Abstract
Background
Epidemiological studies demonstrate that childhood infections, including varicella zoster virus (VZV), are associated with an increased risk of arterial ischemic stroke (AIS). Other herpesviruses have been linked to childhood AIS in case reports. We sought to determine whether herpesvirus infections, which are potentially treatable, increase risk of childhood AIS.
Methods and Results
We enrolled 326 centrally-confirmed cases of AIS and 115 stroke-free controls with trauma (ages 29 days-18 years) with acute blood samples (≤3 weeks after stroke/trauma); cases had convalescent samples (7-28 days later) when feasible. Samples were tested by commercial ELISA kits for IgM/IgG antibodies to herpes simplex virus (HSV) 1 and 2, cytomegalovirus (CMV), Epstein Barr virus (EBV), and varicella zoster virus (VZV). An algorithm developed a priori classified serologic evidence of past and acute herpesvirus infection as dichotomous variables. Median (quartiles) age was 7.7 (3.1-14.3) years for cases and 10.7 (6.9-13.2) for controls (p=0.03). Serologic evidence of past infection did not differ between cases and controls. However, serologic evidence of acute herpesvirus infection doubled the odds of childhood AIS, even after adjusting for age, race, and socio-economic status (OR 2.2; 95% confidence interval, 1.2-4.0; p=0.007). Among 187 cases with acute and convalescent blood samples, 85 (45%) showed evidence of acute herpesvirus infection, with HSV-1 found most often. Most infections were asymptomatic.
Conclusions
Herpesviruses may act as a trigger for childhood AIS, even if the infection is subclinical. Antivirals like acyclovir might have a role in the prevention of recurrent stroke if further studies confirm a causal relationship.
Keywords: ischemic stroke, infection, vaccination, children, pediatric
INTRODUCTION
Arterial ischemic stroke (AIS) affects at least 2.4 per 100,000 US children every year,1, 2 with a high burden of long-term morbidity among survivors.3 The causes of childhood AIS are heterogeneous, and many cases remain idiopathic; established adult stroke risk factors are rarely present. Etiologies include certain chronic conditions, such as congenital heart disease and sickle cell disease, as well as acute disorders, such as arterial dissection and transient cerebral arteriopathy. Acute precipitants of stroke among children with chronic risk factors, as well as precipitants of acute arteriopathies, remain uncertain. Understanding the acute triggers of childhood AIS, and how they relate to chronic risk factors, will help guide strategies for prevention.
While severe infections such as meningitis and endocarditis have long been linked to risk of AIS in adults and children, more recent studies provide evidence for minor clinical infections, such as the common cold, as a trigger for stroke.4–8 Infection could predispose to stroke through systemic inflammation causing a transient hypercoagulable state or endothelial injury. VZV, a member of the herpesvirus family, has been linked to AIS via a much more specific mechanism: by infecting the trigeminal nerve, which provides innervation to the cerebral vasculature, the virus may directly invade vessel walls and cause a focal arteriopathy (“post-varicella arteriopathy”).9–15 In a recent self-controlled case series analysis, children had a four-fold increased risk of receiving a stroke diagnosis in the six months after a chickenpox diagnosis.16 The median time from clinical VZV infection to stroke was 18 weeks in a systematic review of the literature.17 Existing data on the role of VZV in childhood AIS have, however, been limited by single-center design, recall bias in ascertainment of infections, absence of standardized assessment of herpes infections, or small numbers of cases, particularly for children. Data on other herpesviruses (HSV1, EBV) and childhood arteriopathy and AIS are limited to case reports.18–20 A link between other herpesviruses (HSV1, HSV2, EBV, CMV) and atherosclerosis and stroke in adults has also been postulated, but never well established.18, 21–25 Because herpesvirus infections are treatable with antivirals like acyclovir, evidence supporting a role in stroke pathogenesis would have important implications for stroke prevention therapies. More than one in ten children with AIS will suffer a recurrence,26, 27 indicating an urgent need for better secondary stroke prevention strategies.
We therefore assessed the association between serological evidence of past and acute herpesvirus infection and childhood AIS by performing an international, prospective, case-control study: the Vascular effects of Infection in Pediatric Stroke (VIPS) study. We hypothesized that serological evidence of acute herpesvirus infection (VZV, HSV1, HSV2, EBV, CMV) would be associated with risk of AIS among children ages 29 days to 18 years compared to an unmatched population of pediatric controls who had blood drawn while being evaluated for traumatic injury.
METHODS
Setting and Case Enrollment
Between 1/2010 and 3/2014, VIPS prospectively enrolled cases of childhood AIS and stroke-free controls at 37 International Pediatric Stroke Study (IPSS) centers in 9 countries: US, Canada, Australia, Philippines, Chile, United Kingdom, France, Serbia, and China (Figure). Ethics approvals were obtained at the lead institutions (University of California, San Francisco [UCSF], and Hospital for Sick Children, Toronto) and each enrolling site. Details of case ascertainment, confirmation, imaging review, laboratory analyses, and clinical data collection are published in the VIPS methods paper.28 In brief, case inclusion criteria were age 29 days through 18 years at the time of AIS, clinical and imaging evidence of AIS, minimum neuroimaging performed including cerebrovascular imaging, enrollment within three weeks of stroke ictus (a change from two weeks in the published methods), and parental or other legal guardian consent (and patient assent, when appropriate) to participation in the study. Site investigators and/or their local research staff abstracted data from medical records regarding stroke presentation, risk factors, etiologic investigation, and treatment, and mailed neuroimaging studies to the imaging core at UCSF. This analysis was limited to the 326 cases and 115 controls with adequate blood samples (Figure).
Figure 1.
