Introduction
Thirty-three million people have atrial fibrillation (AF), a disorder of heart rhythm.1 Over the past several decades, we have learned that this dysrhythmia originates in the interplay between genetic predisposition, ectopic electrical activity, and abnormal atrial tissue substrate, and then feeds back to remodel and worsen tissue substrate and thereby propagate itself.2 Although the importance of AF partly derives from its strong association with ischemic stroke, there have not been as many advances in our understanding of the mechanisms of stroke in AF. Current views rest on a century-old hypothesis that fibrillation of the atrium produces stasis of blood, which causes thrombus to form and embolize to the brain. When other abnormalities are acknowledged to play a role, the dysrhythmia is still considered the primary cause of thromboembolism.3 While this formulation is intuitively appealing, recent work suggests that the pathogenesis of stroke in AF is more complicated and involves factors in addition to the dysrhythmia.
Possible Stroke Mechanisms in Atrial Fibrillation
AF and stroke have been associated in rigorous studies,4 indicating a true association rather than a spurious finding. Epidemiological logic suggests three explanations: 1) AF causes stroke, 2) stroke causes AF, and/or 3) AF is associated with other factors that cause stroke.
Atrial Fibrillation as a Cause of Stroke
To help judge whether one factor causes another or whether the two are simply correlated, the epidemiologist Bradford Hill proposed the following widely accepted criteria: 1) strength of association, 2) consistency, 3) specificity, 4) temporality, 5) biological gradient, 6) plausibility, 7) coherence, 8) accordance with experimental results, and 9) analogy.5 The relationship between AF and stroke fulfills several of these criteria. Patients with AF face a strongly elevated risk of stroke—about 3- to 5-fold higher after adjustment for risk factors.4 AF has been consistently associated with stroke in different cohorts.6 And a causal association is biologically plausible. Intuitively, uncoordinated myocyte activity would explain the impaired atrial contraction seen in AF, and by Virchow's triad, the resulting stasis of blood should increase thromboembolic risk.
However, several other Hill criteria do not support a straightforward relationship between AF and stroke. Although many studies have found a biological gradient between AF burden and stroke,7-10 this is not consistent across all studies.11 Furthermore, a single brief episode of subclinical AF is associated with a 2-fold higher risk of stroke in older patients with vascular risk factors,12 while young and otherwise healthy patients with clinically apparent AF do not face a significantly increased stroke risk.13 These conflicting data do not suffice to establish a clear biological gradient between the burden of AF and the risk of stroke.
The relationship between AF and stroke also fails Hill's criterion of specificity. If AF causes thromboembolism, it should be specifically associated with embolic strokes. There does appear to be an especially strong association between AF and embolic strokes.14 However, 10% of patients with lacunar strokes have AF,14 and large-artery atherosclerosis is twice as common in patients with AF as those without.15 The link between AF and non-cardioembolic stroke indicates that stroke risk in AF cannot be entirely explained by AF directly causing stroke.
Third, the association between AF and stroke does not fully satisfy Hill's criterion of temporality. A recent case-crossover analysis indicated an increased risk of stroke shortly after the onset of AF.16 On the other hand, two other recent studies found that approximately one-third of patients with both AF and stroke do not manifest any AF until after their stroke, despite undergoing many months of continuous heart-rhythm monitoring before the stroke.17, 18 These findings suggest that although the dysrhythmia itself can cause thromboembolism, the strong association between AF and stroke also involves other factors.
Fourth, a causal interpretation of the association between AF and stroke does not adequately fit the available experimental evidence. If the dysrhythmia is the only cause of thromboembolism, maintaining normal rhythm should eliminate stroke risk. However, in a meta-analysis of eight randomized clinical trials, a rhythm-control strategy had no effect on stroke risk (odds ratio, 0.99; 95% confidence interval, 0.76-1.30).19 It is unlikely that this simply reflected a failure to reliably maintain sinus rhythm, because rhythm-control strategies showed substantial success in maintaining normal sinus rhythm (odds ratio, 4.39; 95% confidence interval, 2.84-6.78). Furthermore, the structural remodeling seen in experimental models of AF occurs after at least a week of sustained rapid pacing,20 so any atrial changes caused by AF are unlikely to explain the association between a single 6-minute episode of AF and a heightened risk of stroke in humans.12 Therefore, robust experimental evidence is lacking to indicate that AF is a necessary step in thrombogenesis.
