Abstract
Aim
Infertility is a concern for young colorectal cancer (CRC) survivors, but this risk is not well quantified. Mismatch repair (MMR) mutation carriers are a useful cohort for studying fertility after CRC as they commonly develop CRC when young, and unaffected family members provide demographically similar controls. The aim of this study was to determine the effect of CRC on fertility in a large cohort of MMR mutation carriers.
Method
MMR mutation carriers identified from the Australasian Colorectal Cancer Family Registry were included. For each year of life within the fertile age range (15 to 49), the number of living individuals and the number of children born to them were determined. Individuals were grouped by whether or not they had had a diagnosis of CRC by that age. Age-specific and total fertility rates were calculated.
Results
1068 subjects (611 women and 457 men) were identified, of whom 467 were diagnosed with CRC. There were 1,192 births during 18674 person- years of follow up of the women and 814 births during 14013 person- years of follow up to the men.
The total fertility rate was decreased in women after a diagnosis of CRC compared who did not have CRC (1.3 vs. 2.2 P=0.0011), but age- specific fertility was only reduced in the 20–24 year age group. In men TFR was similar for both groups (2.0 vs. 1.8) P = 0.27).
Conclusion
Age- specific fertility was decreased in female CRC survivors with Lynch syndrome aged 20–24, but not in older women or in men.
Introduction
Colorectal cancer (CRC) is a common cancer in industrialised nations. In the United States, there are 143,000 new cases per year, of whom approximately 4% are under 45 years of age[1]. Among young CRC survivors, potential infertility is known to be a significant concern [2]. The American Society of Clinical Oncology (ASCO) has published guidelines recommending that oncologists discuss the possibility of infertility with their patients, while acknowledging that in many cases there are insufficient data available to accurately assess this risk[3]. This caveat is especially pertinent in the case of CRC, as only one previous study[4] has attempted to quantify the effect of gastrointestinal cancer on fertility. This was a Swedish population- based cohort study of young (under 45) female cancer survivors. The authors reported a ten per cent reduction in overall (age- adjusted) fertility for female survivors of gastrointestinal cancers when compared with the general population. No previously published studies have documented age- specific fertility rates for female CRC survivors, and fertility rates in male CRC survivors have not been reported.
Lynch syndrome is the commonest known inherited predisposition to colorectal cancer (CRC). It is caused by germline mutations in the DNA mismatch repair (MMR) genes MLH1, MSH2, MSH6 and PMS2, and is characterised by young onset CRC (the median age of onset is around 45 years[5], compared with 69 years in the general population [1]). Lynch syndrome families provide a useful cohort for studying the effect of CRC on fertility, as CRC in this population commonly occurs in subjects who are within the potentially fertile age group, and unaffected family members can be used as demographically similar controls. The aim of this study was to determine the effect of CRC on age- specific fertility rates in a large cohort of subjects who carry MMR gene mutations.
Method
Ethical approval for the study was given by the University of Melbourne Research Ethics Committee. The Australasian Colorectal Cancer Family Registry (ACCFR) includes more than 11,500 subjects from 1800 families in Australia and New Zealand[6]. It contains CRC families recruited through the Victorian Cancer Registry and from family cancer clinics throughout Australia and New Zealand. Personal and family history of cancer and reproductive history were collected by questionnaire. Attempts were made to verify all reports of the diagnosis of CRC and date of death by medical records, pathology reports, death certificates and linkage to national cancer registry and death registry databases. Subjects identified from the ACCFR with proven germline mismatch repair gene mutations were included in this study. Methods for screening and testing for MMR gene mutations have been described in detail elsewhere [7].
