Abstract
Objectives
Spousal caregivers of patients with dementia are in need of interventions to bolster their quality of life. Computer-based, self-administered cognitive training is an innovative approach to targeting spousal caregiver distress and coping. We tested the feasibility of administering one such intervention with minimal clinician intervention.
Methods
Twenty-seven elderly adults (> 64 years old), who each were the primary caregiver for a spouse with dementia, were recruited through the Memory Disorders Clinic of the Alzheimer Disease Research Center in Pittsburgh, PA. Spousal caregivers were instructed to use a handheld computer version of the Adaptive Paced Visual Serial Attention Task (APVSAT) at least three times per week for 4 weeks as part of a larger caregiver intervention trial (P01 AG020677). Feasibility was explored by examining the frequency of APVSAT usage.
Results
Results suggest that self-directed cognitive training is feasible for spousal caregivers of dementia patients. The mean usage of the APSVAT was 42 (SD = 28.58). Performance increased from the beginning to the end of the trial, and usage was not affected by stress, worry, or poor sleep quality.
Conclusion
Findings suggest the potential utility of cognitive training via handheld computer for spousal caregivers of dementia patients to improve problem solving, coping and adaptation, planning, and persevering with goal-directed tasks.
Keywords: dementia spousal caregivers, neurobehavioral intervention, cognitive control training, handheld computer intervention
Introduction
Psychological symptomatology among dementia caregivers is a significant public health issue with multiple negative consequences for the caregivers and patient health (Schulz & Martire, 2004). Yet, mental health treatments are often inaccessible to dementia caregivers due to their inability to leave their family member alone. Interventions that caregivers do receive have typically demonstrated small to moderate effects (Belle et al., 2006). Adjunctive interventions that (1) do not require caregivers to leave their family member alone and (2) address typical treatment moderators may therefore be useful. One identified moderator of psychosocial intervention outcome is executive control deficits (Caswell et al., 2003; De Vugt et al., 2006; Molhman, 2005; Mackenzie, Wiprzycka, Hasher, & Goldstein, 2009). Engagement in psychosocial interventions specifically requires intact features of executive control such as the ability to practice skills and working memory for intervention components; moreover, improving executive control appears to improve psychosocial treatment outcome in the elderly (Mohlman & Gorman, 2005; Mohlman, 2013). Given the moderation of executive control deficits to psychosocial intervention outcomes, we explored the feasibility of a self-administered intervention for executive control administered on a handheld computer in older adult spousal caregivers of dementia patients, which would not only complement other psychological treatments, but also potentially keep the patient in the home for a longer period of time by strengthening the caregiver’s coping abilities.
Determining the feasibility of an intervention that targets executive control is particularly salient, as caring for a spouse with dementia exacerbates cognitive decline (Vitaliano, Murphy, Young, Echeverria, & Borson, 2011). Caregivers appear to be characterized by reduced learning and memory (Mackenzie, et al., 2009) as well as decreased performance on neuropsychological measures of cognitive functioning (Caswell et al., 2003; DeVugt et al., 2006). Improving executive control may thus enhance caregiver functioning and the efficacy of caregiver interventions.
Cognitive training is one approach to improving executive control (Lustig, Shah, Seidler, & Reuter-Lorenz, 2009; Nouchi et al., 2012; Schmiedek, Lovden, & Lindenberger, 2010). Cognitive training typically involves directed training on tasks designed to recruit particular cognitive functions (e.g., memory, attention, or problem-solving). The fundamental supposition is that (1) training will enhance or preserve function in a given area and (2) improvements will generalize outside the training tasks (Clare & Woods, 2003). Initial case study data suggests that cognitive training directed at executive control can improve psychosocial intervention outcome in the elderly (Mohlman et al., 2010).
For the intervention reported here, we followed a specific cognitive training paradigm that targeted a candidate brain mechanism, the dorso-lateral prefrontal cortex, in elderly caregivers. The dorso-lateral prefrontal cortex is highly implicated in executive control and regulating emotions; specifically, depression, anxiety, and stress, which are often elevated in caregivers, are associated with impaired prefrontal function (Croog, Burleson, Sudilovsky, & Baume, 2006; Pinquart & Sorensen, 2003). Following this paradigm is supported by several studies. For example, cognitive training tasks geared towards increasing executive control have been shown to recruit the dorsolateral prefrontal cortex (Price, Paul, Schneider, & Siegle, 2013), decrease rumination (Siegle, Ghinassi, & Thase, 2007), and decrease depressive symptomatology above and beyond active placebo interventions (Calkins, McMorran, Siegle, & Otto, 2014). Moreover, cognitive training tasks geared towards executive control also appear to have sustained impact on health care service use (Siegle, Price, Jones, Ghinassi, & Thase, in press).
One of the component tasks in this intervention was an adaptive version of the Paced Auditory Serial Attention Task (PASAT) (Gronwall, 1977), a working memory task that involves adding serially presented digits in working memory. The PASAT has been shown to employ prefrontal resources (Lazeron, Rombouts, de Sonneville, Barkhof, & Sheltens, 2003) as does an adaptive paced visual serial attention task (APVSAT) variant (Royan, Tombaugh, Rees, & Francis, 2004), which we employed in this intervention. Additionally, we have shown that a single dose of the intervention does not appear to be effective (Calkins et al., 2011), which suggested the specific utility of investigating whether or not caregivers would use the intervention multiple times.