Stroke as a Cause of Atrial Fibrillation
Central nervous system injuries often affect the autonomic nervous system, which plays an important role in the pathogenesis of AF.21 And necrotic cell death from stroke activates a systemic inflammatory response, which also plays a role in the origin of AF.22 Clinical observations support the hypothesis that stroke may trigger AF. Strokes affecting cerebral autonomic centers seem particularly associated with new-onset AF that lacks accompaniments of long-standing AF such as left atrial enlargement.23 However, other clinical findings argue against this hypothesis,24 and even if stroke can trigger AF, this pathway cannot explain the well-documented association between AF and future stroke.4, 6
Atrial Fibrillation-Associated Factors as Causes of Stroke
Besides causing stroke, AF may also be associated with other factors that cause stroke. Age, male sex, hypertension, diabetes mellitus, valvular heart disease, heart failure, coronary heart disease, chronic kidney disease, inflammatory disorders, sleep apnea, and tobacco use are risk factors for both AF and stroke. Confounding in the AF-stroke association is indicated by its attenuation as more shared risk factors are accounted for.4, 6 Nevertheless, AF remains independently associated with stroke even after seemingly thorough adjustment for shared risk factors. And AF is associated not just with stroke in general, but most strongly with strokes whose neuroimaging patterns resemble that of cardiac embolism.25
Even if the origin of stroke in AF is accepted to be the left atrium, other atrial factors in addition to AF may cause thromboembolism. Rather than being the only cause of atrial thromboembolism, could AF sometimes be a marker of other atrial abnormalities that are themselves the actual cause of stroke? AF frequently co-exists with atrial abnormalities such as endothelial dysfunction,26 fibrosis,27 impaired myocyte function,28 chamber dilatation,29 and mechanical dysfunction in the left atrial appendage.30 These abnormalities have been documented in both experimental animal models26 and in humans.27-30 Such factors have been associated with stroke risk in patients with AF31—could these atrial abnormalities also arise independently of AF and cause stroke? If so, they should be associated with stroke even in the absence of AF. Indeed, premature atrial contractions,32 paroxysmal supraventricular tachycardia,33 ECG-defined left atrial abnormality,34-36 and left atrial size37-39 have been associated with stroke independently of AF (Table 1). Markers of atrial dysfunction are specifically associated with cryptogenic or embolic stroke and not with in-situ cerebral small-vessel occlusion,34, 36, 38 indicating that these markers signal a specific risk of atrial thromboembolism rather than general vascular risk.
Table 1.
Studies Demonstrating an Association between Markers of Abnormal Atrial Substrate and Incident Stroke Independently of Atrial Fibrillation
| Marker | Authors | Year | Outcome | Association | |
|---|---|---|---|---|---|
| Not Adjusted for AF | Adjusted for AF | ||||
| ECG markers | |||||
| Frequent PACs | Binici et al40 | 2010 | Stroke | 1.79 (1.14-2.81)* | 1.73 (1.09-2.75)* |
| PSVT | Kamel et al33 | 2013 | Stroke | N/A | 2.10 (1.69-2.62)† |
| PTFV1 | Kamel et al35 | 2014 | Stroke | 1.22 (1.03-1.45)‡ | 1.21 (1.02-1.44)‡ |
| PTFV1 | Kamel et al41 | 2014 | Infarct§ | 1.09 (1.04-1.16)‡ | 1.09 (1.04-1.15)‡ |
| Frequent PACs | Larsen et al32 | 2015 | Stroke | 2.00 (1.16-3.45)‖ | |
| PTFV1 | Kamel et al34 | 2015 | Non-lacunar stroke | 1.44 (1.04-1.99)# | 1.49 (1.07-2.07)# |
| PTFV1 | Kamel et al36 | 2015 | Cryptogenic or cardioembolic stroke | 1.28 (1.07-1.53)‡ | 1.31 (1.08-1.58)‡ |
| Echocardiographic markers | |||||
| Left atrial size | Benjamin et al37 | 1995 | Stroke | N/A | 2.4 (1.6-3.7)** |
| Left atrial size | Di Tullio et al39 | 1999 | Stroke | N/A | 1.47 (1.03-2.11)†† |
| Left atrial size | Karas et al42 | 2012 | Stroke | N/A | 1.35 (1.12-1.62)‡‡ |
| Left atrial size | Yaghi et al38 | 2015 | Cryptogenic or cardioembolic stroke | N/A | 1.55 (1.01-2.37)‡‡ |
| Left atrial volume | Barnes et al43 | 2004 | Stroke | N/A | 1.63 (1.08-2.46)§§ |
| Left atrial volume | Russo et al44 | 2013 | Infarct | N/A | 1.37 (1.04-1.80)‖‖ |
| Left atrial function | Russo et al44 | 2013 | Infarct | N/A | 0.67 (0.50-0.90)## |
Abbreviations: AF, atrial fibrillation; ECG, electrocardiographic; PACs, premature atrial contractions; PSVT, paroxysmal supraventricular tachycardia; PTFV1, P-wave terminal force in lead V1
Hazard ratio (HR) and 95% confidence interval (CI) for the primary outcome of death or stroke.