The period of potential fertility was considered to be between the ages 15 to 49, in keeping with the World Health Organisation[8] and Australian Bureau of Statistics[9] norms for describing population fertility rates. For each year of life within this age range, the total number of subjects known to be alive at that age and the number of children born to them were determined. Subjects in each year of life were grouped according to whether they had been diagnosed with CRC by that age or not. This meant that a subject who, for example was diagnosed with CRC at age 29 would be counted in the ‘no CRC diagnosis’ group from ages 15 to 28 years, and in the ‘after CRC diagnosis’ group for each year of life from age 29 until age 49, death or last follow up.
Age- specific fertility rates (ASFR) were calculated by dividing the total number of children by the total number of subjects in each age group. Age- specific fertility rates are stated as births per 1000 person- years throughout. The total fertility rate (TFR) is the average number of children a hypothetical cohort of women would have had at the end of their reproductive life if they had children at the population age- specific rates during their whole life and survived to the end of their fertile period. It is expressed as children per woman and is calculated as the sum of age- specific fertility for the ages 15 to 49 (multiplied by five if five- yearly age strata are used)[8]. Fertility rates were compared between those subjects who had been diagnosed with CRC and those who had not. Female fertility rates were also compared with the known fertility rates of the Australian female general population (1975 to 2010) as reported by the Australian Bureau of Statistics[9] (male fertility rates for the general population are not recorded).
Statistical Analysis
Age- specific fertility rates were compared using the Chi square test. Confidence intervals (CI) for age- specific fertility rates and total fertility rate were calculated using Byar’s method for direct standardisation as described by Breslow and Day [10]. Confidence intervals for total fertility rates were calculated using the widely accepted method described by Dobson et al [10, 11][12]. A p- value of <0.05 was regarded as showing a statistically significant difference. Statistical analysis was done using Microsoft Excel 2010 (Redmond, Washington) and Medcalc® 2008 (Mariakerke, Belgium) software.
Results
The study included 1068 subjects (611 women and 457 men) with proven germline MMR mutations, of whom 467 were diagnosed with CRC. There were 417 with colonic and 82 with rectal cancer (32 subjects were diagnosed with metachronous cancers in both sites). Among subjects under the age of fifty, there were a total of 322 CRC diagnosed (285 colon and 45 rectum). They were followed up to a median age of 55 (18 to 96) years.
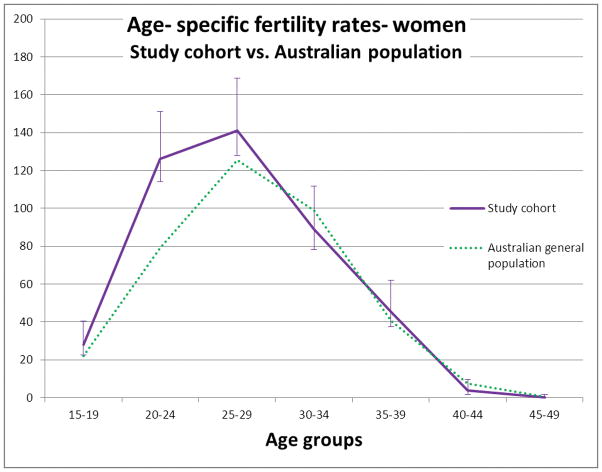
For subjects aged 15 to 49 years, there was a total of 1,192 children born during 18674 person- years of follow up for the women and 814 births during 14013 person- years of follow up for the men. No births occurred in subjects under 15 years of age. No women in this cohort gave birth over the age of 49. Four children were fathered by men over 49 years of age. Age- specific fertility rates for all women from the study cohort, regardless of CRC diagnosis and from the Australian general population are presented in Table 1 and Figure 1. Age- specific fertility rate was higher in the study group than the Australian general population for women in the 15–19, 20–24 and 25–29 age groups, but not in other age groups. Total fertility rate for women in the study group was 2.2 (95% C.I 2.0 to 2.3), which was higher than for women in the Australian general population (TFR 1.87).
TABLE 1. Age-specific fertility rates of women from the entire study group and from the Australian general population.