Related interventions have been helpful for this patient population. For example, a study of cognitively healthy older adults examined Brain Fitness, a cognitive training product, in 487 adults (mean age 75 years) in comparison to an active control condition. Large benefits were seen for auditory processing speed; moderate benefits were observed for everyday cognitive skills; and small but statistically significant benefits were seen for memory performance. Another large (N = 2,832) randomized controlled study of cognitive training interventions (i.e., memory, reasoning, speed of processing) demonstrated improved targeted cognitive abilities over 2 years (Ball et al., 2002). While data regarding home-use cognitive-training interventions has suggested little generalizability in a large sample (i.e., N > 11,000) (Owen et al., 2010), within the subset of individuals over 60 years old, initial data appeared more promising for some domains (BBC, 2009). Thus, in healthy older adults, cognitive training has been beneficial in improving targeted cognitive abilities.
In the intervention presented here, we examined the use of a handheld computer based APVSAT in a home-based intervention with elderly spousal caregivers of individuals with dementia. Our hypotheses were that (1) caregivers would reliably use the APVSAT at home (i.e., feasibility), (2) baseline participant characteristics (e.g., stress, worry, and baseline sleep quality) would not attenuate its use (i.e., moderators), and (3) caregivers’ performance on the intervention task would improve over time (i.e., improvement). We did not examine whether or not performance changes were associated with mood or additional psychological measures, as this feasibility study was conducted with a small subset of participants within a larger, multi-component intervention, preventing an analysis of the APVSAT intervention response.
Methods
Participants
Participants assigned to Stress Management plus Healthy Sleep Practices (SM+HSP) with APVST were 27 primary caregivers for a spouse with dementia (e.g., Dementia Disease, advanced Parkinson’s disease) who indicated that caregiving was a physical or emotional strain. In addition, they needed to report significant subjective sleep complaints (Pittsburgh Sleep Quality Index scores of ≥ 5) (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) or poor sleep efficiency (< 90%) as identified by in-home polysomnography. All participants were required to have a Folstein MMSE (Folstein, Folstein, & McHugh, 1975) at or above 25. Participants were excluded if they (1) anticipated institutionalizing their spouse within 6 months of enrolling in the study, (2) had a neurological disorder (e.g., Parkinson’s disease, seizure disorder, stroke) (3) had a primary sleep disorder (the parent study examined stress-related sleep disruptions in caregivers), and/or (4) had an unstable or acute medical disorder. Participants were not paid for use of the APSVAT. Participant characteristics are shown in Table 1.
Table 1.
Sample Description (N = 27)
| Women % | 81.25% |
| Age in Years (M ± SD) | 74.61 (6.52) |
| Caucasian % | 96.29% |
| Education (% High School or above) | |
| middle school to technical school | 41% |
| some college-college graduate | 52% |
| some post-graduate-post-graduate | 7% |
| Income before Retirement (N = 31) | |
| below $49,999 | 40% |
| $50,000 to $99,999 | 24% |
| $100,000 or greater | 8% |
| didn’t know or declined | 28% |
| Income In Past Year (N = 31) | |
| below $49,999 | 52% |
| $50,000 to $99,999 | 16% |
| $100,000 to $149,000 | 4% |
| didn’t know or declined | 28% |
Procedure
This report is based on a subset of participants enrolled in a randomized clinical trial of Stress Management plus Healthy Sleep Practices (SM+HSP) compared at an Attention Only control condition (i.e., “Aging Well, Sleeping Efficiently: Intervention Studies,” M. Hall, P01 AG020677; NCT00178568). Following informed consent, participants completed the SCID-I to confirm study eligibility. Sixty qualified participants completed baseline measures and were randomly assigned to either experimental or control conditions. Both conditions involved eight weekly private in-home sessions. The experimental SM+HSP condition provided information about dementia, social support, coping skills training, affective self-management, and healthy sleep practices. The Attention-Only control condition targeted nutrition skills and meal planning. On week 4 of the intervention, 27 participants assigned to the SM+HSP condition were given a handheld computer with the APVSAT software installed. Given the amount of information delivered in the beginning of the study, we chose the 4th week as the most appropriate time to begin training for and use of the APSVAT. Thus, from week four through week eight, the use and training of the APSVAT coincided with other study activities and training.
Participants were trained on the use of the APVSAT by reviewing a study brochure and an operational guide with screenshots that included operating the handheld computer, starting the program, and doing the task. Participants then demonstrated and practiced using the device with the nurse interventionist. When participants demonstrated correct usage of the APVSAT, they were instructed to complete three 5-minute blocks per session, ideally at least three times per week.
The total length of the APVSAT component of the SM+HSP intervention was approximately 4 weeks, which facilitated 28 days of potential use, give or take a short time to accommodate scheduling constraints. Twelve participants used it beyond 4 weeks. Participant data was time-stamped each time the program was opened and closed. Baseline assessments were re-administered at post-intervention and again at 6 and 12 months. Participants completed the BDI weekly throughout the intervention period.