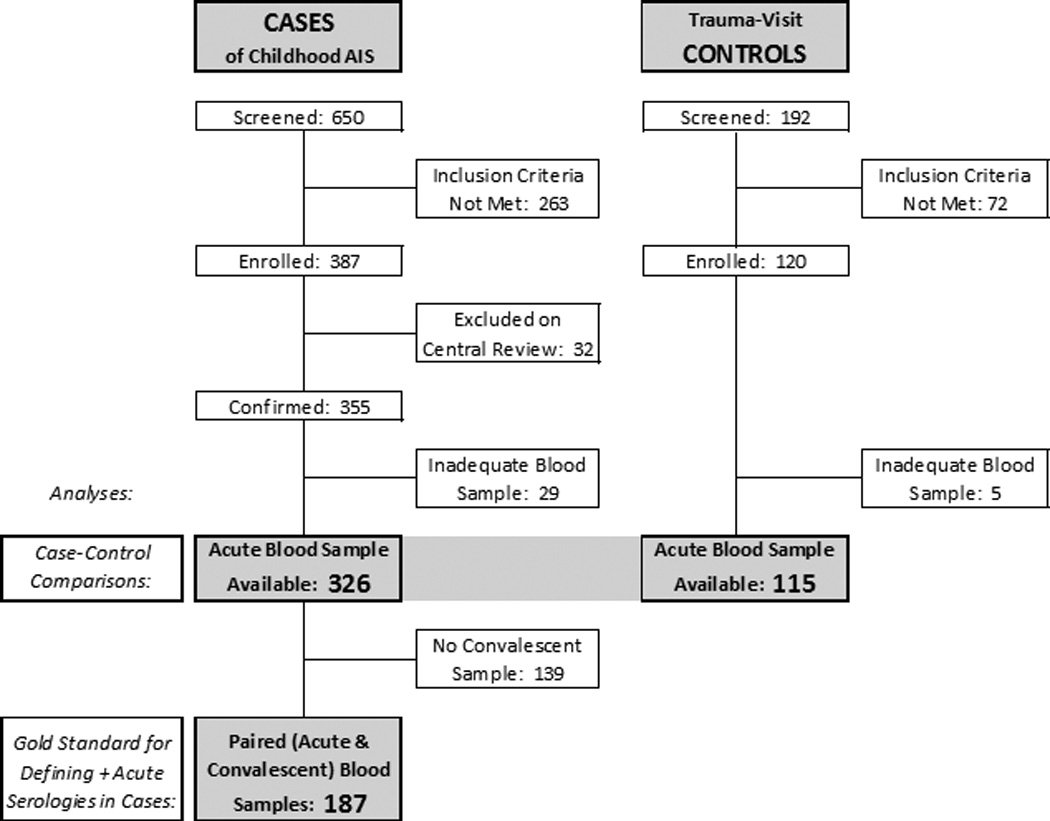
Flow diagram showing VIPS cases of childhood AIS and trauma-visit controls included in the current manuscript. Case-control comparisons are based on assays of the acute blood samples in 326 cases and blood samples collected at time of trauma visit for 115 controls; collection of convalescent samples in the controls was not feasible. Assays of paired blood samples (acute and convalescent) were considered the gold standard for defining positive acute herpesvirus serologies amongst cases (as fully described in the methods). Collection of convalescent samples in cases was performed when feasible.
Case Confirmation and Classification of Stroke Subtype
A neuroradiologist and pediatric stroke neurologist performed centralized review of brain parenchymal imaging and clinical data to confirm that cases met pre-established clinical and imaging criteria for AIS in children: “a focal neurological deficit of acute onset or a seizure,” and “a focal brain infarct conforming to an established arterial territory in a location and of a maturity consistent with the neurological signs and symptoms.”28 Discordant opinions were adjudicated by a second neuroradiologist. Cases were excluded if the central review team agreed that either the clinical or imaging criteria had not been met. All cases were subjected to a rigorous, multi-step process for classifying stroke subtype, as previously described.29 Subtypes of stroke included: arteriopathic (definite or possible), cardioembolic, presumed embolic with hypercoagulable condition, other etiology, and idiopathic.
Control Enrollment
VIPS enrolled stroke-free unmatched controls aged 29 days through 18 years. Inclusion criteria were aimed at selecting children who were comparable to the cases in terms of their likelihood of having had a recent infection. VIPS had two types of controls: “routine-visit” controls enrolled at the time of a routinely scheduled clinic visit; and “trauma-visit” controls enrolled at the time of an acute care visit or admission for trauma. Only the trauma controls could feasibly be consented for a blood sample (at the time of clinical phlebotomy or IV insertion), and therefore are the only controls used in the present analysis. The enrollment target was 120 trauma controls based on a priori power calculations. Although trauma controls were not matched, similar age and geographic distributions were encouraged by providing histograms of these characteristics to the sites throughout the enrollment period. However, sites that did not commonly care for pediatric trauma patients could not feasibly enroll trauma controls.
Parental Interview
To measure clinical history of infection, parents/guardians of both cases and controls participated in structured interviews.8, 28 They were asked whether the child had had a clinical infection in the 6 months preceding the stroke (cases) or enrollment (controls), and were asked detailed questions about the most recent infection including its location, clinical manifestations, and severity. They were also asked about prior vaccinations, including VZV, and markers of socioeconomic status (SES): residence (urban, suburban, rural), maternal education, and household income (converted to US dollars).
Blood Specimen Collection and Laboratory Assays
For ethical reasons, all blood samples were collected at the time of venous or arterial access for clinical purposes. For both cases and controls, an acute blood sample (approximately 16 mL: 10 mL for serum and 6 mL for plasma in ethylenediaminetetraacetic acid) was collected as soon as possible after enrollment, or a maximum of three weeks after the stroke ictus in cases. Exact volumes varied slightly by patient size and individual site institutional review board requirements regarding collection of blood in children. In some cases, blood was drawn on separate successive days to comply with algorithms for pediatric phlebotomy. Convalescent serum and plasma samples were collected seven to 28 days after the initial sample collection when feasible (i.e., when the patient underwent repeat venous or arterial access for clinical purposes within that time window). Convalescent samples could not be feasibly obtained on the controls. Blood samples were centrifuged, aliquotted, and frozen at –70° C at the enrolling site. Samples were then shipped up to twice yearly on dry ice to the core laboratory at the Center for Advanced Laboratory Medicine (CALM) at Columbia University for storage and batched analysis.
Serological assays for HSV 1, HSV 2, VZV, CMV, and EBV were performed using enzyme linked immunosorbent assays (ELISA; Detailed Methods in Supplemental Material). Both IgM and IgG serologies were performed on acute and, where available, convalescent samples. The commercial ELISA kit for VZV may not detect IgG antibody following varicella vaccination.30
Data Analysis and Definitions of Past and Acute Herpesvirus Infection
Baseline characteristics of cases and controls, and of cases with and without paired (acute and convalescent) blood samples, were compared using Wilcoxon rank sum tests for continuous variables and chi-square tests (or Fisher’s exact, when appropriate) for categorical variables. The detailed algorithms for defining serologic evidence of herpesvirus infection were established a priori (Supplemental Tables 1 and 2). Serological evidence of past infection was defined as a positive IgG titer (≥1.1) with a negative IgM titer (<1.1) Serological evidence of acute infection was defined by either positive IgM titers (≥1.1) or, when paired samples were available, by rising IgG titers.
Because convalescent blood samples were not available in controls, all case-control analyses defined herpesvirus infection solely on the basis of the acute blood samples (Figure). For these analyses, we used logistic regression techniques to construct univariate and multivariable models. HSV1 is more common in lower socioeconomic countries, and among lower socioeconomic groups within a country.31, 32 Hence, countries where patients were enrolled were categorized as either lower and middle income (LAMI) countries, including the Philippines, Serbia, and China, or high income countries (all others), as defined by the World Bank in 2014.33 In our final multivariable model, we adjusted for individual-level SES (collected in the parental interview) and for race because of previously reported racial differences in childhood stroke risk.34 We also adjusted for LAMI status, although its estimated odds ratio is imprecise due to the small number of controls enrolled in LAMI countries. In addition, because controls were enrolled predominantly in the USA, Canada, and Chile, we performed sensitivity analyses in which we included only cases and controls enrolled in those three countries.
