Abstract
Importance
This research is the single largest NR2E3 genotype-phenotype correlation study performed to date in autosomal dominant Retinitis Pigmentosa.
Objective
The aim of this study is to analyse the frequency of the p.Gly56Arg mutation in NR2E3 for the largest cohort of autosomal dominant Retinitis Pigmentosa patients to date and its associated phenotype.
Patients and Methods
A cohort of 201 unrelated Spanish families affected by autosomal dominant Retinitis Pigmentosa. The p.Gly56Arg mutation in the NR2E3 (NM_014249.2) gene was analysed in 201 families. In the 24 cases where the mutation had been detected, a haplotype analysis linked to the p.Gly56Arg families was performed, using four extragenic polymorphic markers D15S967, D15S1050, D15S204 and D15S188. Phenotype study included presence and age of onset of night blindness, visual field loss and cataracts; and an ophthalmoscopic examination after pupillary dilation and electroretinogram for the 24 cases.
Results
Seven of the 201 analyzed families were positive for the p.Gly56Arg, leading to a prevalence of 3.5%. Clinical data were available for 24 subjects. Night blindness was the first noticeable symptom (mean 15.9 years). Visual field loss onset was variable (23.3 ± 11.9 years). Loss of visual acuity appeared late in the disease´s evolution. Most of the patients with cataracts (50%) presented it from the third decade of life. Fundus changes showed inter and intrafamiliar variability, but most of the patients showed typical RP changes and it was common to find macular affectation (47.4%). Electroretinogram was impaired from the beginning of the disease. Two families shared a common haplotype. Additionally, all patients shared a 104Kb region between D15S1050 and the NR2E3 gene.
Conclusions
This study highlights the importance of p.Gly56Arg in the NR2E3 gene as a common mutation associated with adRP, and provides new clues to its phenotype, which can allow for a better clinical management and genetic counselling of patients and their families.
Introduction
Retinitis Pigmentosa (RP, MIM# 268000), with a prevalence of approximately one in 4000 [1], is the most common form of inherited retinopathy. RP is a group of clinically and genetically heterogeneous retinal degenerative diseases. Clinically it is characterized by progressive loss of photoreceptors and pigment deposits predominantly in the peripheral retina, and by a relative sparing of the central retina. The diagnostic criteria for RP were established by Marmor [2–5]. To date, sixty-nine genes have been associated with non-syndromic RP (http://www.sph.uth.tmc.edu/RetNet/, data accessed 30/12/2015) and all modes of inheritance have been described in this disease: autosomal dominant, autosomal recessive, X linked, mitochondrial and, in rare cases, digenic [6].
In Spain, autosomal dominant form of RP (adRP) represents approximately 15% of Spanish RP families [7, 8]. The large number of genes involved in adRP disease complicates genetic analysis of these patients. To date, 23 genes (and one mapped locus) have been associated with adRP (http://www.sph.uth.tmc.edu/RetNet/, data accessed 30/12/2015). One of these 23 genes is the NR2E3 gene, which contains eight exons that expand a genomic sequence of around 7.7 kilobases (kb). The open reading frame of this gene encodes for a retinal nuclear receptor protein that acts as a transcriptional regulator, activating rod-specific genes in concert with other transcriptional factors (CRX and NRL), as well as repressing the transcription of cone-specific genes in differentiating rod photoreceptors [9].
Most of the mutations in this gene have been associated with autosomal recessive retinitis pigmentosa (arRP) with variable phenotypes (enhanced S-cone sensitivity syndrome -ESCS- [10, 11], Goldmann-Favre syndrome -GFS- [12], and clumped pigmentary retinal degeneration -CPRD-) [13–16]. However, one mutation (p.Gly56Arg) in the first zinc-finger of the DNA binding domain of the NR2E3 gene has been found in adRP patients [17] associated with RP phenotype (progressive rod degeneration and ulterior cone affectation) [18]. This mutation accounts for approximately 1–2% of North American and 3.4% of European adRP patients [17–19], and, until present time, is the only mutation found in the NR2E3 gene responsible for adRP [17–21].
The aim of this study is to analyse the frequency of the p.Gly56Arg mutation in the NR2E3 gene in our cohort of adRP patients and to determine the associated phenotype.
Patients and Methods
Patients
The adRP diagnosis was based on pedigree data and ophthalmologic examination. Our patients were classified as affected by RP according to the following clinical criteria: night blindness (NB), progressive loss of peripheral vision (mid peripheral scotoma or ring scotoma), fundus compatible with RP [3, 4] (ophthalmoscopic examination after pupillary dilation), and pathologic electroretinogram (ERG) showing a marked reduction in rod or rod and cone signal (full-field electroretinogram according to the standards of the International Society for Clinical Electrophysiology of Vision: http://www.iscev.org) [22]. Autosomal dominant inheritance was considered according to previously established criteria [7, 8].
The severity of visual acuity loss was classified following the WHO criteria (normal vision ≥0.4, moderate low vision <0.4 –>0.1, severe low vision ≤0.1 - ≥0.05, and profound vision loss and blindness <0.05)
Written informed consent was obtained from all individuals included in the study and research protocols were approved by the Ethics committee of the University Hospital Fundación Jiménez Díaz in accordance with the tenets of the Declaration of Helsinki and their reviews.
