Abstract
Rationale
Neuroendocrine-immune regulation is essential for maintaining health. Early-life adversity may cause dysregulation in the neuroendocrine-immune network through repeated activation of the stress response, thereby increasing disease risk.
Objective
This paper examined the extent to which maternal psychological well-being moderates neuroendocrine-immune relations in children.
Methods
We used data from a laboratory-based study of mothers and their five-year old children (n=125 mother-child pairs) conducted from 2011–2013 in Baltimore, Maryland. Child saliva was assayed for markers of immune function (i.e., cytokines: interleukin [IL]-1β, IL-6, IL-8, tumor necrosis factor alpha [TNF-α]) and hypothalamic-pituitary-adrenal activity (i.e., cortisol). A composite score for depressive symptoms, anxiety, and parenting stress characterized maternal psychological distress. Multilevel mixed models examined the relationship between maternal psychological well-being and child neuroendocrine-immune relations.
Results
Significant cytokine × maternal distress interactions indicated that as maternal distress increased, expected inverse cytokine-cortisol relations within children became weaker for IL-1β, IL-6, and TNF-α. Sex-stratified models revealed that these interactions were only significant among girls. Among boys, there were inverse cytokine-cortisol relations for all cytokines, and, while in the same direction as observed among girls, the cytokine × maternal distress interactions were non-significant.
Conclusion
The findings suggest that maternal distress is associated with child neuroendocrine-immune relations in saliva and may alter the sensitivity of inflammatory immune processes to cortisol's inhibitory effects. This desensitization may place the child at risk for inflammatory diseases. The findings support efforts for the early detection and treatment of at-risk mothers to protect maternal and child health and well-being.
Keywords: salivary cortisol, cytokines, children, maternal mental health, acute stress
Introduction
Early-life adversity contributes to poorer mental and physical health throughout life (Danese & McEwen, 2012; J. Shonkoff et al., 2012). Preventing and identifying early-life adversity are increasingly important aspects of social, education, public health and clinical policies and programs aiming to promote healthy development and lifelong well-being (Brent & Silverstein, 2013; Garner et al., 2012). Developing effective interventions, however, requires advanced understanding of the mechanisms linking adversity and disease.
Early-life adversity activates a developing child’s stress response. While acute activation of the stress response protects the child in the short-term and helps the child cope with immediate dangers or threats (McEwen, 2005), repeated activation of the stress response, as a consequence of chronic or frequent childhood adversities, may result in physiologic damage or dysfunction in the system of nervous, endocrine and immune processes involved in the body’s response to stress (Danese & McEwen, 2012; Hertzman & Boyce, 2009; McEwen, 2005). Coordination within the neuroendocrine-immune (NEI) network is essential for maintaining homeostasis (Johnson, Riley, Granger, & Riis, 2013). Adversity-related NEI disruptions early in life may render the body vulnerable to imbalanced NEI activity, and increase disease risk later in life. For example, adversity may make inflammatory immune processes less sensitive to inhibitory signals from the central nervous system (i.e., cortisol), thereby increasing risk for chronic inflammation (Miller et al., 2009).
Previous research suggests, however, that the negative effects of adversity on health may be buffered by a supportive caregiver (Chen, Miller, Kobor, & Cole, 2011; Miller et al., 2011; J. P. Shonkoff, 2012). A sensitive and attentive adult caregiver is one of the most important protective factors supporting child health (Chen et al., 2011; Miller et al., 2011; J. P. Shonkoff, 2012). Animal studies have shown a potential link between the quality of early-life maternal care and later-life immune functioning with dysfunctional maternal behavior associated with an inflammation-prone immune response in offspring (Coe & Lubach, 2003). While the study of these biologic effects in humans is limited, there is evidence that high levels of social support and maternal care are associated with lower and more regulated inflammatory processes in adults otherwise at risk for stress-related increases in inflammatory processes (Chen et al., 2011; Miller, Cohen, & Ritchey, 2002). These findings demonstrate the importance of social-emotional supports in NEI network regulation, particularly under high stress conditions.
Infancy and early childhood, when the hypothalamic-pituitary-adrenal (HPA) axis and immune system are undergoing developmental change, represent sensitive periods during which stress, and a supportive caregiver, may have profound, and possibly lasting, impacts on NEI network functioning (Coe & Lubach, 2003; Miller, Chen, & Zhou, 2007). Studying NEI relations in children, however, has traditionally been limited by the need for blood-based biomeasures. Blood collection can be especially difficult for studies involving children (Djuric et al., 2008). In contrast, saliva collection is minimally-invasive, low-cost and generally more socially acceptable (Djuric et al., 2008; Marques, Silverman, & Sternberg, 2010). Research focused on salivary immune markers in healthy young children is limited; however, there is a growing body of literature, especially examining interleukin-1 beta (IL-1β) and interleukin-6 (IL-6) (El-Sheikh, Buckhalt, Granger, Erath, & Acebo, 2007; Keller, El-Sheikh, Vaughn, & Granger, 2010; Riis, Granger, DiPietro, Bandeen-Roche, & Johnson, 2015). These salivary inflammatory biomarkers open up new opportunities to gain a better understanding of NEI functioning in children and the role caregivers play in moderating stress-related damage within the NEI network. This emerging line of inquiry may help illuminate a link between early-life adversity and health, which, in turn, may contribute to more effective screening tools and interventions.
This study uses salivary biomeasures to examine whether coordination between HPA axis and inflammatory immune system functioning varies by level of maternal distress in five-year old children from diverse socioeconomic backgrounds. This paper addresses two gaps in current knowledge. First, unlike prior studies that do not adequately parse financial and psychosocial aspects of adversity (Slopen, Koenen, & Kubzansky, 2012), we used several markers of maternal psychological well-being and socioeconomic status (SES) to separate the effects of caregivers from SES. Second, rather than examining a single biologic system, we assessed relations between the HPA and immune systems to gain a nuanced understanding of the biologic consequences of early-life adversity. We hypothesized that maternal psychological well-being would moderate HPA-immune system relations regardless of SES.
Methods
This study used data from the Fetus to Five study, supplemented with data collected from a community sample (DiPietro et al., 2010; Riis et al., 2015).