No confidence intervals are given for the Australian population as these are from population census figures and not a sampled population. Age specific fertility rates for the study cohort which are significantly difference from the general population rate are in bold type.
| Study cohort | Australian population | ||||
|---|---|---|---|---|---|
| Age groups | Number of births | Person- years | Fertility rate | 95% C.I. | Fertility rate |
| 15–19 | 86 | 3053 | 28 | 23–35 | 22 |
| 20–24 | 382 | 3028 | 126 | 114–139 | 79 |
| 25–29 | 413 | 2926 | 141 | 128–155 | 125 |
| 30–34 | 244 | 2744 | 89 | 78–101 | 99 |
| 35–39 | 115 | 2544 | 45 | 37–54 | 41 |
| 40–44 | 9 | 2301 | 3.9 | 1.8–7.4 | 7.3 |
| 45–49 | 0 | 2078 | 0 | 0–1.8 | 0.4 |
|
| |||||
| TFR | 2.2 | 2.0–2.3 | 1.9 | ||
Figure 1. Age- specific fertility rates for women from the study cohort vs. the Australian general population.
Error bars are 95% confidence interval (C.I.). No C.I. are presented for the general population data as these are population census figures, not from a sampled population.
Table 2 shows the age- specific fertility rates for study subjects with or without a CRC diagnosis. These are illustrated in Figures 2 and 3. For women in the 20–24 year age group, age specific fertility was lower in those with a CRC diagnosis (0 births/1000 person- years in those who had been diagnosed with CRC vs. 128 births/1000 person- years in subjects who had not been diagnosed with CRC, P=0.019). There was no statistically significant difference in age- specific fertility rates between women with or without a CRC diagnosis in any other age group. There was no statistically significant difference in age- specific fertility rates between men who had been diagnosed with CRC compared with those without a CRC diagnosis in any age group.
TABLE 2. Age- specific fertility rates for subjects with or without a CRC diagnosis.
Age- specific fertility rates are given as births/ 1000 person- years. Total fertility rate (TFR) is given as births/ person. The sample sizes for subjects aged 15–19 with a CRC diagnosis were insufficient to calculate meaningful confidence intervals. Significantly different rates are marked in bold type.
| Age groups | Births | Person- years | Fertility rate | 95% C.I. | Births | Person- years | Fertility rate | 95% C.I. | P-value |
|---|---|---|---|---|---|---|---|---|---|
| No CRC diagnosis (women) | After CRC diagnosis (women) | ||||||||
| 15–19 | 86 | 3045 | 28 | 23–35 | 0 | 8 | 0 | N/A | 1.0 |
| 20–24 | 382 | 2992 | 128 | 116–141 | 0 | 36 | 0 | 0–102 | 0.019 |
| 25–29 | 399 | 2813 | 142 | 128–156 | 14 | 113 | 124 | 68–208 | 0.69 |
| 30–34 | 228 | 2579 | 88 | 77–101 | 16 | 165 | 97 | 55–157 | 0.67 |
| 35–39 | 104 | 2263 | 46 | 38–56 | 11 | 281 | 39 | 20–70 | 0.76 |
| 40–44 | 8 | 1864 | 4.3 | 1.9–8.5 | 1 | 437 | 2.3 | 0.03–13 | 1.0 |
| 45–49 | 0 | 1497 | 0.0 | 0.0–2.45 | 0 | 581 | 0 | 0–6.3 | 1.0 |
|
| |||||||||
| TFR | 2.2 | 2.1–2.3 | 1.3 | 0.9–1.8 | |||||
|
| |||||||||
| No CRC diagnosis (men) | After CRC diagnosis (men) | ||||||||
|
| |||||||||
| 15–19 | 16 | 2284 | 7.0 | 4.0–11 | 0 | 1 | 0 | N/A | 1.0 |
| 20–24 | 132 | 2256 | 59 | 49–69 | 1 | 22 | 45 | 0.6–253 | 1.0 |
| 25–29 | 281 | 2178 | 129 | 114–145 | 9 | 54 | 167 | 76–316 | 0.41 |
| 30–34 | 205 | 1951 | 105 | 91–120 | 12 | 142 | 85 | 44–148 | 0.57 |
| 35–39 | 104 | 1657 | 63 | 51–76 | 13 | 270 | 48 | 26–82 | 0.41 |
| 40–44 | 34 | 1304 | 26 | 18–36 | 5 | 395 | 13 | 4.1–30 | 0.13 |
| 45–49 | 6 | 990 | 6.1 | 2.2–13 | 1 | 509 | 2.0 | 0.03–11 | 0.43 |
|
| |||||||||
| TFR | 2.0 | 1.8–2.1 | 1.8 | 1.1–2.7 | |||||
FIGURE 2. Age- specific fertility rates for women from the study cohort with or without a CRC diagnosis.