Screening for NR2E3 autosomal dominant mutation
DNA was extracted from peripheral blood samples and collected in EDTA tubes using an automated DNA extractor according to manufacturer instructions (model BioRobot EZ1; Qiagen, Hilden, Germany).
The p.Gly56Arg mutation in the NR2E3 gene (NM_014249.2) was analysed in a total of 201 unrelated adRP families. The analysis was performed by direct sequencing, as previously reported [19], or by adRP genotyping Asper Ophthalmics microarray (Asper Biotech [19], http://www.asperbio.com/asper-ophthalmics/autosomal-dominant-retinitis-pigmentosa-ad-rp/autosomal-dominant-retinitis-pigmentosa-targeted-mutation-analysis, versions from February 2008 to July 2014).
Among 201 families, 60 had been studied previously, showing a negative result, using the first version of the adRP genotyping microarray, which did not include the p.Gly56Arg mutation [23]. The remaining 141 families underwent an updated version of the adRP genotyping microarray which included the p.Gly56Arg mutation in the NR2E3 gene. Direct sequencing was used to analyse the 60 previously studied families, to confirm the results obtained with the genotyping microarray and to segregate the disease causative mutation p.Gly56Arg in the NR2E3 gene in the families.
Haplotype Analysis
Haplotype analysis was performed using four extragenic polymorphic markers (NR2E3 genomic position according to Human Genome Assembly GRCh37, Chr15: 72,084,977–72,110,600) strongly linked to this locus: D15S967, D15S1050, D15S204, and D15S188. For the genotyping process, PCR products were electrophoresed in an ABI Prism 3130 Genetic Analyzer and analyzed with the GeneMapper v3.5 software package (Applied Biosystems).
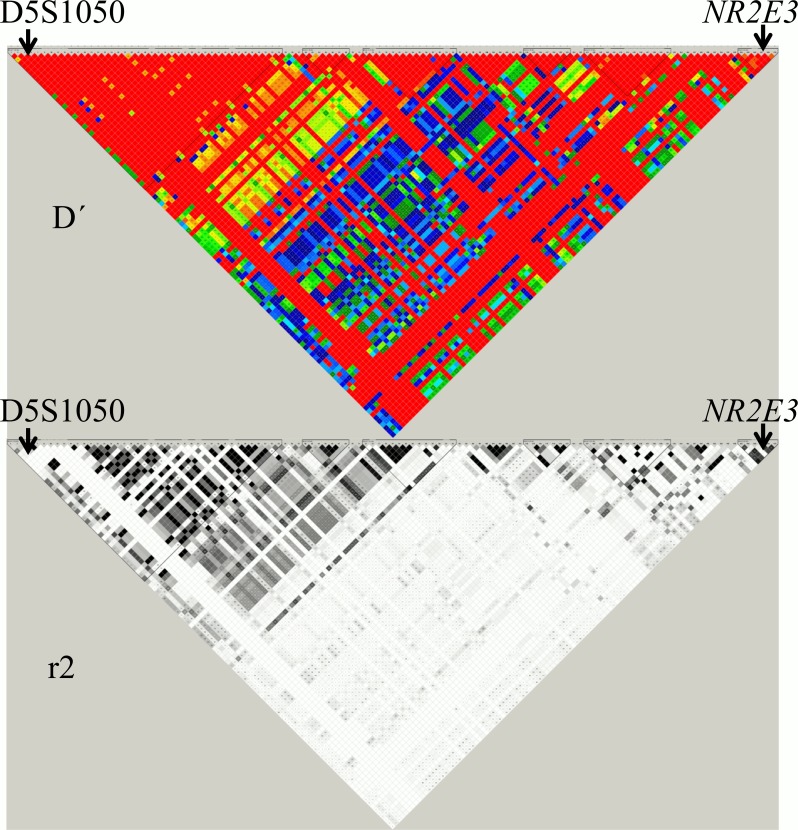
An in silico analysis was performed using the Hapmap/Haploview 4.0 software (http://www.hapmap.org/) to establish the linkage disequilibrium blocks (D´ and r2 parameters) in the genomic region between D15S1050 and the NR2E3 gene (Fig 1).
Fig 1. Linkage disequilibrium for region Chr15: 71,980,969–72,110,600 of the Human Genome Assembly GRCh37 (Hapmap/Haploview 4.0 software: http://www.hapmap.org/).
Colour image: D´ (red: D´ = 1, the lower the D´ the further away from red). Black & white image: r2 (black: r2, the lower the r2 the further away from black).
Results
Seven out of 201 adRP studied families were positive for the p.Gly56Arg mutation in the NR2E3 gene (five of them identified by direct sequencing of the mutation and two by the adRP genotyping microarray).
The detection of the dominant NR2E3 mutation in seven families, gives us a frequency of 3.5% (7/201) in our adRP studied cohort. All families were of Spanish origin except RP-1650 which was from Venezuela (both grandparents from mother´s side–disease origin–were Venezuelan).
Phenotypic characteristics of NR2E3 mutation
Clinical data were available for 24 subjects from the seven NR2E3 mutated adRP families (Table 1).
