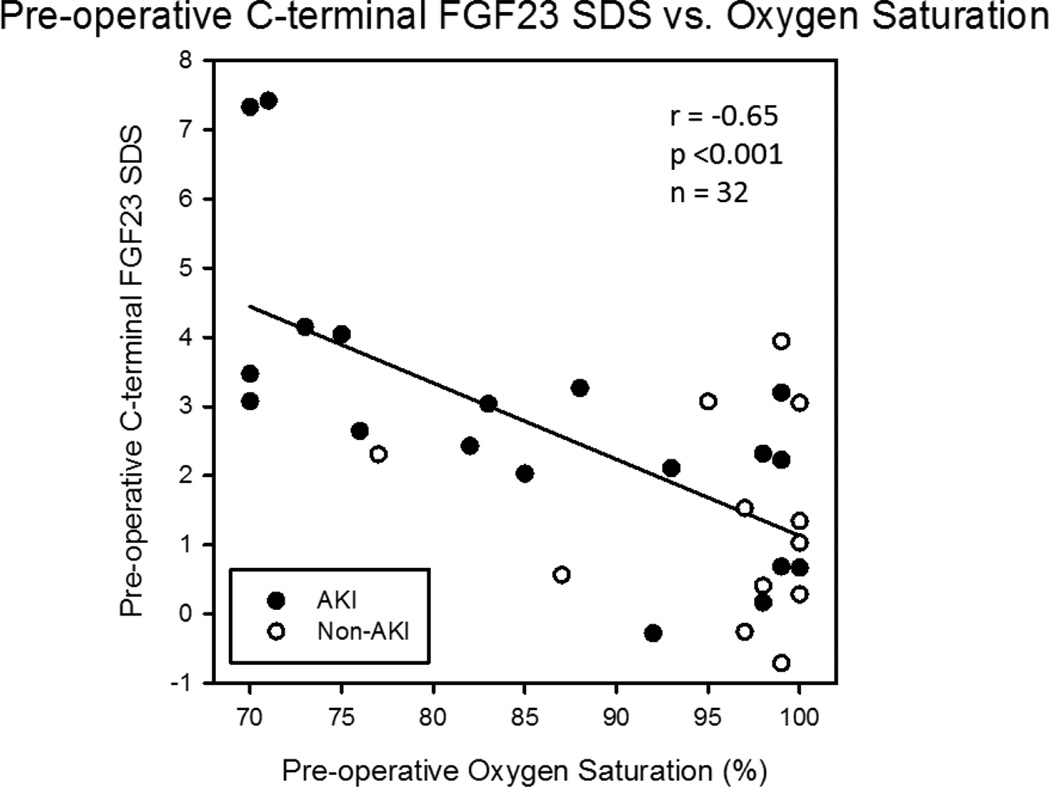
Pre-operative oxygen saturation (SpO2) inversely correlated with cFGF23 SDS (r = −0.65, p<0.001, Figure 3), cFGF23 levels (r = −0.48, p=0.005), and iFGF23 levels (r = −0.35, p=0.047). Previous SpO2 values measured at a median of 37 (24, 54) days prior to the pre-operative SpO2 determinations were highly correlated with the pre-operative measurements (r = 0.96, p<0.001), suggesting stability of the oxygen saturation measurements over time. In multiple linear regression modeling, pre-operative SpO2 remained negatively associated with cFGF23 SDS, independent of serum phosphate (SpO2 p<0.001) or phosphate SDS (SpO2 p=0.002).
Figure 3.
Relationship between pre-operative C-terminal fibroblast growth factor-23 (FGF23) SDS and oxygen saturation. Pre-operative oxygen saturation correlated negatively with pre-operative cFGF23 age-adjusted SDS. Also, whereas 13/15 (87%) of patients with oxygen saturation <95% developed acute kidney injury (AKI), only 7/17 (41%) of patients with oxygen saturation ≥95% developed AKI (p=0.01).
