Summary
Vaccines that protect against viral infections generally induce neutralizing antibodies. When vaccines are evaluated, the need arises to assess the affinity maturation of the antibody responses. Binding titers of polyclonal sera depend not only on the affinities of the constituent antibodies but also on their individual concentrations, which are difficult to ascertain. Therefore an assay based on chaotrope disruption of antibody-antigen complexes was designed for measuring binding strength. This assay works well with many viral antigens but gives differential results depending on the conformational dependence of epitopes on complex antigens such as the envelope glycoprotein of HIV-1. Kinetic binding assays might offer alternatives, since they can measure average off-rate constants for polyclonal antibodies in a serum. Here, potentials and fallacies of these techniques are discussed.
Keywords: vaccine, antibody, virus, HIV Env, neutralization, avidity, chaotrope, surface plasmon resonance, affinity, kinetic constants
Introduction
Vaccination successfully prevents several viral infections and has even eradicated some viruses from the human population (1). Strong evidence points to a key role for neutralizing antibodies (NAbs) in the protection afforded by viral vaccines. And just as NAbs are the best correlate of protection after vaccination, they are markers of immunity, which can be life-long, against reinfection after the clearing of acute viral infections from the organism (2–4). Furthermore, chronic infections with some viruses that cause cancer, e.g., human papilloma virus and hepatitis B virus, can be prevented by vaccines inducing potent NAb responses (1, 2, 4, 5).
HIV establishes a chronic infection, which can be treated effectively but not eradicated from the organism by pharmacological or immunological intervention (6); nor can HIV transmission yet be prevented through vaccination (7). Passive immunization with NAbs does, however, protect against viral challenge in animal models of HIV-1 infection. Cellular immune responses may act in concert with NAbs: in the macaque model of HIV-1 infection, new vaccine candidates profoundly and enduringly suppress viral loads to below detectability by inducing specific effector memory T cells (8). But clinical trials of HIV vaccines, both those focusing on cellular immunity and those aiming at inducing NAbs, have failed, with one exception: the RV144 trial, based on vector priming and Env protein boosts, showed 30% protection, of borderline statistical significance (9). Indeed, the RV144 vaccine had not induced NAbs active against circulating strains of the virus (10). Neither in animal experiments nor in clinical trials have broadly active NAbs against HIV so far been induced (11). Maybe that is why numerous other measurements of the antibody responses have been so extensively sifted for links to viral acquisition rates and viremic control in attempts to find correlates of protection or risk (12). One such measurement is the avidity index of antibody binding, usually involving the treatment of bound antibody in an enzyme-linked immunosorbent assay with a chaotropic ion such as thiocyanate and the subsequent measurement of the effect on the binding titer.
Avidity assays have been used successfully in the context of multiple virus infections. It is often medically important to distinguish ongoing or recent infections from those in the more distant past, for example to determine infectiousness. Chaotrope-based avidity indices differentiate between current and past infections with tick-borne encephalitis virus, West Nile virus, hantavirus, parotitis virus, morbilli virus, rubella virus, hepatitis C virus, parvovirus, human herpes virus 6, cytomegalovirus, and human and simian immunodeficiency virus (SIV) (13–27).
The avidity assay and its variants are described and analyzed in more detail in this Perspective. The reason for this focus is the prominence the assay has gained in HIV vaccine research and the problems stemming from its application to the HIV-1 envelope glycoprotein as the antigen. In addition, though, notable developments in protein chemistry are radically changing the understanding of the chaotropic effect. This new knowledge raises questions of what precise information about affinity that the assay purveys. Because of these problems, it seems worthwhile to contrast the chaotrope-based assay with alternative methods for assessing the binding strength of polyclonal antibodies. This Perspective outlines how techniques for measuring the kinetics of antibody binding might present such alternatives (28). The strengths and weaknesses of all these techniques are discussed in relation to developments in immunology and structural biology that provide deeper insights into antibody-antigen interactions, affinity maturation, and the induction of protective antibody responses.
The maturing immune response and the definitions of affinity and avidity
The use of the terms intrinsic affinity, functional affinity, and avidity in the literature is inconsistent. Therefore some clarifications are needed.
Intrinsic affinity refers to the monovalent interaction of a single paratope with an epitope and can be measured either by the use of Fabs or monomeric antigens, although the affinity of a paratope for an epitope may of course differ depending on how it is presented in monomeric and oligomeric antigenic contexts (29). Through subtle conformational effects, the constant domains of antibodies can also affect the intrinsic affinity (30).
The term functional affinity was introduced to apply to the binding strength of a bi- or polyvalent antibody to antigens that present more than one copy of an epitope, because they are multimeric or conjugated in multiple copies to a solid phase, thus allowing cross-linking by the antibody. The degree of strengthening by bi- or multivalent binding can remain unknown and the functional affinity describes the average strength of mixed valencies of such binding (31, 32).
Avidity, strictly defined, refers specifically to the strengthening of binding through more than one point of interaction. This effect can be quantified as the ratio of the dissociation constant, Kd, for the intrinsic affinity over that for the functional affinity. But sometimes the term avidity is used in a looser sense as a synonym for affinity, in practice particularly for functional affinity, as in the terms avidity assay and avidity index, discussed here.
Still, the aim of the avidity assay is to assess intrinsic affinities: for it is the intrinsic affinity that increases with affinity maturation after an infection or vaccination. In contrast, the switches from one isotype to another generally decrease the potential for avidity (2): the decavalent IgM precedes the bivalent IgG response; within the IgG isotype, IgG3, which tends to emerge early, has a greater hinge flexibility than the other subclasses, and that facilitates bivalent binding.
The affinity of pure monoclonal antibodies can be readily measured by numerous techniques. The problem was to compare the average affinity of antibodies in polyclonal sera in order to assess the maturation of the humoral immune response. The proposed solution was the so-called avidity assay.
Chaotrope-based assays
Both the concentration and affinity of antibodies to viral antigens rise after vaccination and during infection, but they vary independently of each other and reflect different biological processes (33). The problem is how to dissect them and how to assess the affinity of polyclonal antibodies when their concentrations remain unknown (34–37).
One approach has been to disrupt the antibody-antigen complexes by chaotropic ions, such as thiocyanate (SCN−) (38) (Figure 1; the chaotropic mechanism of action is discussed in the section New developments in chaotrope chemistry below). The strength of binding would then be reflected in the resistance to such disruption. The chaotropic effect was applied to an ELISA and the measurement of the relative residual antibody binding capacity was called the avidity index (39).
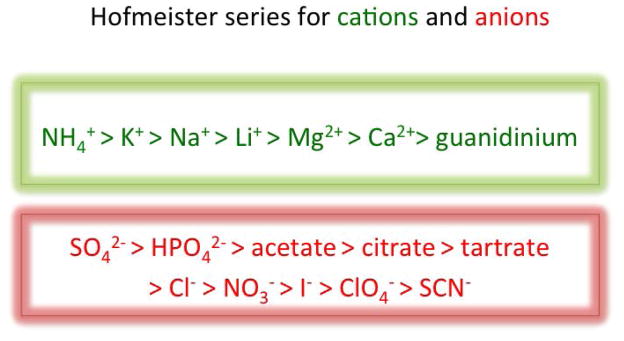
Figure 1.
The Hofmeister series. In 1888 Franz Hofmeister described the ranking of salt solutions for efficacy in precipitating serum globulins (81). By comparing cations paired with the same anion and vice versa he elegantly dissected the effects of the individual ions and ranked them as illustrated in the series. Each series describes a spectrum from the first ion (left), which most decreases globulin solubility (“salting out”) to the last ion (right), which most increases it (“salting in”). Anionic effects tend to be stronger than the cationic ones. The series has also been called lyotropic and the left-hand extreme kosmotropic, whereas the righ-hand extreme is commonly referred to as chaotropic. Those terms are derived from a now much revised view of how the ions work. Thiocynate, the most chaotropic of the anions, is often used in the chaotrope-based avidity assay. Certain non-ionic molecules, e.g., urea, share some properties of the chaotropic ions and have also been used in the assay. The old theory suggested that the effect was general and attributable to how the ions interact with water: the propensity of water molecules to form shells around macromolecules would be diminished by the chaotrope. Part of the binding energy for two interacting protein molecules is explained by how the macromolecular interaction reduces the sizes of those combined water shells. Therefore the binding energy would be unfavorably affected by the chaotrope. The newer insights in Hofmeister solvation science suggest less general mechanisms: instead the solvation effects on proteins partly stem from ionic interactions with the peptidic backbone and the side chains, the latter in particular making the susceptibility of each antibody-antigen complex unique, like the amino-acid composition of the parts contributing to the epitope and the paratope. Already the old view suggested variation in chaotrope resistance according to the relative contributions to the paratope-epitope binding of van der Waals or hydrophobic versus polar interactions. But the new insights further undermine the theoretical basis for using chaotropes to measure binding strength.
Chaotrope-based avidity measurements have been used for assessing affinity maturation of antibody responses to vaccines against rubella and mumps viruses (26, 39). Although those vaccines do induce protective neutralizing antibodies, however, the relationship to neutralization and protection was not analyzed in those studies. A deeper investigation of an influenza-virus vaccine showed that an adjuvant raised both the chaotrope-resistant binding and the neutralization titers among the vaccinees, but direct correlations were only investigated between neutralization and other measurements of antibody binding than chaotrope resistance (discussed in Kinetically based methods, below) (40). It is thus possible but not proven that in some cases the avidity index tracks properties of the antibody response that are directly or indirectly related to maturation of the antibody affinities for neutralization-relevant antigens, thereby improving protection.
The special case of HIV-1 vaccines
HIV-1 differs from many other viruses in that test vaccines against it so far have failed to induce broadly active neutralizing antibodies. Maybe partly because of that failure, other types of immune responses are being extensively scrutinized for any correlations with the limited degrees of protection observed in animal models and in only one out of multiple human clinical trials (9, 11, 12, 41). Among those tests applied in the evaluation of HIV-1 and SIV (simian immunodeficiency virus) vaccine candidates, chaotrope-based avidity assays figure prominently (42–52). Thus, the avidity index has been correlated with protection both from acquisition of infection (43, 48–50) and from high viral loads in breakthrough infections (42, 44, 46), although exceptions to such associations occur (45). Before these intriguing immunochemical phenomena can be discussed, some typological distinctions among epitopes must be adumbrated.