HR (95% CI) associated with a diagnosis of PSVT.
HR (95% CI) per 1-standard deviation (SD) increase in PTFV1.
Infarct refers to silent brain infarcts detected on magnetic resonance imaging.
HR (95% CI) associated with excessive PACs, defined as ≥30 PACs per hour.
HR (95% CI) associated with ECG-defined left atrial abnormality (PTFV1 ≥4,000 ms*μV).
HR (95% CI) per 10-mm increase in left atrial size in men. The association was not significant in women (HR, 1.4; 95% CI, 0.9-2.1).
Odds ratio (OR) and 95% CI per 10 mm/1.7 m2 increase in the left atrial diameter divided by body surface area (left atrial index).
HR (95% CI) per 1-SD increase in left atrial size.
HR (95% CI) for left atrial volume ≥32 ml/m2.
HR (95% CI) per 1-SD increase in left atrial minimum volume.
OR (95% CI) for each 1-SD increase in the left atrial ejection fraction.
Might these associations be mediated by AF? Left atrial abnormalities may reflect abnormal atrial substrate, which causes paroxysmal and difficult-to-detect AF, which then causes stroke.37, 39 However, adjustment for clinically apparent AF does not change the association between left atrial abnormalities and stroke34-36—an unexpected finding if AF mediates their relationship. Another interpretation of these associations is that subclinical AF causes abnormal atrial substrate, which then causes stroke. In this interpretation, AF is again required for downstream changes to occur and result in thrombogenesis. However, structural remodeling seems to require weeks of AF,20 not just the 6 minutes that suffice to signify an increased stroke risk.12 Such inconsistencies undermine the concept of AF as the sole cause of the atrial abnormalities that have been associated with stroke. These associations and their lack of attenuation after adjustment for AF suggest that atrial disease causes thrombogenesis via additional pathways besides AF. Proof of principle is offered by a homozygous mutation of the natriuretic peptide precursor A gene. Even though AF is absent, this disorder leads to atrial dilatation, progressive loss of atrial activity with eventual atrial standstill, and thromboembolism.45
Updated Model for the Mechanisms of Stroke in Atrial Fibrillation
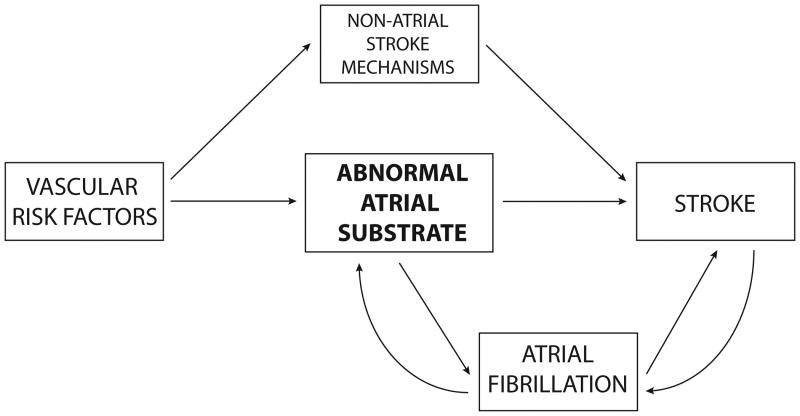
Given the findings above, the mechanistic basis of stroke in patients with AF is likely to be more complex than currently appreciated. An up-to-date model must emphasize systemic and atrial substrate as well as rhythm (Figure 1). Aging and systemic vascular risk factors cause an abnormal atrial tissue substrate, or atrial cardiopathy, that can result in AF and/or thromboembolism. For atrial cardiopathy to play such a role in thrombogenesis would be analogous to the ventricular cardiopathy seen in myocardial infarction and heart failure, two diseases in which thromboembolism can occur even in the absence of dysrhythmia. Once AF develops, the dysrhythmia causes contractile dysfunction and stasis, which further increases the risk of thromboembolism. In addition, over time the dysrhythmia causes structural remodeling of the atrium, thereby worsening atrial cardiopathy and increasing the risk of thromboembolism even further. In parallel, systemic risk factors increase stroke risk via other mechanisms outside the atrium, such as large-artery atherosclerosis, ventricular systolic dysfunction, and in-situ cerebral small-vessel occlusion. Once stroke occurs, autonomic changes and post-stroke inflammation may transiently increase AF risk.
