Abstract
Many researchers are using viruses to deliver genes of interest into the brains of laboratory animals. However, certain target brain cells are not easily infected by viruses. Moreover, the differential tropism of different viruses in monkey brain is not well established. We investigated the cellular tropism of lentivirus and adeno-associated virus (AAV) toward neuron and glia in the brain of cynomolgus monkeys (Macaca fascularis). Lentivirus and AAV were injected into putamen of the monkey brain. One month after injection, monkeys were sacrificed, and then the presence of viral infection by expression of reporter fluorescence proteins was examined. Tissues were sectioned and stained with NeuN and GFAP antibodies for identifying neuronal cells or astrocytes, respectively, and viral reporter GFP-expressing cells were counted. We found that while lentivirus infected mostly astrocytes, AAV infected neurons at a higher rate than astrocytes. Moreover, astrocytes showed reactiveness when cells were infected by virus, likely due to virus-mediated neuroinflammation. The Sholl analysis was done to compare the hypertrophy of infected and uninfected astrocytes by virus. The lentivirus infected astrocytes showed negligible hypertrophy whereas AAV infected astrocytes showed significant changes in morphology, compared to uninfected astrocytes. In the brain of cynomolgus monkey, lentivirus shows tropism for astrocytes over neurons without much reactivity in astrocytes, whereas AAV shows tropism for neurons over glial cells with a significant reactivity in astrocytes. We conclude that AAV is best-suited for gene delivery to neurons, whereas lentivirus is the best choice for gene delivery to astrocytes in the brain of cynomolgus monkeys.
Keywords: virus tropism, lentivirus, AAV, monkey, neuron, astrocyte
INTRODUCTION
Gene therapy has been around for nearly a decade as a modern therapeutics [1]. However, gene therapy for disorders of the nervous system still remains a major medical challenge. Gene therapy for the brain diseases is particularly challenging due to the post-mitotic nature of neuronal cells and the restricted accessibility of the brain itself. Lentiviral vectors and adeno-associated viral vectors (AAV) are increasingly regarded as the two most useful gene therapy vectors for the CNS. Viral vectors based on lentiviruses are particularly attractive vehicles, routinely used in developing gene-based therapies to treat neurological diseases [2]. Both vectors have been successfully used to express various foreign genes in a variety of brain regions and neuronal cell types [3,4,5,6].
There are several factors that should be concerned in using viral vectors, such as efficiency of gene delivery, targeting of expression, duration of expression, and safety [1]. By evaluating a variety of cell lines, it has been revealed that virus efficiency is cell-type dependent, a fact that has significant implications for the preparation of standard virus stocks [7]. The efficiency of virus is different by cell type or region [7]. In order to identify the property of each cell types, many researchers have investigated the tropism of viruses. Moreover, improving the efficiency of viral gene delivery has become increasingly important to increase the success of clinical trials [8].
Many researchers have further advanced the tropism of AAV through pseudotyping, variant serotypes and hybrid capsid from variant serotypes, which also alter viral tropism [9]. AAV serotype 1 through 6 were tested in the substantia nigra of the African green monkey brain [9]. In that study, the authors found that AAV serotype 5 displayed the most efficient transduction of neurons in this brain region. Recently, AAV-DJ vector was generated as one of the recombinogenic hybrid vector from gene shuffling of eight serotypes and has been widely used across a broad range of cell types because it has a higher transduction efficiency both in vitro and in vivo [10,11]. Even though AAV serotype 5 displayed the most efficient transduction of neurons in the African green monkey brain region [9], AAV-DJ has not been tested in the primate. Therefore, in the present study we tested the AAV-DJ in putamen of cynomolgus monkey for the first time. Furthermore, comparison of AAV and lentivirus or analysis of tropism of lentivirus especially in the monkey brain has not been conducted yet. Monkeys are becoming extremely valuable in the field of neuroscientific research. Therefore, in the current study, we set out to investigate the cellular tropism of lentivirus and AAV in cynomolgus monkey.
Another important issue in using virus for brain research is to establish physiological levels of virally delivered gene expression and therefore ways of regulating the expression [1]. Cell body and processes of astrocytes are usually hypertrophied at the sites of injury or surround the lesions [12,13,14]. The hypertrophied morphological change is generally called reactiveness of astrocytes. For example, a previous study examined the harmful effect of astrocyte-specific overexpression of GFP by viral infection of AAV-GFAP (Glial fibril acidic protein, marker for astrocyte)-GFP in mouse brain [15]. The authors found a profound neuroinflammation with infected astrocytes turned from normal astrocytes into reactive astrocytes as evidenced by presence of morphological changes and increased GFAP and vimentin level. The consequence of the appearance of reactive astrocytes by AAV was that in nearby neurons the amplitude of inhibitory synaptic activities was decreased. Therefore, it is important to find a virus type and expression level that do not cause reactivity in astrocytes. The degree of reactivity in astrocytes by viral infection in nonhuman primate is unknown. In this study, we examined which viral vector causes more reactivity in astrocytes in the brain of cynomolgus monkeys.
MATERIALS AND METHODS
Animals
Total of 2 male cynomolgus monkeys from Korea Institute of Toxicology (KIT) were used in these experiments. They were 6~7 years old and bred for 4 years after being imported from China. Mean body weight of the monkeys was 4.6 kg. The procedure of breeding and maintenance of monkeys are followed by the Institutional Animal Care and Use Committee (IACUC) of the KIT (Certification acquisition of Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International in 1998).