Continuous and discontinuous epitopes
The division of protein epitopes into linear and non-linear, continuous and discontinuous, simple and composite, or conformationally independent and dependent is somewhat artificial. For, if a patch on a protein surface is large enough to provide the binding energy of, say, a neutralizing antibody, it is likely to contain elements that are not contiguous in the polypeptide chain (53). Therefore, the proper folding of the protein is often required to bring critical elements of the epitope into proximity, so that the paratope can make contact with them. Nevertheless, the distinction is meaningful in that some epitopes can be mimicked by short peptides (<20 residues) derived from the amino-acid sequence of the protein antigen; others cannot (54–58). The mimicking of epitopes can involve induced fits by the antibody; sometimes, different antibodies to overlapping epitopes can induce different shapes of the same antigenic peptide (59).
Although the dichotomous verbal classifications of epitopes above are largely interchangeable, distinctions exist: no epitope is literally linear, particularly after the induced fit by a paratope; the conformational dependence of epitopes with the same degree of continuity can differ; the term continuous only means that part of the epitope is sufficiently continuous for a peptide to bind to the same paratope as the intact epitope - often with considerably lower affinity; and composite epitopes in some contexts comprise those with glycan constituents in addition to the polypeptide components. As a further specification, the antigenicity of some epitopes depends not only on protomer folding but also on the intact structure of a whole oligomer, either directly because the epitopes span more than one subunit, or indirectly because of conformational influences of the quaternary structure on the conformation of each protomer. Both instances are exemplified by epitopes on the envelope glycoprotein of HIV-1.
The envelope glycoprotein of HIV-1
The envelope glycoprotein (Env) of HIV-1 is a trimer of heterodimers, each consisting of the outer subunit, gp120, non-covalently associated with the transmembrane component, gp41, which anchors the whole protein in the viral envelope (7, 60–62). The gp120 subunit binds to the main receptor for the virus, CD4, which induces conformational changes allowing the interaction with a co-receptor, one of the chemokine receptors CCR5 or more rarely CXCR4 (63, 64). The co-receptor interaction triggers the fusogenic action of gp41, merging the viral envelope with a cellular membrane, whereby the viral core enters the cytoplasm of the host cell. Because of these functions of Env, it is the target of neutralizing antibodies, which block receptor interactions or later steps in the fusogenic cascade (65).
Env is an extremely variable antigen, but the variation is mostly confined to the large variable loops, where epitopes tend towards the continuous; other epitopes are more conserved and discontinuous. The antibody responses to Env can be divided into three categories: non-neutralizing antibodies (non-NAbs), narrow NAbs, and broadly active NAbs (bNAbs) (66). Responses elicited early in infection comprise the non-neutralizing antibodies, directed to epitopes that are not exposed on native Env trimers, and the narrowly neutralizing antibodies, directed to the variable loops and therefore active only against a narrow range of viral strains. But in a subset of infected persons, after several years, antibodies emerge with broad activity against genetically divergent forms of HIV-1 (67–72). These bNAbs are mostly directed to discontinuous epitopes, such as the CD4-binding-site (CD4bs), glycan-dependent, and quaternary-structural epitopes, some of which span the gp120-gp41 interface (11, 68, 73). The affinity maturation of bNAbs involves a high degree of somatic hypermutation, including deletions and insertions in complementarity-determining regions (CDRs) and mutations in the normally conserved framework regions; in many cases bNAbs have unusually long CDR3 loops (68, 73, 74). These particularities potentially influence chatropic effects on the interactions of antibodies with Env epitopes. But in order to analyze those effects, we must first outline the basis for the avidity assay and index.
The chaotrope-based avidity assay and index
In an avidity assay, a serum or plasma is titrated in an ELISA (enzyme-linked immunosorbent assay) with and without a pulse of a chaotrope, e.g., thicyanate, after the antibody-binding step. Thiocyanate is the strongest anionic chaotrope (Figure 1); the non-ionic chaotrope urea is sometimes used instead. The results are presented as an avidity index, which quantifies the effect of the chaotrope on antibody-antigen binding as the reciprocal titer after chaotrope exposure divided by the reciprocal titer without chaotrope, expressed as a percentage (15, 16, 20, 21, 44, 46, 48–50). Analogously, the reciprocal half-maximum binding concentration, 1/EC50, for an antibody with and without chaotrope treatment can be expressed as a percentage (75). The latter measures the relative functional affinity of the antibody for the antigen in the presence of the chaotrope and can therefore be called the functional affinity index, FAI (75). As an alternative or complement, the level of antibody binding, Bmax, after treatment with the chaotrope, relative to without chaotrope treatment, can be calculated (13, 17–20, 22, 24, 25, 39, 45, 76–78). The latter measurement has been dubbed the Bmax index (BMI) (75). The Bmax itself correlated fairly well with the FAI in one study (75), in line with the observation that the amount of antibody bound can be a confounding factor in avidity assays (79). Most important, though, the EC50 of the binding of monoclonal antibodies to HIV-1 gp120 did not correlate well with the measurement of the chaotropic effect, the FAI (75). That demonstrates that the avidity index does not always reflect affinity.
Antibody binding to continuous HIV-1 Env epitopes resists chaotrope-treatment more than binding to discontinuous epitopes does (38, 75, 76). This difference is not only due to a more efficient direct dissociation of the antibodies from the discontinuous epitopes but can also be attributed to a perturbation of the conformation of the antigen (38, 75).
Specifically, antibody binding to epitopes within the highly variable V3 region itself of HIV-1 Env (as opposed to epitopes overlapping with the V3 base and associated glycans) was consistently chaotrope-resistant. In contrast, the chaotrope sensitivity of the antibody binding to the CD4-binding site on gp120 (CD4bs) was variable and pronounced in several cases. Likewise binding to the CD4-induced (CD4i) epitopes ranged from highly chaotrope-sensitive for some to completely resistant in the case of an antibody, A32, directed to an epitope comprising two continuous segments from the N- and C-terminal regions of gp120 (75).
The bNAbs 2G12 and PGT135, both directed to glycan-dependent epitopes around the V3 base, bound with partial chaotrope sensitivity. In contrast, the binding of another bNAb, PGT128, directed to a nearby epitope, was enhanced by chaotrope treatment. Likewise, antibody binding to cluster I epitopes in gp41 was somewhat augmented by chaotrope treatment (75). These variable, sometimes opposing, effects by themselves suggest that the chaotrope-assay results are subject to complex influences other than mere affinity, which agrees with the outcome of the systematic analysis, demonstrating that affinity does not correlate well with chaotrope resistance (FAI) (75). Furthermore, evidence from pre-treatment of the antigen before antibody binding suggested that the chaotropic effect is partly attributable to denaturation of Env. Although not directly investigated, denaturation of the antibody by the chatrope might also contribute, and different paratopes might be differentially sensitive. Therefore, additional variation in chaotropic effects, enhancing the disconnection from affinity, might arise if the antibodies were pre-treated with chaotrope before binding.
The context of the epitope also modulated the chaotropic effect: binding to some epitopes exposed on both the monomeric gp120 and the Env trimers was differentially affected by the chaotrope. Perhaps most significant of the findings, however, was that the binding of bNAbs to the quaternary-structurally dependent epitope at the trimer apex was obliterated by the chaotrope treatment (75).
The biophysics and semantics of avidity and affinity
It is ironic that avidity in a stricter sense than intended in the term avidity index affects chaotrope sensitivity. Higher valency of the interaction of ligands, including antibodies, conferrred greater resistance, although the monovalent binding of some Fabs to linear epitopes, for example in gp41, was so intrinsically chaotrope-resistant that it was not demonstrably weaker than for the IgG (75). Just as the avidity assay was not intended to measure avidity in the strictest sense, but rather intrinsic affinity, it is perfectly rational to try to eliminate the effect of valency when assessing affinity maturation, for example in order to distinguish recent from longstanding viral infections. This elimination can be achieved by reducing the antigen density (24); the longer the average distance between two antigen molecules, the lower the probability that two-point binding can be established, and the purer will be the intrinsic-affinity measurement. Furthermore, simply lowering the pH (less than what denatures either protein) is a valid alternative to the use of chaotropes for inducing antibody-antigen dissociation: the pH can be optimized for maximum dissociation and minimum denaturation and thereby some of the qualitatively complex effects of chaotropes that are difficult to interpret might be avoided, although it should be noted that pH-triggered fusion proteins would react to sufficiently lowered pH with refolding (24, 80). Indeed, these simple approaches, although more convenient, have proven as accurate as the chaotrope-based method for distinguishing recent from long-standing HIV-1 infections (for these purposes neutralization irrelevant antigens, not native gp120 or Env trimers, are used (24, 78)). That finding raises the question whether limiting dilution of antigen in ELISA could replace the chaotrope-based methods for assessing affinity maturation after vaccination, particularly for antibody binding to neutralization-relevant antigens (78). Indeed, the simplification might improve the assessment of intrinsic affinities.
In summary, the rationale for the avidity assay has been to measure binding strength of polyclonal antibodies, but its application to monoclonal antibodies specific for well-defined epitopes reveals that the degree of chaotrope sensitivity is subject to multiple qualitative influences. Those influences would explain the observed lack of a strong correlation between affinity and chaotrope resistance: specificity for continuous epitopes, for certain glycan-containing epitopes, and the degree of bivalent binding may all elevate resistance. Crucially, the HIV-1 Env trimers are particularly complex and fragile; therefore their native structure may be more easily perturbed by chaotropes than that of other viral antigens.
Interpreting the avidity index in vaccine research
The difference in chaotrope sensitivity of antibody binding to continuous and discontinuous epitopes might shed light on some findings from HIV vaccine experiments in the macaque model. When the reactivities of post-immunization sera with different antigens were compared, the avidity index was lowest for a V1V2-loop-deleted gp120 monomer as antigen, intermediate for a full-length gp120 monomer, and highest for a V1V2 peptide (42, 46). Thus the resistance to chaotrope disruption of binding correlated with the relative preponderance of simple, continuous, and conformationally independent epitopes.
These important observations should be coupled with findings of an absence of broad and potent neutralizing responses in these experiments. The vaccines did not prevent infection but sometimes reduced the ensuing viremia (42–44, 46, 49, 50). The absence of bNAb responses, together with the differential chaotropic effects, suggests what chaotrope sensitivities would emerge if bNAbs were successfully induced. Since bNAbs tend to be directed against complex and even quaternary-structural epitopes, their binding would be reduced or completely disrupted by the chaotrope. The greater the proportion of narrowly active antibodies directed to surface-loop epitopes, and the poorer the response to the currently characterized bNAb epitopes, the higher would be the resulting chaotrope index. The index might correlate negatively with broad protection from infection in such situations.
