Abstract
Background and Purpose
Previous studies revealed a close relationship between thrombus length and recanalization rate after intravenous thrombolysis (IVT). As a novel approach, we prospectively adjusted the order of sequence acquisition to obtain delayed gadolinium-enhanced T1 (dGE-T1) and thereby assess thrombus length on dGE-T1 to evaluate its predictive value for recanalization after IVT.
Methods
We reviewed prospectively collected clinical and imaging data from acute ischemic stroke patients with middle cerebral artery (MCA) occlusion who underwent multimodal MRI before and 24 hours after IVT. Perfusion-weighted imaging (PWI) was performed followed by conventional T1. We measured thrombus length on dGE-T1 and examined its association with MCA recanalization.
Results
Of the included 74 patients, the median age was 66 years and 28 (37.8%) were women. Thrombus length was 8.18 ± 4.56 mm on dGE-T1, which was an acceptable predictor for no recanalization (odds ratio 1.196; 95% CI: 1.015 to 1.409; p=0.033), with a receiver-operator characteristic of 0.732 (95% CI: 0.619 to 0.845; p=0.001). The optimal cut-off point was identified at 6.77 mm, which yielded a sensitivity of 77.8%, a specificity of 57.9%, and odds ratio 4.81 (95% CI: 1.742 to 13.292; p=0.002). Moreover, no one achieved recanalization after IVT when length of thrombus exceeded 14 mm on dGE-T1.
Conclusions
The dGE-T1, obtained by simply adjusting scanning order in multimodal MRI protocol, is a useful tool for thrombus length estimation and MCA recanalization prediction after IVT.
Keywords: magnetic resonance imaging, stroke, thrombolytic therapy, middle cerebral artery
Introduction
Arterial recanalization is extremely important in acute ischemic stroke due to middle cerebral artery (MCA) occlusion, where the natural history carries a poor prognosis.1 Target clot burden, measured by thrombus length, has been shown to be an important determinant of recanalization in intravenous thrombolysis (IVT).2,3 Our previous study has already demonstrated that the morphology of susceptibility vessel sign (SVS) on susceptibility weighted imaging (SWI) could predict MCA recanalization after IVT.4 In the patients with MCA occlusion, thrombus location as seen on SWI correlates well with time-of-flight magnetic resonance angiography (TOF-MRA), first-pass gadolinium-enhanced MRA (GE-MRA) and digital subtraction angiography (DSA).5 However, in clinical practice, about 26% to 73% patients with MCA occlusion will not present the sign of SVS due to the clot composition.4,6,7 Moreover, the sign of SVS usually overestimate the true length and volume of clot due to the blooming artifact,8 which may make it difficult to accurately measure the clot burden.
With multimodal MRI, we observed that conventional T1 which is obtained after perfusion weighted image (PWI) (≈ 90s) can generate delayed gadolinium-enhanced T1 (dGE-T1), which can visualize the distal vessels beyond the clot within the artery. Using co-registration of dGE-T1 with TOF-MRA which only displays the arteries devoid of occlusion, it is thus possible to estimate the length of thrombus. This method will be manageable without adding extra scan time and contrast agent, when PWI/diffusion-weighted imaging (DWI) mismatch profile was used to guide IVT for extending the time window (up to 6 hours).9,10 Therefore, in the current study, we assessed for the first time the thrombus length on dGE-T1 in patients with MCA occlusion by adjusting the scanning order of conventional T1 after PWI, and then evaluated its predictive value for MCA recanalization after IVT, in order to find a new measurement of the clot burden, which can be effectively used in all patients with MCA occlusion.
Subjects and Methods
Study Subjects
We retrospectively reviewed our prospectively collected database for consecutive patients with acute ischemic stroke received thrombolytic therapy between June 2009 and March 2015. We then enrolled patients who (i) had a diagnosis of acute ischemic stroke confirmed by DWI; (ii) received IVT within 6 hours from symptom onset; (iii) underwent multimodal MRI in the following sequences: DWI, SWI, TOF-MRA, fluid-attenuated inversion recovery (FLAIR), PWI and then conventional T1; (iv) MCA occlusion without involvement of internal carotid artery demonstrated on baseline TOF-MRA; (v) underwent follow-up TOF-MRA 24 hours after IVT. The “mismatch” profile was defined as a PWI lesion that was 10 ml or more and 120% or more of the DWI lesion.9,10 We excluded patients who were treated with combined endovascular and IVT, and whose image quality was poor due to motion artifacts.