As chronic hypoxemia may adversely affect kidney function (22–24), potentially rendering the kidney less tolerant of CPB, we hypothesized that pre-operative SpO2 may also predict post-operative CPB-associated AKI. Like cFGF23 SDS, pre-operative SpO2 predicted post-operative CPB-associated AKI (Figure 2). In regression analyses, given its high correlation with cFGF23 SDS, pre-operative SpO2 was not included as a predictor covariate in our original models. However, after excluding cFGF23 SDS from the models, and adjusting for age and CPB time, pre-operative SpO2 was negatively associated with AKI in logistic regression (OR = 0.83 (95% CI: 0.71, 0.98), p=0.03), and negatively associated with post-operative percent decrease in eGFR in linear regression (B = −0.5 (95% CI: −1.0, −0.02), p=0.04). The addition of SpO2 as a covariate to a linear regression model that already included age and CPB time increased the adjusted R2 from 0.46 to 0.52.
DISCUSSION
The current results confirm previous observations demonstrating elevated cFGF23 levels in AKI patients (7–10), as well as the ability of cFGF23 to predict the development of AKI (10), as defined by an increase in serum creatinine of at least 50% from baseline. However, we also present the novel finding that, in patients with cardiopulmonary bypass-associated acute kidney dysfunction, cFGF23 levels remain elevated for a longer duration than iFGF23 levels. Furthermore, in pediatric congenital heart disease patients, we show that chronic hypoxemia is associated with elevated FGF23 levels.
In the setting of acute kidney dysfunction, there is an early and marked elevation in cFGF23 levels, as previously described in a murine AKI model (7). C-terminal levels increased considerably in our AKI group, and even increased 5-fold in the non-AKI group, in whom there was only a 21% median peak increase in creatinine levels. The increase of cFGF23 levels in the non-AKI group may be attributable to subclinical kidney dysfunction or to an inflammatory response. The observation that cFGF23 levels increased out of proportion to the degree of acute kidney dysfunction has been previously observed and ascribed, at least in part, to a systemic inflammatory response to surgery (7). Indeed, in CKD patients, higher cFGF23 levels are independently associated with elevated markers of inflammation (25). Proposed mechanisms by which circulating FGF23 levels may increase in AKI include increased production from bone; increased ectopic production, possibly from damaged renal tubules; decreased renal clearance; decreased degradation; and/or release of stored, preformed hormone (7,26). Although hyperphosphatemia may represent a stimulus for FGF23, circulating FGF23 levels increased before phosphate concentrations peaked. Furthermore, in the non-AKI group, FGF23 levels increased by 2 hours post-reperfusion despite a slight decrease in serum phosphate.
Although circulating FGF23 is nearly all intact and biologically active in patients with end-stage kidney disease (27), the correlation between measurements made by C-terminal and intact FGF23 assays is not as strong in individuals with preserved kidney function (28). We measured FGF23 levels using both the C-terminal and intact assays. Our study sample had a median pre-operative cFGF23 level of 295 RU/ml (range 59–2653 RU/ml) and a median iFGF23 level of 56 pg/ml (range 27–129 pg/ml). As cFGF23 levels vary with age, we used pre-operative age-adjusted cFGF23 standard deviation scores in our analyses, calculated as detailed in Fischer et al (19). Although the cFGF23 levels are clearly elevated for age (mean age-adjusted SDS of 2.28) (19), similar age-adjusted normal values do not exist for iFGF23. However, the iFGF23 values are similar to values observed in our institutional cohort of 47 healthy children (mean age 11.3 years). Although this cohort is older than our study sample, given that serum phosphate levels are higher in younger children, it seems unlikely that normal iFGF23 levels in younger children would be so much lower as to suggest that our observed pre-operative iFGF23 values were dramatically elevated. Indeed, in our healthy cohort, iFGF23 tended to correlate negatively with age (r = −0.28, p=0.06); similarly, cFGF23 levels tend to be higher, not lower, in younger children (19). Therefore, the study sample had unexpectedly high pre-operative cFGF23 levels that were elevated out of proportion to iFGF23 levels. These observations suggest that, although pre-operative FGF23 production may have been increased, proteolytic cleavage mechanisms (29) may have been concurrently upregulated, maintaining levels of intact FGF23 that are closer to the normal range, similar to what is thought to occur in the setting of iron deficiency (30–32)
Post-operatively, both cFGF23 and iFGF23 levels increased by 2 hours post-reperfusion; however, cFGF23 remained elevated for a longer duration than iFGF23 levels. This discrepancy suggests that different factors may affect FGF23 transcription and post-translational cleavage. Group status (AKI vs. non-AKI) modified the effect of time on changes in iFGF23 levels, but not cFGF23 levels, suggesting that overall FGF23 transcription, of which cFGF23 levels may be considered a surrogate measure, was affected less by decreased GFR and more by other factors such as inflammation. Moreover, the generally similar trends in FGF23 levels over time between the AKI and non-AKI groups suggest that factors besides kidney function affected circulating FGF23 concentrations. At 12 hours post-reperfusion, a relative decrease in iFGF23 levels is observed, the etiology of which is unclear. In the AKI group, this decrease occurred in the setting of phosphate levels that were increased from baseline; in the non-AKI group, this decrease occurred in the setting of phosphate levels that were similar to baseline. These observations suggest that other factors besides serum phosphate affected iFGF23 levels, as previously suggested by Christov et al (7).