An algorithm using paired blood samples to measure acute herpesvirus infection is inherently more sensitive than one using a single acute blood sample. We measured the sensitivity (and specificity) of the single-sample algorithm within the subset of cases with paired samples, using the paired-sample algorithm as the gold standard. After demonstrating low sensitivity (and hence high false negative rate) for the single-sample algorithm, we included only cases with paired samples in all case-specific analyses, such as analyses of stroke characteristics and parental report of clinical infections preceding the stroke.
Because herpesviruses establish lifelong latency in humans, serologic evidence of acute herpesvirus infection could represent either a primary infection (i.e., first infection in a susceptible, seronegative individual) or a recurrent infection (i.e., reactivation of a latent infection). IgG antibodies are negative at the time of a primary infection, become detectable within 2 weeks to 3 months, and then remain elevated indefinitely.35 Among cases with paired samples and serologic evidence of acute herpesvirus infection, we defined “serologies consistent with primary infection” as either positive IgM antibodies with negative acute IgG antibody titers, or rapidly-rising IgG antibody titers (4-fold rise). Because of our wide time window for acute blood sample collection (up to 3 weeks after stroke ictus), children with positive IgM and IgG antibody titers could have either late primary infection (blood sample drawn after IgG antibodies being produced) or recurrent infection. Hence, the proportion with “serologies consistent with primary infection” is an underestimate of the true proportion with primary infection.
All analyses were conducted using Stata v12 (Stata Corp., College Station, TX) with alpha set at 0.05. The level of significance used and the p-values are two-sided.
RESULTS
Description of the cases and controls
The present analysis includes a case-control study of 326 childhood AIS cases and 115 stroke-free trauma controls, as well as a case-only study of 187 childhood AIS cases for whom both acute and convalescent (paired) blood samples were available (Figure). Compared to controls, cases were younger and of lower SES; race and ethnicity did not differ significantly (Table 1). Stroke co-morbidities and risk factors (derived from chart review), presentation, and infarct characteristics have been published elsewhere.29 Characteristics of those cases with and without convalescent blood samples are shown in Supplemental Table 3. Controls had a variety of traumatic injuries: 42% had a single bone fracture; 25% had head or neck injury, 10% had an abdominal injury, and the remainder had a variety of injuries, mostly lacerations and dislocations.
Table 1.
Characteristics of VIPS childhood AIS cases and stroke-free trauma controls with acute blood samples available for testing
| Cases | Controls | ||||
|---|---|---|---|---|---|
| N=326 |
N=115 |
||||
| Characteristic | n | (%) | n | (%) | P-value* |
| Demographics | |||||
| Age in years, median (quartiles) | 7.7 | (3.1, 14.3) | 10.7 | (6.9, 13.2) | 0.03** |
| Male gender | 186 | (57.1) | 77 | (67.0) | 0.06 |
| Race | 0.54 | ||||
| White | 212 | (65.0) | 81 | (70.4) | |
| Black | 37 | (11.3) | 7 | (6.1) | |
| East Asian | 7 | (2.1) | 3 | (2.6) | |
| Indian/South Asian | 24 | (7.4) | 5 | (4.3) | |
| Middle Eastern | 3 | (0.9) | 2 | (1.7) | |
| First Nations/Aboriginal | 3 | (0.9) | 1 | (0.9) | |
| Pacific Islander | 1 | (0.3) | 0 | (0.0) | |
| Mixed or other | 34 | (10.4) | 14 | (12.2) | |
| Unknown | 5 | (1.5) | 2 | (1.7) | |
| Ethnicity | 0.17 | ||||
| Non-Hispanic | 264 | (81.0) | 87 | (75.7) | |
| Hispanic | 45 | (13.8) | 15 | (13.0) | |
| Mixed or other | 17 | (5.2) | 13 | (11.3) | |
| Country | <0.0001 | ||||
| USA | 205 | (62.9) | 32 | (27.8) | |
| Canada | 56 | (17.2) | 69 | (60.0) | |
| Australia | 13 | (4.0) | 0 | (0.0) | |
| Philippines | 16 | (4.9) | 0 | (0.0) | |
| Chile | 14 | (4.3) | 13 | (11.3) | |
| United Kingdom | 10 | (3.1) | 0 | (0.0) | |
| France | 6 | (1.8) | 0 | (0.0) | |
| Serbia | 5 | (1.5) | 0 | (0.0) | |
| China | 1 | (0.3) | 1 | (0.9) | |
| Socioeconomic Status | |||||
| Residence | 0.001 | ||||
| Urban | 109 | (33.4) | 62 | (53.9) | |
| Suburban | 142 | (43.6) | 34 | (29.6) | |
| Rural | 75 | (23.0) | 19 | (16.5) | |
| Household income (in US dollars) | <0.0001 | ||||
| <$10,000 | 53 | (16.3) | 10 | (8.7) | |
| $10,000–30,000 | 59 | (18.1) | 18 | (15.7) | |
| $31,000–50,000 | 41 | (12.6) | 17 | (14.8) | |
| $50,000–100,000 | 86 | (26.4) | 22 | (19.1) | |
| >100,000 | 60 | (18.4) | 46 | (40.0) | |
| Missing | 27 | (8.3) | 2 | (1.7) | |
| Maternal education, highest level | 0.02 | ||||
| Less than high school | 41 | (12.6) | 6 | (5.2) | |
| High school graduate | 78 | (23.9) | 19 | (16.5) | |
| Some college education | 97 | (29.8) | 37 | (32.2) | |
| Bachelor's degree | 60 | (18.4) | 25 | (21.7) | |
| Some graduate education | 8 | (2.5) | 8 | (7.0) | |
| Graduate degree | 29 | (8.9) | 17 | (14.8) | |
| Missing | 13 | (4.0) | 3 | (2.6) | |
| Routine visits to an MD office, past 12 months, median (quartiles) |
2 | (2, 4) | 2 | (1, 2) | 0.0005** |
Chi-square (or Fisher’s exact) unless otherwise noted
Wilcoxon rank sum test
Case-Control Analysis: Evidence of past herpesvirus infection
There was no difference between cases and controls in serologic evidence of past herpesvirus infection (Table 2). To control for differences in geography, we conducted a sensitivity analysis limited to the 275 cases and 114 controls enrolled in the USA, Canada, and Chile; the findings were unchanged (Supplemental Table 4). Although commercial VZV kits (as used for this study) are insensitive for detecting IgG antibodies after VZV vaccination,30 it is possible that some of the positive IgG titers for VZV could be explained by prior VZV vaccination: 59.2% of cases and 71.3% of controls had a parental report of vaccination against VZV. Excluding children with prior VZV vaccination or unknown VZV vaccination status, 43% of 107 cases and 59.3% of 27 controls had positive VZV IgG titers (p=0.13).