We retrieved demographic, clinical, laboratory, and radiological data including age, sex; comorbid conditions such as history of hypertension, diabetes mellitus, coronary heart disease and atrial fibrillation; prior antiplatelet use; time interval from stroke onset to treatment; National Institutes of Health stroke scale (NIHSS) score, serum platelet and international normalized ratio (INR) level before IVT; baseline infarct volume, occlusion site, recanalization 24 hours after IVT, and modified Rankin Scale (mRS) score after 3 months.
MRI parameters
All subjects underwent MRI on a 3.0T system (Signa Excite HD, General Electric Medical System, Milwaukee, USA) equipped with an 8-channel phased array head coil. DWI sequence was used to measure the infarct volume (TR = 4000 ms; TE = 69.3 ms; b-value = 1000 s/mm2; slice thickness = 5.0 mm; interslice gap= 1.0 mm). SWI used 11 equally spaced echoes: echo time = 4.5 ms [first echo]; inter-echo spacing = 4.5 ms; repetition time = 58 ms; flip angle = 20°; slice thickness = 2.0 mm with no gap between slices. TOF-MRA consisted of 3 slabs with TR = 20 ms; TE = 3.2 ms; flip angle = 15°; slice thickness = 1.4 mm. FLAIR parameters were TR = 9000 ms; TE = 150 ms; TI = 2250 ms; slice thickness = 5.0 mm. PWIs were obtained using the standard bolus passage of contrast method by injecting gadolinium (0.1 mmol/kg dose via power injector). PWI parameters were TR = 1500 ms; TE = 30 ms; slice thickness = 5.0 mm. Conventional T1 parameters were TR = 1900 ms; TE = 25 ms; slice thickness = 5.0 mm.
Imaging analysis
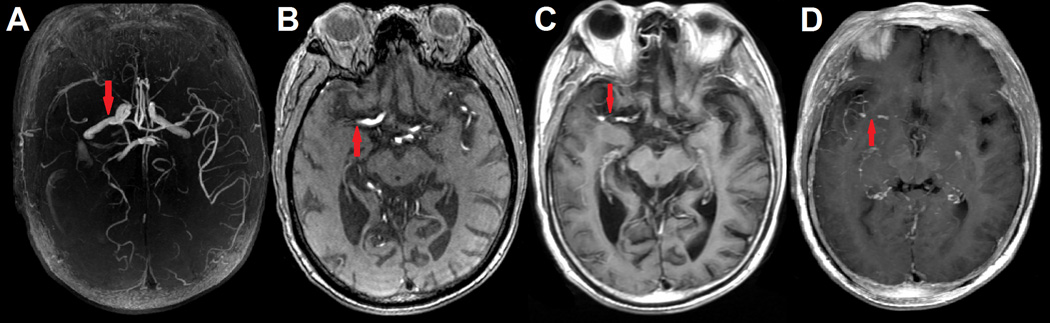
Acute arterial occlusion was determined by the invisibility of the artery on MRA with corresponding symptoms compatible with the involved artery. Occluded arteries on initial MRA were identified as M1 occlusion or M2 occlusion in the current study. Two neurologists, blinded to patients’ clinical information, independently assessed the thrombus length on dGE-T1 and SWI, respectively. SVS was defined as presence of hypointensity in MCA with a blooming artifact, i.e., the diameter exceeded the hypointense signal in the homologous contralateral vessel diameter on GRE or SWI scans.11,12 Distal vessels beyond the clot can be visible on dGE-T1 clearly, while TOF-MRA only displayed the arteries without occlusions (supplemental Figure I, please see http://stroke.ahajournals.org), so that we co-registered the source images of TOF-MRA and dGE-T1 with MRIcron (Chris Rorden, University of South Carolina, Columbia, USA) and then manually measured the distance between proximal and distal end of MCA occlusion as thrombus length by using a straight line (Image J, National Institutes of Health, USA) (Figure 1). When the proximal and distal end of the thrombus were not shown on a single slice, maximum intensity projection (MIP) images (20–30 mm thick) was used. In case of continuous M1 and M2 segment occlusions, the length was summed. When thrombus branched into two or more M2 vessels, the longest thrombus length was measured.