In general, mechanistic analyses of the chaotrope-based avidity assay show that the epitope specificity of an antibody can affect the results more than its affinity does. In particular, because of the fragility of the HIV-1 Env trimer as a soluble antigen and the complexity of its cross-reactive neutralization epitopes, the avidity index is liable to under-represent bNAbs and over-represent non-NAbs.
New developments in chaotrope chemistry
According to a now thoroughly revised view, chaotropes reduce protein-protein interactions primarily by disrupting the ordered shell of water that juxtaposes non-polar patches on the protein surfaces. Thereby the chaotrope would render binding less entropically favorable. But already this view, which is over-simplistic, implies that the balance between van der Waals and polar interactions between paratope and epitope would affect how individual affinities are affected by chaotropes (81–83). Thus two antibodies with the same affinity but different numbers of polar interaction and different areas of non-polar contact surfaces would be differentially affected by chaotropes. Affinity is the net result of entropic and enthalpic contributions to the binding energy: the interaction of two different antibodies with an antigen can show the same affinity, i.e., have the same binding energy, while one interaction has a large entropic contribution (for the sake of the argument assumed to stem only from the effect on water), making it chaotrope-sensitive according to the old view, and the other interaction has a small entropic contribution, making it relatively chaotrope-resistant.
The revised view of chaotropic effects on proteins explains why all chaotropes are not the same. Thus, ionic chaotropes, such as SCN−, also interact directly with the protein backbone and thereby shift the equilibrium towards the unfolded state (84), the kind of denaturing effect that was invoked to explain some disruption of binding in the avidity assay (75). And chaotropic anions can pair with cationic side-chain groups, i.e. the guanidinium of arginine, the ammonium of lysine, and the imidazolium of histidine (79, 81, 83). There is now a renaissance in research into why proteins differ in the ranking of how chaotropes affect their solubility, their so-called salting in and salting out, i.e., why they have different Hofmeister series (81) (Figure 1).
Studying the antigenic effects of systematic substitutions of residues in peptides in conjunction with comparisons of antibody-peptide binding in large numbers of buffers, including those that contain chaotropes, may elucidate aspects of the chemistry of antigenicity: such studies have indeed demonstrated dramatically different effects of urea and thiocyanate on antibody binding (85, 86). It would, however, not only be impractical to use multiple chaotropes (except perhaps as a pool), but variable profiles of resistant and sensitive binding would probably ensue, each specific for the chemical make-up of each epitope-paratope interface; such finger-printing would be too complex to interpret and would have no rationally predictable bearing on the protective potential of the antibodies. Qualitatively distinct effects that depend on the amino-acid composition of particular proteins would hardly translate into quantitatively uniform measurements proportional to intrinsic affinities of ligand interactions with the respective proteins.
Indeed, it may be less surprising that interactions with different epitopes on HIV-1 Env are differentially affected than that the chaotrope-based assays can differentiate recent infections from those in the more distant past for many viruses (13–24, 75, 76). Other disconnections between chaotrope resistance and affinity than those demonstrated for HIV-1 Env should be expected. As a case in point, chaotrope resistance did not correlate inversely with EC50 values for monovalent antibody binding of phosphocholine (87).
Increased affinity can result from various combinations of improved spatial fit, expanded hydrophobic interactions, and polar complementarities between paratope and epitope. Still, it has been suggested that affinity maturation generally entails reduced flexibility in constituent elements of the paratope (88–91). Thus affinity maturation may specifically affect the entropic control of the association rate (89). It should be noted, however, that these entropic effects are distinct from those of the water shell previously suggested to be the main target of the chaotrope according to the over-simplistic view. Hence the generalizations about flexibility, even if valid, do not imply simple quantitative relationships between chaotrope sensitivity and affinity.
The emerging knowledge of bNAbs directed to HIV-1 may cast further light on variable effects of chaotropes. Not only do several bNAbs have long CDR3 loops, which might be perturbed by chaotropes, some have sulfated tyrosines in these loops that confer specific affinity-enhancing polar interactions, further complicating the possible chaotropic effects (7, 92). For example, the commonly used anionic chaotrope thiocyante may interact with the residues that otherwise partner with the sulfated tyrosines.
A hypothesis that epitopes are chemically special patches on the protein surface implies that certain agents would selectively disrupt antibody-antigen interactions. But careful analyses suggest that the amino-acid composition of epitopes does not on average deviate from that of protein surfaces in general (30). Indeed, when known overlapping and non-overlapping epitopes of a well-studied antigen are added up more and more of the surface is found to be antigenic: the antigenic area may indeed approach the entire surface (Figure 2) (93). In contrast, CDRs deviate from the average protein surface in amino-acid composition, each CDR having its own preferences for residues. But this does not in general add up to special amino-acid compositions for the entire paratopes; rather the preferences of the six different CDRs average out. On the epitope side there may thus be the corresponding constituent patches, i.e., components of epitopes, with idiosyncrasies in amino-acid composition, although contact residues on the paratope side and CDRs are not exactly synonymous (30). The problem with specific chaotropic effects designed to disrupt these interactions is the vast complexity that precludes any general predictions.
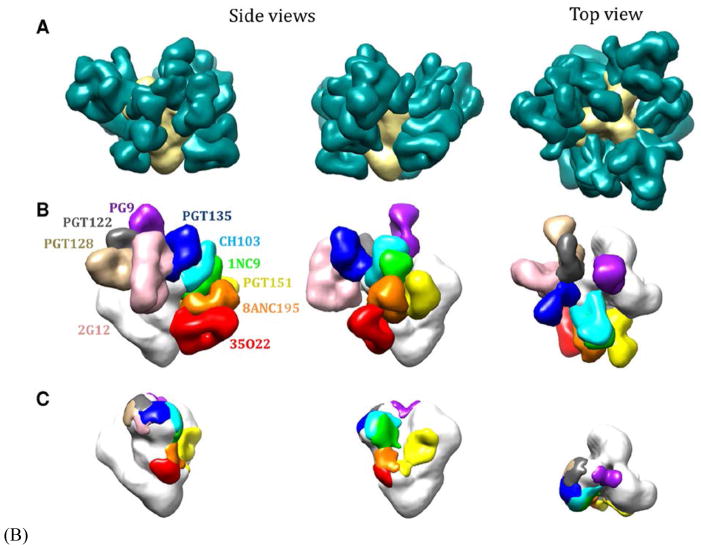
Figure 2. Antibodies (Fabs) bound to neutralization epitopes of an HIV-1 Env trimer.
(A) The Fabs (green) directed to various known neutralization epitopes on each of the three Env protomers nearly cover the trimer (beige). (B) Fabs directed to different epitopes are shown in different colors. Most antibodies bind with a stoichiometry of up three paratopes per trimer; some directed to quaternary-structural epitopes at the trimer apex (PG9) bind to only one epitope per trimer; but here only one Fab of each antibody is represented, thereby revealing the unoccupied surfaces on the other protomers (white). (C) The unoccupied epitopes, corresponding to those occupied by the Fabs in B, are shown in the same colors as the corresponding Fabs in B. The figure shows simultaneous binding of multiple paratopes, a situation that resembles the binding of polyclonal antibodies in a serum although in some sera antibodies to a single epitope dominate. Because the antibodies will have varying affinities and unknown concentrations, only the average off-rate constant can be assessed by kinetic techniques. Furthermore, the binding of one Fab may positively or negatively affect the binding of another (93). The images are reproduced from Derking et al. PLoSPath 2015 11: e1004767 (93).
In conclusion, the differential effects of different chaotropes on the polypeptide backbone as well as on particular amino-acid sides and their interactions with each other are complex, conferring to each paratope-epitope interaction a unique sensitivity. These effects are furthermore expected to be subject to the prevalent phenomenon of hysteresis: the scalar energy change for the initial macromolecular association can be smaller than that of the dissociation. This difference arises from secondary, stabilizing interactions and the concomitant extrusion of solvent molecules from the binding cleft (94, 95). Hysteresis comprises conformational changes that stabilize antibody-antigen binding over time. It can be directly demonstrated by the kinetic methods discussed below: hysteresis would manifest itself as a reduction in the dissociation rate when the association time is prolonged. The degree of this time dependence of the strength of the interaction adds another dimension to the variable influences of chaotropes.
Antigenic perturbation and fixation
Enveloped viruses enter susceptible cells by fusing with the cell-surface of endocytic membranes, a feat mediated by their envelope glycoproteins, which in the process undergo drastic functional conformational changes, sometimes even refolding. Some of these glycoproteins have developed conformational masking as a general means of escaping neutralizing antibodies (7, 96). Even before the drastic functional changes set in, triggered by receptor interactions or a drop in the endosomal pH, viral envelope glycoproteins can transit between distinct conformations. As elucidated by single-molecule fluorescence-resonance-energy transfer, HIV-1 Env fluctuates between three different states, the relative prevalence of each being shifted by receptor (CD4) and antibody binding (97). The research into which conformations best elicit broadly neutralizing antibodies and how to stabilize these conformations is intense (96). Indeed, that approach has already led to the identification of an immunogen that more effectively than un-mutated protein induces neutralizing antibodies to the respiratory syncytial virus (98). Here, the disruptive effects of chaotropes on the interactions and conformations of antibodies and antigens have been considered. Other studies have addressed the degree of antigen perturbation involved in antibody binding. The question is then how the chaotropic effects might relate to conformational flexibility and antigenic perturbation.
In one study, the degree of perturbation in HIV-1 Env required for Ab binding was measured in a cell-based ELISA as the ratio of binding to unfixed Env over the binding to glutaraldehyde-fixed antigen (99). This ratio was called the perturbation factor. How does this measurement relate to the chaotrope-based avidity index? Since chaotrope treatment acts not only by perturbing conformations but apparently also more drastically by denaturing the antigen, whereas the fixation should merely lock the most sampled conformations, it might be farfetched to posit any relatedness of the chaotropic and fixation effects. Upon scrutiny of the few Env ligands included in studies by both approaches, no general relationship emerges, although it should be noted that the binding assays and the forms of Env differed. Thus binding of PGT128 to gp120 was enhanced by the chaotrope but its perturbation factor was among the lowest, ≪1. In other cases the match was better between both the conditions and the results of the two assays: the binding of some antibodies to trimers gave low BMIs in the chaotrope assay (FAIs were too low to measure), as well as low perturbation factors (≪1) in the fixation-based assay, the latter being equivalent to preferential binding of glutaraldehyde-fixed Env. In summary, although no strong agreement should be expected, both the chaotrope- and fixation-based measurements are influenced by other factors on binding than mere functional affinity.