Participants
Mother-child pairs were recruited in 2011–2013 as detailed previously (DiPietro et al., 2010; Riis et al., 2015). Approximately a third of the sample (58 pairs) was recruited from mothers who participated in a fetal development study in 2006–2007. Enrollment in the fetal development study was limited to low-risk, healthy women (DiPietro et al., 2010). To increase the diversity of the sample, an additional 93 participant pairs were enrolled from Baltimore, Maryland via community postings. English fluency was required, and child participants had to be five-years old. Mother-child pairs were excluded if mothers reported that the child had health or developmental conditions impairing cognitive, motor, or regulatory functioning.
Procedures
The Johns Hopkins Bloomberg School of Public Health Institutional Review Board approved the study protocol. Mothers provided written consent. During a 90-minute study visit, five-year old children (Mean [M]=5.45 years, standard deviation [SD]=0.29) completed neuropsychological and behavioral assessments and participated in emotional stressor tasks. Four saliva samples were collected from children; two before and two after the stressor tasks (M [SD] minutes from sample 1–2=12.15 [2.87]; 2–3=14.58 [2.94]; 3–4=23.36 [6.06]). Mothers provided sociodemographic and child health information, and completed a battery of psychological assessments.
Emotional stressor tasks
Children participated in three age-appropriate emotional stressor tasks including: the Disappointing Gift Game, the Not Sharing Game, and Mischel’s Delay of Gratification Task (Mischel & Mischel, 1983). These tasks elicit negative emotions and challenge behavioral and emotional regulation (Cole, Zahn-Waxler, & Smith, 1994; Gagne, Hulle, Aksan, Essex, & Goldsmith, 2011; Jahromi & Stifter, 2008; Kieras, Tobin, Graziano, & Rothbart, 2005; Mischel & Mischel, 1983; Spinrad et al., 2009). The Not Sharing Game also elicits an increase in salivary cortisol in young children (Spinrad et al., 2009). Detailed descriptions of the tasks were previously published (Riis et al., 2015).
Family sociodemographic data
Sociodemographic data included: family income, maternal education, marital status, and the number of moves during the child’s life. Financial stress was measured using a six-item instrument adapted from Essex and colleagues (Essex, Klein, Cho, & Kalin, 2002) assessing the frequency of financial stressors in the last three months (e.g., difficulty paying bills, fears of losing home/job).
Maternal psychological measures
Maternal psychological well-being was measured using three commonly-used, validated instruments. Maternal depressive symptoms in the last week were measured using the Center for Epidemiologic Studies Depression Scale (CESD-20; Radloff, 1977). Trait-anxiety was assessed using the Speilberger State-Trait Anxiety Inventory-Form Y2 (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). Maternal parenting and life stress were measured using the Parenting Stress Index-Short Form (PSI; Aibidin, 1995).
Biomeasure data
Whole saliva was collected using passive drool. Samples were stored at −20 degrees Celsius until assayed. Saliva was assayed for cortisol, a marker of HPA activity, and for four proinflammatory cytokines: IL-1β, IL-6, interleukin-8 (IL-8) and tumor necrosis factor alpha (TNF-α). IL-1β, IL-6, IL-8 and TNF-α are critical signaling molecules involved in initiating and maintaining inflammation (Clough & Roth, 1998; Owen, Punt, & Stranford, 2012). Although limited, prior studies have found associations between inflammatory cytokines and HPA activity in the oral cavity (including previously reported data from the current study) (Izawa et al., 2013; Moons, Eisenberger, & Taylor, 2010; Riis et al., 2015). While salivary cytokine activity likely reflects local immune processes, interactions between inflammatory cytokine and HPA activity in the oral cavity have been found to mirror immune-HPA relations previously identified in serum (Riis et al., 2015). Furthermore, in studies of adults, salivary IL-1β, IL-6 and TNF-α have exhibited acute stress sensitivity (Slavish, Graham-Engeland, Smyth, & Engeland, 2015).
Determination of salivary analytes
Saliva assays were performed at the Center for Interdisciplinary Salivary Bioscience Research at the Johns Hopkins School of Nursing. Cortisol was analyzed in duplicate using a commercially available enzyme immunoassay (Salimetrics, Carlsbad, California). Cortisol assay sensitivity ranged from 0.007 to 3.0 µg/dL. The intra-assay coefficient of variation was less than 5%, and the inter-assay coefficient of variation was less than 10%. Eight percent of cortisol concentrations were below the lower limit of detection; these values were substituted with half the lower limit of detection (0.0035 µg/dL).
Salivary cytokines were measured following Riis and colleagues (Riis et al., 2013) using proinflammatory cytokine 4-plex electrochemiluminescence immunoassays by Meso Scale Discovery (Gaithersburg, Maryland). Cytokine concentrations (pg/mL) were determined with MSD Discovery Workbench Software (v. 3.0.17) using curve fit models (4-PL with a weighting function option of 1/y2). Lower limits of detection and intra-assay coefficients of variation were: IL-1β: 0.06 pg/mL, 4.4%; IL-6: 0.09 pg/mL, 4.8%; IL-8: 0.05 pg/mL, 2.1%; and TNF-α: 0.11 pg/mL, 6.6%. All IL-1β and IL-8 concentrations were measureable above the lower limit of detection. A small number of non-detects for IL-6 (<1%) and TNF-α (5%) were replaced with laboratory-estimated concentrations. Associations between cytokine concentrations and flow rate were tested using Spearman’s rank correlations and regression analyses with robust variance. No consistent relations were found, so data were not adjusted for salivary flow rate.
Descriptive statistics of raw salivary cortisol data by saliva sample are shown in Supplemental Table 1; descriptive statistics of salivary cytokine data were previously published (Riis et al., 2015). All analyte data were positively skewed. Data were log-transformed and Winsorized (Tabachnik & Fidell, 2001) to bring values within three standard deviations of sample means and improve the normality of the distributions. For each analyte, ≤1% of concentrations were Winsorized. Transformed and Winsorized data were used in all statistical analyses.
Key covariates
Salivary biomarkers may be affected by illness and contamination with blood. Thus, mothers provided information on whether children were currently experiencing symptoms of illness and/or whether they had loose or recently shed teeth, or other dental issues. Information about other potential confounders of cortisol and cytokine concentrations, including child age, sex, and maternal smoking status (smoking/non-smoking), was based on maternal report. In addition, because body mass index (BMI) may influence neuroendocrine and inflammatory functioning, BMI was determined using evaluators’ measurements of child height and weight (Centers for Disease Control and Prevention, 2001). Weight status (underweight/healthy weight versus overweight/obese) was assigned to each child using the Centers for Disease Control and Prevention’s BMI percentile ranks by age and sex (Centers for Disease Control and Prevention, 2011).