Interestingly, pre-operative serum phosphate concentrations were relatively low for age in the study sample (mean age-adjusted phosphate SDS of −1.49). Given that preoperative iFGF23 values were similar to what we observed in the cohort of healthy children, it seems unlikely that iFGF23 was causing the relative hypophosphatemia. The effects of PTH and 1,25(OH)2 vitamin D may have played a role. However, relative malnutrition may have been more contributory, as evidenced by the cohort’s below-average growth parameters (mean age-adjusted weight SDS of −1.49, and mean age-adjusted height SDS of −1.22).
Post-operatively, phosphate values decreased to below pre-operative levels by 37 hours post-reperfusion. It is unlikely that this post-operative relative hypophosphatemia was entirely caused by elevated iFGF23 levels, which peaked at 2 hours post-reperfusion. Nutritional status may have been contributory, as many patients in the early post-operative period remained on intravenous fluids and had yet to be advanced to full enteral feeds or parenteral nutrition. It is possible that the elevated PTH levels, at least in the AKI group, also contributed to the hypophosphatemia. Decreased 1,25(OH)2 vitamin D levels may also have contributed, although in the setting of nearly recovered renal function, lower iFGF23 levels and higher PTH levels would be expected to increase 1,25(OH)2 vitamin D production. However, the relative hypophosphatemia may have contributed to the decrease in iFGF23 levels to below baseline at 48 hours post-reperfusion.
Similar to a previous study of FGF23 levels in the pediatric CPB population (10), we observed that pre-operative cFGF23 levels and cFGF23 SDS were markedly higher in those patients who went on to develop AKI. It is unclear why higher pre-operative FGF23 levels are associated with worse post-operative renal function. It is unknown whether higher FGF23 levels have some direct pathologic effect promoting renal injury, or if FGF23 represents a surrogate marker for another factor that is associated with worse post-operative renal function. It has been hypothesized that subclinical kidney dysfunction may contribute to both high pre-operative FGF23 levels and post-operative CPB-associated AKI (10). Cardiac status may also be contributory. In a study of pediatric heart failure patients with normal mean eGFR, FGF23 levels were elevated compared to controls and correlated with heart failure severity (11). As such, high preoperative FGF23 levels and post-operative kidney dysfunction may, in part, be attributable to poor cardiac function. However, our study sample did not have heart failure, as evidenced by low serum BNP values, which is a sensitive marker of heart failure (33,34), and qualitatively normal ventricular function on echocardiogram.
An interesting observation from the present study is the potential role of hypoxemia in the association between high pre-operative cFGF23 levels and post-operative kidney dysfunction. Pre-operative cFGF23 levels and oxygen saturation negatively correlated, and both variables predicted kidney dysfunction, suggesting that elevated cFGF23 levels possibly represented a surrogate marker for hypoxemia. Chronic hypoxia affects renal cellular function (22–24), and small studies of pediatric patients with cyanotic congenital heart disease have demonstrated evidence of tubular and glomerular damage (35–37). As such, some degree of cyanotic nephropathy-associated renal compromise may have rendered the kidneys of our hypoxemic patients less tolerant of CPB and possibly more susceptible to AKI.
The associations between hypoxemia and FGF23 levels in humans have not been previously described; however, increased FGF23 expression has been observed in osteoblast-like cell lines under hypoxic conditions (12,13), and elevated circulating levels have been observed in hypoxic rats (13). Compared to controls, the hypoxic rats had much higher cFGF23 levels, but comparable iFGF23 levels (13). In the current study, SpO2 negatively correlated with FGF23 levels, suggesting an association between chronic hypoxemia and increased FGF23 production.