Measures
The Adaptive Paced Visual Serial Addition Task (APVSAT)
Feasibility was measured by the participants’ (1) frequency of and (2) performance in using a pocket-PC based APVSAT (Figure 1). When using the APVSAT, participants see a series of digits on the screen and are asked to add each new digit to the digit that preceded it (i.e., calculate the sum of the most recently seen two digits—not a running sum). Difficulty is varied by changing the speed with which items are presented—increasing speed presents increasing difficulty. Participants are instructed to get as many items right as they can and to resume the task as quickly as possible when they get something wrong.
Figure 1. Handheld APVSAT.
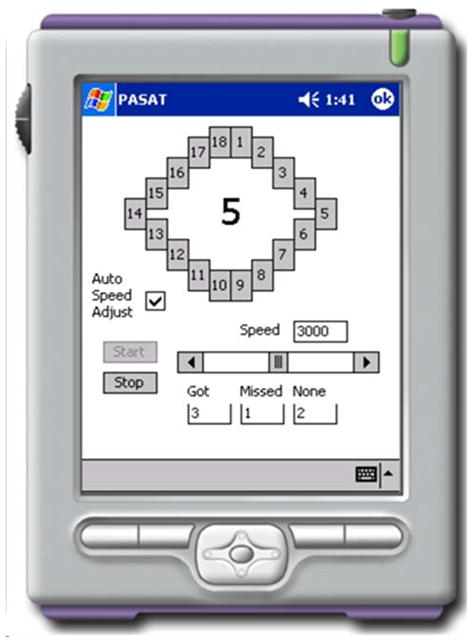
The Adaptive Paced Visual Serial Addition Task (APVSAT). When using the APVSAT, a pocket-PC based application, participants see a series of digits and are asked to add each new digit to the digit that preceded it (i.e., sum the most recently seen two digits—not keep a running sum). Difficulty is manipulated by increasing the speed with which items are presented.
A non-adaptive PASAT can be frustrating (Holdwick & Wingenfeld, 1999). In the non-adaptive task, the speed with which items are presented remains constant, despite incorrect responses. To keep the task tolerable and reduce “giving up” among participants, we modified the task to adapt to the participants’ performance (Siegle et al., 2007). Whereas the original version had fixed inter-stimulus intervals (ISIs), this version speeds up by 100 milliseconds (ms) when participants get four consecutive items correct. It slows down by 100 ms when participants miss four consecutive items. This technique ideally equated the task with difficulty across participants and sessions. An adaptive variant of the PASAT has been used previously in other protocols, and the speed on the task was positively correlated with performance on other tests of executive function (Royan et al., 2004). Thus, the inter-stimulus interval was a measure of performance on the task—faster ISIs were associated with more correct responses. All participants started at an ISI of 3,000 ms (slower than the slowest ISI on the standard PASAT), and they were instructed to resume each day beginning with their final ISI from the previous day. In addition to measuring ISI, we also tracked the raw number of APVSAT uses during the study and mean weekly uses (i.e., regularity).
The following instruments, administered prior to the intervention, were used to assess diagnostic eligibility and presence of dementia. Baseline measures of level of worry, subjective stress, depression, anxiety, intrusive thoughts, and rumination, and sleep quality were included to further describe the sample.
The Structured Clinical Interview for DSM-IV with Psychotic Screen (SCID-I/P w/PSY Screen) (First, Spitzer, & Gibbon,1995) is a structured diagnostic interview that assesses DSM-IV criteria to determine study eligibility.
The Mini Mental Status Exam (MMSE) (Folstein et al.,1975) is a formalized mental status examination that was used to screen for dementia.
The Penn State Worry Questionnaire (PSWQ) (Meyer, Miller, Metzger, & Borkovec, 1990) is a 16-item questionnaire used to measure the dispositional tendency for generalized worry.
The Perceived Stress Scale (PSS) (Cohen, Kamarck, & Mermelstein, 1983) is a 14-item questionnaire used to quantify subjective stress associated with ongoing events or circumstances. The domains measured are unpredictability, lack of control, burden overload, and stressful life circumstances.
The Pittsburgh Sleep Quality Index (PSQI) (Buysse et al.,1989) is a 19-item self-rated questionnaire that assesses sleep quality over the previous month. The PSQI evaluates (1) sleep quality, (2) estimates of sleep duration, latency, and frequency, and (3) severity of sleep-related difficulties. The seven subscales are rated on a 0 to 3 score, and the global PSQI is rated from 0 to 21. Higher scores, (i.e., ≥ 5) indicate worse sleep quality.
The Beck Depression Inventory (BDI-II) (Beck, Steer, & Brown, 1996) is a 21-item measure that was used to assess the severity of symptoms of depression as listed in the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 1994).
The original Hamilton Rating Scale for Depression (Ham-D) (Hamilton, 1960) is a 17-item clinician rated scale that assessed the severity of depressive symptoms. A 25-item version of the Ham-D was used in this study, which included the original 17 items along with additional questions related to diurnal variation, weight gain, and cognitive and melancholic features (Miller, Bishop, Norman, & Maddever, 1985).