Table 1. Clinical data of 24 affected cases presenting the p.Gly56Arg mutation in the NR2E3 gene.
| Individual | Family | Year of birth | Age at diagnostic (years) | Age of NB onset (years) | Age of VF loss onset (years) | Age of cataracts onset (years)* | Age at VA measurement | VA | VA classificationa | Age at VF measurement | VF | VF classificationb | Age at evaluation of fundus | Fundus | ERG / Age (yr) | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I:2 | RP-0030 | 1915 | - | 9yr | 9yr | Yes at 74yr | 78yr | perception of light | 3 | - | - | - | 78yr | typical + pigment deposit in macula | NR / 78yr | - |
| II:2 | RP-0030 | 1938 | - | 5yr | - | Yes (age unknown) | 6yr | - | 71yr | <10° ct | 3 | 71yr | typical (few spicules) + white yellowish spots in posterior pole | NR / 71yr | - | |
| II:5 | RP-0030 | 1943 | 15yr | 8yr | 34yr | 36yr | 46yr & 66yr | BE = 0.2 → hand movement | 1→2 | 48yr | 10° ct | 2 | 66yr | typical + macular atrophy and hypopigmented lesions | NR / 66yr | - |
| III:2 | RP-0030 | 1968 | - | 7yr | 15yr | 32yr | 40yr | BE = 0.8 | 0 | 40yr | 10° ct | 2 | 40yr | Normal vessels, optic disc and macula. Nummular pigment deposits Vitreous floaters | Rods and mix: NR, cones and flicker: reduced amplitude / 40yr | Hyperopia. Progressive hearing loss 36yr |
| III:4 | RP-0030 | 1971 | - | 5yr | 20yr | 38yr | 20yr & 38yr | RE = 0.7→0.6LE = 1→0.7 | 0 | 20yr | peripheral contraction with temporal islet | 1 | 20yr | typical (few spicules) + hypopigmented lesions and vitreous floaters | Sco and Pho: reduced amplitude of b wave / 20yr. NR / 38yr | Progressive myopia |
| III:6 | RP-0030 | 1981 | 10yr | 3yr | 9yr | - | 6yr | RE = 0.7; LE = 1 | 0 | 10yr | Ring scotoma | 1 | - | - | Sco and Pho: reduced amplitude of b wave / 10yr | Progressive myopia |
| III:2 | RP-0711 | 1967 | 22yr | 14yr | 20yr | No at 42yr | 30yr & 42yr | RE = 0.85→0.6 LE = 0.7→0.5 | 0 | 42yr | 10° ct | 2 | 25yr & 40yr | 1st evaluation: tapetoretinal degeneration sine pigmenti 2nd evaluation: typical + with chorioretinal degeneration | Rods and mix: reduced amplitude, cones and flicker: normal / 25yr | - |
| III:4 | RP-0711 | 1973 | - | 16yr | - | - | 38yr | RE = 0.7 LE = 0.5 | 0 | 20yr | 10° ct | 2 | 38yr | Typical + mild degeneration of macular RPE | - | - |
| III:5 | RP-0711 | 1979 | 30yr | - | - | No at 30yr | 30yr | BE = 0.8 | 0 | - | - | - | 30yr | Normal vessels, and optic disc, few peripheral and perivascular spicules + degeneration of macular RPE | NR /30yr | - |
| III:6 | RP-0711 | 1954 | 18yr | Childhood | 40yr | 54yr | 54yr | RE = 0.8 LE = 0.6 | 0 | - | - | - | - | - | NR / 54yr | - |
| III:7 | RP-0711 | 1952 | 24yr | 20yr | - | 45yr | 57yr | RE = 0.8 | 0 | 57yr | 10° ct | 2 | 57yr | (only RE) typical (few spicules, nummular pigment deposits) + degeneration of macular RPE | NR / 57yr | Enucleation of LE in childhood due to glioblastoma. Photopsia |
| III:8 | RP-0711 | 1958 | - | - | - | No at 50yr | 50yr | RE = 0.9 LE = 0.8 | 0 | 50yr | 10° ct | 2 | - | Typical with conserved optic disc + degeneration of macular RPE | NR / 50yr | - |
| IV:2 | RP-0711 | 1991 | 12yr | 11yr | 11yr | No at 12yr | 11yr &18yr | RE = 0.8→0.6 LE = 0.7→0.5 | 0 | 11yr | 30° ct | 1 | 11yr & 18yr | 1st evaluation: tapetoretinal degeneration sine pigmenti Vitreous floaters 2nd evaluation: Normal vessels, optic disc, macula and vitreous. Hypopigmented lesions in mid periphery. | Rods: NR, mix: reduced amplitude of a and b waves, cones and flicker: normal / 12yr. Rods and mix: NR, cones and flicker very reduced amplitude / 18yr | - |
| II:6 | RP-1182 | 1950 | 51yr | 12yr | - | 51yr | 54yr | RE = 0.3 LE = 0.6 | 1 | 54yr | Central & superior hemifield scotoma | 1 | 58yr | Typical + degeneration of macular RPE and vitreous floaters | NR / 58yr | - |
| III:8 | RP-1182 | 1976 | 26yr | 26yr | 26yr | 31yr | 31yr | RE = 0.7 LE = 1 | 0 | 26 | RE = Nasal hemifield scotoma LE = Normal | 1 | 33yr | Normal vessels, optic disc, macula. Nummular pigment deposits in periphery. | Rods, mix and flicker: NR, cones: very reduced amplitude / 33yr | Myopia. Unilateral onset (RE) |