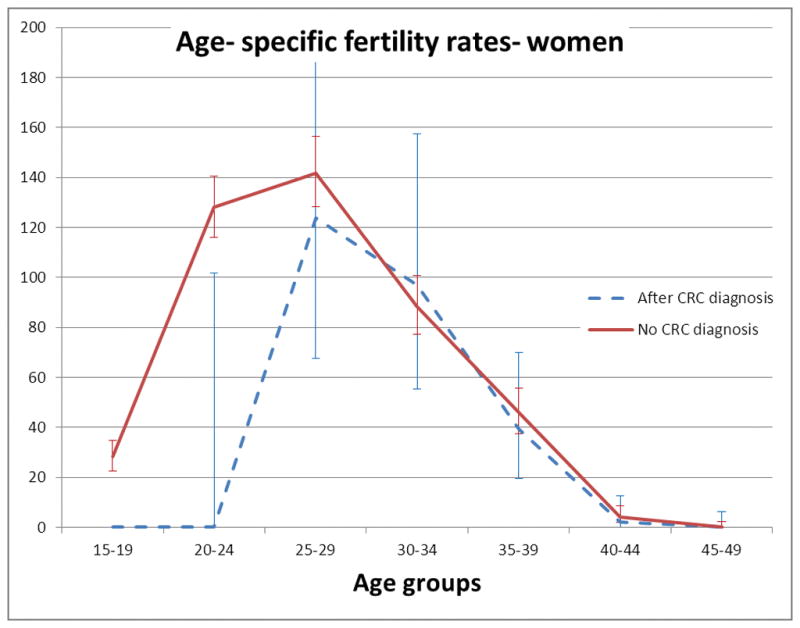
Age- specific fertility rates are given as births/ 1000 person- years. Error bars are 95% C.I. The upper C.I. for subjects with CRC in age group 25–29 has been truncated.
FIGURE 3. Age- specific fertility rates for men with or without a CRC diagnosis.