Kinetically based methods
Because of the complications of chaotrope-based assessments of the binding strength of antibodies directed to HIV-1 Env as well as to other viral antigens, it is rational to explore alternative methods. Kinetically based methods are candidates because from the binding constants they measure, affinities can be derived. Surface plasmon resonance (SPR, Biacore) is a label-free technique based on the reflection of polarized light converted to a resonance signal that allows the amount of, e.g., antibody bound to an antigen to be followed over time (28, 100). By fitting a model function to the binding data for a titrated antibody, one can obtain the on- and off-rate constants of antibody binding to the immobilized antigen, and thereby the affinity of the interaction, because the equilibrium constants are the ratios of the kinetic ones: Kd=koff/kon [M] and Ka=kon/koff [1/M]. Although subject to some uncertainties, the stoichiometry of the interaction, i.e., the number of paratopes maximally bound to an oligomeric antigen, can also be derived from the binding data (Figure 3) (28, 29).
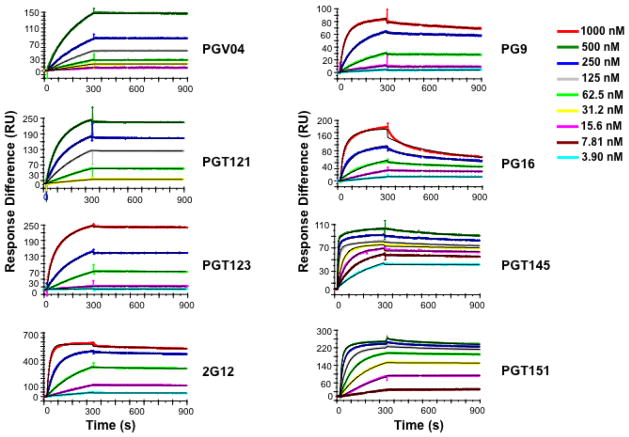
Figure 3.
The kinetics of antibody binding to immobilized trimeric HIV-1 envelope glycoprotein (Env) analyzed by SPR. The sensorgrams, i.e., response units (RU) after background subtraction as a function of time (s), show the binding curves for titrated antibodies as indicated in the legend. The same color code applies to all diagrams but the titration ranges start and end at different concentrations and also differ in the dilution steps. The data were fitted with a model for bivalent binding except in the case of the functionally monovalent antibody, 2G12, the binding of which was fitted with the simple Langmuir model. The curves generated by the modeling are depicted in black but are only visible where they diverge somewhat from the data. Note how the binding of the antibodies, all broadly neutralizing (bNAbs), differs both in on-rate (association phase, 5 min) and off-rate (dissociation phase, 10 min). The on-rate is the product of the on-rate constant, kon, and the antibody concentration; the off-rate is concentration-independent and hence its constant, koff, can be determined when the antibody concentrations are unknown, as is the case for polyconal sera. Therefore it has been possible to apply SPR and another kinetic method, biolayer interferometry, to the monitoring of increased binding strength of antibodies during affinity maturation (10, 104). The diagrams are reproduced from Yasmeen et al. 2014, Retroviology 11:41 (29).
The SPR instrument contains a microfluidic system, allowing a molecule in solution (the analyte) to flow past and bind to the immobilized molecule (the ligand) without any significant changes in the concentration of free analyte, provided the flow rate is sufficiently high. When the flow rate is too low, e.g., if the amount of ligand so high that no feasible flow rate is sufficient, the data will be compromised by the problem of mass-transport limitation or rebinding, which will distort the kinetic and therefore potentially also the equilibrium constants. But the high sensitivity of the detection obviates the untoward effects by allowing of low ligand densities; as another remedy, the flow rate can be increased. And the absence of mass-transoprt limitation can be ascertained by a battery of diagnostic tests. The non-turbulent laminar flow provides a theoretically satisfactory basis for the validation of the binding data.
Another label-free approach, biolayer interferometry (Octet), for studying the kinetics of antibody binding is increasingly used: it can also yield on- and off-rate constants and thereby the affinity of the interactions. This system is not based on laminar flow of the analyte solution but involves the dipping of sensor tips into different wells and mixing by vigorous shaking. It may be hard to avoid mass-transport limitation, although cumbersome schemes have been designed to do so; it may be even harder to ascertain validity of the kinetic data (101). Instead, the advantages of this system are speed of analysis and the large numbers of samples that can be processed.
The reason these techniques are briefly introduced here is that both have been applied as potential alternatives to the chaotrope-based avidity assays (10, 46). Indeed, the kinetic approach has also been dubbed an avidity assay for analyzing plasma (10, 46). This use raises the question of what information the kinetic techniques can provide and how that differs form the avidity index.
When the binding of polyclonal antibodies is studied, the relevant concentrations are unknown and only the off-rate constant, which is concentration-independent, can be analyzed kinetically. It should be noted, however, that affinity maturation also involves increases in the on-rate constant. Furthermore, the antigen density on the SPR chip will determine the avidity, i.e., the degree of bivalency of IgG binding, which will have a dominant influence on the off-rate in an arbitrary manner that may not be relevant to neutralization or other protective mechanisms (29). Just as in ELISA, however, the antigen density on the SPR chip can be reduced until IgG binding is overwhelmingly monovalent (29). Then the measured off-rate constant would provide partial information about the affinity maturation of an antibody response. Not all maturation will be measurable though, since many bNAbs bind with so low off-rate constants that they fall below the limit of detection.
In promising studies of the antibody responses to vaccines against influenza virus, the off-rate constant of binding to haemagglutinin correlated inversely with neutralization titers of sera from human vaccinees and with haemagglutination-inhibition titers and reduction in viral load in a ferret infection model (40, 102). The off-rate constant may be more closely related to the persistent fraction of viral infectivity than is either the on-rate constant or the affinity (29); the off-rate constant for the binding of antibodies to short peptides representing a variable epitope cluster on HIV-1 Env has also bee found to correlate inversely with their capacity to neutralize narrow ranges of HIV-1 isolates (103). In another study, development of bNAb activity against HIV-1 occurred simultaneously with an increased strength of binding as assessed by biolayer interferometry (104). That study went further than assessing the off-rate constant: by using the total IgG concentration as a substitute for the unknown concentration of the Env-specific antibodies, it derived not only the straightforward koff values but also the apparent kon and thereby also Kd values. It should be borne in mind that increases in the relative concentrations of specific antibodies would raise the on-rate and appear as apparent increases in the on-rate constant under those circumstances. Nevertheless, this approach may yield valuable complementary information that is considerably easier to rationalize than that stemming from the multi-factorial influences on the avidity index.
An ambitious scheme would be to harness the capacity of SPR to measure the concentrations of active molecules mixed with inert and irrelevant different species. This application intriguingly builds on the mass-transport limitation that invalidates kinetic measurements (105, 106). The concentrations of active antibodies in purified IgG (serum, containing mixed classes of antibodies, would not work since the analytes must have homogeneous mass; the binding of IgM would be too complex to model) could be measured under conditions that favor mass transport limitation, i.e., large amounts of immobilized antigen, and with varied flow-rate. From the fitted binding parameters, the concentration of active antibodies can then be derived. Thus to determine the proportion of a class of antibodies in a post-vaccination serum or plasma that can simultaneously bind to the immunogen, or to another relevant form of the viral antigen, could in itself help characterize antibody response. Subsequently, however, the IgG could be titrated against lower amounts of antigen to avoid mass transport limitation so that both on- and off-rate constants and thereby the affinities could be measured by the incorporation of the previously determined concentrations of specific IgG; the monovalent and bivalent binding components would be separated by the appropriate modeling. Note that at best the affinity information obtained would be averages for all simultaneously binding antibodies, i.e., potentially directed to several epitopes (Figure 2). Obviously, the procedure would be laborious and consume large amounts of serum. Whether it would have any practical use remains uncertain.
The few examples of particular antibodies that have been studied both with the HIV-1 Env trimer as antigen in the chatrope-based assay and with immobilized trimers by SPR illustrate that the highest affinities of the bNAbs, including trimer-specific ones, (Kd values around or below 1nM) are compatible with the highest chaotrope sensitivities (29, 75). Again this suggests that the chaotropic effect is no substitute for affinity measurements and must at least partly be attributed to a disruption of the fragile antigenic structure, which is particularly delicate for quaternary-structural epitopes (75).
Avidity in a stricter sense
SPR enables the dissection of intrinsic affinity and avidity. Flowing a Fab over an immobilized antigen is the most direct way of measuring the intrinsic kinetic and, thereby, affinity constants by SPR: with IgG as the analyte instead, a bivalent model must be applied unless the antigen density is sufficiently low to exclude bivalent binding by antibodies that cannot cross-link two epitopes intra-oligomerically. How should we then best express the effects of strictly defined avidity that affect the binding? The densities of viral surface proteins on the SPR chip can be made to simulate those on the surface of virions; avidity dependence on antigen density can be measured, and avidity can be quantitatively expressed either as the ratio of Kd for Fab over IgG, both Langmuir-modeled, or of the Kd for modeled monovalent IgG binding over the approximate Langmuir-modeled binding of the whole IgG (29). The agreement between the monovalent binding in the bivalent modeling and simple Langmuir-modeled Fab binding is good (29). Theoretically these do not have to coincide exactly: the Fc portion can have indirect, allosteric effects on the paratopes as well as on the accessibility of the epitopes (30). Furthermore, Fc portions can interact with each other and this would enhance the valency of binding further (107).
Avidity in the stricter sense of binding valency has been studied by SPR in another manner that is also relevant to neutralization and virion aggregation (cf. (108)). The method involves the immobilization of virions, the subsequent binding of antibodies to the virions, and the ultimate detection of free paratopes on the bound antibodies by another round of virion injection: if the IgG molecules are not engaged bivalently, the virions in the second round will be captured, causing a strong SPR signal (109).
Notably, the contribution of strict avidity to neutralization varies among viruses. It is particularly feeble for HIV-1, probably because the Env trimer spikes on the viron surface are sparse, which counteracts cross-linking (110). As mentioned, this density can be mimicked in the SPR system and the avidity effect can be determined by comparing the binding of Fabs and IgG to Env at different densities; then the Fab/IgG functional affinitiy ratios can be compared with the Fab/IgG neutralization ratios (29). The Fab/IgG neutralization IC50 ratio tends to be around 10 for HIV-1 and can be orders of magnitude higher for other viruses (29, 110). Of course, an antibody that could bind bivalently to two epitopes on the same trimer would not depend on Env density for its functional affinity, but spatial constraints militate against that sort of intra-trimeric binding by natural antibodies to HIV-1 Env (60, 61). Binding agents with optimal spacers between the two paratopes have been created and these antibody-derived molecules neutralize HIV-1 with orders of magnitude greater potency than the corresponding regular IgG (111). The concept of avidity remains central to an understanding of virus neutralization.