Table 2.
Evidence of past herpesvirus infection (positive IgG and negative IgM antibody titers) in childhood AIS cases* versus stroke-free trauma controls
| Cases | Controls | ||||||
|---|---|---|---|---|---|---|---|
| N=326 |
N=115 |
||||||
| Herpes Virus | n | (%) | n | (%) | OR† | (95% CI) | P-value |
| HSV1 or 2 | 53 | (16.3) | 24 | (20.9) | 0.78 | (0.45, 1.35) | 0.38 |
| VZV | 182 | (55.8) | 68 | (59.1) | 0.93 | (0.60, 1.44) | 0.74 |
| CMV | 95 | (29.1) | 42 | (36.5) | 0.74 | (0.47, 1.17) | 0.20 |
| EBV | 176 | (54.0) | 58 | (50.4) | 1.26 | (0.82, 1.95) | 0.29 |
| Any herpes virus | 260 | (79.8) | 97 | (84.3) | 0.83 | (0.46, 1.50) | 0.54 |
Analysis based on acute blood samples only to allow case/control comparison
Odds ratio (OR), age adjusted
Case-Control Analysis: Evidence of acute herpesvirus infection
Cases were more likely than controls to have serologic evidence of acute herpesvirus infection: 30.1% of cases versus 18.3% of controls had IgM antibody to a herpesvirus (Table 3). The most prevalent antibodies were for HSV. Of the 80 cases with positive HSV IgM antibodies, HSV type could not be determined (based on concomitant IgG antibodies to HSV1 and HSV2) in 48 (60%); however, 32 (40%) had evidence of HSV1 infection, and none had HSV2.
Table 3.
Evidence of acute herpesvirus infection (positive IgM antibody titers) in childhood AIS cases* versus stroke-free trauma controls
| Prevalence of Acute Herpes Infection (IgM +) |
|||||||
|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||
| N=326 |
N=115 |
||||||
| Herpes Virus | n | (%) | n | (%) | OR† | (95% CI) | P-value |
| HSV 1 or 2 | 80 | (24.5) | 19 | (16.5) | 1.68 | (0.98, 3.00) | 0.07 |
| HSV 1 | 32 | (9.8) | 4 | (3.5) | 3.42 | (1.31, 11.8) | 0.04 |
| HSV 2 | 0 | (0.0) | 0 | (0.0) | - | - | - |
| HSV, indeterminate | 48 | (14.7) | 15 | (13.0) | 1.23 | (0.67, 2.37) | 0.31 |
| VZV | 37 | (11.3) | 3 | (2.6) | 4.43 | (1.55, 18.7) | 0.02 |
| CMV | 18 | (5.5) | 2 | (1.7) | 2.85 | (0.79, 18.2) | 0.17 |
| EBV | 4 | (1.2) | 1 | (0.9) | 1.44 | (0.21, 28.4) | 0.75 |
| Any herpesvirus | 98 | (30.1) | 21 | (18.3) | 1.91 | (1.14, 3.31) | 0.02 |
Analysis based on acute blood samples only to allow case/control comparison
Odds ratio (OR), age adjusted
In our final multivariable model that included other predictors of stroke—residence (urban, suburban, rural), age (as a categorical variable), and race—serological evidence of acute herpesvirus infection was an independent predictor of stroke risk among children (Table 4); the model estimates were similar with and without adjustment for LAMI country status. We performed a sensitivity analysis limited to the 275 cases and 114 controls enrolled in the USA, Canada, and Chile. There was minimal impact on the model, with an adjusted OR for positive acute herpesvirus serologies of 1.8 (95% confidence interval 1.0-3.3, p=0.05, without adjustment for LAMI status).
Table 4.
Multivariate model showing independent predictors of childhood AIS using all 326 cases of childhood AIS and 115 stroke-free trauma controls*
| Model with LAMI Country |
Model without LAMI Country |
|||||
|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Acute herpesvirus infection† | 1.9 | (1.1, 3.6) | 0.02 | 2.2 | (1.2, 4.0) | 0.007 |
| Age | ||||||
| 0–3 yrs | Ref | Ref | ||||
| 4–7 yrs | 0.2 | (0.1, 0.5) | <0.0001 | 0.2 | (0.1, 0.5) | <0.0001 |
| 8–11 yrs | 0.1 | (0.05, 0.2) | <0.0001 | 0.1 | (0.05, 0.2) | <0.0001 |
| 12–15 yrs | 0.2 | (0.1, 0.4) | <0.0001 | 0.2 | (0.1, 0.4) | <0.0001 |
| 16+ yrs | 0.5 | (0.2, 1.5) | 0.24 | 0.5 | (0.2, 1.5) | 0.25 |
| Race | ||||||
| White | Ref | Ref | ||||
| Black | 2.6 | (1.0, 6.6) | 0.04 | 2.5 | (1.0, 6.3) | 0.05 |
| Asian | 0.8 | (0.3, 2.0) | 0.63 | 1.2 | (0.5, 2.9) | 0.62 |
| Other/mixed/unknown | 0.9 | (0.5, 1.8) | 0.78 | 0.9 | (0.5, 1.7) | 0.71 |
| Residence | ||||||
| Urban | Ref | Ref | ||||
| Suburban | 3.8 | (2.2, 6.6) | <0.0001 | 3.6 | (2.1, 6.3) | <0.0001 |
| Rural | 2.7 | (1.4, 5.2) | 0.003 | 2.6 | (1.3, 4.9) | 0.004 |
| LAMI country | 9.6 | (1.1, 85) | 0.04 | |||
All variables in the logistic regression model are shown.
Positive IgM antibody titers to any herpesvirus; using acute blood samples only to allow case/control comparison
Ref=reference category; LAMI=lower and middle income
Prevalence of acute herpesvirus infection among cases with paired samples
Among those with paired samples available for testing, we found that the single-sample algorithm, when compared to the gold standard paired-sample algorithm, underestimated the actual prevalence of acute herpesvirus infection (Table 5). Although 100% specific, the single-sample algorithm had a sensitivity of less than 50% for acute herpesvirus infection overall, and approximately 60% for both HSV and VZV. The true prevalence of acute herpesvirus infection among childhood AIS cases, based on the paired-sample algorithm, could be estimated as 45.5% (85/187). Antibodies against HSV were again the most prevalent, identified in75 (88%) of the 85 cases that were positive (Table 5).
Table 5.