| II:4 | RP-0124 | 1935 | 57yr | 10yr | 10yr | 57yr ** | 57yr → 61yr | RE = 0.1 LE = 0.1 | 2 | 57yr | RE 10° ct LE = absolute scotoma | 3 | 57yr | Typical | NR / 57yr | Astigmatism |
| I:2 | RP-0576 | 1957 | 38yr | 24yr | 28yr | 52yr | 40yr & 52yr | RE = 0.7→0.2 LE = 0.9→0.2 | 0→1 | 38yr | 10° ct | 2 | 38yr | Typical | Rods, mix and flicker: NR, cones: very reduced amplitude / 38yr. NR / 40yr | - |
| I:2 | RP-1650 | - | - | - | - | - | 60yr | - | 2 | - | - | - | - | - | - | - |
| II:2 | RP-1650 | 1958 | 42yr | - | - | - | - | - | - | - | - | - | - | - | - | - |
| III:1 | RP-1650 | 1978 | 27yr | 29yr | 25yr | No at 25yr | 25yr | BE = 0.6 | 0 | 32 | <20° ct | 2 | 32yr | Typical | Abnormal / 32yr | - |
| I:2 | RP-1996 | 1924 | - | - | - | - | 53yr | (Neither perception nor projection of light) | - | - | - | - | - | - | - | High IOP |
| II:4 | RP-1996 | 1953 | 48yr | 38yr | 48yr | 59yr | 57yr→59yr | RE = 0.8→0.5; LE = 0.2 | 1 | 59 | <10° ct | 3 | 59 | Degeneration of macular RPE + macular edema + peripheral spicules | Sco, Pho and flicker: reduced amplitude of b wave / 57yr | High IOP |
| III:3 | RP-1996 | 1980 | 30yr | 20yr | 20yr | 32yr | 32yr | BE = 1 | 0 | 30 | BE = temporal hemifield scotoma | 1 | 32 | Choroidal hypopigmentation (no spicules) | - | FA: choroidal silence with granular alteration and macular pattern / 32yr |
Yr: Years. VA: Visual Acuity. VF: Visual Field. Ct: Central. ERG: Electroretinogram. VEP: Visual Evoked Potential. FA: Fluorescein Angiography RPE: retinal pigment epithelium. RE: Right eyes. LE: Left eye. BE = both eyes. Sco = Scotopic. Pho = Photopic.
* All patients with cataracts (except family RP-1996) presented: subcapsular posterior cataracts in BE.
** Patients also presented cortical anterior cataract in BE.
*** VA after cataract surgery.
# The age at onset of cataracts is not available (mature cataract at diagnosis).
## Legal blindness. VA classificationa: 0 = Normal vision (normal and near normal vision) (≥0.4), 1 = Moderate low vision (<0.4 –>0.1), 2 = Severe low vision (≤0.1–≥0.05, legal blindness), 3 = profound vision loss and blindness (blindness and near blindness, <0.05). VF classificationb 0 = normal, 1 = peripheral and ring scotoma, peripheral constriction with VF ≥20°, 2 = <20°–≥10° central, 3 = <10° central. Typical fundus: optic disc pallor, attenuation of the retinal vessels and pigmentary deposits resembling bone spicules. IOP: Intraocular pressure. NR: Non recordable.
In most cases NB was the first noticeable symptom with an onset in pre-adolescence or early twenties (mean age 15.9 ± 10.1 years). Visual field (VF) loss onset was variable, although tubular vision onset tended to occur around the third decade of life (mean age 23.2 ± 11.9 years) and stayed stable until an advanced age. Loss of visual acuity (VA) appeared later in the disease´s evolution, being normal (≥0.4) until the fifth decade of life (Table 2).
Table 2. Phenotypic characteristics (means and standard deviation) of patients with the p.Gly56Arg mutation in NR2E3.
| Age at diagnosis (yr) | NB Onset (yr) | VF loss Onset (yr) | Cataract (yr) | VA | VA (WHO severity) | Fundus | ERG | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Typical | Macular alteration | Impaired (yr) | Non recordable (yr) | ||||||||
| 30.2 ± 14 | 15.9 ± 10.1 | 23.3 ± 11.9 | Yes = 14, No = 5, NA = 5 | <50yr RE = 0.70 ± 0,17; LE = 0.72 ± 0.23 | >50yr RE = 0.3 ± 0,3; LE = 0.3 ± 0,3; | <50yr Normal vision | >50yr Moderate to profound vision loss | 63.2% (12/19) | 47.4% (9/19) | 29.2 ± 13.9 | 54.55 ± 14.4 |
Yr: Years. VA: Visual Acuity. VF: Visual Field. ERG: Electroretinogram. NA: Not Available. RE: Right Eye. LE: Left Eye. VA classificationa: 0 = Normal vision (normal and near normal vision) (≥0.4), 1 = Moderate low vision (<0.4 –>0.1), 2 = Severe low vision (≤0.1–≥0.05, legal blindness), 3 = profound vision loss and blindness (blindness and near blindness, <0.05). Typical fundus: optic disc pallor, attenuation of the retinal vessels and pigmentary deposits resembling bone spicules.