The impact of diurnal patterns in cortisol was adjusted for using elapsed time from child waking to study appointment using maternal report. To account for the differential timing of stressor tasks relative to saliva samples across children, a task timing variable, defined as the time between the beginning of the stressor tasks and each sample, was calculated.
Statistical Analyses
Maternal psychological measures
Cronbach’s alpha (α) was used to assess the internal consistency of the CES-D, STAI and PSI in the sample. Correlations between the scale scores were also examined using Spearman’s rho.
Factor analyses
The first step in data analysis was to determine whether the eight SES indicators and maternal psychological scale scores (Table 1) could be combined into distinct measures. This was achieved by polychoric exploratory factor analysis with maximum likelihood estimation and robust standard errors (SEs), oblique rotation, and one to four factors. A two factor solution was selected based on information criteria, factor loadings, and residual variances; one factor represented financial hardship and one represented maternal distress (Table 1; Supplemental Table 2). Oblique rotation resulted in two factors with correlation=0.57 (p<.001). Greater financial hardship was associated with greater maternal distress. Factor scores, derived using the posterior mode method, provided a score for financial hardship and maternal distress for each child.
Table 1.
Family sociodemographic and maternal psychological characteristics (n=125)
| Sociodemographic Characteristics a | Number (%)/ Mean ± SD |
Range |
|---|---|---|
| Family income in last 12 months | ||
| <$5,000–$11,999 | 30 (24) | |
| $12,000–$24,999 | 29 (23) | |
| $25,000–$49,999 | 11 (9) | |
| $50,000–$99,999 | 14 (11) | |
| ≥$100,000 | 41 (33) | |
| Maternal education | ||
| <High school | 8 (6) | |
| High school degree/GED | 33 (26) | |
| Some college | 31 (25) | |
| 2-Year degree | 7 (6) | |
| 4-Year degree | 20 (16) | |
| Master’s degree | 11 (9) | |
| Doctoral/professional degree | 15 (12) | |
| Maternal marital status | ||
| Married | 56 (45) | |
| Moves during child's life | ||
| 0 | 35 (28) | |
| 1 | 38 (30) | |
| 2 | 17 (14) | |
| 3 | 21 (17) | |
| 4 | 7 (6) | |
| 5 | 2 (2) | |
| 6+ | 5 (4) | |
| Maternal financial stress | 14.62 ± 6.87 | 6–30 |
| Maternal Psychological Characteristics b | ||
| Maternal depressive symptoms | 13.62 ± 10.91 | 0–47 |
| Maternal anxiety | 37.39 ± 9.62 | 20–62 |
| Maternal parenting and life stress | 67.67 ± 17.26 | 36–113 |
Note. SD=Standard deviation. GED=General equivalency diploma.
Variables included in financial hardship factor. There were no missing data for maternal education, marital status, or moves during child's life. Family income data were missing or classified as “don’t know” for 10% of the sample; incomplete data were substituted with the mean of the sample group. Financial stress data were missing for 6% of the sample, but no participant was missing more than one item. Missing items were imputed with the mean score of the completed items.
Variables included in maternal distress factor. Missing data on psychological measures ranged from 10–18%, but only six participants were missing more than one item per instrument. Missing data were imputed with mean scores of completed items in the scale (or subscale for the Parenting Stress Index-Short Form).
Multilevel mixed models
The next step was to examine the effect of cytokine concentrations, maternal distress, and cytokine × maternal distress interactions on cortisol values. To do this, cortisol was modeled as an outcome using multilevel mixed models with a random intercept and a random slope to account for repeated sampling within individuals and varying cortisol trajectories between individuals. Robust variance and an independent covariance and residual errors structure were specified. Residual variance was modeled separately for each sample to allow for unequal variance across samples.
First, bivariate analyses explored relations between cortisol and each independent variable. Next, four separate fully-adjusted models for cortisol were performed, each with only one cytokine predictor. The independent variables in fully-adjusted models included: the cytokine of interest, the maternal distress score, the cytokine × maternal distress interaction, all key covariates, and the financial hardship score. Fully-adjusted models also included a time since waking × saliva sample interaction based on literature showing blunted cortisol responses to acute stress in the morning versus afternoon (Dickerson & Kemeny, 2004). We previously reported sex differences in cytokine-cortisol relations (Riis et al., 2015), so a sex × cytokine interaction was also included in fully-adjusted models. The main independent variables in fully-adjusted models were the cytokine of interest, the maternal distress score, and the cytokine × maternal distress interaction. Four parsimonious final models were created by subjecting fully-adjusted models to backwards stepwise selection of covariates using a p<.05 retention criterion. The main independent variables were retained for final models and not subjected to the backwards stepwise selection. Model fit was assessed for each final model using residual and added variable plots.
In addition to including financial hardship as a covariate, post-hoc models examined the specificity of the moderating effect of maternal distress on cytokine-cortisol relations by testing the significance of cytokine × financial hardship interaction terms in final models with maternal distress included as a covariate.
All analyses, except factor analyses, were conducted in Stata/SE 12.1 (StataCorp LP, College Station, Texas). Factor analyses were conducted in MPlus Version 7.11 (Muthen and Muthen, Los Angeles, California). Sensitivity analyses tested the robustness of the interaction between cytokine concentrations and maternal distress by using a three-level categorical maternal distress variable (created using tertiles) instead of a continuous factor score. In the final models, sensitivity analyses also examined the association between individual maternal distress and family financial variables, rather than factor scores.
Results
Data were collected from 151 mother-child pairs. Three children were unable to complete the appointment, and 23 children had insufficient salivary data. Analyses were performed on the remaining 125 pairs. Sociodemographic and health measures did not differ between children with and without full salivary data. All children were five-years old, approximately half were female, and slightly more than a third were overweight (Table 2). On average, children were awake for seven hours when the study appointment began (Table 2). The families in our sample represented a wide range in SES families; annual incomes ranged from less than $5,000 to greater than $100,000 (Table 1). There was also a wide range of maternal psychological distress among our sample, as assessed by the CES-D, STAI and PSI (Table 1).
Table 2.