Sensitivity for detection of acute herpesvirus infection of algorithm based on single acute blood samples versus paired samples (gold standard) among 187 childhood AIS cases with paired samples
| Positive acute herpesvirus infection |
||||||
|---|---|---|---|---|---|---|
| Based on Single Sample |
Based on Paired Samples |
Sensitivity of Single Sample |
Specificity of Single Sample |
|||
| Herpesvirus | n | (%) | n | (%) | ||
| HSV 1 or 2 | 44 | (23.5) | 75 | (40.1) | 59% | 100% |
| VZV | 11 | (5.9) | 18 | (9.6) | 61% | 100% |
| CMV | 3 | (1.6) | 10 | (5.3) | 30% | 100% |
| EBV | 1 | (0.5) | 5 | (2.7) | 20% | 100% |
| Any herpesvirus | 49 | (26.2) | 85 | (45.5) | 46% | 100% |
Evidence of primary herpesvirus infection
Of the 75 cases with positive acute serologies for HSV, 43 (57%) had either negative IgG or a 4-fold rise in IgG titers, suggesting that more than half were primary HSV infections (i.e., first-time infection in a seronegative child). Similarly, 5 of 10 with positive acute CMV serologies, and 3 of 5 with positive acute EBV serologies, had serologies consistent with primary infection. For VZV, on the other hand, the minority (7/18, 39%) had serologies consistent with primary infection. As noted in the methods section, these proportions are likely underestimates, as children positive for both IgM and IgG could have either late primary or recurrent infection.
Clinical and stroke characteristics among cases with evidence of acute herpesvirus infection
AIS cases with acute herpesvirus infection were more likely to be from a LAMI country than cases without acute herpesvirus infection (Supplemental Table 5). There were no differences in stroke subtype (classified on central review), presentation, or infarct characteristics. Based on parental interview, only a minority of the 85 children with serological evidence of acute herpesvirus infection had a clinical infection in the preceding week (17.6%) or month (42.4%). Conversely, acute herpesvirus serologies were positive in only 15 (43%) of 35 children with a clinical infection in the prior week, and 36 (46%) of 78 children with a clinical infection in the prior month. These results suggest that many of the clinical infections were not herpesvirus related, and many of the acute herpesvirus infections were subclinical.
DISCUSSION
In this international epidemiological study, we found serological evidence that recent, but not past, herpesvirus infection is associated with increased risk of AIS among children, even after adjusting for age and socioeconomic status. Acute HSV infection was the most common, present in 40% of AIS cases; the majority had serologies suggestive of a primary HSV infection, as opposed to reactivation of a latent infection. Most of the herpes infections were subclinical, and the single acute blood samples had low sensitivity, indicating challenges to early diagnosis of a recent herpesvirus infection: clinical history and a single set of serologies are not adequate for ruling out infection.
Other studies of pediatric AIS have found infection to be common prior to stroke. In a prior analysis of the VIPS cohort, 39% of cases (versus 22% of controls, p<0.0001) had parental report of a clinical infection in the prior 4 weeks, and 18% of cases (3.4% of controls, p<0.0001) in the prior 1 week.8 In a retrospective population-based study of children enrolled in Kaiser Permanente Northern California, 33% of 126 cases (versus 13% of controls) had a medical encounter for infection in the prior 4 weeks,6 and 14% of cases (1.7% of controls) had an encounter in the prior 7 days.5 The International Pediatric Stroke Study reported preceding infection in 24% of 676 AIS cases enrolled in 10 countries.36 These studies were primarily based on clinical history of infection, however, and were not able to provide serological evidence of infection, nor did they specify types of infections.
Our study provides novel epidemiological evidence that acute herpesvirus infection, in particular, may play a role in childhood AIS. Herpesviruses occur worldwide, and are unique in that they can establish latency in sensory ganglia or other cells, resulting in a lifelong infection.31, 32, 37 Initial infection with HSV, CMV, and EBV is often subclinical. All herpesviruses have been implicated in neurological diseases, including meningitis, encephalitis, and transverse myelitis. Prior studies of herpesviruses and stroke have focused predominantly on VZV. Observational studies provide evidence that children can develop a cerebral arteriopathy and AIS after chickenpox.10, 11, 13, 15 Other analyses similarly found evidence that VZV is an independent risk factor for stroke in adults <40 years old,38 and that some adults with unexplained stroke and arteriopathy have chronic VZV infection.9 Although adult studies typically sought serological and genomic evidence of VZV in the CSF, this was not feasible in VIPS because lumbar puncture is not a routine part of childhood stroke evaluation. Nonetheless, our data provide epidemiologic evidence that VZV contributes to AIS risk in children.
Our study is the first to address the role of other herpesvirus infections in childhood AIS. In fact, we found that positive acute titers for HSV were the most common: among children with AIS and paired blood samples, 40% had serologic evidence of acute HSV infection (mostly HSV1). The seroprevalence of HSV1 (i.e., prevalence of IgG antibodies indicating past infection) increases rapidly with age, reaching 44% in young adults and 90% among those >70 years old.31 Published data on acute infection rates with HSV in the general population are not available, however. Of note, in our present study, most children with serological evidence of acute herpesvirus infection did not have a parental report of recent illness, consistent with published serologic studies suggesting that a large proportion of herpesvirus infections are subclinical.32
Although causation cannot be inferred from an association in an epidemiological study like VIPS, other published studies suggest herpesvirus infections could lead to stroke through several different mechanisms, and that these mechanisms may vary by type of herpes virus. While infection with VZV is known to directly invade cerebral arteries and cause a focal arteritis,9 other herpesvirus infections may have an indirect pathologic effect on arteries by causing inflammatory injury to the endothelium. In the late 1970s, herpesviruses were demonstrated to induce atherosclerosis-like changes in animal models,39 and HSV has been found in early aortic atherosclerotic lesions from humans.40 Herpesviruses cause increased expression of cytokines, including interleukin 2 and TNF alpha, as well as vascular smooth muscle cell proliferation and migration.41–43 Our data suggest herpesvirus infection is similarly prevalent across pediatric stroke subtypes, including arteriopathic and cardioembolic, suggesting a common mechanism. Cardioembolic strokes may be explained by inflammatory injury to cardiac endothelium promoting thrombus formation in the heart. Effects of herpesviruses on endothelium and smooth muscle of arteries might predispose to arterial dissection or the development of transient cerebral arteriopathy (TCA). Although VZV is an established cause of TCA (called “post-varicella arteriopathy” in those cases), the role of other herpesviruses could explain why this continues to occur in children vaccinated against VZV.