Only two patients presented legal blindness (VA ≤0.1 or VF ≤10°) at 60 and 71 years of age, respectively. Additionally, one patient with RP, who also presented glaucoma, was diagnosed with complete blindness (neither perception nor projection of light) at the age of 53.
More than half of the patients (58.3%) with available ophthalmological data presented cataract (73.7%), most of them (50%) since the third decade of life (Table 2).
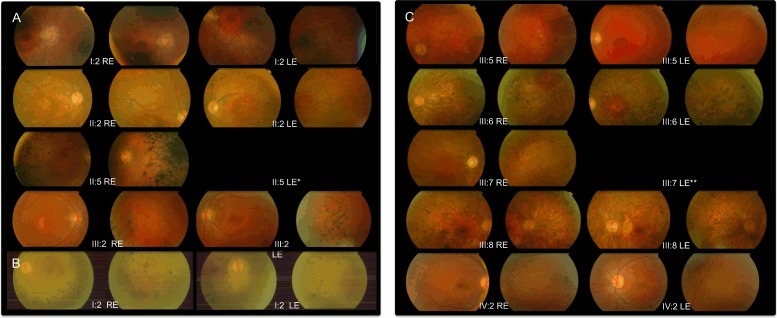
All ophthalmoscopic examination data available (19 patients) showed fundus alterations (Fig 2).
Fig 2. Fundus Images: Each patient presents two fundus images per eye.
A) Family RP-0030, B) Family RP-0576, C) Family RP-0711. *Fundus image not available. **Enucleation of the LE due to glioblastoma. RE = Right eye. LE = Left eye.
These fundus alterations could be detected since the beginning of the vision impairment, although visual acuity was not affected (Table 1).
Although fundus changes showed inter and intrafamiliar variability, most of the patients showed typical RP changes with the progression of the disease. It was also frequent to find macular affectation (47.4% -Table 2-) with preserved visual acuity, even when patients were studied in the first stages of the disease (family RP-0711, IV:2 -Table 1-).
ERG recordings were available for 19 cases. All available ERG recordings (scotopic, photopic and flicker) showed alterations from the beginning of the disease, and these changes could be detected around the first decade of life, when studied at that age (family RP-0711 patient IV:2; and family RP-0030 patient III:6—Table 1 and S1 Fig). Non recordable ERG occurred around the age of fifty (Table 2). Similarly as for fundus changes, the initial impairment in ERG recordings did not seem to alter visual acuity.
Haplotype analysis
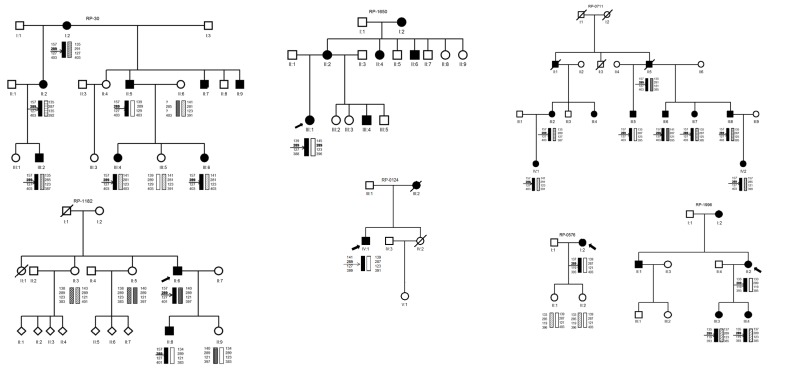
Four of the families (RP-0030, RP-0576, RP-0711 and RP-1182) belonged to the same town in Spain (in the Toledo region, with 2755 inhabitants in 1920 and 3511 inhabitants in 2010, according to the demographic data of the Instituto Nacional de Estadística -INE- http://www.ine.es). In two of these families, RP-0030 and RP-1182, a common ancestor (four generations above the respective family probands) could be identified, but not in RP-0711 and RP-0576 (family trees investigated five generations above the respective probands, Fig 3).
Fig 3. Pedigrees and Haplotype analysis of adRP families with p.Gly56Arg mutation in NR2E3.
Haplotype analysis: → marks the NR2E3 gene position. The NR2E3 extragenic polymorphic markers used are D15S967, D15S1050, D15S204 and D15S188.
Inferring haplotypes in families RP-1650 and RP-0124 (and even in RP-1996) is very speculative. However, the two large families belonging to the same village (RP-0030, RP-0711) shared a common haplotype for the four flanking biomarkers [D15S976 (157bp allele)—D15S1050 (289 bp allele)—D15S204 (127 bp allele)—D15S188 (403 bp allele)] linked to the mutation and RP-1182 shared the same haplotype for the markers D15S976-D15S1050-D15S204. Besides, RP-0124 and RP-1650 families shared a common haplotype defined by the two flanking markers D15S1050 (289bp allele) and D15S204 (127bp allele) whereas the other two families, one from the same city (RP-0576) and RP1996 showed the same proximal marker (D15S1050 289bp) but differed in the distal one (D15S204 119bp allele) indicating that at least two independent mutational events or alternatively a postmutational recombination event, might have occurred (Fig 3).
So, D15S1050 (289bp allele), the closest microsatellite to NR2E3 gene, is shared by all patients, leaving a small region of 104Kb comprised between D15S1050 (ensemble genomic position Crh15: 71,980,969–71,981,252) and the NR2E3 gene (ensemble genomic position Crh15: 72,084,977–72,110,600), which could be common to all of the patients carrying p.Gly56Arg mutation.