The Hamilton Anxiety Scale (Ham-A) (Hamilton, 1959) is a 14-item clinician administered assessment that was used to measure symptoms of anxiety.
The Impact of Events Schedule (IES) (Horowitz, Wilner, & Alvarez, 1979) is a 15-item questionnaire that quantified the frequency of intrusive thoughts and avoidance behaviors associated with stressful events.
The Multidimensional Rumination Questionnaire (MRQ) (Fritz, 1999) is a 27-item questionnaire that assessed four potential subtypes of rumination in response to a stressful event: (1) symptoms of depression—MORMOTS; (2) anger/aggression—MORMOTA; (3) instrumental behavior—MORINST; (4) searching for a meaning—MORSRCH).
Statistical Analyses
Feasibility
Since an empirically established indicator of feasibility for this novel approach was not available (La Rue, 2010), we examined APSVAT use from this study to illustrate overall feasibility. APVSAT use was assessed by examining descriptive statistics that included the mean, median, standard deviation, and range of use across the 28-day intervention period. Frequency of use was tested using Fisher’s exact tests to examine whether the proportion of participants using the device at each use threshold was significantly more than 50% (i.e., whether or not there were significantly more than 14/27 participants with each number of uses). The regularity of usage was examined as the number of weeks of usage out of 4 weeks of usage (e.g., 3/4 to 4/4) and tested against 0 weeks of usage via a z-test. Assessing regularity of use, instead of examining total number of uses, addressed subject adherence with the handheld computer (i.e., whether or not participants used it as recommended).
Use moderators
We correlated baseline ratings of perceived stress (i.e., PSS) and worry (i.e., PSWQ) with the number of uses of the APVSAT during the 28-day intervention period. These measures were considered especially important to assess because worry and stress may impact participants’ ability or willingness to engage in self-help activities. Given the focus of poor sleep quality in the parent intervention, we also correlated baseline PSQI total scores with number of uses of the APSVAT during the 28-day intervention period.
Performance Improvement
A paired t-test was used to determine whether or not participants improved on their APVSAT performance, measured by mean ISI from day 1 to day 28. To understand whether the pattern of change on APVSAT performance across participants improved, we used a generalized estimating equation (GEE) model with both linear and quadratic effects for time, accounting for the correlated responses within participants.
Due to the variability in time that participants had possession of the APVSAT, sensitivity analyses were performed on (1) all participants (i.e., the entire sample, including those that adhered to the 28-day timeline plus those who used it beyond that timeline) and (2) participants who adhered to the 28-day intervention timeline.
Results
Socio-demographic features and baseline psychological measures of this sample are presented in Table 1 and Table 2. The sample was predominantly female, with a mean age of 75 years. Most participants were Caucasian, and all considered themselves non-Latino.
Table 2.
Baseline Psychological Measures (N = 19–27)
| Variable | Baseline |
|---|---|
|
| |
| MMSE | 28.90 (1.12) |
|
| |
| PSWQ | 44.62 (17.60) |
|
| |
| PSS | 4.81 (3.02) |
|
| |
| BDI | 6.53 (5.68) |
|
| |
| HAM-25 | 8.72 (6.10) |
|
| |
| HAM-A | 5.16 (3.00) |
|
| |
| I-Thought | 14.08 (9.26) |
|
| |
| I-Avoidance | 14.41 (9.14) |
|
| |
| MRQ | |
| MOREMOTS | 17.20 (10.10) |
| MOREMOTA | 6.87 (4.2525 |
| MORINST | 12.52 (5.32) |
| MORSRCH | 2.57 (3.98) |
Abbreviations: MMSE, Mini-Mental Status Exam; PSWQ, Penn State Worry Questionnaire; PSS, Perceived Stress Scale; BDI, Beck Depression Inventory; HAM-25, 25 -item Hamilton Depression Rating Scale; HAM-A, Hamilton Anxiety Scale; I-Thought & Avoidance, Impact Events Schedule thought and avoidance subscales; MRQ, Multidimensional Rumination Questionnaire; MOREMOTS, depression subscale of MRQ; MOREMOTA, anxiety subscale of MRQ; MORINST, instrumental response in MRQ; MORSRCH, searching for meaning response in MRQ
Feasibility: Will Dementia Caregivers Follow the Handheld APVSAT Intervention?
The minimum usage of the APVSAT was 4 and the maximum was 113. The mean usage for the entire sample was 43.59 times (SD = 30.29). The mean length of time that participants had the handheld computer was 28.08 days, which corresponded to an average of approximately 1.55 uses per day. The median was 45.0 uses for the entire sample. In participants who had the device for 28 or fewer days (N = 15), the mean usage was 42.07 times (SD = 28.58).