We did not find evidence that past infection with herpesviruses is associated with childhood AIS risk. In adults, there has been conflicting evidence that past infection as determined by serology is associated with stroke risk. While case-control studies have suggested that serologies against certain bacterial and viral pathogens, such as Chlamydia pneumoniae, HSV1, and CMV, are associated with stroke risk, prospective cohort studies are less convincing.7, 44 Data from the Northern Manhattan Study, however, provide evidence that overall infectious burden, using a summative measure of multiple serologies, rather than individual pathogens, may be a better marker of risk.45 Our failure to find an association with stroke for past herpesvirus infections in children may indicate that the increased risk that accumulates over the lifetime has not yet reached a critical point in childhood. However, the cases in our study were younger than the controls; hence, we may have missed an association between past infection and childhood AIS because the cases had less time to acquire an infection.
Our study has additional limitations. First, our pool of controls was restricted to otherwise healthy children from whom blood could feasibly be collected. Our choice of children evaluated for trauma, while carefully chosen to be as comparable as possible to our cases in terms of their likelihood of infection, nonetheless limited the number of potential controls available. Because controls were primarily enrolled in the USA, Canada, and Chile, our case-control findings are most generalizable to those countries. And although we collected convalescent blood samples on cases where possible, our controls did not have parallel “convalescent” blood samples. Because single blood samples had low sensitivity for acute herpesvirus infection, this likely resulted in some misclassification in our case-control analyses. However, the specificity of single blood samples was high, limiting the misclassification to false negatives which should bias the association between herpesviruses and AIS towards the null. In addition, because we had paired samples in a large subset of cases, we had a more accurate estimate of the true prevalence of acute herpesvirus infection in the population of interest. Second, cases and controls were not matched by site, and some sites were able to contribute only cases. Because childhood stroke is uncommon, enrollment of cases required a large number of centers, yet not all could feasibly enroll trauma controls. We were able to adjust for SES—which is linked to herpesvirus seroprevalence—and perform sensitivity analyses limited to the three countries enrolling the majority of subjects. However, there may have been residual confounding related to variables we did not measure, or small numbers in important subgroups like LAMI countries. Third, we tested blood, but not CSF, for evidence of herpesvirus infection. It is not clear, however, that viral particles need to cross the blood brain barrier for herpesviruses to trigger stroke; the pathology could be limited to endothelial injury. Fourth, we cannot be certain that the positive serologies detected were not reflective of non-specific immune activation due to stroke itself. Immunological activation after stroke is a complex process, and current evidence suggests that stroke leads to immunodepression, although individuals with concurrent infection may have an increase in immune activation against brain proteins.46, 47 There is no evidence, however, that antibodies against herpes viruses increase after stroke. Finally, serology for herpesviruses can be problematic because of lack of specificity and sensitivity of assays as well as differences in individual host response. Because commercial IgM ELISA tests are particularly insensitive for VZV, we have likely under-estimated the subjects with acute VZV infection; as this misclassification is likely nondifferential (affecting cases and controls), we expect it would have biased our results towards the null. In addition, serologies alone cannot adequately distinguish between primary versus recurrent herpesvirus infection; we will seek additional funding to perform IgG avidity tests to better make this distinction.
The strengths of our study are considerable. This is the largest prospective study of childhood AIS, a rare and poorly understood condition, and our study represents a collaboration of a number of international centers. The potentially inconsistent lab procedures, conditions, and interpretation that could result from such diversity was anticipated and minimized through use of a central core laboratory to standardize serological testing. A protocol which implemented centralized imaging review with a clinical adjudication process likewise maximized standardization and consistency in the considerable challenge of categorizing stroke and arteriopathy subtypes. Finally, we used serological testing in addition to parental interview as a source of information about infection.
CONCLUSIONS
Herpesviruses may act as a trigger for childhood AIS. The absence of infectious signs or symptoms does not rule out an acute herpesvirus infection, as most children with positive acute serologies in our study lacked a history of recent clinical infection. A single acute sample is insensitive for detecting herpesvirus infection, so convalescent samples are needed to accurately measure infection. Antivirals like acyclovir might have a role in secondary stroke prevention strategies, but further evidence in support of a causal role is needed.
Supplementary Material
Clinical Perspectives.
In this prospective, multicenter study, investigators from the International Pediatric Stroke Study consortium found serologic evidence that herpesviruses act as a trigger for childhood arterial ischemic stroke. Varicella zoster virus (VZV) is an established cause of stroke, known to directly invade the distal internal carotid artery and its major branches, and cause a focal vasculitis. It has also recently been implicated as a cause of giant cell arteritis. This study suggests a role for other herpesvirus, particularly herpes simplex virus type 1 (HSV1). Though cases with stroke were similar to controls in rates of past herpesvirus infection, cases were significantly more likely to have an acute herpesvirus infection, even after controlling for age and socio-economic status. Among cases with serologic analyses of acute and convalescent blood samples, almost half had an acute herpesvirus infection, mostly HSV (40% of cases), with fewer cases of VZV, cytomegalovirus (CMV), and Epstein Barr virus (EBV). Most had serologies consistent with a primary infection, meaning a first-ever infection in a previously seronegative child. The majority of acute herpesvirus infections were subclinical, suggesting that a negative clinical history cannot rule out herpesvirus infection. A single acute blood sample detected fewer than half of the acute herpesvirus infections, suggesting that a convalescent blood sample should be tested if an acute sample is negative. Because herpesvirus infections are potentially treatable, these findings may have important implications for secondary stroke prevention in children, but further evidence in support of a causal role is needed.
Acknowledgments
The authors wish to acknowledge the important contributions of the research coordinators at VIPS sites, and of the patients and their families.
Funding Sources: This work was funded by NIH R01 NS062820 (Fullerton/DeVeber) and the Bellaflies Foundation gift for biostatistical support.
All authors receive NIH funding for this project except for Dr. Glaser.
Appendix
VIPS Investigators: Dowling MM(University of Texas Southwestern Medical Center, Dallas), Benedict SL (Primary Children's Medical Center, Salt Lake City), Bernard TJ (Children's Hospital Colorado), Fox CK (University of California San Francisco), deVeber GA (The Hospital for Sick Children, Toronto), Friedman NR (Cleveland Clinic Children's Hospital), Lo WD (The Ohio State University and Nationwide Children's Hospital, Columbus OH), Ichord RN (Children's Hospital of Philadelphia), Tan MA (University of the Philippines-Philippine General Hospital, Manila), Mackay MT (Royal Children's Hospital Melbourne), Kirton A (Alberta Children's Hospital), Hernandez Chavez MI (Pontificia Universidad Catolica de Chile), Humphreys P (Children's Hospital of Eastern Ontario), Jordan LC (Vanderbilt University Medical Center, Nashville), Sultan SM (Columbia University Medical Center, New York), Rivkin MJ (Boston Children's Hospital), Rafay MF (Children's Hospital, Winnipeg, University of Manitoba), Titomanlio L (Hôpital Robert Debré, Paris), Kovacevic GS (Mother and Child Health Care Institute, Serbia), Yager JY (Stollery Children's Hospital), Amlie-Lefond C (Seattle Children's Hospital), Dlamini N (Evelina London Children's Hospital), Condie J (Phoenix Children's Hospital), Yeh EA (Children's Hospital of Buffalo), Kneen R (Alder Hey Children's Hospital), Bjornson BH (British Columbia Children's Hospital), Pergami P (West Virginia University), Zou LP (Chinese PLA General Hospital, Beijing), Elbers J (Stanford Children’s Health, Palo Alto), Abdalla A (Akron Children's Hospital), Chan AK (McMaster University Medical Centre, Hamilton), Farooq O (Women & Children's Hospital of Buffalo), Lim MJ (Evelina London Children's Hospital), Carpenter JL(Children's National Medical Center, Washington, D.C.), Pavlakis S (Maimonides Medical Center, Brooklyn), Wong VCN (Queen Mary Hospital, The University of Hong Kong), Forsyth R (Institute of Neuroscience, Newcastle University, UK)
Footnotes
Disclosures: The authors have no commercial interests related to this project.