Child health and demographic characteristics (n=125)
| Characteristics a | Number (%)/ Mean ± SD |
|---|---|
| Age (years) | 5.45 ± 0.29 |
| Female | 64 (51) |
| Current illness b | 24 (19) |
| Recent periodontal/dental issues c | 14 (11) |
| Mother smokes | 28 (22) |
| Weight status category d | |
| Underweight | 5 (4) |
| Body mass index (kg/m2) | 13.28 ± 0.51 |
| Healthy weight | 76 (61) |
| Body mass index (kg/m2) | 15.75 ± 0.82 |
| Overweight | 25 (20) |
| Body mass index (kg/m2) | 17.56 ± 0.44 |
| Obese | 19 (15) |
| Body mass index (kg/m2) | 20.19 ± 2.84 |
| Time from waking to study appointment (hours) | 7.05 ± 2.41 |
Note. SD=Standard deviation.
Missing data were <3% for all variables except for child age. All children were five years old, however, age in months was missing for 9% of the sample; missing data were imputed with mean values.
Current illness based on maternal response to the question: “Is your child currently feeling sick or ill (i.e., runny nose, fever, cough aching, etc)? (yes/no)”
Child periodontal/dental issues based on maternal response to the question: “Does your child have any current dental problems such as cuts or sores in his or her mouth, very loose teeth, a tooth lost in the last 48 hours, bleeding gums while brushing, or untreated cavities? (yes/no)”
Weight status categories were determined using the Centers for Disease Control and Prevention’s standards for body mass index percentile ranges by age and sex; underweight= < 5th percentile; normal/healthy weight= 5th–85th percentile; overweight= 85th–<95th percentile; obese= ≥95th percentile.
Maternal Psychological Measures
There was strong internal consistency for the CES-D, STAI and PSI in the sample (Cronbach’s α= .90, .85, .90, respectively). Scores on the psychological assessments were also positively correlated: CES-D with STAI: ρ(123)= .84; CES-D with PSI: ρ(123)= .53; STAI with PSI ρ(123)= .60 (all p’s<.001).
Multilevel Mixed Models for Cortisol
Backward stepwise selection of covariates
Seven covariates were retained in each of the four final models (i.e., saliva sample, child sex, sex × cytokine, current illness, financial hardship, time since waking, time since waking × sample). In addition to these covariates, child BMI category was significantly associated with cortisol in models with TNF-α as the main cytokine predictor. Thus, BMI category was included in this final model. In all models, there were significant inverse relations between financial hardship and cortisol indicating that as financial hardship increased, cortisol levels decreased (Table 3).
Table 3.
Adjusted relations between cortisol, cytokines, and maternal distress among five-year old children (n=125)
| Cytokine Main Independent Variable | ||||||||
|---|---|---|---|---|---|---|---|---|
| IL-1β | IL-6 | IL-8 | TNFα | |||||
| Fixed Effectsa | Parameter (SE) | p | Parameter (SE) | p | Parameter (SE) | p | Parameter(SE) | p |
| Intercept | −3.00 (0.13) | <.001 | −3.00 (0.13) | <.001 | −2.97 (0.13) | <.001 | −2.88 (0.13) | <.001 |
| Main Independent Variables | ||||||||
| Cytokine concentration b | −0.13 (0.10) | .19 | −0.15 (0.07) | .04 | −0.27 (0.12) | .03 | −0.11 (0.09) | .22 |
| Maternal distress b | 0.04 (0.13) | .76 | 0.04 (0.13) | .75 | 0.07 (0.13) | .57 | 0.07 (0.13) | .59 |
| Cytokine × maternal distress b | 0.21 (0.05) | <.001 | 0.14 (0.06) | .02 | 0.13 (0.08) | .09 | 0.13 (0.05) | .02 |
| Covariates | ||||||||
| Sample | −0.10 (0.03) | .001 | −0.12 (0.02) | <.001 | −0.13 (0.03) | <.001 | −0.11 (0.03) | <.001 |
| Child sex | −0.09 (0.15) | .56 | −0.06 (0.15) | .69 | −0.08 (0.15) | .58 | −0.07 (0.14) | .64 |
| Child sex × cytokine | 0.27 (0.12) | .03 | 0.28 (0.11) | .01 | 0.40 (0.15) | .007 | 0.20 (0.10) | .04 |
| Child overweight or obese c | - | - | - | - | - | - | −0.38 (0.18) | .03 |
| Child currently sick | 0.46 (0.16) | .005 | 0.44 (0.15) | .004 | 0.47 (0.16) | .003 | 0.51 (0.16) | .001 |
| Family financial hardship | −0.08 (0.02) | .001 | −0.08 (0.02) | .001 | −0.08 (0.02) | <.001 | −0.07 (0.02) | .002 |
| Time since waking | −0.05 (0.03) | .12 | −0.03 (0.03) | .30 | −0.05 (0.03) | .11 | −0.03 (0.03) | .29 |
| Time since waking × sample | −0.02 (0.01) | .01 | −0.02 (0.01) | .009 | −0.02 (0.01) | .009 | −0.02 (0.01) | .01 |
Note. SE= robust standard error; IL-1β= interleukin-1 beta; IL-6= interleukin-6; IL-8= interleukin-8; TNF-α= tumor necrosis factor alpha.
Random effects results shown in Supplemental Table 3.
Main study findings.
Child body mass index category was only significantly associated with cortisol in models with TNF-α, so body mass index category was only retained in the final model with TNF-α as the cytokine main independent variable.