Figure 1.
Updated Model of Thromboembolic Stroke. This model emphasizes the importance of systemic and atrial substrate as well as rhythm in explaining the relationship between atrial fibrillation (AF) and stroke. In this model, aging and systemic vascular risk factors cause an abnormal atrial tissue substrate, or atrial cardiopathy, that can result in AF and/or thromboembolism. Once AF develops, the dysrhythmia causes contractile dysfunction and stasis, which further increases the risk of thromboembolism. In addition, over time the dysrhythmia causes structural remodeling of the atrium, thereby worsening atrial cardiopathy and increasing the risk of thromboembolism even further. In parallel, systemic risk factors increase stroke risk via other mechanisms outside the atrium, such as large-artery atherosclerosis, ventricular systolic dysfunction, and in-situ cerebral small-vessel occlusion. Once stroke occurs, autonomic changes and post-stroke inflammation may transiently increase AF risk.
This updated model largely resolves the inconsistencies between the Hill criteria and recent data on the association between AF and stroke. If AF and thromboembolism occur as parallel but separate downstream effects of atrial cardiopathy, then AF can increase thromboembolic risk but is not necessary for thromboembolism to occur, so the timing and burden of dysrhythmia need not be coupled with the timing and burden of stroke. Under this construct, it would not be surprising that a brief period of AF is associated with stroke months later12 or that one-third of patients with AF and stroke do not manifest AF until after their stroke.17 An atrial substrate model also explains the lack of specificity between AF and embolic stroke. AF patients often have non-embolic strokes because AF serves as a marker of upstream systemic vascular risk factors. Lastly, a substrate model accords with experimental evidence and explains the otherwise puzzling observation that rhythm-control treatments do not eliminate stroke risk.19 If AF is only a secondary contributor to abnormal atrial tissue substrate, successful elimination of the dysrhythmia will not eliminate the thrombogenic potential of the underlying atrial cardiopathy.
Implications of an Updated Model of Stroke
By placing atrial cardiopathy alongside AF as a cause of thromboembolic stroke, an updated model may help explain why one-third of strokes have no known cause.46 Many cryptogenic strokes are suspected to arise from cardiac embolism, but only one-third of these patients manifest AF even after 3 years of continuous heart-rhythm monitoring.47 Since we currently conceive of AF as the sine qua non of atrial thromboembolism, we may be failing to recognize cases that occur in the absence of AF and incorrectly labeling these strokes cryptogenic.
An updated model of stroke and AF may lead to better strategies for identifying thromboembolic risk in patients with established AF. Assessing markers of abnormal atrial tissue substrate in addition to the burden of upstream vascular risk factors may better identify the few patients with truly lone AF who do not face a substantial risk of stroke.13 An updated model may also allow better screening for thromboembolic risk in the general population without known AF. AF screening is important but has been hampered by the difficulty of prolonged heart-rhythm monitoring. Assessment of atrial substrate by a standard ECG or echocardiogram can be done at a single point in time, and may help augment AF screening efforts.