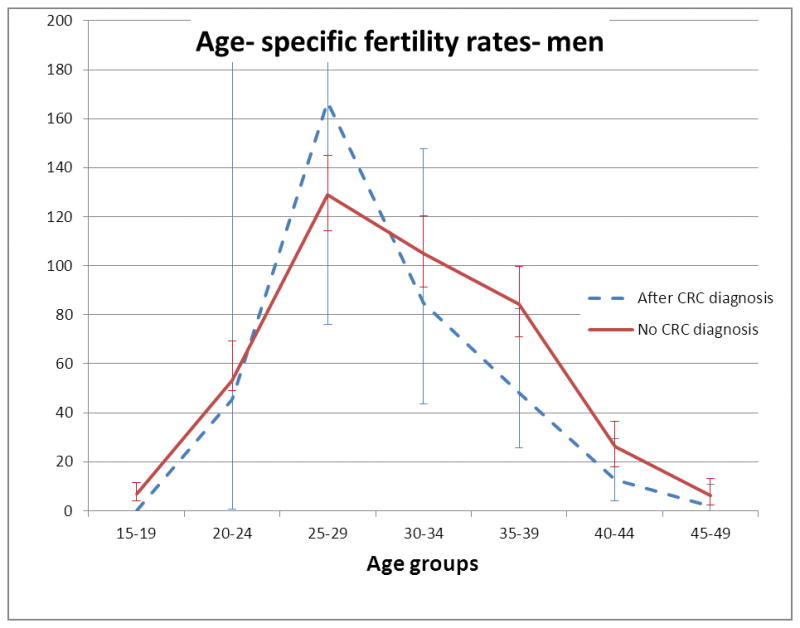
Age- specific fertility rates are given as births/ 1000 person- years. Error bars are 95% C.I. The upper C.I. for subjects with CRC in age groups 20–24 and 25–29 have been truncated
Overall, total fertility rate was lower in women with a CRC diagnosis compared with those without (1.3 (95% C.I 0.90 to 1.8) vs. 2.2 (95% C.I. 2.1 to 2.3) P=0.0011. In men, there was no significant difference in TFR between the two groups (2.0 (95% C.I. 1.8 to 2.1) for men without a CRC diagnosis vs. 1.8 (95% C.I. 1.1 to 2.7) for those with a CRC diagnosis. P = 0.27).
Subjects diagnosed with colon or rectal cancers were also analysed separately, and these results are shown in Table 3. For women with a colon cancer diagnosis, the results were similar to the findings for CRC overall. Age- specific fertility rate for women with a colon cancer diagnosis in the 20–24 year age group was reduced, with 0 births/ 1000 person- years compared with 128 births/ 1000 person years for women without a CRC diagnosis(P=0.017). Age- specific fertility rates for women with a colon cancer diagnosis for all other age groups were similar to those for women without a CRC diagnosis. Total fertility rate for women with a colon cancer diagnosis was reduced compared to women without a CRC diagnosis (1.5 (95% C.I. 1.1–2.1) vs. 2.2 (95% C.I 2.1–2.3), P=0.016). For women who were diagnosed with rectal cancer, there was no significant difference in any of the age- specific fertility rates compared with women without a CRC diagnosis, but there was an overall reduction in total fertility rate (0.72 (95% C.I 0.2–1.7) vs. 2.2 (95% C.I. 2.1–2.3), P=0.015). There were no significant differences in age- specific fertility rates for men with a diagnosis of colon or rectal cancer compared with subjects without a CRC diagnosis. Total fertility rate for men after colon cancer was similar to that for men without a CRC diagnosis (1.7 (95% C.I. 1.0 to 2.5) vs. 1.8 (95% C.I. 1.1–2.7), P= 0.72). TFR for men after a rectal cancer diagnosis was also similar to men without a CRC diagnosis (1.84 (95% C.I. 0.39–5.0) vs. 1.8 (95% C.I. 1.1–2.7), P=0.38.
TABLE 3. Age- specific fertility rates of subjects after a diagnosis of CRC, grouped by site of tumour (colon vs. rectum).
Age- specific fertility rates are given as births/ 1000 person- years. Total fertility rate (TFR) is given as births/ person. There were no men who developed rectal CA under the age of 20, so the age- specific fertility rate for the 15–19 year old subjects in this group could not be calculated. The sample sizes were too small to calculate meaningful confidence intervals for age- specific fertility rates for a number of groups.