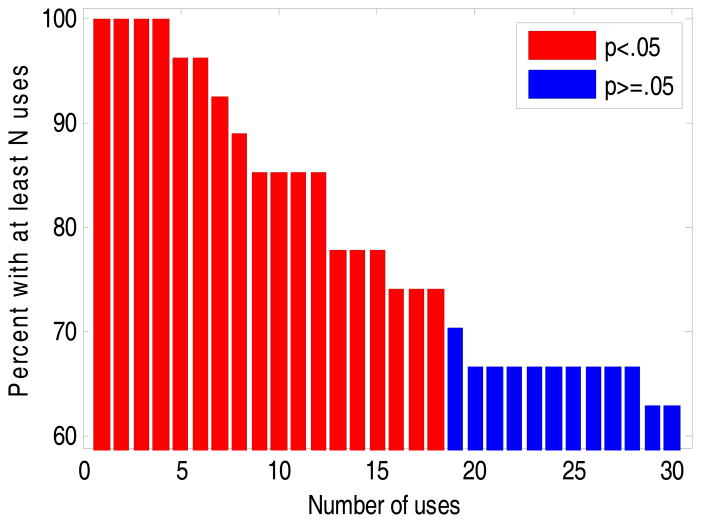
Figure 2 shows the number of participants who used the handheld computer to complete the task N times for each possible threshold from a single use through 30 uses. Numbers of uses for which the proportion of participants was greater than 50% (statistically significant; p <. 05), using a Fisher’s exact test, are shown in red, whereas numbers of uses for which the proportion of participants was less than 50% (statistically significant; p >.05) are plotted in blue. As shown in the figure, the maximally significant threshold was 18 uses; 74% of participants used APSVAT 18 or more times during the 4-week exposure.
Figure 2. Distribution of APVSAT Use.
The distribution of uses of the APVSAT throughout the 28-day study period. Red bars indicate that significantly more than half of the participants (N>14) used the intervention at the represented frequency (Fisher’s exact p<.05).
In addition to the primary measure of feasibility, a secondary measure examined the regularity of use (i.e., the number of weeks that participants used APSVAT at least once). The gold standard of “good adherence” is considered 80% (Haynes et al., 1976); thus, if a participant used the handheld computer approximately 3 of 4 weeks, the standard is achieved. Of the 15 participants who kept the handheld computer for only 4 weeks, 10 (66.67%) used it at least 3 of those weeks. Of the entire sample of 27 participants, 22 used it at least 3 weeks (81.48%), and 15 used it at least 4 weeks (55.56%), demonstrating there was considerable regularity of use.
Use Moderators: Are Baseline Participant Characteristics Associated with Use of the Intervention?
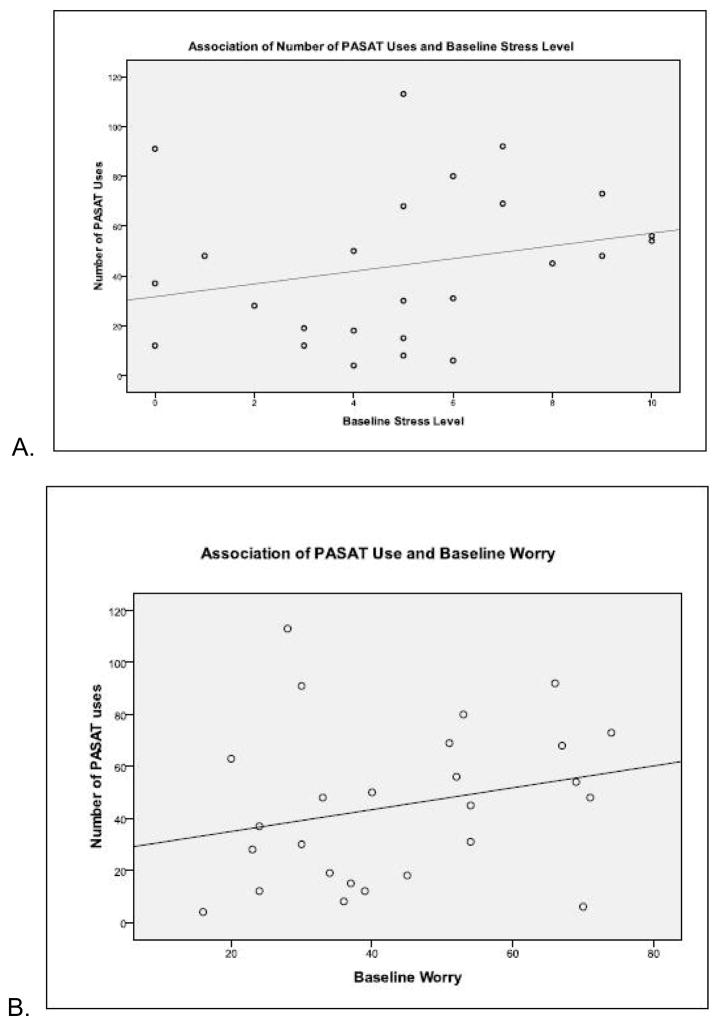
Use of the handheld computer was not adversely associated with stress and worry. That is, usage was not significantly associated with scores on the Penn State Worry Questionnaire (r = 0.25; p = 0.22) or scores on the Perceived Stress Scale (r = 0.25, p = 0.23). Moreover, for each questionnaire, there were one (PSS) or two (PSWQ) outliers. When these values were removed, participants with the highest levels of perceived stress had the highest levels of handheld computer use (r = .41; p = .05); so too did participants with the highest level of worry (r =.38. 38; p =.07). Figure 3 illustrates these relationships.
Figure 3.