Limitations of our study include the small sample size, the relatively short duration of post-operative measurement, incomplete biochemical assessment (including lack of 1,25(OH)2 vitamin D levels and inflammatory markers), and the observational nature of our findings. Furthermore, this study sample developed only mild AKI; therefore, the changes in iFGF23 may be underestimated given the lesser degree of renal dysfunction.
In summary, although elevated FGF23 levels have been previously described in CPB-associated AKI, the current findings provide new insights into how AKI may affect FGF23 levels. In mild AKI, cFGF23 levels are increased for a longer duration than iFGF23 levels, possibly suggesting alterations in cleavage mechanisms. Preoperatively, age-adjusted cFGF23 SDS may predict CPB-associated kidney dysfunction. Furthermore, for the first time in humans, we have identified an association between chronic hypoxemia and FGF23 levels. Further studies will be needed to elucidate the mechanisms by which hypoxemia and acute kidney dysfunction may result in elevated FGF23 levels.
ACKNOWLEDGEMENTS
This work was supported in part by USPHS Grants DK-67563, DK-35423, DK-80984; CTSI Grant UL1 TR-000124; NIH K12 Child Health Research Career Development Award K12-HD-034610; NIH Training Grant T32-DK-07789; funds from the UCLA Children’s Discovery and Innovation Institute, and funds from the American Society of Nephrology Norman Siegel Foundation. This work was presented as a poster abstract at the American Society of Nephrology’s Kidney Week, November 7, 2013, Atlanta, Georgia. FGF23 and PTH kits were kindly provided by Immutopics International.
Footnotes
Ethics statement: The study was approved by the UCLAInstitutional Review Board, and informed consent and assent were obtained from parents and patients accordingly.
Conflict of Interest: The authors declare no conflict of interest.
REFERENCES
- 1.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;4:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;6:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;7:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 4.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P MMKD Study Group. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;9:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 5.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M Chronic Renal Insufficiency Cohort (CRIC) Study Group. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;19:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christov M, Waikar SS, Pereira RC, Havasi A, Leaf DE, Goltzman D, Pajevic PD, Wolf M, Jüppner H. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int. 2013;4:776–785. doi: 10.1038/ki.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M, Hsu R, Hsu CY, Kordesch K, Nicasio E, Cortez A, McAlpine I, Brady S, Zhuo H, Kangelaris KN, Stein J, Calfee CS, Liu KD. FGF-23 and PTH levels in patients with acute kidney injury: A cross-sectional case series study. Ann Intensive Care. 2011;1:21. doi: 10.1186/2110-5820-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leaf DE, Wolf M, Waikar SS, Chase H, Christov M, Cremers S, Stern L. FGF-23 evels in patients with AKI and risk of adverse outcomes. Clin J Am Soc Nephrol. 2012;8:1217–1223. doi: 10.2215/CJN.00550112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali FN, Hassinger A, Price H, Langman CB. Preoperative plasma FGF23 levels predict acute kidney injury in children: results of a pilot study. Pediatr Nephrol. 2013;6:959–962. doi: 10.1007/s00467-012-2395-2. [DOI] [PubMed] [Google Scholar]
- 11.Isakova T, Houston J, Santacruz L, Schiavenato E, Somarriba G, Harmon WG, Lipshultz SE, Miller TL, Rusconi PG. Associations between fibroblast growth factor 23 and cardiac characteristics in pediatric heart failure. Pediatr Nephrol. 2013;10:2035–2042. doi: 10.1007/s00467-013-2515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirao M, Hashimoto J, Yamasaki N, Ando W, Tsuboi H, Myoui A, Yoshikawa H. Oxygen tension is an important mediator of the transformation of osteoblasts to osteocytes. J Bone Miner Metab. 2007;5:266–276. doi: 10.1007/s00774-007-0765-9. [DOI] [PubMed] [Google Scholar]