Discussion
This paper is an extensive report of frequency of the mutation (p.Gly56Arg) in the NR2E3 gene in 24 cases diagnosed of adRP, and the associated phenotype.
The prevalence for this mutation in our present cohort of adRP families is 3.5% which is similar to the rates described in Europe (3.4%) [17], but higher than those described in North America [19].
In our cohort of adRP families, the p.Gly56Arg mutation is the second most common single mutation detected [24] after the p.Pro347Leu mutation in the RHO gene which was found in nine families out of 200, representing a 4.4% [25].
The results of the haplotype analysis are conclusive for the two most informative families belonging to the same small village (Fig 3). We found a common haplotype in those families for the four markers and for three of them in the family RP-1182. These data support an ancient founder effect for the p.Gly56Arg in our families. However one must be cautious because i) one out of four families coming from the same small geographical area did not share the common haplotype. This fact is unexpected and despite there being an association between the 289 allele of D15S1050 and p.Gly56Arg, D15S1050 and NR2E3 are in different disequilibrium blocks, we have to consider that the Spanish population is underrepresented in the Hapmap project and that the allele 289 has a frequency of 0.519 (http://www.genoscope.cns.fr/externe/gmap/Nature-1995/), so the probability that both the allele 289 and the mutation are located in the chromosome is very high. Furthermore, there are no hot spot recombination sites like the common motif CCNCCNTNNCCNC [26] within the NR2E3 gene and their 5’ and 3’ UTR regions that could explain the lack of a common ancestral haplotype by a high recombination rate, ii) The frequency of p.Gly56Arg in populations that have evolutionary diverged, such as American, European and Chinese, are high and quite similar (1.2% in American [19], 3.4% in European [17] and 1.2% in China [27]) and iii) the mutation c.166G>A is a change GGG>AGG in codon 56 of NR2E3. This change lies on a CpG dinucleotide and has been reported as high de novo mutation site. In fact, the most common de novo mutated codon associated with human disease is a GGG>AGG or CGG mutation in codon 380 of FGFR3 gene [28, 29] which results in achondroplasia. In summary, it is tempting to speculate that the p.Gly56Arg arose once in our population as a very ancient hit giving time for a number of recombination events that have led to the change in the ancient haplotype.
The NR2E3 gene is associated with both autosomal recessive and dominant retinitis pigmentosa. The NR2E3 gene recessive mutations present variable phenotypes (ESCS, GFS and CPRD) with variable ophthalmological findings, but all showing night blindness, rudimental or absent rod function, and hyperfunction of the "blue" S-cones [14, 20, 30]. Dominant NR2E3 gene mutation has been associated with RP phenotype [17–20] and to date, the phenotype has only been described in five European [17, 18, 20] and four North American [19] families characterized by presenting RP, but not to other phenotypes associated to NR2E3 gene recessive mutations [16, 17]. However, some affected members of those families displayed phenotypic similarities to ESCS [17, 20].
Although we observe, as it has been previously described [18], inter and intrafamiliar phenotype variability for the p.Gly56Arg mutation, some genotype-phenotype correlation appears to be quite apparent, not only homogeneous progression [19], but occurrence of vision impairment milestones. Patients with NR2E3 dominant mutation showed a moderate form of retinal dystrophy. Thus, patients in our cohort present early NB onset around puberty, preservation of VF>10° until the 4th-5th decade of life, normal or moderately low VA (WHO criteria) to an advanced age and preservation of photoreceptor function (recordable ERG) until the 4th decade of life (Table 2).
It is important to notice that this phenotype is milder than the one found in recessive forms [18] and to point that the fundus alterations (additionally to typical RP fundus, atypical findings are present as macular changes and choriocapillaris atrophy) and ERG findings (impaired photopic and flicker records in the first stages of the disease) observed in some of our patients, are not the classical RP changes previously described in the European and North American NR2E3 dominant patients [17, 19, 20].
Moreover these changes did not seem to correlate with visual acuity impairment, at least during the first stages of the disease (impaired ERG and fundus changes with normal VA). However the absence of correlation between ERG and VA is common in RP patients [1].
These fundus changes have recently been described on the ophthalmoscopic findings of NR2E3 ESCS (recessive) patients. These changes can lead to a misdiagnosis if family history, initial symptoms reported by patients and progression of the disease in the early stages are not queried when performing the clinical history. Besides, other ophthalmological signs such as double concentric autofluorescence ring (not performed in our cohort), that has been described as an initial sign of retinal degeneration in patients carrying the NR2E3 dominant mutation [20], could help in the molecular diagnostic orientation of adRP patients.
Furthermore, some of these changes such as macular edema, can, at least partially, be treated [31, 32], which is important for prognosis, disease outcome and follow up of these patients. Although this event was only observed in one of our patients by ophthalmoscopic examination, we cannot discard that it could be present in other NR2E3 adRP patients, as no OCT data was available for the present cohort.
Conclusion
We believe that the relevance of this study is not only being the single largest NR2E3 genotype-phenotype correlation study performed to date, but also highlighting the importance of p.Gly56Arg in the NR2E3 gene associated to adRP.