The biological importance of binding kinetics
The kinetics of antibody-antigen interactions are at the heart of immunological processes: the on- and off-rate constants are intricately and asymmetrically related to the iterative process of B-cell-receptor ligation and affinity maturation (112). Ever greater on-rate constants would enhance presentation to and stimulation of B-cells. Such rate increases would thus be selected for until they reach the physico-chemical limit. But mass-transport limitation makes on-rates difficult to detect correctly by SPR above 105 (M−1s−1). The off-rate constant selection, in contrast, is cell-biologically limited for every cycle of selection in the germinal centers of lymph nodes: when the off-rate, which is concentration-independent, is lower than the endocytic rate of the B-cells, a further reduction confers no selective advantage. How can then extremely low off-rate constants that are typical, e.g., for bNAbs against HIV-1, arise? Presumably, there is a cross-antigenic effect of mutations that reduce the off-rate for antibodies down to the endocytic rate for the presented antigen, and in parallel reduces it further for the same antibody in relation to another antigen for which the off-rate is already lower than the endocytic threshold. When the virus mutates again, that raises the off-rate and the cycle of selection resumes; the cross-antigenic kinetics readjust anew.
Thus SPR and interferometry provide more direct evidence that is more readily rationalized and biologically more important than does the chaotrope-based assay. It is an advantage that the same techniques can be used with poly- and monoclonal antibodies. Thus, the kinetic methods bring unity to the study of the B-cell response and will allow comparisons between the cruder polyclonal and the more finely dissected monoclonal levels of antibody responses.
Discrepancies between antibody binding and neutralization
The premise all through this discussion has been that measurements of antibody binding should have maximum relevance to neutralization and thereby to protection from virus infections. One complication is that many antibodies are polyspecific or polyreactive, i.e., they bind different, even unrelated, antigens (33, 113). This capacity can sometimes be explained by the conformational flexibility of the paratopes (114). For viruses that display few NAb epitopes, because of a paucity of the entry-mediating viral proteins on the virion surface, this capacity can have profound effects on neutralization (115, 116). If an IgG molecule can rarely bridge two relevant viral protein oligomers, it may instead cross-link one of those to a host-derived passenger antigen and thereby achieve a lower off-rate. As a result, neutralization is potentiated (115, 116). But this effect would reduce the correlation between the affinity measured against native-like viral proteins and the neutralizing potency.
In other cases, the epitope can consist of, e.g., different constellations of glycans. Such constellations may occur in different numbers on glycoproteins of different strains of same virus (117, 118), but they may be differentially antigenic and the affinities measured will therefore be mere averages. Heterogeneity in glycosylation may leave some viral proteins non-antigenic, thereby affecting the slope and plateaus of neutralization curves (29, 119, 120). Again, discrepancies would arise between binding measurements obtained with homogeneous viral proteins and neutralization of heterogeneous viral populations. When the viral populations are antigenically heterogeneous, polyclonal mixtures of antibodies can achieve stronger neutralization efficacy than individual ones (119, 120). The molecular details, including glycosylation, of the antigens in the binding assays determine the relevance of the binding constants to protection.
As the binding measurements become more precise and sophisticated, sometimes correlating well with neutralization of matching viruses in experiments (121–123), it should be born in mind that protection induced by vaccination requires antibody binding to heterologous viral antigens with various degrees of differences from the immunogens.
Outlook and conclusions
The chaotrope-based avidity assays have been applied in clinical settings; they have helped to identify recent infections and to demonstrate the maturation of responses to vaccines. These assays have also been used in experimental HIV-vaccine research. In the absence of neutralizing responses, the avidity assays have been included in wide arrays of tests in the search for statistical correlates with the limited protection observed (12). Hence the problem of how to interpret what these assays really measure arose. In the study of bNAbs to HIV-1 it has emerged that some of these antibodies only bind to native-like trimers, which are conformationally delicate antigens. When soluble Env trimers were applied in the chatrope-based assays, bNAb binding was abrogated, whereas non-NAb binding to more linear epitopes on subunits or peptides was preserved (75). This bias in the chaotrope-based assays points to the need for an altenative technique to assess affinity maturation relevant to protection.
Since concentrations and affinities both rise after initial infection and vaccination, and since both are relevant to protection, it might be asked why assays aimed at determining binding strength would be superior to simple binding titrations, which yield half-maximal binding dilutions that are the net result of concentrations and affinities. Indeed, during infections with acutely cytopathic viruses, such as vesicular stomatitis virus, the antibody response may mature rapidly and thereafter the degree of protection is determined by changes in antibody concentration (33). Regular titrations, particularly against low densities of antigen that exclude bivalent binding, as well as the kinetic methods, i.e., SPR and biolayer interferometry as outlined here, can give valuable information about the development of polyclonal antibody responses. The information provided by all of these measurements is more direct than that contained in the chaotrope-based avidity indices, and it is better understood biophysically and more comparable with the measurements of choice for monoclonal antibodies.
The comparison with monoclonal antibodies is crucial. Even if some studies of polyclonal sera after experimental vaccination can be useful, a deeper understanding requires a dissection of those responses into their component antibody specificities and indeed further into the germline lineages from which the effective responses originate. Great advances in viral vaccine research are due to the new methods for isolating human monoclonal antibodies through immortalization of memory B-cells and plasma cells (124).
Essential are also the methods for determining the neutralizing capacity of both monoclonal antibodies and the post-immunization polyclonal sera. The epitope mapping of the neutralizing antibodies, the structural studies of the antibody-antigen complexes, the thermodynamic and kinetic analyses of the binding, and the measurement of potency and breadth of neutralization together guide the further development of immunogens. Some of those techniques allow comparable measurements for single antibodies and polyclonal sera; others are restricted to individual antibodies but instrumental in improving the understanding of neutralizing responses. Of note, it was analyses of the chaotropic effects on the binding by well-characterized monoclonal antibodies that revealed some of the obfuscating aspects of the traditional avidity assay.
Independently of developments in immunology and virology, the basic chemistry of chaotropic and other ionic effects on proteins - the solvation science that started with Hofmeister’s ingenious experiments - is undergoing a renaissance and will no doubt improve our understanding of protein interactions. That improvement will benefit the study of immunologically and virologically important proteins, viz. antigens and antibodies. The opposite concurrent trend towards simplicity might seem ironic: determining the kinetics of antibody binding, once pursued within the arcane sub-domain of immuno-chemistry, is becoming routine and provides affinity measurements as a welcome byproduct. What was a simple tool is becoming an intricate topic, and what was an intricate topic has produced a simple tool.
Expert commentary
Anti-viral vaccines are among the greatest successes in medical history. Neutralizing antibodies are known to mediate vaccine-induced protection against viral infections. But many effective vaccines against important viral pathogens remain to be developed. When vaccines are studied experimentally and in clinical trials, we need the most informative assays to assess the elicited immune responses. Chaotrope-based avidity assays for measuring antibody binding strength have served well for distinguishing current from past infections with many viruses. It has transpired, however, that these assays preferentially detect antibodies of certain specificities while missing others. Antibody binding to important neutralization epitopes on the complex and fragile HIV-1 envelope glycoprotein trimer is hyper-sensitive to the disruptive effects of the chaotrope, although the interactions are of high affinity. Furthermore, the understanding of the multifaceted effects of the chaotrope on proteins is still evolving and this increasing complexity undermines simple interpretations of chaotrope-based avidity indices. It is therefore warranted to explore alternative techniques that can measure antibody-binding properties relevant to protection. Thus, surface plasmon resonance and biolayer interferometry, which measure the kinetics of binding, are emerging tools for evaluating polyclonal vaccine-induced antibody responses. The kinetics of antibody binding to native-like viral antigens are intricately related to affinity maturation and neutralization.
Five-year view
Over the next few years alternatives to the inadequately understood chaotrope-based avidity assays for assessing the strength of antibody interactions with viral antigens will be developed further for better vaccine evaluations. Among those alternatives are surface plasmon resonance and biolayer interferometry that measure the concentration-independent off-rate constants of antibodies in polyclonal preparations and give precise affinity and kinetic information for monoclonal antibodies. Optimization of these techniques for the particular purpose of evaluating polyclonal antibody binding to complex viral antigens is likely to lead to a consensus for standard measurements. No doubt the renaissance of the study of chaotropic and other ionic interactions with proteins will meanwhile furnish exciting new insights with relevance to virology and immunology. But so far, such insights have increased the complexity and thereby the difficulties in interpreting the chaotrope avidity assays.
Key issues.
Chaotrope-based antibody binding assays have been used widely for assessing the strength of the binding of anti-viral antibodies but are not mechanistically understood.
Binding to continuous epitopes is more resistant to chaotrope treatment than binding to discontinuous epitopes.
Binding to important quaternary neutralization epitopes on HIV-1 Env trimers is particularly vulnerable to chaotrope disruption.
The effects of chaotropes and other ions in Hofmeister’s series are complex; these effects are the subject of continuing advanced studies in protein chemistry.
New B-cell and plasma-cell cloning techniques allow the dissection of antibody responses and facilitate detailed studies of paratope-epitope interface structures as well as of binding kinetics, thermodynamics, and stoichiometry.
Surface plasmon resonance and biolayer interferometry measure the binding kinetics of monoclonal antibodies but can also give some biophysically interpretable information about the binding strength of polyclonal antibodies.
Pseudovirus-based neutralization assays allow the screening of post-vaccination sera against multiple viral variants mutants and thereby the determination of the salient neutralization epitopes recognized by vaccine-elicited antibodies.
Neutralization and binding titers have great predictive value in vaccine analyses, provided the antigens used in the assays are similar to functional entry-mediating proteins on the surfaces of circulating viruses.
Chaotropic effects on proteins in general and antibody-antigen interactions in particular may become better understood through developments in physical chemistry while their usefulness in routine immunological assays recedes.
Acknowledgments
The author’s work in this area is supported by the NIH grant R37AI36082.