Neuroendocrine-immune relations and moderation by maternal distress
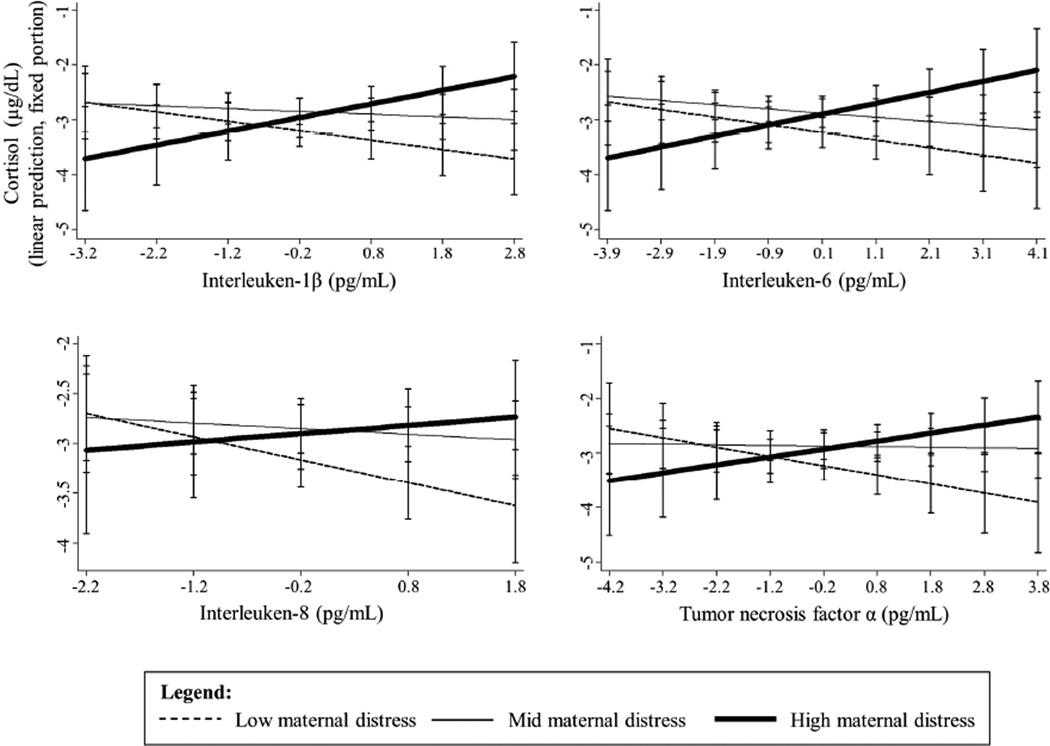
IL-6 and IL-8 were inversely associated with cortisol (Table 3). While there was no main effect of maternal distress on cortisol in any model, there were significant interactions between cytokines and maternal distress for IL-1β, IL-6 and TNF-α (Table 3). Inverse relations between cortisol and IL-1β, IL-6 and TNF-α became less strong with increasing maternal distress (these relationships are illustrated in Figure 1 using the three-category maternal distress variable created using tertiles).
Figure 1.
Maternal distress moderates salivary cytokine-cortisol relations in five-year old children (n=125).a
aCortisol and cytokines are Winsorized and log-transformed. Cytokines are centered. Models were adjusted for child health and demographic factors and family socioeconomic status. Maternal distress categorized using tertiles.
Post-hoc analyses tested the specificity of cytokine × maternal distress interaction effects on cortisol by examining the significance of cytokine × financial hardship in the final models with maternal distress included as a covariate. The cytokine × financial hardship interaction term did not significantly predict cortisol for IL-6, IL-8 and TNF-α. The interaction between IL-1β and financial hardship was significantly and positively associated with cortisol (β=0.03, SE=0.01, p=.02), indicating that the inverse relation between IL-1β and cortisol became less strong as financial hardship increased, a similar pattern to the relation observed for maternal distress.
The role of child sex
There were significant sex × cytokine interactions in every model that indicated stronger, inverse cytokine-cortisol relations among boys than girls (Table 3). To explore these sex-specific relations, we performed stratified analyses for each final model using a homogeneous, independent residual structure and regular variance (i.e., not robust) given the smaller sample sizes.
Cytokine-cortisol relations varied by sex with significant inverse relations between cortisol and all cytokines among boys (β=−0.18 to −0.34, SE=0.05 to0.08, p=<.001 to .004), but not girls. Among girls, IL-1β, IL-6 and IL-8 were positively related to cortisol, although these relations were only marginally significant (β=0.09 to 0.12, SE=0.05 to 0.07, p=.06 to .07). Similarly, cytokine by maternal distress interactions varied by sex for IL-1β, IL-6 and TNF-α with significant positive interactions among girls (β=0.18–0.27, SE=0.07, p=<.001–.009), but not boys, despite the fact t-tests of model coefficients revealed no significant differences between the interaction effects for boys and girls. Among girls, as maternal distress increased, the positive cytokine-cortisol relations became stronger, mirroring the relations in the full sample. Again, we tested the specificity of cytokine × maternal distress interactions in boys and girls separately in post-hoc analyses. No significant cytokine × financial hardship interactions were found among boys. Among girls, the only significant cytokine × financial hardship interaction was seen for IL-1β (β=0.03, SE=0.01, p=.02) and the direction of this effect was the same as that observed in the full sample.
Discussion
Findings from this study provide insight into the sensitivity of child NEI relations to adversity. In our sample of young children from varied SES families, relations between inflammatory immune and HPA activity in saliva varied by maternal distress. Regardless of SES, as maternal distress increased, inverse relations between salivary cytokine and cortisol activity in children became weaker. Systemically, cortisol regulates inflammation by inhibiting the activity of inflammatory cytokines (Irwin & Cole, 2011). This regulatory mechanism is important for preventing excessive and potentially dangerous inflammation (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Irwin & Cole, 2011). Our findings suggest that maternal distress is associated with less efficient regulation of salivary inflammatory mechanisms in children. These findings are consistent with the notion that adversity can result in a “defensive phenotype” characterized by an over-active inflammatory response (Miller et al., 2009; Zhang et al., 2006). While potentially adaptive in the short-term, prolonged up-regulation of inflammatory mechanisms damages cells and increases disease risk (Dantzer et al., 2008).