References
- 1.Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke. 2009;40:3415–3421. doi: 10.1161/STROKEAHA.109.564633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleindorfer D, Khoury J, Kissela B, Alwell K, Woo D, Miller R, Schneider A, Moomaw C, Broderick JP. Temporal trends in the incidence and case fatality of stroke in children and adolescents. J Child Neurol. 2006;21:415–418. doi: 10.1177/08830738060210050301. [DOI] [PubMed] [Google Scholar]
- 3.Lanthier S, Carmant L, David M, Larbrisseau A, de Veber G. Stroke in children: The coexistence of multiple risk factors predicts poor outcome. Neurology. 2000;54:371–378. doi: 10.1212/wnl.54.2.371. [DOI] [PubMed] [Google Scholar]
- 4.Elkind MS, Carty CL, O'Meara ES, Lumley T, Lefkowitz D, Kronmal RA, Longstreth WT., Jr Hospitalization for infection and risk of acute ischemic stroke: The Cardiovascular Health Study. Stroke. 2011;42:1851–1856. doi: 10.1161/STROKEAHA.110.608588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hills NK, Sidney S, Fullerton HJ. Timing and number of minor infections as risk factors for childhood arterial ischemic stroke. Neurology. 2014;83:890–897. doi: 10.1212/WNL.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hills NK, Johnston SC, Sidney S, Zielinski BA, Fullerton HJ. Recent trauma and acute infection as risk factors for childhood arterial ischemic stroke. Ann Neurol. 2012;72:850–858. doi: 10.1002/ana.23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grau AJ, Urbanek C, Palm F. Common infections and the risk of stroke. Nat Rev Neurol. 2010;6:681–694. doi: 10.1038/nrneurol.2010.163. [DOI] [PubMed] [Google Scholar]
- 8.Fullerton HJ, Hills NK, Elkind MS, Dowling MM, Wintermark M, Glaser CA, Tan M, Rivkin MJ, Titomanlio L, Barkovich AJ, DeVeber G. Infection, vaccination, and childhood arterial ischemic stroke: Results of the VIPS study. Neurology. 2015;85:1459–1466. doi: 10.1212/WNL.0000000000002065. Epub 2015 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagel MA, Cohrs RJ, Mahalingam R, Wellish MC, Forghani B, Schiller A, Safdieh JE, Kamenkovich E, Ostrow LW, Levy M, Greenberg B, Russman AN, Katzan I, Gardner CJ, Häusler M, Nau R, Saraya T, Wada H, Goto H, de Martino M, Ueno M, Brown WD, Terborg C, Gilden DH. The varicella zoster virus vasculopathies: Clinical, CSF, imaging, and virologic features. Neurology. 2008;70:853–860. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miravet E, Danchaivijitr N, Basu H, Saunders DE, Ganesan V. Clinical and radiological features of childhood cerebral infarction following varicella zoster virus infection. Dev Med Child Neurol. 2007;49:417–422. doi: 10.1111/j.1469-8749.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 11.Lanthier S, Armstrong D, Domi T, deVeber G. Post-varicella arteriopathy of childhood: Natural history of vascular stenosis. Neurology. 2005;64:660–663. doi: 10.1212/01.WNL.0000151851.66154.27. [DOI] [PubMed] [Google Scholar]
- 12.Alehan FK, Boyvat F, Baskin E, Derbent M, Ozbek N. Focal cerebral vasculitis and stroke after chickenpox. Eur J Paediatr Neurol. 2002;6:331–333. doi: 10.1016/s1090-3798(02)90622-7. [DOI] [PubMed] [Google Scholar]
- 13.Sebire G, Meyer L, Chabrier S. Varicella as a risk factor for cerebral infarction in childhood: A case-control study. Ann Neurol. 1999;45:679–680. doi: 10.1002/1531-8249(199905)45:5<679::aid-ana22>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Melanson M, Chalk C, Georgevich L, Fett K, Lapierre Y, Duong H, Richardson J, Marineau C, Rouleau GA. Varicella-zoster virus DNA in CSF and arteries in delayed contralateral hemiplegia: Evidence for viral invasion of cerebral arteries. Neurology. 1996;47:569–570. doi: 10.1212/wnl.47.2.569. [DOI] [PubMed] [Google Scholar]
- 15.Askalan R, Laughlin S, Mayank S, Chan A, MacGregor D, Andrew M, Curtis R, Meaney B, deVeber G. Chickenpox and stroke in childhood: A study of frequency and causation. Stroke. 2001;32:1257–1262. doi: 10.1161/01.str.32.6.1257. [DOI] [PubMed] [Google Scholar]
- 16.Thomas SL, Minassian C, Ganesan V, Langan SM, Smeeth L. Chickenpox and risk of stroke: A self-controlled case series analysis. Clin Infect Dis. 2014;58:61–68. doi: 10.1093/cid/cit659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciccone S, Faggioli R, Calzolari F, Sartori S, Calderone M, Borgna-Pignatti C. Stroke after varicella-zoster infection: Report of a case and review of the literature. Pediatr Infect Dis J. 2010;29:864–867. doi: 10.1097/inf.0b013e3181ddefb6. [DOI] [PubMed] [Google Scholar]
- 18.Sas AM, Niks EH, Lequin MH, Catsman-Berrevoets CE, de Wit MC. Herpes simplex virus type-1 encephalitis and occipital ischemic stroke. Pediatr Neurol. 2009;41:294–296. doi: 10.1016/j.pediatrneurol.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Terlizzi V, Improta F, Di Fraia T, Sanguigno E, D'Amico A, Buono S, Raia V, Boccia G. Primary herpes virus infection and ischemic stroke in childhood: A new association? J Clin Neurosci. 2014;21:1656–1658. doi: 10.1016/j.jocn.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Weeks JK, Helton KJ, Conley ME, Onciu M, Khan RB. Diffuse cns vasculopathy with chronic Epstein-barr virus infection in X-linked lymphoproliferative disease. Am J Neuroradiol. 2006;27:884–886. [PMC free article] [PubMed] [Google Scholar]
- 21.Snider SB, Jacobs CS, Scripko PS, Klein JP, Lyons JL. Hemorrhagic and ischemic stroke secondary to herpes simplex virus type 2 meningitis and vasculopathy. J Neurovirol. 2014;20:419–422. doi: 10.1007/s13365-014-0253-7. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Hennekens CH, Stampfer MJ, Wang F. Prospective study of herpes simplex virus, cytomegalovirus, and the risk of future myocardial infarction and stroke. Circulation. 1998;98:2796–2799. doi: 10.1161/01.cir.98.25.2796. [DOI] [PubMed] [Google Scholar]