Figures 3a and 3b. Association between Use and Stress/Worry
Illustrates the relationship between perceived stress and worry and APVSAT usage.
Usage was also not adversely affected by sleep quality, as measured by the Pittsburgh Sleep Quality Index (PSQI) (r = .40; p = .05). The mean PSQI was 4.88 (SD = 2.24), a level slightly below the PSQI poor sleep quality cutoff of 5.
Improvement: Do Dementia Caregivers Improve on the APVSAT over Time?
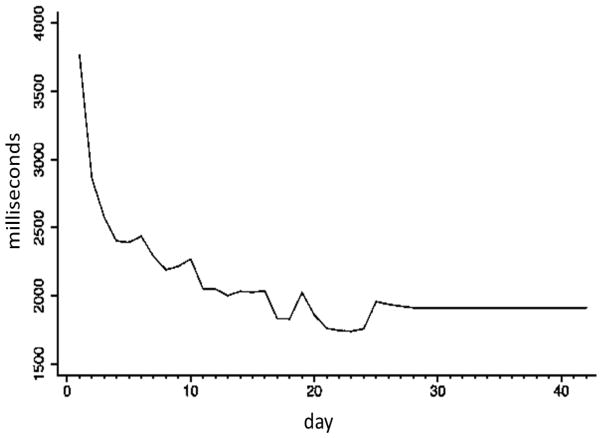
The mean ISI significantly decreased from the beginning to the end of the intervention (t [26] = 8.14; p < 0.001, mean difference [D] = 1,672 ms [SD = 1066.83]; relative change [RC], calculated as ISIlast day − ISIfirst day/ISIfirst day = .45 [SD = .22]), suggesting that participants improved on the task. Figure 4 illustrates this change. A GEE model assuming a first order autoregressive covariance structure with both linear and quadratic terms for use suggested that APVSAT performance increased quadratically with day of use (linear B = −32.58 [p < 0.001]; quadratic B = 2.18 [p < 0.001]). When the analysis was restricted to the 15 participants who had the device for just 4 weeks, the results of the GEE model were similar: linear B = −34.37 (p < 0.001) and quadratic B = 2.53 (p < 0.001), with a mean reduction in the interstimulus interval of 1,853 ms (t [14] = 9.43 [p < 0.001]); RC = .49 [SD = .16]).
Figure 4. Change in Mean Interstimulus Interval among the 28 Day Adherence Group.
The mean ISI (ms) significantly decreased from the beginning to the end of the intervention, suggesting that participants improved on the task.
Discussion
This study examined the feasibility of using a cognitive training task (APSVAT) implemented on a handheld computer in the home to target specific neuropsychological vulnerabilities in older adult caregivers of spouses with dementia. Results are aligned with our hypotheses, suggesting that (1) this strategy is feasible, (2) sleep quality, stress, and worry did not attenuate its use, and (3) the intervention task improved over time. Specifically, 74% percent of participants used the APSVAT at least 18 times. This exceeds the minimum training rates of a large cognitive training study, which identified two device uses a minimum threshold (Owen et al., 2010). This is significant because this large (i.e., >11,000 participants) study of cognitive training tasks was primarily directed at healthy 18 to 60 year-old volunteers. Moreover, participants from this study exceeded the Owen study threshold, despite being stressed (and/or worried) and carrying out caregiving tasks.
Throughout our intervention, participants improved their task performance, in terms of speed, by nearly 50%. Our data are encouraging because older adults of this generation have had less exposure to similar electronic devices, and the use of technology is frequently seen as a barrier to many elders due to wide individual differences in age-related decline in cognitive abilities (Mollenkopf & Fozard, 2003). Over 80% of the participants used the APVSAT for at least 3 weeks. As can be seen in Figure 4, there is a decrease in improvement in speed at use 17, indicating early skill acquisition. Therefore, strict guidelines, such as using the handheld computer every week, may not be necessary for performance improvements. Given that task difficulty increased with correct responses, perhaps participants expended less cognitive effort per trial at the same level of difficulty of previous sessions.
There were no socio-demographic predictors associated with using the handheld computer. This differs from studies that have shown that age and intelligence level negatively affect the APVSAT; this also is consistent with other studies that have found no gender effect on performance (Tombaugh, 2006). This initial finding merits future study with a larger, more gender-varied sample. Lower sleep quality was associated with increased number of uses. Levels of worry and stress did show a trend toward being associated with increased use of the APVSAT, suggesting that poor sleep quality, anxiety, and worry were not barriers to performance and/or use.
Because (1) the APVSAT was administered in the context of multiple other intervention components and (2) participants with psychiatric disorder were excluded from the study, we were unable to interpret the unique effects of APSVAT on mood. Additionally, other intervention components (e.g., time required to review educational components) may have affected participants’ willingness to use the APSVAT more regularly. Examining adherence to the APSVAT and improvements in mood and coping in a sample selected for mood vulnerability would be a promising strategy for future research.