Interestingly, the significant interactions between cytokines and maternal distress were driven by significant interactions among girls. While cytokine × maternal distress interactions among boys were in the same direction as those in girls, and there were no significant differences in interaction effects by sex, all interaction terms were non-significant among boys. These differences may be related to sex differences in cytokine-cortisol relations. As previously reported (Riis et al., 2015), there were inverse relations between cortisol and every cytokine for boys but not girls. Among girls, cytokines were positively associated with cortisol (although relations were non-significant). Similar sex differences in serum cytokine-cortisol relations have been reported in adults (Dickerson, Gable, Irwin, Aziz, & Kemeny, 2009; Prather et al., 2009; Rohleder, Kudielka, Hellhammer, Wolf, & Kirschbaum, 2002; Rohleder, Schommer, Hellhammer, Engel, & Kirschbaum, 2001). These studies suggest that inflammatory cytokines in males show increased sensitivity to the inhibitory effects of cortisol after acute stress, while the same cytokines in females show no change or decreased sensitivity (Dickerson et al., 2009; Rohleder et al., 2002, 2001). The findings are also consistent with animal studies, which have found the anti-inflammatory effects of glucocorticoids to be less potent in female rats compared to males (Duma, Collins, Chou, & Cidlowski, 2010; Quinn, Ramamoorthy, & Cidlowski, 2014). Studies in mice have also found similar sex differences in the effects of early-life stress on NEI relations. In a series of experiments, Avitsur and colleagues found that postnatal stress was associated with increased production of IL-1β and TNF-α in response to immune challenge in female mice, but not males (Avitsur, Hunzeker, & Sheridan, 2006). Furthermore, enhanced cytokine responses were related to reduced glucocorticoid production, suggesting postnatal stress altered NEI relations in the mice (Avitsur et al., 2006). Taken together, these findings support the notion that females may be more vulnerable to the effects of maternal distress on NEI regulation. If cytokines are predisposed to exhibit decreased sensitivity to cortisol, the desensitization of inflammatory mechanisms associated with adversity may be magnified or observed at lower levels of adversity or earlier in the lifecourse among females.
We observed that financial hardship was inversely associated with cortisol, indicating that as financial hardship increased cortisol levels decreased. These results are consistent with studies showing blunted physiologic stress responses among low-SES children (Blair, Berry, Mills-koonce, Granger, & The FLP Investigators, 2013; Evans & Kim, 2007). SES-related differences in cortisol likely reflect a combination of psychosocial and environmental effects. While we accounted for the distinct effects of maternal distress, other unmeasured SES-related factors likely contributed to the observed financial hardship relations.
Importantly, the moderating effect of maternal distress on cytokine-cortisol relations was significant after adjusting for financial hardship. Also, financial hardship did not moderate NEI relations for IL-6, IL-8 or TNF-α. The only cytokine-cortisol relation moderated by financial hardship was the IL-1β-cortisol relation, and the IL-1b × financial hardship interaction was in the same direction as the cytokine by maternal distress interactions. Additional research is needed to fully understand the role of financial, psychosocial, and environmental factors in modifying NEI relations in children. However, these results suggest that maternal psychological well-being plays a unique role in moderating child NEI relations in saliva and these effects cannot be explained by SES.
Strengths and Limitations
There are several strengths to our study. First, the sample size is large and includes children with diverse SES backgrounds. Also, we measured and adjusted for several aspects of SES, and we examined the discrete effects of maternal psychological well-being using validated instruments. The findings are further strengthened by repeated within-subject sampling and tightly controlled laboratory procedures. Finally, our use of salivary biomeasures represents a novel approach to studying the effects of adversity which allowed us to examine several measures of NEI activity. By examining immune and HPA activity simultaneously, we gained a new, more complex understanding of the physiologic effects of adversity. This study demonstrates the potential of using minimally-invasive methods to study, detect, and treat the physiologic effects of early-life adversity.
Despite these strengths, limitations of the study warrant discussion. While the order of study tasks was constant, the time between saliva samples and stressor tasks depended on the speed at which the child completed the tasks. Therefore, changes in analytes across the visit are considered cumulative responses to all tasks. Variation in cortisol trajectories after acute stressors is typical for young children (Gunnar, Talge, & Herrera, 2009), and our statistical models accounted for varying cortisol trajectories across the visit. We also tested the effect of task timing on cortisol and found non-significant relations in every model. Preliminary models also explored the effects of three-way interactions for cytokine × maternal distress × saliva sample and found no evidence that the interaction between cytokine and maternal distress changed across the study visit.
Our sample included families that represented a wide range in SES, however, income and maternal education were not evenly distributed. There were large proportions of low- and high-income families and fewer middle-income families. Studies with equal representation across socioeconomic strata are needed to fully understand relations between SES and child NEI functioning. Also, we used a maternal distress factor score to examine the combined effect of various dimensions of functioning without overloading models with covariates. This approach, however, limits the clinical significance of the findings as accepted cut-offs for individual instruments are not distinguished. Sensitivity analyses that examined the effect of individual maternal psychological well-being and family SES variables, rather than factor scores, found similar results to those reported here. In addition, all measures, with the exception of biomeasure and BMI data, were based on maternal report and could have introduced the possibility of reporter bias. Finally, longitudinal studies are needed to clarify the direction of the observed relations and the meaning of altered NEI relations in saliva for health and disease risk. Additional research of salivary cytokines, including serum-saliva studies, is also needed to increase our understanding of the meaning of salivary cytokines in healthy children. Furthermore, our findings suggest that maternal distress is related to differences in some, but not all, salivary inflammatory mechanisms examined; further research is needed to understand the sensitivity of specific NEI relations to psychosocial factors. Future research should investigate NEI relations using other markers of inflammation, such as C-reactive protein, which has been found to have stronger serum-saliva correlations than other inflammatory markers (Slavish et al., 2015).
Conclusion
This study advances understanding of potential mechanisms linking early-life adversity and later-life disease and aids in the identification of adversity risk factors. While prior studies reported significant relations between maternal mental health and altered HPA activity in children (Essex et al., 2002; Fernald, Burke, & Gunnar, 2008; Palmer et al., 2013), to our knowledge, this is the first study examining the moderating effect of maternal distress on cross-system NEI relations. The findings suggest that maternal distress can impact child NEI functioning in saliva by altering the sensitivity of inflammatory immune processes to the inhibitory effects of cortisol. While additional research on salivary NEI functioning is needed, this desensitization may indicate increased risk for chronic inflammation and disease (Danese & McEwen, 2012). Currently little is known about the meaning of salivary markers of immune and NEI relations in children for overall health, however, higher levels of salivary inflammatory activity have been associated with sleep disruptions (El-Sheikh et al., 2007) and behavioral and mood disorders in young children (Keller et al., 2010). If left unchecked, poorly regulated inflammatory activity in adulthood is associated with a range of health conditions including depression, obesity, and heart disease (Barnes & Adcock, 2009; Danese & McEwen, 2012; Dantzer et al., 2008).