NR2E3 is responsible for two main retinal phenotypes ESCS (including Goldmann-Favre syndrome) only for recessive forms and RP (including CPRD) related to both dominant and recessive forms. However, in our cohort there is a wide range of phenotypic characteristics that differ from typical RP phenotype and resemble other NR2E3 phenotypes such as ESCS and CPRD, mainly at a fundus level where we found macular disturbance, which is frequently seen in ESCS, in 50% of our evaluated patients and two patients with nummular pigmentation (typical of CPRD phenotype).
In this report, we are providing new clues of a characteristic phenotype for this mutation that allows for making an estimated prognosis of autosomal dominant RP due to p.Gly56Arg mutation in the NR2E3 gene. Besides, we believe that this study can improve molecular diagnosis approach, clinical management, risk assessment, and genetic counselling of adRP patients and their families.
Supporting Information
A) ERG at 11years of age. B) ERG at 18 years of age.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files. The adRP genotyping Asper Ophthalmics microarray (Asper Biotech) is a commercially available genotyping microarray chip based on APEX technology (Asper Biotech, Tartu, Estonia). The Asper Biotech array checks for the presence of 414 markers in 16 genes related to adRP. The list of these mutations is available to all the interested researchers using the hyperlink: http://www.asperbio.com/asper-ophthalmics/autosomal-dominant-retinitis-pigmentosa-genetic-testing.
Funding Statement
Support was provided by FIS (PI: 13/00226), the Centre for Biomedical Network Research on Rare Diseases—CIBERER (06/07/0036), the Biobank of Fundación Jiménez Díaz University Hospital (RD09/0076/00101), ONCE 2014, Feder (Fondos Europeo de Desarrollo Regional) and Fundaluce (4019-002). Patricia Fernandez-San Jose´s work is supported by a Rio Hortega grant (CM12/00013), Marta Corton by a Miguel Servet grant (CP/03256) all from Instituto de Salud Carlos III. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006. October;1:40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gawande AA, Donovan WJ, Ginsburg AP, Marmor MF. Photoaversion in retinitis pigmentosa. Br J Ophthalmol 1989. February;73:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marmor MF. Visual acuity and field loss in retinitis pigmentosa. Arch Ophthalmol 1991. January;109(1):13–4. [DOI] [PubMed] [Google Scholar]
- 4.Marmor MF. Visual loss in retinitis pigmentosa. Am J Ophthalmol 1980. May;89(5):692–8. [DOI] [PubMed] [Google Scholar]
- 5.Marmor MF. The electroretinogram in retinitis pigmentosa. Arch Ophthalmol 1979. July;97(7):1300–4. [DOI] [PubMed] [Google Scholar]
- 6.Haim M. Retinitis pigmentosa: problems associated with genetic classification. Clin Genet 1993. August;44(2):62–70. [DOI] [PubMed] [Google Scholar]
- 7.Ayuso C, Garcia-Sandoval B, Najera C, Valverde D, Carballo M, Antiñolo G. Retinitis pigmentosa in Spain. The Spanish Multicentric and Multidisciplinary Group for Research into Retinitis Pigmentosa. Clin Genet 1995. September;48(3):120–2. [PubMed] [Google Scholar]
- 8.Ayuso C, editor. Estudio de la Retinosis Pigmentaria en España. Capitulo XI: La Retinosis Pigmentaria en España: estudio clínico y genético. ONCE (Organización Nacional de Ciegos Españoles), 2001.
- 9.Roduit R, Escher P, Schorderet DF. Mutations in the DNA-binding domain of NR2E3 affect in vivo dimerization and interaction with CRX. PLoS One 2009. October;4(10):e7379 10.1371/journal.pone.0007379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson SG, Marmor MF, Kemp CM, Knighton RW. SWS (blue) cone hypersensitivity in a newly identified retinal degeneration. Invest Ophthalmol Vis Sci. May 1990;31(5):827–838. [PubMed] [Google Scholar]
- 11.Marmor MF, Jacobson SG, Foerster MH, Kellner U, Weleber RG. Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and enhanced S cone sensitivity. Am J Ophthalmol. August 15 1990;110(2):124–134. [DOI] [PubMed] [Google Scholar]
- 12.Nasr YG, Cherfan GM, Michels RG, Wilkinson CP. Goldmann-Favre maculopathy. Retina. 1990;10(3):178–180. [PubMed] [Google Scholar]
- 13.Sharon D, Sandberg MA, Caruso RC, Berson EL, Dryja TP. Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch Ophthalmol. 2003. September;121(9):1316–23. [DOI] [PubMed] [Google Scholar]
- 14.Schorderet DF, Escher P. NR2E3 mutations in enhanced S-cone sensitivity syndrome (ESCS), Goldmann-Favre syndrome (GFS), clumped pigmentary retinal degeneration (CPRD), and retinitis pigmentosa (RP). Hum Mutat 2009. November;30(11):1475–85. 10.1002/humu.21096 [DOI] [PubMed] [Google Scholar]
- 15.Haider NB, Jacobson SG, Cideciyan AV, Swiderski R, Streb LM, Searby C, et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet 2000. February;24(2):127–31. [DOI] [PubMed] [Google Scholar]