A substrate model has several implications for therapeutic strategies to prevent stroke. The perception that the dysrhythmia is the only cause of thromboembolism often makes providers and patients reluctant to continue anticoagulant therapy during stretches of normal sinus rhythm.3 A greater emphasis on the atrial cardiopathy that led to AF in the first place, and which persists even if sinus rhythm returns, may reinforce the importance of continuing proven anticoagulant treatments. Similarly, recognition of atrial cardiopathy highlights the findings from randomized clinical trials that rhythm-control therapies, such as catheter ablation of AF, should not be viewed as a stand-alone form of thromboprophylaxis.19
An updated model implies that treatments to reverse abnormal atrial substrate, not just to restore normal rhythm, may be beneficial in reducing thromboembolic risk. Underlying risk factors such as obesity and the metabolic syndrome promote AF and atrial cardiopathy through numerous mechanisms.48 Local epicardial fat is increasingly recognized as a contributor to local inflammation in the atrium, while obesity-induced obstructive sleep apnea raises intra-atrial pressures.49 Intensive vascular risk factor management after AF ablation appears to improve the underlying atrial substrate.50 Therefore, future trials may be warranted to assess whether treatment of atrial substrate reduces stroke risk. In addition, if AF is a downstream marker of vascular risk factors that separately produce non-atrial stroke mechanisms such as carotid atherosclerosis or cerebral small-vessel disease, a comprehensive approach to stroke prevention should explore and emphasize intensive management of all risk factors, rather than just focusing on recommendations regarding anticoagulant therapy. Current guidelines on AF do not emphasize global risk factor management.51
A substrate model also has implications for stroke prevention in patients without AF. If AF serves as a marker of thrombogenic atrial substrate, the benefit seen with anticoagulant drugs in AF may extend to patients with atrial cardiopathy but no AF. Randomized trials comparing anticoagulant versus antiplatelet therapies may be warranted in patients with markers of atrial cardiopathy and no evidence of AF.
Many of the studies that found associations between atrial cardiopathy and stroke used consensus definitions of biomarker thresholds, but more work is required to determine whether additional markers, such as cardiac magnetic resonance imaging of tissue fibrosis and computed tomographic assessment of left atrial appendage morphology, may better identify the risk of atrial thromboembolism. Combined with further research on the benefits of anticoagulation for varying degrees of atrial cardiopathy, such work would allow for a consensus definition of atrial cardiopathy to aid clinical decision making.
Conclusion
The prevailing model of AF and thromboembolism is likely incomplete. A straightforward association between AF and stroke does not convincingly demonstrate temporality, specificity, or a biological gradient, and it is not concordant with the totality of the available experimental evidence. A model in which thromboembolism is caused by both AF and abnormal systemic and atrial tissue substrate better fits the available data. Such a model has several important implications for stroke prevention strategies. By emphasizing systemic and atrial substrate in addition to rhythm, it points to new strategies for identifying and treating patients at risk of thromboembolism. Further research to test this model and the various strategies it suggests may result in improvements in stroke care and a reduction in the burden of this disabling disease, which accounts for 10% of deaths worldwide.
Acknowledgments
None.
Funding Sources: This work was supported by grant K23NS082367 (Kamel) from the National Institutes of Health/National Institute of Neurological Disorders and Stroke.
Footnotes
Disclosures: Dr. Kamel and Dr. Iadecola report no potential conflicts of interest. Dr. Okin serves as a consultant to and has received research grants from Novartis Pharmaceuticals (modest). Dr. Elkind receives compensation for providing consultative services for Biotelemetry/Cardionet, BMS-Pfizer Partnership, Biogen IDEC, Boehringer-Ingelheim, Daiichi-Sankyo, and Janssen Pharmaceuticals (all modest); receives royalties from UpToDate for a chapter related to cryptogenic stroke (modest); and participates in the American Heart Association's Cryptogenic Stroke Initiative.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–1499. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- 3.Zimetbaum P, Waks JW, Ellis ER, Glotzer TV, Passman RS. Role of atrial fibrillation burden in assessing thromboembolic risk. Circ Arrhythm Electrophysiol. 2014;7:1223–1229. doi: 10.1161/CIRCEP.114.001356. [DOI] [PubMed] [Google Scholar]