Footnotes
Financial and competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Hilleman MR. Vaccines in historic evolution and perspective: a narrative of vaccine discoveries. Vaccine. 2000;18:1436–1447. doi: 10.1016/s0264-410x(99)00434-x. [DOI] [PubMed] [Google Scholar]
- 2.Klasse PJ, Sanders RW, Cerutti A, Moore JP. How can HIV-type-1-Env immunogenicity be improved to facilitate antibody-based vaccine development? AIDS Res Hum Retroviruses. 2012;28:1–15. doi: 10.1089/aid.2011.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337:183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 5.Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006;24(Suppl 3):S3/106–113. doi: 10.1016/j.vaccine.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 6.Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio PM, Incesu RB, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West AP, Jr, Scharf L, Scheid JF, Klein F, Bjorkman PJ, Nussenzweig MC. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 10.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R. A Blueprint for HIV Vaccine Discovery. Cell Host Microbe. 2012;12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klasse PJ, Moore JP. Good CoP, bad CoP? Interrogating the immune responses to primate lentiviral vaccines. Retrovirology. 2012;9:80. doi: 10.1186/1742-4690-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward KN, Dhaliwal W, Ashworth KL, Clutterbuck EJ, Teo CG. Measurement of antibody avidity for hepatitis C virus distinguishes primary antibody responses from passively acquired antibody. J Med Virol. 1994;43:367–372. doi: 10.1002/jmv.1890430409. [DOI] [PubMed] [Google Scholar]
- 14.Hedman K, Seppala I. Recent rubella virus infection indicated by a low avidity of specific IgG. J Clin Immunol. 1988;8:214–221. doi: 10.1007/BF00917569. [DOI] [PubMed] [Google Scholar]
- 15.Soderlund M, Brown CS, Cohen BJ, Hedman K. Accurate serodiagnosis of B19 parvovirus infections by measurement of IgG avidity. J Infect Dis. 1995;171:710–713. doi: 10.1093/infdis/171.3.710. [DOI] [PubMed] [Google Scholar]
- 16.Ward KN, Gray JJ, Joslin ME, Sheldon MJ. Avidity of IgG antibodies to human herpesvirus-6 distinguishes primary from recurrent infection in organ transplant recipients and excludes cross-reactivity with other herpesviruses. J Med Virol. 1993;39:44–49. doi: 10.1002/jmv.1890390109. [DOI] [PubMed] [Google Scholar]
- 17.Lutz E, Ward KN, Gray JJ. Maturation of antibody avidity after primary human cytomegalovirus infection is delayed in immunosuppressed solid organ transplant patients. J Med Virol. 1994;44:317–322. doi: 10.1002/jmv.1890440402. [DOI] [PubMed] [Google Scholar]
- 18.Monsalvo AC, Batalle JP, Lopez MF, Krause JC, Klemenc J, Hernandez JZ, Maskin B, Bugna J, Rubinstein C, Aguilar L, Dalurzo L, Libster R, Savy V, Baumeister E, Cabral G, Font J, Solari L, Weller KP, Johnson J, Echavarria M, Edwards KM, Chappell JD, Crowe JE, Jr, Williams JV, Melendi GA, Polack FP. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med. 2011;17:195–199. doi: 10.1038/nm.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med. 2003;9:1209–1213. doi: 10.1038/nm918. [DOI] [PubMed] [Google Scholar]
- 20.Gassmann C, Bauer G. Avidity determination of IgG directed against tick-borne encephalitis virus improves detection of current infections. J Med Virol. 1997;51:242–251. [PubMed] [Google Scholar]
- 21.Hedman K, Vaheri A, Brummer-Korvenkontio M. Rapid diagnosis of hantavirus disease with an IgG-avidity assay. Lancet. 1991;338:1353–1356. doi: 10.1016/0140-6736(91)92235-t. [DOI] [PubMed] [Google Scholar]
- 22.Wreghitt TG, Gray JJ, Aloyisus S, Contreras M, Barbara JA. Antibody avidity test for recent infection with hepatitis C virus. Lancet. 1990;335:789. doi: 10.1016/0140-6736(90)90902-h. [DOI] [PubMed] [Google Scholar]
- 23.Cole KS, Alvarez M, Elliott DH, Lam H, Martin E, Chau T, Micken K, Rowles JL, Clements JE, Murphey-Corb M, Montelaro RC, Robinson JE. Characterization of neutralization epitopes of simian immunodeficiency virus (SIV) recognized by rhesus monoclonal antibodies derived from monkeys infected with an attenuated SIV strain. Virology. 2001;290:59–73. doi: 10.1006/viro.2001.1144. [DOI] [PubMed] [Google Scholar]
- 24*.Wei X, Liu X, Dobbs T, Kuehl D, Nkengasong JN, Hu DJ, Parekh BS. Development of two avidity-based assays to detect recent HIV type 1 seroconversion using a multisubtype gp41 recombinant protein. AIDS Res Hum Retroviruses. 2010;26:61–71. doi: 10.1089/aid.2009.0133. An alternative to the chaotrope-avidity assay for assessing intrinsic affinity was based on low antigen density. [DOI] [PubMed] [Google Scholar]
- 25.Narita M, Matsuzono Y, Takekoshi Y, Yamada S, Itakura O, Kubota M, Kikuta H, Togashi T. Analysis of mumps vaccine failure by means of avidity testing for mumps virus-specific immunoglobulin G. Clin Diagn Lab Immunol. 1998;5:799–803. doi: 10.1128/cdli.5.6.799-803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narita M, Yamada S, Matsuzono Y, Itakura O, Togashi T, Kikuta H. Immunoglobulin G avidity testing in serum and cerebrospinal fluid for analysis of measles virus infection. Clin Diagn Lab Immunol. 1996;3:211–215. doi: 10.1128/cdli.3.2.211-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox JL, Hazell SL, Tobler LH, Busch MP. Immunoglobulin G avidity in differentiation between early and late antibody responses to West Nile virus. Clin Vaccine Immunol. 2006;13:33–36. doi: 10.1128/CVI.13.1.33-36.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hearty S, Conroy PJ, Ayyar BV, Byrne B, O’Kennedy R. Surface plasmon resonance for vaccine design and efficacy studies: recent applications and future trends. Expert Rev Vaccines. 2010;9:645–664. doi: 10.1586/erv.10.52. [DOI] [PubMed] [Google Scholar]
- 29**.Yasmeen A, Ringe R, Derking R, Cupo A, Julien JP, Burton DR, Ward AB, Wilson IA, Sanders RW, Moore JP, Klasse PJ. Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits. Retrovirology. 2014;11:41. doi: 10.1186/1742-4690-11-41. Approaches for studying the kinetics of antibody binding to native-like HIV-1 Env trimers by surface plasmon resonance were optimized in this study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sela-Culang I, Kunik V, Ofran Y. The structural basis of antibody-antigen recognition. Front Immunol. 2013;4:302. doi: 10.3389/fimmu.2013.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Underwood PA. Problems and pitfalls with measurement of antibody affinity using solid phase binding in the ELISA. J Immunol Methods. 1993;164:119–130. doi: 10.1016/0022-1759(93)90282-c. [DOI] [PubMed] [Google Scholar]
- 32.Underwood PA, Steele JG, Dalton BA. Effects of polystyrene surface chemistry on the biological activity of solid phase fibronectin and vitronectin, analysed with monoclonal antibodies. J Cell Sci. 1993;104( Pt 3):793–803. doi: 10.1242/jcs.104.3.793. [DOI] [PubMed] [Google Scholar]
- 33.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 34.Butler JE, Feldbush TL, McGivern PL, Stewart N. The enzyme-linked immunosorbent assay (ELISA): a measure of antibody concentration or affinity. Immunochemistry. 1978;15:131–136. doi: 10.1016/0161-5890(78)90053-6. [DOI] [PubMed] [Google Scholar]
- 35.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972;109:129–135. [PubMed] [Google Scholar]
- 36.Katz FE, Steward MW. Studies on the genetic control of antibody affinity: the independent control of antibody levels and affinity in Biozzi mice. J Immunol. 1976;117:477–479. [PubMed] [Google Scholar]
- 37.Klasse PJ, Sattentau QJ. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol. 2002;83:2091–2108. doi: 10.1099/0022-1317-83-9-2091. [DOI] [PubMed] [Google Scholar]
- 38.Edgington TS. Dissociation of antibody from erythrocyte surfaces by chaotropic ions. J Immunol. 1971;106:673–680. [PubMed] [Google Scholar]
- 39*.Pullen GR, Fitzgerald MG, Hosking CS. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986;86:83–87. doi: 10.1016/0022-1759(86)90268-1. This study introduced the chaotrope disruption of antibody binding in ELISA for the monitoring of affinity maturation of the antibody response after Rubella vaccination. [DOI] [PubMed] [Google Scholar]
- 40.Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011;3:85ra48. doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 42.Gordon SN, Doster MN, Kines RC, Keele BF, Brocca-Cofano E, Guan Y, Pegu P, Liyanage NP, Vaccari M, Cuburu N, Buck CB, Ferrari G, Montefiori D, Piatak M, Jr, Lifson JD, Xenophontos AM, Venzon D, Robert-Guroff M, Graham BS, Lowy DR, Schiller JT, Franchini G. Antibody to the gp120 V1/V2 Loops and CD4+ and CD8+ T Cell Responses in Protection from SIVmac251 Vaginal Acquisition and Persistent Viremia. J Immunol. 2014;193:6172–6183. doi: 10.4049/jimmunol.1401504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai L, Kwa S, Kozlowski PA, Montefiori DC, Ferrari G, Johnson WE, Hirsch V, Villinger F, Chennareddi L, Earl PL, Moss B, Amara RR, Robinson HL. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J Infect Dis. 2011;204:164–173. doi: 10.1093/infdis/jir199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai L, Vodros D, Kozlowski PA, Montefiori DC, Wilson RL, Akerstrom VL, Chennareddi L, Yu T, Kannanganat S, Ofielu L, Villinger F, Wyatt LS, Moss B, Amara RR, Robinson HL. GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology. 2007;369:153–167. doi: 10.1016/j.virol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, Whitney JB, Seoighe C, Lacerda M, Keating S, Norris PJ, Hudgens MG, Gilbert PB, Buzby AP, Mach LV, Zhang J, Balachandran H, Shaw GM, Schmidt SD, Todd JP, Dodson A, Mascola JR, Nabel GJ. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci Transl Med. 2011;3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, Ferrari G, Pal R, Ferrari MG, Whitney S, Hudacik L, Billings E, Rao M, Montefiori D, Tomaras G, Alam SM, Fenizia C, Lifson JD, Stablein D, Tartaglia J, Michael N, Kim J, Venzon D, Franchini G. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol. 2013;87:1708–1719. doi: 10.1128/JVI.02544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross TM, Xu Y, Green TD, Montefiori DC, Robinson HL. Enhanced avidity maturation of antibody to human immunodeficiency virus envelope: DNA vaccination with gp120-C3d fusion proteins. AIDS Res Hum Retroviruses. 2001;17:829–835. doi: 10.1089/088922201750252025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, DiPasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF, Robert-Guroff M. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol. 2012;86:4644–4657. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao J, Lai L, Amara RR, Montefiori DC, Villinger F, Chennareddi L, Wyatt LS, Moss B, Robinson HL. Preclinical studies of human immunodeficiency virus/AIDS vaccines: inverse correlation between avidity of anti-Env antibodies and peak postchallenge viremia. J Virol. 2009;83:4102–4111. doi: 10.1128/JVI.02173-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, Hidajat R, Demberg T, Robert-Guroff M. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2010;84:7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta S, Clark ES, Termini JM, Boucher J, Kanagavelu S, LeBranche CC, Abraham S, Montefiori DC, Khan WN, Stone GW. DNA vaccine molecular adjuvants SP-D-BAFF and SP-D-APRIL enhance anti-gp120 immune response and increase HIV-1 neutralizing antibody titers. J Virol. 2015 doi: 10.1128/JVI.02904-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richmond JF, Lu S, Santoro JC, Weng J, Hu SL, Montefiori DC, Robinson HL. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J Virol. 1998;72:9092–9100. doi: 10.1128/jvi.72.11.9092-9100.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barlow DJ, Edwards MS, Thornton JM. Continuous and discontinuous protein antigenic determinants. Nature. 1986;322:747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- 54.Atassi MZ. Antigenic structures of proteins. Their determination has revealed important aspects of immune recognition and generated strategies for synthetic mimicking of protein binding sites. Eur J Biochem. 1984;145:1–20. doi: 10.1111/j.1432-1033.1984.tb08516.x. [DOI] [PubMed] [Google Scholar]
- 55.Berzofsky JA. Intrinsic and extrinsic factors in protein antigenic structure. Science. 1985;229:932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- 56.Goudsmit J, Meloen RH, Brasseur R. Map of sequential B cell epitopes of the HIV-1 transmembrane protein using human antibodies as probe. Intervirology. 1990;31:327–338. doi: 10.1159/000150169. [DOI] [PubMed] [Google Scholar]
- 57.Klasse PJ, Pipkorn R, Blomberg J. A cluster of continuous antigenic structures in the transmembrane protein of HIV-1: individual patterns of reactivity in human sera. Mol Immunol. 1991;28:613–622. doi: 10.1016/0161-5890(91)90130-c. [DOI] [PubMed] [Google Scholar]
- 58.Laver WG, Air GM, Webster RG, Smith-Gill SJ. Epitopes on protein antigens: misconceptions and realities. Cell. 1990;61:553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- 59.Rosen O, Chill J, Sharon M, Kessler N, Mester B, Zolla-Pazner S, Anglister J. Induced fit in HIV-neutralizing antibody complexes: evidence for alternative conformations of the gp120 V3 loop and the molecular basis for broad neutralization. Biochemistry. 2005;44:7250–7258. doi: 10.1021/bi047387t. [DOI] [PubMed] [Google Scholar]
- 60.Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, Ward AB. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Checkley MA, Luttge BG, Freed EO. HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J Mol Biol. 2011;410:582–608. doi: 10.1016/j.jmb.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klasse PJ. Structural biology. A new bundle of prospects for blocking HIV-1 entry. Science. 2013;341:1347–1348. doi: 10.1126/science.1245384. [DOI] [PubMed] [Google Scholar]
- 64.Weiss RA. Thirty years on: HIV receptor gymnastics and the prevention of infection. BMC Biol. 2013;11:57. doi: 10.1186/1741-7007-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klasse PJ. The molecular basis of HIV entry. Cell Microbiol. 2012;14:1183–1192. doi: 10.1111/j.1462-5822.2012.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat Immunol. 2015;16:571–576. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore JP, Ho DD. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993;67:863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, Birungi J, Pozniak A, McPhee DA, Manigart O, Karita E, Inwoley A, Jaoko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Walker LM, Poignard P, Wrin T, Fast PE, Burton DR, Koff WC. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doria-Rose NA, Klein RM, Daniels MG, O’Dell S, Nason M, Lapedes A, Bhattacharya T, Migueles SA, Wyatt RT, Korber BT, Mascola JR, Connors M. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol. 2010;84:1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 2011;7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piantadosi A, Panteleeff D, Blish CA, Baeten JM, Jaoko W, McClelland RS, Overbaugh J. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J Virol. 2009;83:10269–10274. doi: 10.1128/JVI.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medina-Ramirez M, Sanders RW, Klasse PJ. Targeting B-cell germlines and focusing affinity maturation: the next hurdles in HIV-1-vaccine development? Expert Rev Vaccines. 2014;13:449–452. doi: 10.1586/14760584.2014.894469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancera M, Zhou T, Incesu RB, Fu BZ, Gnanapragasam PN, Oliveira TY, Seaman MS, Kwong PD, Bjorkman PJ, Nussenzweig MC. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75**.Alexander MR, Ringe R, Sanders RW, Voss JE, Moore JP, Klasse PJ. What do chaotrope-based avidity assays for antibodies to HIV-1 envelope glycoproteins measure? J Virol. 2015 doi: 10.1128/JVI.00320-15. This study of antibody binding to HIV-1 Env shows that sensitivity to chaotrope disruption is greater for complex than linear epitopes and particularly pronounced for quaternary neutralization epitopes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Binley JM, Arshad H, Fouts TR, Moore JP. An investigation of the high-avidity antibody response to glycoprotein 120 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1997;13:1007–1015. doi: 10.1089/aid.1997.13.1007. [DOI] [PubMed] [Google Scholar]
- 77.Curtis KA, Kennedy MS, Luckay A, Cong ME, Youngpairoj AS, Zheng Q, Smith J, Hanson D, Heneine W, Owen SM, Garcia-Lerma JG. Delayed maturation of antibody avidity but not seroconversion in rhesus macaques infected with simian HIV during oral pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2011;57:355–362. doi: 10.1097/QAI.0b013e3182234a51. [DOI] [PubMed] [Google Scholar]
- 78.Duong YT, Qiu M, De AK, Jackson K, Dobbs T, Kim AA, Nkengasong JN, Parekh BS. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One. 2012;7:e33328. doi: 10.1371/journal.pone.0033328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dimitrov JD, Lacroix-Desmazes S, Kaveri SV. Important parameters for evaluation of antibody avidity by immunosorbent assay. Anal Biochem. 2011;418:149–151. doi: 10.1016/j.ab.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 80.Hardie G, van Regenmortel MH. Isolation of specific antibody under conditions of low ionic strength. J Immunol Methods. 1977;15:305–314. doi: 10.1016/0022-1759(77)90092-8. [DOI] [PubMed] [Google Scholar]
- 81**.Jungwirth P, Cremer PS. Beyond Hofmeister. Nat Chem. 2014;6:261–263. doi: 10.1038/nchem.1899. This perspective discusses the ground-breaking ranking of ionic effects on protein solubility in the light of the new developments in this area, showing that chaotropic effects cannot be generalized because they involve both backbone and side-chain interactions. [DOI] [PubMed] [Google Scholar]
- 82.Kamoun PP. Denaturation of globular proteins by urea: breakdown of hydrogen or hydrophobic bonds? Trends Biochem Sci. 1988;13:424–425. doi: 10.1016/0968-0004(88)90211-3. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Cremer PS. Interactions between macromolecules and ions: The Hofmeister series. Curr Opin Chem Biol. 2006;10:658–663. doi: 10.1016/j.cbpa.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 84.Baldwin RL. How Hofmeister ion interactions affect protein stability. Biophys J. 1996;71:2056–2063. doi: 10.1016/S0006-3495(96)79404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andersson K, Choulier L, Hamalainen MD, van Regenmortel MH, Altschuh D, Malmqvist M. Predicting the kinetics of peptide-antibody interactions using a multivariate experimental design of sequence and chemical space. J Mol Recognit. 2001;14:62–71. doi: 10.1002/1099-1352(200101/02)14:1<62::AID-JMR520>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 86.Choulier L, Andersson K, Hamalainen MD, van Regenmortel MH, Malmqvist M, Altschuh D. QSAR studies applied to the prediction of antigen-antibody interaction kinetics as measured by BIACORE. Protein Eng. 2002;15:373–382. doi: 10.1093/protein/15.5.373. [DOI] [PubMed] [Google Scholar]
- 87.Hall TJ, Heckel C. Thiocyanate elution estimation of relative antibody affinity. J Immunol Methods. 1988;115:153–155. doi: 10.1016/0022-1759(88)90324-9. [DOI] [PubMed] [Google Scholar]
- 88.Eisen HN, Chakraborty AK. Evolving concepts of specificity in immune reactions. Proc Natl Acad Sci U S A. 2010;107:22373–22380. doi: 10.1073/pnas.1012051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manivel V, Sahoo NC, Salunke DM, Rao KV. Maturation of an antibody response is governed by modulations in flexibility of the antigen-combining site. Immunity. 2000;13:611–620. doi: 10.1016/s1074-7613(00)00061-3. [DOI] [PubMed] [Google Scholar]
- 90.Wedemayer GJ, Patten PA, Wang LH, Schultz PG, Stevens RC. Structural insights into the evolution of an antibody combining site. Science. 1997;276:1665–1669. doi: 10.1126/science.276.5319.1665. [DOI] [PubMed] [Google Scholar]