| Age groups | Births | Person- years | Fertility rate | 95% C.I. | Births | Person- years | Fertility rate | 95% C.I. |
|---|---|---|---|---|---|---|---|---|
| After Colon CA diagnosis (women) | After Rectal CA diagnosis (women) | |||||||
| 15–19 | 0 | 8 | 0 | N/A | 0 | 3 | 0 | N/A |
| 20–24 | 0 | 34 | 0 | 0–108 | 0 | 8 | 0 | N/A |
| 25–29 | 14 | 90 | 156 | 85–261 | 4 | 28 | 143 | 38–366 |
| 30–34 | 16 | 151 | 106 | 61–172 | 0 | 29 | 0 | 0–126 |
| 35–39 | 10 | 251 | 40 | 19–73 | 1 | 53 | 20 | 0.25–105 |
| 40–44 | 1 | 401 | 2.5 | 0.03–14 | 0 | 71 | 0 | 0–52 |
| 45–49 | 0 | 532 | 0 | 0–6.9 | 0 | 91 | 0 | 0–40 |
|
| ||||||||
| TFR | 1.5 | 1.1–2.1 | 0.72 | 0.2–1.7 | ||||
|
| ||||||||
| After Colon CA diagnosis (men) | After Rectal CA diagnosis (men) | |||||||
|
| ||||||||
| 15–19 | 0 | 1 | 0 | N/A | 0 | 0 | N/A | N/A |
| 20–24 | 0 | 15 | 0 | 0–245 | 1 | 7 | 143 | N/A |
| 25–29 | 8 | 44 | 182 | 78–358 | 1 | 10 | 100 | N/A |
| 30–34 | 11 | 131 | 84 | 42–150 | 1 | 12 | 83 | N/A |
| 35–39 | 12 | 242 | 50 | 26–87 | 1 | 42 | 24 | 0.3–132 |
| 40–44 | 5 | 358 | 14 | 4.5–33 | 0 | 61 | 0 | 0–60 |
| 45–49 | 1 | 462 | 2.2 | 0–12 | 0 | 87 | 0 | 0–42 |
|
| ||||||||
| TFR | 1.7 | 1.0–2.5 | 1.84 | 0.39–5.0 | ||||
Discussion
We have previously documented a reduction in the total (lifetime) number of children born to subjects from the ACCFR cohort who are diagnosed with CRC at a young age [13], but it was not known whether this was due to a reduction in fertility among survivors, or if it was simply due to decreased survival. In this study of colorectal cancer survivors who carry germline MMR mutations, we found that total fertility for women was decreased by approximately 40% after a diagnosis of CRC. The reduction in age- specific fertility rates could only be detected in younger women (age 20 to 24), and was not apparent in other age groups. A CRC diagnosis did not adversely affect age- specific fertility in men. Both colonic and rectal cancer diagnoses were associated with decreased TFR in women, but no difference was detected for men with cancers in either site.
In two recent reviews, Spanos et al[14] and O’Neill et al[15] both found no evidence that colon cancer surgery or 5-fluorouracil chemotherapy affected fertility, but reported concerns about the effects of rectal surgery and radiotherapy and newer chemotherapy agents. Both emphasised the importance of adequate pre- treatment fertility counselling and discussed the merits of fertility preservation options that a CRC survivor could be offered. These include embryo preservation and oocyte vitrification. Kumar et al [16] reported that discussion about fertility occurred in only one third of CRC patients under the age of 40 at their institution. They concluded that it was important to educate health care professionals about the importance of fertility risk, while acknowledging the lack of direct evidence for risk to fertility associated with colon surgery or chemotherapy.
The only previously published study directly comparing fertility rates of women with or without gastrointestinal cancer was by Hartman et al [4]. They reported a ten per cent reduction in fertility for women less than 45 years of age compared with the Swedish general population. Age- specific fertility rates in cancer survivors for a number of different tumours, including gastro- intestinal, were compared with those of the general Swedish population using the technique of indirect standardisation. This involves determining age- specific birth rates for the reference population, and applying these to the study population to calculate the expected number of events for that population. The authors calculated a standardised birth rate (SBR, an indirectly standardised rate) for gastrointestinal cancer survivors of 0.90 (95% C.I. 0.83 to 0.97), but did not quote age- specific birth rates for the study population. Indirectly standardised rates can give misleading results, however, in any situation where the age profiles of the study and reference populations are not similar[11]. In the present study, we found the age distributions of subjects with or without a CRC diagnosis were markedly different. For this reason we used the TFR (calculated by the process of direct standardisation), in which the age distribution of the sample populations do not affect the calculation, and so groups with disparate age distributions[11] can be compared. This is discussed in full in the appendix.