- 23.Visser MR, Vercellotti GM. Herpes simplex virus and atherosclerosis. Eur Heart J. 1993;14(Suppl K):39–42. [PubMed] [Google Scholar]
- 24.Huang ZR, Yu LP, Yang XC, Zhang F, Chen YR, Feng F, Qian XS, Cai J. Human cytomegalovirus linked to stroke in a Chinese population. CNS Neurosci Ther. 2012;18:457–460. doi: 10.1111/j.1755-5949.2012.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Tuo HZ, Wang RJ, Yi L, Wang JW, Wang DX. Human cytomegalovirus-IgM seropositivity is not associated with atherogenic alterations of lipid profiles and inflammatory status in ischemic stroke patients: A preliminary study. Neurol Res. 2011;33:473–481. doi: 10.1179/016164111X13007856084007. [DOI] [PubMed] [Google Scholar]
- 26.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: The importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- 27.Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation. 2006;114:2170–2177. doi: 10.1161/CIRCULATIONAHA.105.583690. [DOI] [PubMed] [Google Scholar]
- 28.Fullerton HJ, Elkind MS, Barkovich AJ, Glaser C, Glidden D, Hills NK, Leiva-Salinas C, Wintermark M, Deveber GA. The Vascular effects of Infection in Pediatric Stroke (VIPS) study. J Child Neurol. 2011;26:1101–1110. doi: 10.1177/0883073811408089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wintermark M, Hills NK, deVeber GA, Barkovich AJ, Elkind MS, Sear K, Zhu G, Leiva-Salinas C, Hou Q, Dowling MM, Bernard TJ, Friedman NR, Ichord RN, Fullerton HJ. Arteriopathy diagnosis in childhood arterial ischemic stroke: Results of the Vascular effects of Infection in Pediatric Stroke study. Stroke. 2014;45:3597–3605. doi: 10.1161/STROKEAHA.114.007404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen JI, Ali MA, Bayat A, Steinberg SP, Park H, Gershon AA, Burbelo PD. Detection of antibodies to varicella-zoster virus in recipients of the varicella vaccine by using a luciferase immunoprecipitation system assay. Clin Vaccine Immunol. 2014;21:1288–1291. doi: 10.1128/CVI.00250-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J Infect Dis. 2002;186(Suppl 1):S3–S28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 32.Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 33.The World Bank: Data: Countries and economies. 2014 [Google Scholar]
- 34.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: Ethnic and gender disparities. Neurology. 2003;61:189–194. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- 35.LeGoff J, Pere H, Belec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83. doi: 10.1186/1743-422X-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackay MT, Wiznitzer M, Benedict SL, Lee KJ, Deveber GA, Ganesan V. Arterial ischemic stroke risk factors: The International Pediatric Stroke Study. Ann Neurol. 2011;69:130–140. doi: 10.1002/ana.22224. [DOI] [PubMed] [Google Scholar]
- 37.Alter SJ, Bennett JS, Koranyi K, Kreppel A, Simon R. Common childhood viral infections. Curr Probl Pediatr Adolesc Health Care. 2015;45:21–53. doi: 10.1016/j.cppeds.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Breuer J, Pacou M, Gautier A, Brown MM. Herpes zoster as a risk factor for stroke and TIA: A retrospective cohort study in the UK. Neurology. 2014;83:e27–e33. doi: 10.1212/WNL.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 39.Minick CR, Fabricant CG, Fabricant J, Litrenta MM. Atheroarteriosclerosis induced by infection with a herpesvirus. Am J Pathol. 1979;96:673–706. [PMC free article] [PubMed] [Google Scholar]
- 40.Benditt EP, Barrett T, McDougall JK. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983;80:6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geist LJ, Monick MM, Stinski MF, Hunninghake GW. The immediate early genes of human cytomegalovirus upregulate tumor necrosis factor-alpha gene expression. J Clin Invest. 1994;93:474–478. doi: 10.1172/JCI116995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geist LJ, Monick MM, Stinski MF, Hunninghake GW. The immediate early genes of human cytomegalovirus upregulate expression of the interleukin-2 and interleukin-2 receptor genes. Am J Respir Cell Mol Biol. 1991;5:292–296. doi: 10.1165/ajrcmb/5.3.292. [DOI] [PubMed] [Google Scholar]
- 43.Yonemitsu Y, Kaneda Y, Komori K, Hirai K, Sugimachi K, Sueishi K. The immediate early gene of human cytomegalovirus stimulates vascular smooth muscle cell proliferation in vitro and in vivo. Biochem Biophys Res Commun. 1997;231:447–451. doi: 10.1006/bbrc.1997.6035. [DOI] [PubMed] [Google Scholar]
- 44.Ridker PM, Hennekens CH, Buring JE, Kundsin R, Shih J. Baseline igg antibody titers to chlamydia pneumoniae, helicobacter pylori, herpes simplex virus, and cytomegalovirus and the risk for cardiovascular disease in women. Ann Intern Med. 1999;131:573–577. doi: 10.7326/0003-4819-131-8-199910190-00004. [DOI] [PubMed] [Google Scholar]
- 45.Elkind MS, Ramakrishnan P, Moon YP, Boden-Albala B, Liu KM, Spitalnik SL, Rundek T, Sacco RL, Paik MC. Infectious burden and risk of stroke: The Northern Manhattan Study. Arch Neurol. 2010;67:33–38. doi: 10.1001/archneurol.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emsley HC, Smith CJ, Hopkins SJ. Infection and brain-induced immunodepression after acute ischemic stroke. Stroke. 2008;39:e7. doi: 10.1161/STROKEAHA.107.500447. author reply e8. [DOI] [PubMed] [Google Scholar]
- 47.Becker KJ, Kalil AJ, Tanzi P, Zierath DK, Savos AV, Gee JM, Hadwin J, Carter KT, Shibata D, Cain KC. Autoimmune responses to the brain after stroke are associated with worse outcome. Stroke. 2011;42:2763–2769. doi: 10.1161/STROKEAHA.111.619593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