- 91.Zimmermann J, Oakman EL, Thorpe IF, Shi X, Abbyad P, Brooks CL, 3rd, Boxer SG, Romesberg FE. Antibody evolution constrains conformational heterogeneity by tailoring protein dynamics. Proc Natl Acad Sci U S A. 2006;103:13722–13727. doi: 10.1073/pnas.0603282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Julien JP, Lee JH, Cupo A, Murin CD, Derking R, Hoffenberg S, Caulfield MJ, King CR, Marozsan AJ, Klasse PJ, Sanders RW, Moore JP, Wilson IA, Ward AB. Asymmetric recognition of the HIV-1 trimer by broadly neutralizing antibody PG9. Proc Natl Acad Sci U S A. 2013;110:4351–4356. doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Derking R, Ozorowski G, Sliepen K, Yasmeen A, Cupo A, Torres JL, Julien JP, Lee JH, van Montfort T, de Taeye SW, Connors M, Burton DR, Wilson IA, Klasse PJ, Ward AB, Moore JP, Sanders RW. Comprehensive antigenic map of a cleaved soluble HIV-1 envelope trimer. PLoS Pathog. 2015;11:e1004767. doi: 10.1371/journal.ppat.1004767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Absolom DR, van Oss CJ. The nature of the antigen-antibody bond and the factors affecting its association and dissociation. CRC Crit Rev Immunol. 1986;6:1–46. [PubMed] [Google Scholar]
- 95.van Oss CJ, Good RJ, Chaudhury MK. Nature of the antigen-antibody interaction. Primary and secondary bonds: optimal conditions for association and dissociation. J Chromatogr. 1986;376:111–119. [PubMed] [Google Scholar]
- ************96**.Do Kwon Y, Pancera M, Acharya P, Georgiev IS, Crooks ET, Gorman J, Joyce MG, Guttman M, Ma X, Narpala S, Soto C, Terry DS, Yang Y, Zhou T, Ahlsen G, Bailer RT, Chambers M, Chuang GY, Doria-Rose NA, Druz A, Hallen MA, Harned A, Kirys T, Louder MK, O’Dell S, Ofek G, Osawa K, Prabhakaran M, Sastry M, Stewart-Jones GB, Stuckey J, Thomas PV, Tittley T, Williams C, Zhang B, Zhao H, Zhou Z, Donald BR, Lee LK, Zolla-Pazner S, Baxa U, Schon A, Freire E, Shapiro L, Lee KK, Arthos J, Munro JB, Blanchard SC, Mothes W, Binley JM, et al. Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat Struct Mol Biol. 2015;22:522–531. doi: 10.1038/nsmb.3051. Soluble native-like HIV-1 trimers were further engineered to limit their conformational flexibility and optimize the presentation of neutralization epitopes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Munro JB, Gorman J, Ma X, Zhou Z, Arthos J, Burton DR, Koff WC, Courter JR, Smith AB, 3rd, Kwong PD, Blanchard SC, Mothes W. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science. 2014;346:759–763. doi: 10.1126/science.1254426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang GY, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko SY, Todd JP, Rao S, Graham BS, Kwong PD. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haim H, Salas I, McGee K, Eichelberger N, Winter E, Pacheco B, Sodroski J. Modeling virus- and antibody-specific factors to predict human immunodeficiency virus neutralization efficiency. Cell Host Microbe. 2013;14:547–558. doi: 10.1016/j.chom.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karlsson R, Michaelsson A, Mattsson L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J Immunol Methods. 1991;145:229–240. doi: 10.1016/0022-1759(91)90331-9. [DOI] [PubMed] [Google Scholar]
- 101.Abdiche Y, Malashock D, Pinkerton A, Pons J. Determining kinetics and affinities of protein interactions using a parallel real-time label-free biosensor, the Octet. Anal Biochem. 2008;377:209–217. doi: 10.1016/j.ab.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 102.Khurana S, Coyle EM, Verma S, King LR, Manischewitz J, Crevar CJ, Carter DM, Ross TM, Golding H. H5 N-terminal beta sheet promotes oligomerization of H7-HA1 that induces better antibody affinity maturation and enhanced protection against H7N7 and H7N9 viruses compared to inactivated influenza vaccine. Vaccine. 2014;32:6421–6432. doi: 10.1016/j.vaccine.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 103.VanCott TC, Bethke FR, Polonis VR, Gorny MK, Zolla-Pazner S, Redfield RR, Birx DL. Dissociation rate of antibody-gp120 binding interactions is predictive of V3-mediated neutralization of HIV-1. J Immunol. 1994;153:449–459. [PubMed] [Google Scholar]
- 104**.Sather DN, Carbonetti S, Malherbe DC, Pissani F, Stuart AB, Hessell AJ, Gray MD, Mikell I, Kalams SA, Haigwood NL, Stamatatos L. Emergence of Broadly Neutralizing Antibodies and Viral Coevolution in Two Subjects during the Early Stages of Infection with Human Immunodeficiency Virus Type 1. J Virol. 2014;88:12968–12981. doi: 10.1128/JVI.01816-14. In order to assess the development of the broadly neutralizing antibody response in HIV-1-infected humans, the authors used biolayer interferometry to measure the binding to Env of polyclonal IgG and thereby to obtain apparent dissociation constants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Richalet-Secordel PM, Rauffer-Bruyere N, Christensen LL, Ofenloch-Haehnle B, Seidel C, Van Regenmortel MH. Concentration measurement of unpurified proteins using biosensor technology under conditions of partial mass transport limitation. Anal Biochem. 1997;249:165–173. doi: 10.1006/abio.1997.2183. [DOI] [PubMed] [Google Scholar]
- 106.Zeder-Lutz G, Benito A, Van Regenmortel MH. Active concentration measurements of recombinant biomolecules using biosensor technology. J Mol Recognit. 1999;12:300–309. doi: 10.1002/(SICI)1099-1352(199909/10)12:5<300::AID-JMR467>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 107.Greenspan NS. Affinity, complementarity, cooperativity, and specificity in antibody recognition. Curr Top Microbiol Immunol. 2001;260:65–85. doi: 10.1007/978-3-662-05783-4_5. [DOI] [PubMed] [Google Scholar]
- 108.Alexander MR, Sanders RW, Moore JP, Klasse PJ. Virion Aggregation by Neutralizing and Nonneutralizing Antibodies to the HIV-1 Envelope Glycoprotein. AIDS Res Hum Retroviruses. 2015 doi: 10.1089/AID.2015.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schofield DJ, Dimmock NJ. Determination of affinities of a panel of IgGs and Fabs for whole enveloped (influenza A) virions using surface plasmon resonance. J Virol Methods. 1996;62:33–42. doi: 10.1016/0166-0934(96)02086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klein JS, Bjorkman PJ. Few and far between: how HIV may be evading antibody avidity. PLoS Pathog. 2010;6:e1000908. doi: 10.1371/journal.ppat.1000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Galimidi RP, Klein JS, Politzer MS, Bai S, Seaman MS, Nussenzweig MC, West AP, Jr, Bjorkman PJ. Intra-spike crosslinking overcomes antibody evasion by HIV-1. Cell. 2015;160:433–446. doi: 10.1016/j.cell.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112*.Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity. 1998;8:751–759. doi: 10.1016/s1074-7613(00)80580-4. This study shows the influence and thresholds of kinetic constants in affinity maturation of antibodies. [DOI] [PubMed] [Google Scholar]
- 113.Van Regenmortel MH. Specificity, polyspecificity, and heterospecificity of antibody-antigen recognition. J Mol Recognit. 2014;27:627–639. doi: 10.1002/jmr.2394. [DOI] [PubMed] [Google Scholar]
- 114.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003;299:1362–1367. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 115.Mouquet H, Scheid JF, Zoller MJ, Krogsgaard M, Ott RG, Shukair S, Artyomov MN, Pietzsch J, Connors M, Pereyra F, Walker BD, Ho DD, Wilson PC, Seaman MS, Eisen HN, Chakraborty AK, Hope TJ, Ravetch JV, Wardemann H, Nussenzweig MC. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mouquet H, Warncke M, Scheid JF, Seaman MS, Nussenzweig MC. Enhanced HIV-1 neutralization by antibody heteroligation. Proc Natl Acad Sci U S A. 2012;109:875–880. doi: 10.1073/pnas.1120059109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dunlop DC, Bonomelli C, Mansab F, Vasiljevic S, Doores KJ, Wormald MR, Palma AS, Feizi T, Harvey DJ, Dwek RA, Crispin M, Scanlan CN. Polysaccharide mimicry of the epitope of the broadly neutralizing anti-HIV antibody, 2G12, induces enhanced antibody responses to self oligomannose glycans. Glycobiology. 2010;20:812–823. doi: 10.1093/glycob/cwq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sok D, Doores KJ, Briney B, Le KM, Saye-Francisco KL, Ramos A, Kulp DW, Julien JP, Menis S, Wickramasinghe L, Seaman MS, Schief WR, Wilson IA, Poignard P, Burton DR. Promiscuous glycan site recognition by antibodies to the high-mannose patch of gp120 broadens neutralization of HIV. Sci Transl Med. 2014;6:236ra263. doi: 10.1126/scitranslmed.3008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ketas TJ, Holuigue S, Matthews K, Moore JP, Klasse PJ. Env-glycoprotein heterogeneity as a source of apparent synergy and enhanced cooperativity in inhibition of HIV-1 infection by neutralizing antibodies and entry inhibitors. Virology. 2012;422:22–36. doi: 10.1016/j.virol.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCoy LE, Falkowska E, Doores KJ, Le K, Sok D, van Gils MJ, Euler Z, Burger JA, Seaman MS, Sanders RW, Schuitemaker H, Poignard P, Wrin T, Burton DR. Incomplete Neutralization and Deviation from Sigmoidal Neutralization Curves for HIV Broadly Neutralizing Monoclonal Antibodies. PLoS Pathog. 2015;11:e1005110. doi: 10.1371/journal.ppat.1005110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parren PW, Mondor I, Naniche D, Ditzel HJ, Klasse PJ, Burton DR, Sattentau QJ. Neutralization of human immunodeficiency virus type 1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J Virol. 1998;72:3512–3519. doi: 10.1128/jvi.72.5.3512-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pugach P, Ozorowski G, Cupo A, Ringe R, Yasmeen A, de Val N, Derking R, Kim HJ, Korzun J, Golabek M, de Los Reyes K, Ketas TJ, Julien JP, Burton DR, Wilson IA, Sanders RW, Klasse PJ, Ward AB, Moore JP. A native-like SOSIP.664 trimer based on an HIV-1 subtype B env gene. J Virol. 2015;89:3380–3395. doi: 10.1128/JVI.03473-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, Kim HJ, Blattner C, de la Pena AT, Korzun J, Golabek M, de Los Reyes K, Ketas TJ, van Gils MJ, King CR, Wilson IA, Ward AB, Klasse PJ, Moore JP. A next-generation cleaved, soluble HIV-1 Env Trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Corti D, Lanzavecchia A. Efficient Methods To Isolate Human Monoclonal Antibodies from Memory B Cells and Plasma Cells. Microbiol Spectr. 2014:2. doi: 10.1128/microbiolspec.AID-0018-2014. [DOI] [PubMed] [Google Scholar]