- 16.Yzer S, Barbazetto I, Allikmets R, van Schooneveld MJ, Bergen A, Tsang SH, et al. Expanded clinical spectrum of enhanced S-cone syndrome. JAMA Ophthalmol 2013. October;131(10):1324–30. 10.1001/jamaophthalmol.2013.4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppieters F, Leroy BP, Beysen D, Hellemans J, De Bosscher K, Haegeman G, et al. Recurrent mutation in the first zinc finger of the orphan nuclear receptor NR2E3 causes autosomal dominant retinitis pigmentosa. Am J Hum Genet 2007. July;81(1):147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escher P, Gouras P, Roduit R, Tiab L, Bolay S, Delarive T, et al. Mutations in NR2E3 can cause dominant or recessive retinal degenerations in the same family. Hum Mutat 2009. March;30(3):342–51. 10.1002/humu.20858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gire AI, Sullivan LS, Bowne SJ, Birch DG, Hughbanks-Wheaton D, Heckenlively JR, et al. The Gly56Arg mutation in NR2E3 accounts for 1–2% of autosomal dominant retinitis pigmentosa. Mol Vis 2007. October;13:1970–5. [PubMed] [Google Scholar]
- 20.Escher P, Tran HV, Vaclavik V, Borruat FX, Schorderet DF, Munier FL. Double Concentric Autofluorescence Ring in NR2E3-p.G56R-Linked Autosomal Dominant Retinitis Pigmentosa. Invest Ophthalmol Vis Sci 2012. July;53(8):4754–64. 10.1167/iovs.11-8693 [DOI] [PubMed] [Google Scholar]
- 21.Sullivan LS, Bowne SJ, Reeves MJ, Blain D, Goetz K, Ndifor V, et al. Prevalence of Mutations in eyeGENE Probands With a Diagnosis of Autosomal Dominant Retinitis Pigmentosa. Invest Ophthalmol Vis Sci 2013. September;54(9):6255–61. 10.1167/iovs.13-12605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, et al. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015. February:130(1):1–12 10.1007/s10633-014-9473-7 [DOI] [PubMed] [Google Scholar]
- 23.Tonisson N, Kurg A, Lohmussaar E, Metspalu A. Arrayed primer extension on the DNA chip-method & applications In: Schena M, ed. Microarray Biochip Technology. Natick, MA: Eaton Publishing; 2000; 247–63. [Google Scholar]
- 24.Blanco-Kelly F, García-Hoyos M, Cortón M, Avila-Fernández A, Riveiro-Álvarez R, Giménez A, et al. Genotyping microarray: mutation screening in Spanish families with autosomal dominant retinitis pigmentosa. Mol Vis 2012;18:1478–83. [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-San Jose P, Blanco-Kelly F, Corton M, Trujillo-Tiebas MJ, Gimenez A, Avila-Fernandez A, et al. Prevalence of Rhodopsin mutations in autosomal dominant Retinitis Pigmentosa in Spain: clinical and analytical review in 200 families. Acta Ophthalmol. 2015. Feb;93(1):e38–44. 10.1111/aos.12486 [DOI] [PubMed] [Google Scholar]
- 26.Myers S, Freeman C, Auton A, Donnelly P, McVean G. A common sequence motif associated with recombination hos spots and genome instability in humans. Nat Genet 2008. September;40(9):1124–29. 10.1038/ng.213 [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Zhang X, Chen LJ, Chiang SW, Tam PO, Lai TY, et al. Association of NR2E3 but not NRL mutation with retinitis pigmentosa in the Chinese population. Invest Ophthalmol Vis Sci 2010. April; 51(4):2229–35. 10.1167/iovs.09-4299 [DOI] [PubMed] [Google Scholar]
- 28.Crow JF. The high spontaneous mutation rate: is it a health risk?. Proc Natl Acad Sci 1997. August; 94(16):8380–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drake JW, Charlesworth B, Charlesworth D, Crow JF. Rates of spontaneous mutation. Genetics 1998. April;148(4):1667–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Audo I, Michaelides M, Robson AG, Hawlina M, Vaclavik V, Sandbach JM, et al. Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci 2008. May;49(5):2082–93. 10.1167/iovs.05-1629 [DOI] [PubMed] [Google Scholar]
- 31.Ganesh A, Stroh E, Manayath GJ, Al-Zuhaibi S, Levin AV. Macular cysts in retinal dystrophy. Curr Opin Ophthalmol 2011. September;22(5):332–9. 10.1097/ICU.0b013e328349229e [DOI] [PubMed] [Google Scholar]
- 32.Ikeda Y, Yoshida N, Notomi S, Murakami Y, Hisatomi T, Enaida H, et al. Therapeutic effect of prolonged treatment with topical dorzolamide for cystoid macular oedema in patients with retinitis pigmentosa. Br J Ophthalmol 2013. September;97(9):1187–91. 10.1136/bjophthalmol-2012-303005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) ERG at 11years of age. B) ERG at 18 years of age.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. The adRP genotyping Asper Ophthalmics microarray (Asper Biotech) is a commercially available genotyping microarray chip based on APEX technology (Asper Biotech, Tartu, Estonia). The Asper Biotech array checks for the presence of 414 markers in 16 genes related to adRP. The list of these mutations is available to all the interested researchers using the hyperlink: http://www.asperbio.com/asper-ophthalmics/autosomal-dominant-retinitis-pigmentosa-genetic-testing.