FIGURE 1.
Occlusion site on delayed gadolinium-enhanced T1 (dGE-T1) and time-of-flight magnetic resonance angiography (TOF-MRA) of a patient with right middle cerebral artery (MCA) occlusion. (A) was reconstructed TOF-MRA indicated right MCA occlusion. (B) was source axial image of TOF-MRA at the occlusion site (red arrow). (C) was axial image of dGE-T1 that coregistered and then merged to raw axial image of TOF-MRA.
Evaluation of Outcome
We used the Arterial Occlusive Lesion (AOL) scale (Grade 0: complete occlusion of the target artery; Grade 1: incomplete occlusion or partial local recanalization at the target artery with no distal flow; Grade 2: incomplete occlusion or partial local recanalization at the target artery with any distal flow; Grade 3: complete recanalization and restoration of the target artery with any distal flow) to define recanalization or no recanalization based on the presence (Grades 2 or 3) or absence (Grades 0 or 1) of any downstream flow.13 Clinical outcome at 3 months was assessed with mRS and dichotomized into good outcome (0 to 2) and poor outcome (3 to 6).
Reliability and validity of the radiological data
The two investigators who jointly evaluated the MRI findings were blinded to the patients’ clinical data. A single trained observer (S.Y.) measured thrombus length of all patients twice, at an interval of 3 months apart. Another observer (Q.C.) independently made the same evaluation.
Statistical analysis
Fisher's exact test was used to compare the dichotomous variables between groups, while independent samples two-tailed t-test or Mann-Whitney U test was used for the continuous variables, as appropriate. Variables with a P value of < 0.1 in univariate analyses were included in the binary logistic regression model. Pearson's or Spearman's correlation analysis was used depending on the normality of the distribution. Receiver operating characteristics (ROC) curve analysis was used to determine predictive value. Statistical significance was set at a P value of < 0.05. All statistical analysis was performed with SPSS package.
Results
The inter-observer and intra-observer reliabilities about the measurements of thrombus length on dGE-T1 were excellent (intraclass correlation coefficients = 0.977 and 0.941, respectively). A total of 74 remaining patients were included for the final analysis. Demographic, clinical and laboratory data were not different between included and excluded subjects. Of the included patients, the median age was 66 years (mean 66 ± 13 years, range 43–94 years) and 28 (37.8%) were women. Median baseline NIHSS score was 13 (IQR 6–17). Mean time from onset to treatment was 240 ± 85 minutes.
Follow-up MRA 24 hours after IVT revealed recanalization in 38 (51.4%) patients and no recanalization in 36 (48.6%). SVS sign on SWI existed in 78.4% (58 in 74) of the included patients. The mean length of SVS was 12.62 ± 7.59 mm (range 3.83–47.23 mm). Thrombus length on dGE-T1 was 8.18 ± 4.56 mm (range 1.63–30.26 mm). MIP images were used in 21 subjects, mainly because their thrombus length was longer than those measured on a single slice (12.50 ± 5.15 mm versus 6.47 ± 2.91 mm, p<0.001). The thrombus length measured by SVS was longer than that measured on dGE-T1, with the overestimation ratio ranging from 1.03 to 3.47. However, these two measurements had a good association (Pearson r = 0.805, p<0.001) in the 58 patients with SVS.
Table 1 shows the characteristics of patients with and without recanalization for comparison. Patients with recanalization had a lower rate of M1 occlusion and poor outcome. Thrombus length on dGE-T1 was significantly longer in patients without recanalization in univariate analysis. Moreover, age was marginally associated with recanalization. Thus, age, M1 occlusion, and thrombus length were included in the binary logistic regression model. Both thrombus length and M1 occlusion were independent predictors for no recanalization of MCA (Table 2). ROC analysis revealed acceptable predictive value of thrombus length for recanalization (AUC = 0.732, 95% CI: 0.619–0.845, p=0.001). The cut-off point of thrombus length measured on dGE-T1 for no recanalization was 6.77 mm, and this yielded a sensitivity of 77.8% and a specificity of 57.9%, and odds ratio 4.81 (95% CI: 1.742 to 13.292; p=0.002). Moreover, no one achieved recanalization after IVT when length of thrombus exceeded 14 mm on dGE-T1.
TABLE 1.