Other limitations of this study include a lack of qualitative data. Since our research question was whether or not caregivers would use the handheld computer, given their multiple caregiving responsibilities, objective data (e.g., actual APSVAT usage) with time codes and response frequencies were deemed adequate to answer this question. Nonetheless, we do have anecdotal evidence that participants were initially concerned about the amount of time they were encouraged to spend using the handheld computer. For example, some participants viewed this as a burden—just “one more thing to do.” The research coordinator worked with these participants to identify a time that was good for using the handheld computer. Many of these participants reported that adding the use of the handheld computer to daily activities that prepared them for the day, such as crossword puzzles, meditation, reading, or prayer, was a useful approach for incorporating device usage into their daily routines. The collection of more qualitative data of this sort would benefit a future study of this type by clarifying any challenges facing the participants in implementation.
The results of this study are important in light of meta-analytic data, which suggests that other types of cognitive training are associated with positive outcomes in interventions targeting, for example, the prevention of dementia onset (Valenzuela & Sachdev, 2009). The extent to which the current demonstration of feasibility and improvement will transfer to other aspects of executive functioning and broader outcomes must be demonstrated in future studies. Nonetheless, our results lend promise to the idea that elderly caregivers can, and will, use handheld computer interventions as a critical part of efforts to improve their mental health.
Acknowledgments
The project described was supported by Award Number KL2TR000146 from the National Center for Research Resources and P01 AG020677 (NCT00178568) from the National Institute on Aging (PI: M. Hall), and K01 MH01554 from the National Institute of Mental Health (PI: M. Hall). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The authors are grateful to the staff of the AgeWise study for the assistance with data collection and APVSAT training. In particular, we would like to thank Annette Wood for her frequent help with fielding questions and compiling the datasets for analysis. Additionally, the authors would like to thank James Moorehead for all of the administrative help he provided to prepare this manuscript for submission.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4. The American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Willis SL. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BBC. Brain Test Britain—Further research into participants aged 60 and over. BBC Lab UK; 2009. Retrieved from https://www.bbc.co.uk/labuk/results/braintestbritain/8_o60s.html. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D, Gitlin LN, Zhang S. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: A randomized, controlled trial. Annals of Internal Medicine. 2006;145(10):727–738. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Calkins AW, McMorran CG, Siegle GJ, Otto MW. The effects of computerized cognitive control training on community adults with depressed mood. Behavioural and Cognitive Psychotherapy. 2015;1:1–12. doi: 10.1017/S1352465814000046. [DOI] [PubMed] [Google Scholar]
- Calkins AW, Deveney CM, Weitzman ML, Hearon BA, Siegle GJ, Otto MW. The effects of prior cognitive control task exposure on responses to emotional tasks in healthy participants. Behavioural and Cognitive Psychotherapy. 2011;39(2):205–220. doi: 10.1017/S1352465810000652. S1352465810000652. [DOI] [PubMed] [Google Scholar]
- Caswell LW, Vitaliano PP, Croyle KL, Scanlan JM, Zhang J, Daruwala A. Negative associations of chronic stress and cognitive performance in older adult spouse caregivers. Experimental Aging Research. 2003;3(29):303–318. doi: 10.1080/03610730303721. [DOI] [PubMed] [Google Scholar]
- Clare L, Woods B. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia (Cochrane review) Cochrane Database of Systematic Reviews. 2003;2008:4. doi: 10.1002/14651858.CD003260. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Kamarck TW, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Croog SH, Burleson JA, Sudilovsky A, Baume RM. Spouse caregivers of Alzheimer patients: Problem responses to caregiver burden. Aging & Mental Health. 2006;10(2):87–100. doi: 10.1080/13607860500492498. [DOI] [PubMed] [Google Scholar]
- De Vugt ME, Jolles J, van Osch L, Stevens F, Aalten P, Lousberg R, Verhey FRJ. Cognitive functioning in spousal caregivers of dementia patients: Findings from the prospective MAASBED study. Age and Aging. 2006;35:160–166. doi: 10.1093/ageing/afj044. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) Biometric Research; New York, NY: 1995. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ”Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fritz H. The role of rumination in adjustment to a first coronary event. Carnegie Mellon University; Pittsburgh, Pennsylvania: 1999. Unpublished manuscript. [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: A measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The Assessment of Anxiety States by Rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A Rating Scale for Depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;12:52–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BR, Gibson ES, Hackett BC, Sackett DL, Taylor DW, Roberts RS, Johnson AL. Improvement of medication compliance in uncontrolled hypertension. The Lancet. 1976;307(7972):1265–1268. doi: 10.1016/S0140-6736(76)91737-2. [DOI] [PubMed] [Google Scholar]