Our findings add to a large body of research indicating the critical role of caregivers in child health (e.g., Goodman et al., 2011; Halligan, Herbert, Goodyer, & Murray, 2004; Shankardass et al., 2009; Surkan, Kennedy, Hurley, & Black, 2011; Wright, 2007). Our finding that maternal distress is associated with changes in child NEI relations regardless of family SES highlights the importance of identifying and treating maternal mental health problems in families of all socioeconomic strata. The American Academy of Pediatrics recommends screening for post-partum depression as part of standard well child care (Tanski, Garfunkel, Duncan, & Weitzman, 2010). However, recently, pediatricians have been called upon to play a larger role in identifying and treating maternal mental health issues (Chemtob, Gudiño, & Laraque, 2013; Garner et al., 2012). Our findings support screening for maternal depression, and underscore the potential benefit of clinical, family and community-based programs and policies that support mothers and help protect maternal as well as child health and well-being.
Supplementary Material
Research highlights.
Stress may alter child neuroendocrine-immune (NEI) function, increasing disease risk.
We examined whether maternal psychological distress moderates child NEI relations.
Child NEI functioning was measured using salivary biomarkers.
Maternal distress moderated child NEI relations in saliva.
The effects of maternal distress on NEI relations were stronger in girls than boys.
Acknowledgments
We are grateful to Jessica Bayer, Tracey Hand, Jessica Parker, and Jessica Acevedo at Johns Hopkins and Arizona State University for technical support with salivary assays. This research was supported by KO1DA027229-01 from NIH/NIDA to SBJ. The funding source was not involved in the study design, data collection, analysis and interpretation of data, nor in the writing of the manuscript and the decision to submit it for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure:
DAG has a financial relationship with Salimetrics LLC (Carlsbad, CA). All other authors have nothing to disclose.
Conflict of interest:
DAG is founder and Chief Scientific and Strategy Advisor at Salimetrics LLC (Carlsbad, CA), and this relationship is managed by the policies of the committees on conflict of interest at the Johns Hopkins University School of Medicine and the Office of Research Integrity and Adherence at Arizona State University. The other authors have no conflicts of interests.
Contributor Information
Jenna L. Riis, Email: jriis1@jhu.edu.
Douglas A. Granger, Email: Douglas.Granger@asu.edu.
Cynthia S. Minkovitz, Email: cmink@jhu.edu.
Karen Bandeen-Roche, Email: kbandee1@jhu.edu.
Janet A. DiPietro, Email: jdipiet1@jhu.edu.
Sara B. Johnson, Email: sjohnson@jhu.edu.
References
- Aibidin RR. Parenting Stress Index. Professional Manual. 3rd. Odessa, Fl 33556: Psychological Assessement Resources, Inc; 1995. [Google Scholar]
- Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain, Behavior, and Immunity. 2006;20(4):339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Barnes P, Adcock I. Glucocorticoid resistance in inflammatory diseases. The Lancet. 2009;373(9678):1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- Blair C, Berry D, Mills-koonce R, Granger DA The FLP Investigators. Cumulative effects of early poverty on cortisol in young children : Moderation by autonomic nervous system activity. Psychoneuroendocrinology. 2013;2013 doi: 10.1016/j.psyneuen.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D, Silverstein M. Shedding light on the long shadow of childhood adversity. JAMA : The Journal of the American Medical Association. 2013;309(17):1777–1778. doi: 10.1001/jama.2013.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Data Table of BMI-for-age Charts. 2001 Retrieved from http://www.cdc.gov/growthcharts/html_charts/bmiagerev.htm.
- Centers for Disease Control and Prevention. About BMI for Children and Teens. 2011 Retrieved from http://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html.
- Chemtob CM, Gudiño OG, Laraque D. Maternal posttraumatic stress disorder and depression in pediatric primary care: association with child maltreatment and frequency of child exposure to traumatic events. JAMA Pediatrics. 2013;167(11):1011–1018. doi: 10.1001/jamapediatrics.2013.2218. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2011;16(7):729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough N, Roth J. Understanding Immunology. St. Louis, Missouri: Mosby-Year Book, Inc.; 1998. [Google Scholar]
- Coe CL, Lubach GR. Critical periods of special health relevance for psychoneuroimmunology. Brain, Behavior, and Immunity. 2003;17:3–12. doi: 10.1016/s0889-1591(02)00099-5. [DOI] [PubMed] [Google Scholar]
- Cole PM, Zahn-Waxler C, Smith KD. Expressive Control During a Disappointment: Variations Related to Preschoolers’ Behavior Problems. Developmental Psychology. 1994;30(6):835–846. [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews. Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME. Social-Evaluative Threat and Proinflammatory Cytokine Regulation: An Experimental Laboratory Investigation. Psychological Science. 2009;20(10):1237–1244. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- DiPietro J, Kivlighan K, Costigan K, Rubin S, Shiffler D, Henderson J, Pillion J. Prenatal Antecedents of Newborn Neurological Maturation. Child Development. 2010;81(1):115–130. doi: 10.1111/j.1467-8624.2009.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric Z, Bird CE, Furumoto-Dawson A, Rauscher GH, Ruffin MT, Stowe RP, Masi CM. Biomarkers of Psychological Stress in Health Disparities Research. The Open Biomarkers Journal. 2008;1(1):7–19. doi: 10.2174/1875318300801010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duma D, Collins J, Chou J, Cidlowski J. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Science Signaling. 2010;3(143):ra74. doi: 10.1126/scisignal.2001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Granger DA, Erath SA, Acebo C. The association between children’s sleep disruption and salivary interleukin-6. Journal of Sleep Research. 2007;16(2):188–197. doi: 10.1111/j.1365-2869.2007.00593.x. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biological Psychiatry. 2002;52(8):776–784. doi: 10.1016/s0006-3223(02)01553-6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12372649. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood Poverty and Health: Cumulative Risk Exposure and Stress Dysregulation. 2007;18(11):953–958. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Fernald LCH, Burke HM, Gunnar MR. Salivary cortisol levels in children of low-income women with high depressive symptomatology. Development and Psychopathology. 2008;20(2):423–436. doi: 10.1017/S0954579408000205. [DOI] [PubMed] [Google Scholar]