Univariate comparison between patients with and without recanalization
| Variable | MCA Recanalization | P value | |
|---|---|---|---|
| No (n = 36) | Yes (n = 38) | ||
| Age (year) | 63.2 ± 13.8 | 68.9 ± 12.1 | 0.065 |
| Female | 15(41.7%) | 13(34.2%) | 0.633 |
| Comorbid conditions | |||
| Hypertension | 23(63.9%) | 27(71.1%) | 0.621 |
| Diabetes mellitus | 3(8.3%) | 6(15.8%) | 0.481 |
| Coronary heart disease | 3(8.3%) | 6(15.8%) | 0.481 |
| Atrial fibrillation | 16(44.4%) | 22(57.9%) | 0.352 |
| Clinical variables | |||
| NIHSS score | 14(8–17) | 11(6–16) | 0.260# |
| Onset to treatment (min) | 242.3 ± 101.3 | 236.8 ± 66.7 | 0.786 |
| Prior antiplatelet use | 6(16.7%) | 5(13.2%) | 0.751 |
| Platelet (109/L) | 192.4 ± 70.0 | 173.5 ± 52.5 | 0.193 |
| INR | 1.030(0.990–1.080) | 1.035(1.005–1.090) | 0.762# |
| Radiologic data | |||
| Baseline infarct volume (ml) | 10.8(3.9–55.1) | 7.5(2.4–33.5) | 0.265# |
| M1 occlusion | 32(88.9%) | 17(44.7%) | <0.001 |
| Thrombus length (mm) | 9.99 ± 5.20 | 6.47 ± 3.06 | 0.001 |
| Poor clinical outcome, mRS ≥ 3 | 28(77.8%) | 14(36.8%) | <0.001 |
MCA = middle cerebral artery; NIHSS = National Institutes of Health Stroke Scale; INR = international normalized ratio.
Mann-Whitney U test.
TABLE 2.
Multivariate regression analysis of independent predictors for no recanalization in the patients with MCA occlusions
| OR | 95% CI | P value | |
|---|---|---|---|
| Age (year) | 0.994 | 0.952–1.037 | 0.773 |
| M1 occlusion | 6.555 | 1.828–23.501 | 0.004 |
| Thrombus length (mm) | 1.196 | 1.015–1.409 | 0.033 |
MCA = middle cerebral artery; OR = odds ratio; CI = confidence interval.
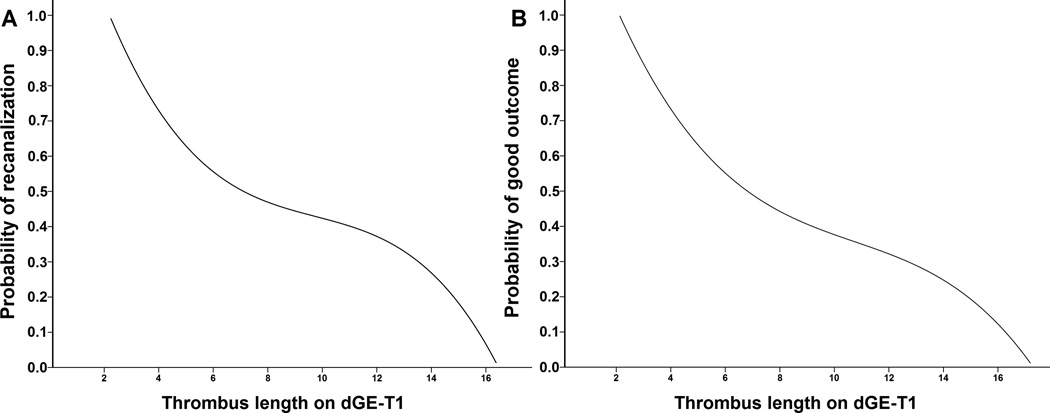
Patients with poor outcome were older, had higher frequency of M1 occlusion, higher baseline NIHSS score, larger DWI volume and longer thrombus length than those with good outcome. The thrombus length on dGE-T1 was an significant independent predictor for poor outcome (odds ratio 1.253; 95% CI: 1.026 to 1.529; p=0.027) after adjusting for baseline NIHSS score, age, baseline DWI volume and M1 occlusion in the binary logistic regression model (Table 3). The cut-off point of thrombus length measured on dGE-T1 for poor outcome was 7.71 mm, and this yielded a sensitivity of 59.5% and a specificity of 75.0%, and odds ratio 4.41 (95% CI: 1.607 to 12.112; p=0.004). The relationship between thrombus lengths on dGE-T1 and probability of recanalization and good outcome were shown in Figure 2.