- 4.Wolf PA, Dawber TR, Thomas HE, Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28:973–977. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 5.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 6.Manolio TA, Kronmal RA, Burke GL, O'Leary DH, Price TR. Short-term predictors of incident stroke in older adults. The Cardiovascular Health Study. Stroke. 1996;27:1479–1486. doi: 10.1161/01.str.27.9.1479. [DOI] [PubMed] [Google Scholar]
- 7.Al-Khatib SM, Thomas L, Wallentin L, Lopes RD, Gersh B, Garcia D, et al. Outcomes of apixaban vs. warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J. 2013;34:2464–2471. doi: 10.1093/eurheartj/eht135. [DOI] [PubMed] [Google Scholar]
- 8.Vanassche T, Lauw MN, Eikelboom JW, Healey JS, Hart RG, Alings M, et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J. 2015;36:281–287a. doi: 10.1093/eurheartj/ehu307. [DOI] [PubMed] [Google Scholar]
- 9.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 10.Nuotio I, Hartikainen JE, Gronberg T, Biancari F, Airaksinen KE. Time to cardioversion for acute atrial fibrillation and thromboembolic complications. JAMA. 2014;312:647–649. doi: 10.1001/jama.2014.3824. [DOI] [PubMed] [Google Scholar]
- 11.Flaker G, Ezekowitz M, Yusuf S, Wallentin L, Noack H, Brueckmann M, et al. Efficacy and safety of dabigatran compared to warfarin in patients with paroxysmal, persistent, and permanent atrial fibrillation: results from the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) study. J Am Coll Cardiol. 2012;59:854–855. doi: 10.1016/j.jacc.2011.10.896. [DOI] [PubMed] [Google Scholar]
- 12.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 13.Chao TF, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, et al. Atrial fibrillation and the risk of ischemic stroke: does it still matter in patients with a CHA2DS2-VASc score of 0 or 1? Stroke. 2012;43:2551–2555. doi: 10.1161/STROKEAHA.112.667865. [DOI] [PubMed] [Google Scholar]
- 14.Lodder J, Bamford JM, Sandercock PA, Jones LN, Warlow CP. Are hypertension or cardiac embolism likely causes of lacunar infarction? Stroke. 1990;21:375–381. doi: 10.1161/01.str.21.3.375. [DOI] [PubMed] [Google Scholar]
- 15.Chesebro JH, Fuster V, Halperin JL. Atrial fibrillation--risk marker for stroke. N Engl J Med. 1990;323:1556–1558. doi: 10.1056/NEJM199011293232209. [DOI] [PubMed] [Google Scholar]
- 16.Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, et al. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015;8:1040–1047. doi: 10.1161/CIRCEP.114.003057. [DOI] [PubMed] [Google Scholar]
- 17.Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 18.Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GY, et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015;36:1660–1668. doi: 10.1093/eurheartj/ehv115. [DOI] [PubMed] [Google Scholar]
- 19.Al-Khatib SM, Allen LaPointe NM, Chatterjee R, Crowley MJ, Dupre ME, Kong DF, et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2014;160:760–773. doi: 10.7326/M13-1467. [DOI] [PubMed] [Google Scholar]
- 20.De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. 2011;89:754–765. doi: 10.1093/cvr/cvq357. [DOI] [PubMed] [Google Scholar]
- 21.Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez Toledo ME, Klein FR, Riccio PM, Cassara FP, Munoz Giacomelli F, Racosta JM, et al. Atrial fibrillation detected after acute ischemic stroke: evidence supporting the neurogenic hypothesis. J Stroke Cerebrovasc Dis. 2013;22:e486–491. doi: 10.1016/j.jstrokecerebrovasdis.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Abboud H, Berroir S, Labreuche J, Orjuela K, Amarenco P, Investigators G. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann Neurol. 2006;59:691–699. doi: 10.1002/ana.20806. [DOI] [PubMed] [Google Scholar]
- 25.Boiten J, Lodder J. Lacunar infarcts. Pathogenesis and validity of the clinical syndromes. Stroke. 1991;22:1374–1378. doi: 10.1161/01.str.22.11.1374. [DOI] [PubMed] [Google Scholar]
- 26.Cai H, Li Z, Goette A, Mera F, Honeycutt C, Feterik K, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106:2854–2858. doi: 10.1161/01.cir.0000039327.11661.16. [DOI] [PubMed] [Google Scholar]