- Gagne JR, Hulle CA Van, Aksan N, Essex MJ, Goldsmith HH. Deriving Childhood Temperament Measures From Emotion-Eliciting Behavioral Episodes: Scale Construction and Initial Validation. Psychological Assessment. 2011;23(2):337–353. doi: 10.1037/a0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner A, Shonkoff J, Siegel B, Dobbins M, Earls M, McGuinn L, Wood D. Early childhood adversity, toxic stress, and the role of the pediatrician: translating developmental science into lifelong health. Pediatrics. 2012;129(1):e224–e231. doi: 10.1542/peds.2011-2662. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clinical Child and Family Psychology Review. 2011;14(1):1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34(7):953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry. 2004;55(4):376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Boyce T. How Experience Gets Under the Skin to Create Gradients in Devleopmental Health. Annual Review of Public Health. 2009;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature Reviews. Immunology. 2011;11(9):625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S, Sugaya N, Kimura K, Ogawa N, Yamada KC, Shirotsuki K, Nomura S. An increase in salivary interleukin-6 level following acute psychosocial stress and its biological correlates in healthy young adults. Biological Psychology. 2013;94(2):249–254. doi: 10.1016/j.biopsycho.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Jahromi LB, Stifter CA. Individual Differences in Preschoolers’ Self-Regulation and Theory of Mind. Merrill-Palmer Quarterly. 2008;54(1):125–150. [Google Scholar]
- Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319–327. doi: 10.1542/peds.2012-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller PS, El-Sheikh M, Vaughn B, Granger DA. Relations between mucosal immunity and children’s mental health: the role of child sex. Physiology & Behavior. 2010;101(5):705–712. doi: 10.1016/j.physbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieras JE, Tobin RM, Graziano WG, Rothbart MK. You Can’t Always Get What You Want. Effortful Control and Children's Response to Undesirable Gifts. Psychological Science. 2005;16(5):391–396. doi: 10.1111/j.0956-7976.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Marques AH, Silverman MN, Sternberg EM. Evaluation of stress systems by applying noninvasive methodologies: measurements of neuroimmune biomarkers in the sweat, heart rate variability and salivary cortisol. NeuroImmunoModulation. 2010;17:205–208. doi: 10.1159/000258725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stressed or stressed out: What is the difference? Journal of Psychiatry and Neuroscience. 2005;30(5):315–318. [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If It Goes Up Must It Come Down? Chronic Stress and the Hypothalamic-Pituitary-Adrenocortical Axis in Humans. Psychological Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002;21(6):531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychological Science. 2011;22(12):1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel HN, Mischel W. The Development of Children’s Knowledge of Self-control Strategies. Child Development. 1983;54:603–619. [Google Scholar]
- Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stress have different biological profiles. Brain, Behavior, and Immunity. 2010;24(2):215–219. doi: 10.1016/j.bbi.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Owen J, Punt J, Stranford S. Kuby Immunology. 7th. New York, NY: W. H. Freeman; 2012. [Google Scholar]
- Palmer FB, Anand KJS, Graff JC, Murphy LE, Qu Y, Völgyi E, Tylavsky Fa. Early adversity, socioemotional development, and stress in urban 1-year-old children. The Journal of Pediatrics. 2013;163(6):1733.e1–1739.e1. doi: 10.1016/j.jpeds.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Prather AA, Carroll JE, Fury JM, McDade KK, Ross D, Marsland AL. Gender differences in stimulated cytokine production following acute psychological stress. Brain Behavior and Immunity. 2009;23(5):622–628. doi: 10.1016/j.bbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M, Ramamoorthy S, Cidlowski Ja. Sexually dimorphic actions of glucocorticoids: Beyond chromosomes and sex hormones. Annals of the New York Academy of Sciences. 2014;1317(1):1–6. doi: 10.1111/nyas.12425. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Riis JL, Granger DA, DiPietro JA, Bandeen-Roche K, Johnson SB. Salivary Cytokines as a Minimally-Invasive Measure of Immune Functioning in young children: Correlates of Individual Differences and Sensitivity to Laboratory Stress. Developmental Psychobiology. 2015;57(2):153–167. doi: 10.1002/dev.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis JL, Out D, Dorn LD, Beal SJ, Denson L, Pabst S, Granger DA. Salivary cytokines in healthy adolescent girls: Intercorrelations, stability, and associations with serum cytokines, age, and pubertal stage. Developmental Psychobiology. 2013 Jun; doi: 10.1002/dev.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Kudielka BM, Hellhammer DH, Wolf JM, Kirschbaum C. Age and sex steroid-related changes in glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Journal of Neuroimmunology. 2002;126(1–2):69–77. doi: 10.1016/s0165-5728(02)00062-0. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12020958. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosomatic Medicine. 2001;63(6):966–972. doi: 10.1097/00006842-200111000-00016. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11719636. [DOI] [PubMed] [Google Scholar]
- Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(30):12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J, Garner A, Siegel B, Dobbins M, Earls M, McGuinn L, Wood D. The Lifelong Effects of Early Childhood Adversity and Toxic Stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP. Leveraging the biology of adversity to address the roots of disparities in health and development. Proc.Natl.Acad.Sci.U.S.A. 2012;109(Supplement 2):17302–17307. doi: 10.1073/pnas.1121259109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavish DC, Graham-Engeland JE, Smyth JM, Engeland CG. Salivary markers of inflammation in response to acute stress. Brain Behavior and Immunity. 2015 Sep;44:253–269. doi: 10.1016/j.bbi.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: a systematic review. Brain, Behavior, and Immunity. 2012;26(2):239–250. doi: 10.1016/j.bbi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Spinrad TL, Eisenberg N, Granger DA, Eggum ND, Sallquist J, Haugen RGG, Hofer C. Individual differences in preschoolers’ salivary cortisol and alpha-amylase reactivity: relations to temperament and maladjustment. Hormones and Behavior. 2009;56(1):133–139. doi: 10.1016/j.yhbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surkan PJ, Kennedy CE, Hurley KM, Black MM. Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bulletin of the World Health Organization. 2011;89(8):608–615. doi: 10.2471/BLT.11.088187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnik BG, Fidell LS. Using Multivariate Analysis. 4th. Boston, MA: Allyn and Bacon; 2001. [Google Scholar]
- Tanski S, Garfunkel LC, Duncan PM, Weitzman M. Performing Preventive Services: A Bright Futures Handbook. 2010. [Google Scholar]
- Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatric and Perinatal Epidemiology. 2007;21(Suppl 3):8–14. doi: 10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- Zhang T-Y, Bagot R, Parent C, Nesbitt C, Bredy TW, Caldji C, Meaney MJ. Maternal programming of defensive responses through sustained effects on gene expression. Biological Psychology. 2006;73(1):72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.