TABLE 3.
Multivariate regression analysis of independent predictors for poor clinical outcome
| OR | 95% CI | P value | |
|---|---|---|---|
| Age (year) | 1.087 | 1.020–1.159 | 0.010 |
| NIHSS score | 1.174 | 1.052–1.311 | 0.004 |
| Baseline infarct volume (ml) | 1.007 | 0.991–1.023 | 0.450 |
| M1 occlusion | 5.748 | 1.338–23.803 | 0.016 |
| Thrombus length (mm) | 1.253 | 1.026–1.529 | 0.027 |
OR = odds ratio; CI = confidence interval; NIHSS = National Institutes of Health Stroke Scale.
FIGURE 2.
Regression curve representing an estimate of recanalization (A) and good functional outcome (B) probability by intravenous thrombolysis depending on the thrombus length measured on delayed gadolinium-enhanced T1 (dGE-T1).
Discussion
In the current study, we found that about 20% patients of acute ischemic stroke with MCA occlusion will not present SVS sign on SWI. We thus established a novel approach for estimation of thrombus length with dGE-T1, which can be obtained in all patients of MCA occlusion by changing the scanning order of conventional T1 after PWI. We further demonstrated that this measurement of thrombus length could independently predict MCA recanalization and clinical outcome after IVT.
The length of large vessel occlusion is considered a major influencing factor for the prognosis after IVT in patients with acute ischemic stroke.2,3 On non-enhanced CT (NCCT), it was found that IVT had nearly no potential to recanalize the occluded vessels if the length of thrombus exceeds 8 mm, which was depicted as hyperdense middle cerebral artery sign (HMCAS).3 The HMCAS may be absent in approximately 50–70% patients with acute MCA occlusion using standard 3 mm NCCT slice thicknesses,14,15 while the sensitivity would become higher by using thin-slice NCCT reconstructions.3 However, Rohan et al’s study did not confirm the specificity of a threshold of 8 mm for recanalization,2,3 which may be due to the different thrombus constituents with the presence of HMCAS.15 Recently, Mortimer et al also found that delayed contrast enhanced CT can reliably be used to estimate thrombus length,16 which indicates that the use of contrast may improve the visualization of thrombus.
Obviously, delineating the distal end of thrombus is the key to accurately measure the length of thrombus. Considering that retrograde flow from the patent pial collaterals is the dominant source to reach the distal end of occlusion, we prefer to utilize the images which can fully display the retrograde flow of contrast, to determine the distal end. Usually, the maximal enhancement in a non-occluded artery was observed around 30s after the administration of contrast, with a plateau at around 80–120s after contrast injection.17 As we can see, a delayed phase of gadolinium-enhanced T1, which occurred when conventional T1 was obtained after PWI (usually taking 90s after contrast injection), allows several passes of contrast, which can even visualize the slow retrograde flow of contrast, making the face of distal clot fully delineated.
This evaluation of thrombus length on dGE-T1 in our study was confirmed to be comparable to SVS on SWI. While SVS on SWI is limited in the clinical practice due to its blooming effect and invisibility in about 26% to 73% of patients, the application of thrombus length on dGE-T1 will be wide in all patients with MCA occlusion. It is worthwhile to note that, in contrast to SWI, dGE-T1 is an indirect technique to visualize the thrombus length. Due to partial volume effects, each bright T1-weighted structure will simulate an open vessel, even in the close surrounding area of the thrombus. A thrombus may not be detected in its full length as the high signal of gadolinium in the open vessel dominates the signal of the whole voxel. In addition, similar false results can be produced by enhancement of the vessel wall, enhancement of adjacent veins or penetration/diffusion of gadolinium into the thrombus. Therefore, the possibility also exists that there is an underestimation of thrombus length by the indirect visualization on dGE-T1 due to the above mentioned technical factors. Furthermore, slice thickness on SWI sequences was 2.0 mm, whereas slice thickness on dGE-T1 was 5.0 mm, which might also explain some differences in thrombus length estimation.