A surprising finding in this study was that TFR for women with Lynch syndrome from the ACCFR was higher than that of the Australian population generally (fertility rates for men in the general population are not reported). The probable reason for this is selection bias, as larger families are more likely to be recruited by family cancer clinics. This example highlights the kind of sampling bias that can cause misleading interpretation of fertility rates in an affected population when compared with the general population. It is unlikely to have affected the main result of this study, however, as the CRC and control groups were sampled from the same families.
The decision to have children is influenced by many psychological and social factors, which may bias studies of fertility between groups. We studied subjects from a large familial cancer database, so that unaffected family members could act as a control group (that would be as demographically and socially similar as possible to the affected individuals) and because of the high number of young CRC patients in this cohort. We only included proven germline MMR mutation carriers (as controls as well as patients post CRC) as individuals with Lynch syndrome may modify their reproductive behaviour to avoid passing on their mutation. They may decide not to have children, or even consider techniques such as pre- implantation genetic testing. For this study we did not attempt to collect information from subjects as to whether they had chosen to have children or not and what the reasons were for the decision. The data presented in this paper cannot determine whether the observed changes in fertility were related to CRC or to reproductive decisions made following the genetic diagnosis of Lynch syndrome (which often occurs after the CRC is diagnosed). Although MMR proteins are essential in DNA replication, there is no evidence in the current literature that Lynch syndrome directly affects fertility, and the high fertility rate in this cohort does not suggest that this occurs.
It is uncertain whether these findings are generalizable to patients with sporadic cancers. Colorectal cancers that display microsatellite instability (as in Lynch syndrome) are known to have a better prognosis than sporadic CRC and may be unresponsive to 5-fluorouracil based chemotherapy regimes [17]. In addition, CRC detected in subjects with Lynch syndrome, who are offered intensive surveillance, may be detected at an earlier stage than sporadic tumours.
It is expected that rectal cancer would have a greater impact on fertility than colon cancer (as a greater number receive radiotherapy, and pelvic surgery may be more likely to have a deleterious effect on fertility). In Lynch syndrome, rectal cancers are less common (as a percentage of CRC overall), and in this cohort there were only 82 subjects with rectal cancer (of whom 45 were diagnosed under the age of 50), so this study was not powered to detect differences in age- specific fertility rates in subjects with rectal cancer. These results of this should therefore be interpreted with caution in for patients with rectal cancer, and may only be applicable to those with tumours of the colon. Another limitation of this study is the small numbers of subjects with CRC in the age groups under 25, and our data are underpowered to detect differences in fertility (if they exist) in this age group. Such patients are rare, and so it would be difficult to recruit large numbers of these subjects in any setting.
In conclusion, we found that age- specific fertility was decreased in women with Lynch syndrome in the 20 to 24 year age group after CRC diagnosis. Subjects of both genders who survived into their late twenties and beyond had no detectable reduction in age- specific fertility, which may be reassuring to colon cancer survivors who hope to have children.
What does this paper add to the literature?
This is the first study to report the effect of colorectal cancer on age- specific fertility in women stratified by age groups, and the first to study the effect on fertility overall in men.
Contributor Information
Douglas Stupart, Department of Surgery, Deakin University, Geelong, Victoria, Australia.
Aung Ko Win, Centre for Molecular, Environmental, Genetic and Analytic Epidemiology, Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia.
Mark Jenkins, Centre for Molecular, Environmental, Genetic and Analytic Epidemiology, Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia.