- Holdwick DJ, Jr, Wingenfeld SA. The subjective experience of PASAT testing. Does the PASAT induce negative mood? Archives of Clinical Neuropsychology. 1999;14(3):273–284. doi: 10.1016/S0887-6177(98)00021-3. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Wilner N, Alvarez W. Impact of event scale: A measure of subjective stress. Psychosomatic Medicine. 1979;41(3):209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- La Rue A. Healthy brain aging: Role of cognitive reserve, cognitive stimulation, and cognitive exercises. Clinics in Geriatric Medicine. 2010;26:99–111. doi: 10.1016/j.cger.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Lazeron RH, Rombouts SA, de Sonneville L, Barkhof F, Sheltens P. A paced visual serial addition test for fMRI. Journal of Neurological Science. 2003;213:29–34. doi: 10.1016/s0022-510x(03)00144-8. [DOI] [PubMed] [Google Scholar]
- Lustig C, Shah P, Seidler R, Reuter-Lorenz PA. Aging, training, and the brain: A review and future directions. Neuropsychology Review. 2009;19:504–522. doi: 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie CS, Wiprzycka UJ, Hasher L, Goldstein D. Associations between psychological distress, learning, and memory in spouse caregivers of older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2009;64B(6):742–746. doi: 10.1093/geronb/gbp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the penn state worry questionnaire. Behaviour Research & Therapy. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Miller IW, Bishop S, Norman WH, Maddever H. The Modified Hamilton Rating Scale for Depression: reliability and validity. Psychiatry Research. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Mohlman J. Does executive dysfunction affect treatment outcome in late-life mood and anxiety disorders? Journal of Geriatric Psychiatry and Neurology. 2005;18(2):97–108. doi: 10.1177/0891988705276061. [DOI] [PubMed] [Google Scholar]
- Mohlman J. Executive skills in older adults with GAD: relations with clinical variables and CBT outcome. Journal of Anxiety Disorders. 2013;27(1):131–139. doi: 10.1016/j.janxdis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Mohlman J, Gorman JM. The role of executive functioning in CBT: A pilot study with anxious older adults. Behaviour Research and Therapy. 2005;43(4):447–465. doi: 10.1016/j.brat.2004.03.007. S0005-7967(04)00086-5. [DOI] [PubMed] [Google Scholar]
- Mohlman J, Reel DH, Chazin D, Ong D, Georgescu B, Tiu J, Dobkin RD. A novel approach to treating anxiety and enhancing executive skills in an older adult with Parkinson’s disease. Clinical Case Studies. 2010;9(1):74–90. doi: 10.1177/1534650109351305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenkopf H, Fozard JL. Environments, gerontology and old age. Annual Review of Geriatrics and Gerontology. 2003:250–267. [Google Scholar]
- Nouchi R, Taki Y, Takeuchi H, Hashizume H, Akitsuki Y, Shigemune Y, Kawashima R. Brain training game improves executive functions and processing speed in elderly: A randomized controlled trial. Plos one. 2012;7(1):1–9. doi: 10.1371/journal.pone.0029676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS, Ballard CG. Putting brain training to the test. Macmillan Publishers Limited; 2010. Retrieved from http://www.nature.com/nature/journal/v465/n7299/full/nature09042.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M, Sorensen S. Associations of stressors and uplifts of caregiving with caregiver burden and depressive mood: A meta-analysis. Journal of Gerontology: Psychological Sciences. 2003;58b(2):112–128. doi: 10.1093/geronb/58.2.p112. [DOI] [PubMed] [Google Scholar]
- Price RB, Paul B, Schneider W, Siegle GJ. Neural correlates of three neurocognitive intervention strategies: A preliminary step towards personalized treatment for psychological disorders. Cognitive Therapy and Research. 2013;37(4):657–672. doi: 10.1007/s10608-012-9508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royan J, Tombaugh TN, Rees L, Francis M. The adjusting-paced serial addition test (Adjusting-PSAT): Thresholds for speed of information processing as a function of stimulus modality and problem complexity. Archives of Clinical Neuropsychology. 2004;19(1):131–143. [PubMed] [Google Scholar]
- Schulz R, Martire LM. Family caregiving of persons with dementia: Prevalence, health effects, and support strategies. American Journal of Geriatric Psychiatry. 2004;12(3):240–249. doi: 10.1097/00019442-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Lovden M, Lindenberger U. Hundred days of cognitive training enhance broad cognitive abilities in adulthood: Findings from the COGITO study. Frontiers in Aging Neuroscience. 2010;2:1–10. doi: 10.3389/fnagi.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Ghinassi F, Thase ME. Neurobehavioral therapies in the 21st century: Summary of an emerging field and an extended example of cognitive control training for depression. Cognitive Therapy & Research. 2007;31:235–262. doi: 10.1007/s10608-006-9118-6. [DOI] [Google Scholar]
- Siegle GJ, Price RB, Jones NP, Ghinassi F, Painter T, Thase ME. You Gotta Work at It Pupillary Indices of Task Focus Are Prognostic for Response to a Neurocognitive Intervention for Rumination in Depression. Clinical Psychological Science. 2014;2(4):455–471. doi: 10.1177/2167702614536160. [DOI] [Google Scholar]
- Tombaugh TN. A comprehensive review of the paced auditory serial addition test (PASAT) Archives of Clinical Neuropsychology. 2006;21:53–76. doi: 10.1016/j.acn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Valenzuela M, Sachdev P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. American Journal of Geriatric Psychiatry. 2009;17:179–187. doi: 10.1097/JGP.0b013e3181953b57. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Murphy M, Young HM, Echeverria D, Borson S. Does caring for a spouse with dementia promote cognitive decline? A hypothesis and proposed mechanisms. Journal of the American Geriatrics Society. 2011;59(5):900–908. doi: 10.1111/j.1532-5415.2011.03368.x. [DOI] [PubMed] [Google Scholar]