Recently, Friedrich et al demonstrated an inverse correlation between distance from carotic terminus to thrombus (DT) and a poor clinical outcome,18 which indicated the importance of thrombus location, since a long DT might represent proximal MCA occlusion along with the occlusion of lenticulostriate perforator, which would have a great impact on clinical outcome. However, the relationship between DT and recanalization still remains unclear. It would be interesting in future studies to investigate whether a comprehensive measurement of thrombus, including length and DT, could improve patient triage and finally improve overall patient outcomes when reperfusion therapy is needed.
Intra-arterial thrombectomy has been established as an effective treatment in patients with documented occlusion of the distal internal carotid or proximal MCA.19–23 Our results may not directly affect clinical decision-making, however, it is still worth exploring whether IVT with rt-PA might be omitted in patients who can be quickly moved to the endovascular suite in future investigations, if the rate of recanalization with IVT is considered very low based on the thrombus length. On the other hand, the prediction of recanalization after IVT would also help the clinicians in the hospitals without endovascular suites to evaluate the cost effectiveness and the quick decision of transference to the comprehensive stroke center for endovascular treatment. Recently, Weisstanner et al have shown that recanalization rates are independent from the length of the thrombus, if modern endovascular recanalization techniques are applied with highly increased rate of recanalization.5 Certainly, it is also worth exploring the relationship between thrombus length on dGE-T1 and recanalization or clinical outcome in patients received combined IVT and endovascular treatment in the future.
Most excitingly, a recent study has showed that it was feasible and practical to achieve the benchmark of door-to-needle (DTN) time ≤ 60 minutes, by using MRI as the routine screening modality before reperfusion therapy.24 MRI is unlikely to be faster than CT currently, not only because of the longer acquisition duration, but also because of the time taken for safety screen, access to the scanner and image acquisition. However, multimodal MRI was proved to easily exclude stroke mimics, make diagnosis of small stroke even located in posterior circulation, and provide comprehensive information that is useful for clinical decision-making. Further studies may need to investigate whether the benefits of multimodal MRI outweigh the harms of the time delay. On the other hand, we would like to note that our novel protocol provides more information about clot, not needing to add extra scan time and contrast agent.
Our study had several limitations. First, although we prospectively collected data using a stroke registry and MRI protocol, our study had a retrospective design and might have a potential risk of selection bias. Second, there is a lack of information about early recanalization since the thrombus length was determined 24 hours after IVT. Third, thrombus length is hard to be accurately measured via real vascular pathways on dGE-T1. However, this is also difficult to be achieved on DSA. Fourth, the use of dGE-T1 could theoretically differentiate slow flow, incomplete stenosis from occlusion, however, this has not yet been confirmed by DSA. Fifth, we only included patients with MCA occlusion to reduce the heterogeneity. It would be clinically important to determine the specific threshold of thrombus length for both anterior cerebral artery and internal carotid artery in the future. Finally, the sample size was modest and was performed at a single center. Confirmation and extension in larger and multicenter cohorts is needed.
In summary, we presented a novel approach to estimate the thrombus length on dGE-T1, which can be easily obtained by adjusting scanning order in multimodal MRI protocol. The thrombus length measured on dGE-T1 was proved to independently predict MCA recanalization and clinical outcome after IVT.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the Science Technology Department of Zhejiang Province (2013C03043-3), the National Natural Science Foundation of China (81171095 & 81471170).
Disclosure
Dr Liebeskind: consultant/advisory board (modest)-Stryker and Medtronic; supported by NIH/NINDS.