Ingrid M. Winship, Genetic Medicine and Family Cancer Clinic, Royal Melbourne Hospital, Victoria, Australia
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CLY, Ruhl MJ, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2010, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. 2013 cited 2013 4 September 2013 ; Available from: http://seer.cancer.gov/csr/1975_2010/
- 2.Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer. A pilot survey of survivors’ attitudes and experiences. Cancer. 1999;86(4):697–709. doi: 10.1002/(sici)1097-0142(19990815)86:4<697::aid-cncr20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. Journal Of Clinical Oncology: Official Journal Of The American Society Of Clinical Oncology. 2006;24(18):2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 4.Hartman M, Liu J, Czene K, Miao H, Chia K, Salim A, Verkooijen HM. Birth rates among female cancer survivors: a population-based cohort study in Sweden. Cancer. 2013;119(10):1892–9. doi: 10.1002/cncr.27929. [DOI] [PubMed] [Google Scholar]
- 5.Watson P, Riley B. The tumor spectrum in the Lynch syndrome. Fam Cancer. 2005;4(3):245–8. doi: 10.1007/s10689-004-7994-z. [DOI] [PubMed] [Google Scholar]
- 6.Winship I, Win AK. The Australasian Colorectal Cancer Family Registry. Med J Aust. 2012;197(9):480–1. doi: 10.5694/mja12.11395. [DOI] [PubMed] [Google Scholar]
- 7.Win AK, Lindor NM, Winship I, Tucker KM, Buchanan DD, Young JP, Rosty C, Leggett B, Giles GG, Goldblatt J, Macrae FA, Parry S, Kalady MF, Baron JA, Ahnen DJ, Marchand LL, Gallinger S, Haile RW, Newcomb PA, Hopper JL, Jenkins MA. Risks of colorectal and other cancers after endometrial cancer for women with Lynch syndrome. doi: 10.1093/jnci/djs525. Electronic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Statistics 2013 - Indicator compendium. World Health Organization; 2013. [Google Scholar]
- 9.Age-specific fertility rates and total fertility rate, Single year of age of mother–Australia–1975 to 2010. Australian Bureau of Statistics; 2011. [Google Scholar]
- 10.Breslow N, Day N. IARC Scientific Publications. International Agency for Research on Cancer, World Health Organisation; Lyon: 1987. Statistical methods in cancer research, volume II: The design and analysis of cohort studies. [PubMed] [Google Scholar]
- 11.Eayres D. Technical Briefing 3: Commonly Used Public Health Statistics and their Confidence Intervals. Association of Public Health Observatories, Public Health England; 2008. [Google Scholar]
- 12.Dobson AJ, Kuulasmaa K, Eberle E, Scherer J. Confidence intervals for weighted sums of Poisson parameters. Stat Med. 1991;10(3):457–62. doi: 10.1002/sim.4780100317. [DOI] [PubMed] [Google Scholar]
- 13.Stupart D, Win A, Jenkins M, Winship I, GP, Ramesar R. Fertility and apparent genetic anticipation in Lynch syndrome. Fam Cancer. 2014 doi: 10.1007/s10689-014-9714-7. Electronic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spanos CP, Mamopoulos A, Tsapas A, Syrakos T, Kiskinis D. Female fertility and colorectal cancer. Int J Colorectal Dis. 2008;23(8):735–743. doi: 10.1007/s00384-008-0483-3. [DOI] [PubMed] [Google Scholar]
- 15.O’Neill MT, Dhonnchu TN, Brannigan AE. Topic update: effects of colorectal cancer treatments on female fertility and potential methods for fertility preservation. Dis Colon Rectum. 2011;54(3):363–9. doi: 10.1007/DCR.0b013e31820240b3. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Merali A, Pond GR, Zbuk K. Fertility risk discussions in young patients diagnosed with colorectal cancer. Current oncology. 2012;19(3):155–9. doi: 10.3747/co.19.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46(15):2788–98. doi: 10.1016/j.ejca.2010.05.009. [DOI] [PubMed] [Google Scholar]