- 13.Clinkenbeard EL, Farrow EG, Summers LJ, Cass TA, Roberts JL, Bayt CA, Lahm T, Albrecht M, Allen MR, Peacock M, White KE. Neonatal iron deficiency causes abnormal phosphorus metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res. 2014;2:361–369. doi: 10.1002/jbmr.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008;6:807–813. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW, Parikh CR TRIBE-AKI Consortium. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;6:1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;2:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;3:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2002;3:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer DC, Mischek A, Wolf S, Rahn A, Salweski B, Kundt G, Haffner D. Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase. Ann Clin Biochem. 2012;6:546–553. doi: 10.1258/acb.2012.011274. [DOI] [PubMed] [Google Scholar]
- 20.Ardeshirpour L, Cole DE, Carpenter TO. Evaluation of bone and mineral disorders. Pediatr Endocrinol Rev. 2007;5:584–598. [PubMed] [Google Scholar]
- 21.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;1:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 22.Sahai A, Mei C, Schrier RW, Tannen RL. Mechanisms of chronic hypoxia-induced renal cell growth. Kidney Int. 1999;4:1277–1281. doi: 10.1046/j.1523-1755.1999.00703.x. [DOI] [PubMed] [Google Scholar]
- 23.Eckardt KU, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger C, Wiesener MS, Willam C. Role of hypoxia in the pathogenesis of renal disease. Kidney Int. 2005:S46–S51. doi: 10.1111/j.1523-1755.2005.09909.x. [DOI] [PubMed] [Google Scholar]
- 24.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;1:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 25.Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, Hamm LL, Negrea L, Leonard MB, Raj D, Wolf M Chronic Renal Insufficiency Cohort. Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol. 2012;7:1155–1162. doi: 10.2215/CJN.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neyra JA, Moe OW, Hu MC. Fibroblast growth factor 23 and acute kidney injury. Pediatr Nephrol. 2015;30(11):1909–1918. doi: 10.1007/s00467-014-3006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Jüppner H. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;2:578–585. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith ER, Cai MM, McMahon LP, Holt SG. Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab. 2012;9:3357–3365. doi: 10.1210/jc.2012-1811. [DOI] [PubMed] [Google Scholar]
- 29.Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, Koller A, Nizet V, White KE, Dixon JE. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci USA. 2014;15:5520–5525. doi: 10.1073/pnas.1402218111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, Garringer HJ, Vidal R, Chan RJ, Goodwin CB, Hui SL, Peacock M, White KE. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci USA. 2011;46:E1146–E1155. doi: 10.1073/pnas.1110905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab. 2011;11:3541–3549. doi: 10.1210/jc.2011-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;8:1793–1803. doi: 10.1002/jbmr.1923. [DOI] [PubMed] [Google Scholar]
- 33.Dao Q, Krishnaswamy P, Kazanegra R, Harrison A, Amirnovin R, Lenert L, Clopton P, Alberto J, Hlavin P, Maisel AS. Utility of B-type natriuretic peptide in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol. 2001;2:379–385. doi: 10.1016/s0735-1097(00)01156-6. [DOI] [PubMed] [Google Scholar]
- 34.Strunk A, Bhalla V, Clopton P, Nowak RM, McCord J, Hollander JE, Duc P, Storrow AB, Abraham WT, Wu AH, Steg G, Perez A, Kazanegra R, Herrmann HC, Aumont MC, McCullough PA, Maisel A. Impact of the history of congestive heart failure on the utility of B-type natriuretic peptide in the emergency diagnosis of heart failure: results from the Breathing Not Properly Multinational Study. Am J Med. 2006;1:69.e1–69.e11. doi: 10.1016/j.amjmed.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Burlet A, Drukker A, Guignard JP. Renal function in cyanotic congenital heart disease. Nephron. 1999;3:296–300. doi: 10.1159/000045296. [DOI] [PubMed] [Google Scholar]
- 36.Agras PI, Derbent M, Ozcay F, Baskin E, Turkoglu S, Aldemir D, Tokel K, Saatci U. Effect of congenital heart disease on renal function in childhood. Nephron Physiol. 2005;1:10–15. doi: 10.1159/000081797. [DOI] [PubMed] [Google Scholar]
- 37.Zheng J, Yao Y, Han L, Xiao Y. Renal function and injury in infants and young children with congenital heart disease. Pediatr Nephrol. 2013;1:99–104. doi: 10.1007/s00467-012-2292-8. [DOI] [PubMed] [Google Scholar]