References
- 1.Hernandez-Perez M, Perez de la Ossa N, Aleu A, Millan M, Gomis M, Dorado L, et al. Natural history of acute stroke due to occlusion of the middle cerebral artery and intracranial internal carotid artery. J Neuroimaging. 2014;24:354–358. doi: 10.1111/jon.12062. [DOI] [PubMed] [Google Scholar]
- 2.Rohan V, Baxa J, Tupy R, Cerna L, Sevcik P, Friesl M, et al. Length of occlusion predicts recanalization and outcome after intravenous thrombolysis in middle cerebral artery stroke. Stroke. 2014;45:2010–2017. doi: 10.1161/STROKEAHA.114.005731. [DOI] [PubMed] [Google Scholar]
- 3.Riedel CH, Zimmermann P, Jensen-Kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: Successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42:1775–1777. doi: 10.1161/STROKEAHA.110.609693. [DOI] [PubMed] [Google Scholar]
- 4.Yan S, Hu H, Shi Z, Zhang X, Zhang S, Liebeskind DS, et al. Morphology of susceptibility vessel sign predicts middle cerebral artery recanalization after intravenous thrombolysis. Stroke. 2014;45:2795–2797. doi: 10.1161/STROKEAHA.114.006144. [DOI] [PubMed] [Google Scholar]
- 5.Weisstanner C, Gratz PP, Schroth G, Verma RK, Kochl A, Jung S, et al. Thrombus imaging in acute stroke: Correlation of thrombus length on susceptibility-weighted imaging with endovascular reperfusion success. Eur Radiol. 2014;24:1735–1741. doi: 10.1007/s00330-014-3200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho KH, Kim JS, Kwon SU, Cho AH, Kang DW. Significance of susceptibility vessel sign on t2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke. 2005;36:2379–2383. doi: 10.1161/01.STR.0000185932.73486.7a. [DOI] [PubMed] [Google Scholar]
- 7.Kimura K, Sakamoto Y, Aoki J, Iguchi Y, Shibazaki K, Inoue T. Clinical and mri predictors of no early recanalization within 1 hour after tissue-type plasminogen activator administration. Stroke. 2011;42:3150–3155. doi: 10.1161/STROKEAHA.111.623207. [DOI] [PubMed] [Google Scholar]
- 8.Naggara O, Raymond J, Domingo Ayllon M, Al-Shareef F, Touze E, Chenoufi M, et al. T2* "susceptibility vessel sign" demonstrates clot location and length in acute ischemic stroke. PLoS One. 2013;8:e76727. doi: 10.1371/journal.pone.0076727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 10.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (epithet): A placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 11.Flacke S, Urbach H, Keller E, Traber F, Hartmann A, Textor J, et al. Middle cerebral artery (mca) susceptibility sign at susceptibility-based perfusion mr imaging: Clinical importance and comparison with hyperdense mca sign at ct. Radiology. 2000;215:476–482. doi: 10.1148/radiology.215.2.r00ma09476. [DOI] [PubMed] [Google Scholar]
- 12.Rovira A, Orellana P, Alvarez-Sabin J, Arenillas JF, Aymerich X, Grive E, et al. Hyperacute ischemic stroke: Middle cerebral artery susceptibility sign at echo-planar gradient-echo mr imaging. Radiology. 2004;232:466–473. doi: 10.1148/radiol.2322030273. [DOI] [PubMed] [Google Scholar]
- 13.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abul-Kasim K, Selariu E, Brizzi M, Petersson J. Hyperdense middle cerebral artery sign in multidetector computed tomography: Definition, occurrence, and reliability analysis. Neurol India. 2009;57:143–150. doi: 10.4103/0028-3886.51282. [DOI] [PubMed] [Google Scholar]
- 15.Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, et al. Ct and mri early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortimer AM, Little DH, Minhas KS, Walton ER, Renowden SA, Bradley MD. Thrombus length estimation in acute ischemic stroke: A potential role for delayed contrast enhanced ct. J Neurointerv Surg. 2014;6:244–248. doi: 10.1136/neurintsurg-2013-010769. [DOI] [PubMed] [Google Scholar]
- 17.Na DG, Byun HS, Lee KH, Chung CS, Kim EY, Ro DW, et al. Acute occlusion of the middle cerebral artery: Early evaluation with triphasic helical ct--preliminary results. Radiology. 1998;207:113–122. doi: 10.1148/radiology.207.1.9530306. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich B, Gawlitza M, Schob S, Hobohm C, Raviolo M, Hoffmann KT, et al. Distance to thrombus in acute middle cerebral artery occlusion: A predictor of outcome after intravenous thrombolysis for acute ischemic stroke. Stroke. 2015;46:692–696. doi: 10.1161/STROKEAHA.114.008454. [DOI] [PubMed] [Google Scholar]
- 19.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 20.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 21.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 22.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-pa-vs. T-pa alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 23.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 24.Shah S, Luby M, Poole K, Morella T, Keller E, Benson RT, et al. Screening with mri for accurate and rapid stroke treatment: Smart. Neurology. 2015;84:2438–2444. doi: 10.1212/WNL.0000000000001678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.