- 27.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 28.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 29.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–730. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 30.Warraich HJ, Gandhavadi M, Manning WJ. Mechanical discordance of the left atrium and appendage: a novel mechanism of stroke in paroxysmal atrial fibrillation. Stroke. 2014;45:1481–1484. doi: 10.1161/STROKEAHA.114.004800. [DOI] [PubMed] [Google Scholar]
- 31.Stollberger C, Chnupa P, Kronik G, Brainin M, Finsterer J, Schneider B, et al. Transesophageal echocardiography to assess embolic risk in patients with atrial fibrillation. ELAT Study Group. Embolism in Left Atrial Thrombi. Ann Intern Med. 1998;128:630–638. doi: 10.7326/0003-4819-128-8-199804150-00004. [DOI] [PubMed] [Google Scholar]
- 32.Larsen BS, Kumarathurai P, Falkenberg J, Nielsen OW, Sajadieh A. Excessive atrial ectopy and short atrial runs increase the risk of stroke beyond incident atrial fibrillation. J Am Coll Cardiol. 2015;66:232–241. doi: 10.1016/j.jacc.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Kamel H, Elkind MS, Bhave PD, Navi BB, Okin PM, Iadecola C, et al. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke. 2013;44:1550–1554. doi: 10.1161/STROKEAHA.113.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamel H, O'Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in the Atherosclerosis Risk In Communities study. Ann Neurol. 2015;78:670–678. doi: 10.1002/ana.24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT, Jr, Nazarian S, et al. P-wave morphology and the risk of incident ischemic stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:2786–2788. doi: 10.1161/STROKEAHA.114.006364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamel H, Hunter M, Moon YP, Yaghi S, Cheung K, Di Tullio MR, et al. Electrocardiographic left atrial abnormality and risk of stroke: Northern Manhattan Study. Stroke. 2015;46:3208–3212. doi: 10.1161/STROKEAHA.115.009989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 38.Yaghi S, Moon YP, Mora-McLaughlin C, Willey JZ, Cheung K, Di Tullio MR, et al. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke. 2015;46:1488–1493. doi: 10.1161/STROKEAHA.115.008711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30:2019–2024. doi: 10.1161/01.str.30.10.2019. [DOI] [PubMed] [Google Scholar]
- 40.Binici Z, Intzilakis T, Nielsen OW, Kober L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. doi: 10.1161/CIRCULATIONAHA.109.874982. [DOI] [PubMed] [Google Scholar]
- 41.Kamel H, Bartz TM, Longstreth WT, Jr, Okin PM, Thacker EL, Patton KK, et al. Association between left atrial abnormality on ECG and vascular brain injury on MRI in the Cardiovascular Health Study. Stroke. 2015;46:711–716. doi: 10.1161/STROKEAHA.114.007762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karas MG, Devereux RB, Wiebers DO, Whisnant JP, Best LG, Lee ET, et al. Incremental value of biochemical and echocardiographic measures in prediction of ischemic stroke: the Strong Heart Study. Stroke. 2012;43:720–726. doi: 10.1161/STROKEAHA.111.631168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR, et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79:1008–1014. doi: 10.4065/79.8.1008. [DOI] [PubMed] [Google Scholar]
- 44.Russo C, Jin Z, Liu R, Iwata S, Tugcu A, Yoshita M, et al. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) study. JACC Cardiovasc Imaging. 2013;6:313–323. doi: 10.1016/j.jcmg.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Disertori M, Quintarelli S, Grasso M, Pilotto A, Narula N, Favalli V, et al. Autosomal recessive atrial dilated cardiomyopathy with standstill evolution associated with mutation of natriuretic peptide precursor A. Circ Cardiovasc Genet. 2013;6:27–36. doi: 10.1161/CIRCGENETICS.112.963520. [DOI] [PubMed] [Google Scholar]
- 46.Marnane M, Duggan CA, Sheehan OC, Merwick A, Hannon N, Curtin D, et al. Stroke subtype classification to mechanism-specific and undetermined categories by TOAST, A-S-C-O, and Causative Classification system. Stroke. 2010;41:1579–1586. doi: 10.1161/STROKEAHA.109.575373. [DOI] [PubMed] [Google Scholar]
- 47.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 48.Magnani JW, Lopez FL, Soliman EZ, Maclehose RF, Crow RS, Alonso A. P wave indices, obesity, and the metabolic syndrome: the Atherosclerosis Risk In Communities study. Obesity (Silver Spring) 2012;20:666–672. doi: 10.1038/oby.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magnani JW, Hylek EM, Apovian CM. Obesity begets atrial fibrillation: a contemporary summary. Circulation. 2013;128:401–405. doi: 10.1161/CIRCULATIONAHA.113.001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 51.January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Jr, Cigarroa JE, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]