Recombinant virus
For the lentiviral vector, Lenti-GFP, we used a previously reported lentivirus-based shRNA expression vector system, pSicoR that contains scrambled shRNA under the U6 promoter and CMV-GFP cassette (Addgene Co.) [16]. To design the equivalent adeno-associated virus (AAV) vector, AAV-GFP (AAVU6-scrambled shRNA-CMV-EGFP vector), we digested out the U6 promoter, scrambled shRNA and EGFP fragment from the pSicoR lentiviral vector and cloned it into the transfer plasmid AAV containing multiple cloning site (Agilent Technologies cat. no. 240071). Using this AAV-GFP viral vector, AAV virus was packaged with serotype DJ at KIST Virus Facility (http://virus.kist.re.kr). The titer of AAV-GFP was 1×1012 genome copy/ml. The Lenti-GFP was also packaged at KIST Virus Facility at the titer of 3.6×109 genome copy/ml.
Stereotaxic surgery
Animals were anesthetized with approximately 8~10 mg/kg of zoletil 50 (Virbac Korea) 30 min before clamped to the stereotaxic instrument (Kopf instrument, model 1404). Total 25 µl virus (1/3 diluted by saline) was injected using the Hamilton syringe and infusion pump at a speed of 2.5 µl per minutes. After surgery, monkeys were prescribed Cephalosporin-C twice per day for 5 days, Ketopropen once a day for 3 days and Prednisolone acetate once a day for 5 days. AAV-GFP and Lenti-GFP virus were injected in putamen (AP +0.9, ML +0.7~1.3, DV -2.5 from bregma). Viruses were diluted with 0.9% saline in the proportion of 1:3.
Immunohistochemistry
The monkeys were terminated by exsanguination while under deep anesthesia (Thiopental sodium, 250 mg/head). After being dissected, brains are post-fixed in 4% PFA for 24 hours and then incubated in 30% sucrose for 2~3 days. They are embedded in frozen section compound (Leica Biosystems) and frozen tissues are sliced in a thickness of 50 µ m. Sliced sections were blocked with blocking solution (2% donkey serum, 2% goat serum) for one and half hour and incubated with mouse anti-NeuN (MAB377; 1:1000, Millipore), chicken anti-GFAP (AB5541; 1:500, Millipore) antibodies at 4℃ overnight. Tissues are rinsed 3 times and incubated with donkey anti chicken Alexa Fluor 594 conjugated IgG (1:200, Jackson Immuno Research Laboratories, INC), donkey anti mouse Alexa Fluor 647 conjugated IgG (1:200, Jackson Immuno Research Laboratories, INC). Tissues are counterstained with DAPI (1:3000, 46190, Pierce) before they are mounted on the slide glass. Images are taken with Nikon A1 confocal microscopy.
Statistical analysis
Student t-test [17] was used to analyze the difference of the branches. All values indicated are means±SEM and the statistical significance is presented with n.s. (non-significant) or asterisks, p values of <0.05(*), <0.01(**), and <0.001(***).
RESULTS
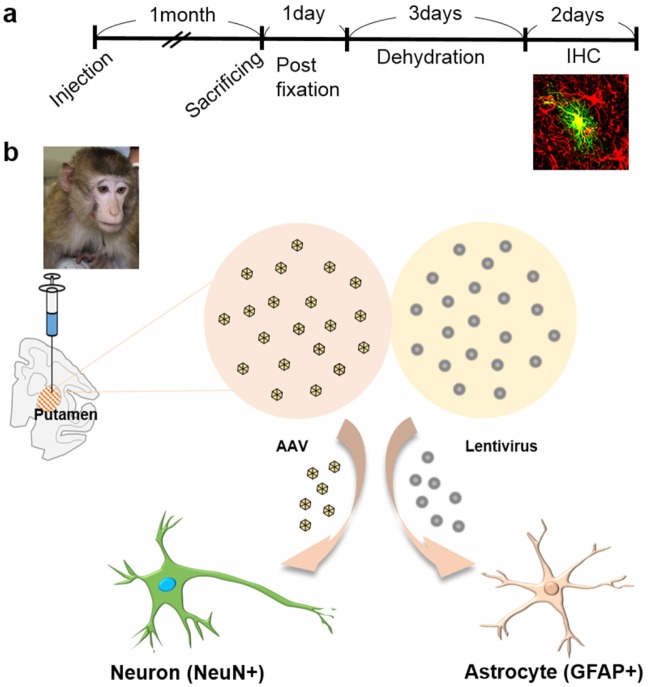
To investigate the cellular tropism, lentivirus and AAV were injected into putamen of 6~7 year-old cynomolgus monkeys with Hamilton syringe with 22 gauge needle (Fig. 1). The animals survived for 30 days in good health with postoperative clinical evaluation showing no evidence of behavioral abnormalities, loss of appetite, weight loss, or other abnormalities. 1 month after injection, monkeys were sacrificed and fixed in 4% paraformaldehyde solution. For sectioning frozen tissues, brains were incubated for 3 days in sucrose solution for dehydration (Fig.1a). Tissues were sliced with thickness of 50 µm, and stained with antibodies against GFAP, or NeuN (neuronal nuclei, marker for neuron) and DAPI. Colocalization with GFAP or NeuN positive cells and GFP expressing cells were counted (Fig. 1b).
Fig. 1. Timeline and schematic diagram of virus injection in cynomolgus monkey. (a) Protocol for stereotaxic surgery and post-mortem experiments. After post-fixation and incubation in sucrose, immunohistochemistry (IHC) was performed. (b) Cynomolgus monkey. Adeno-associated virus (AAV) and lentivirus were infected in putamen of cynomolgus monkeys. AAV preferred to infect neuron (NeuN+), lentivirus preferred to infect astrocytes (GFAP+).
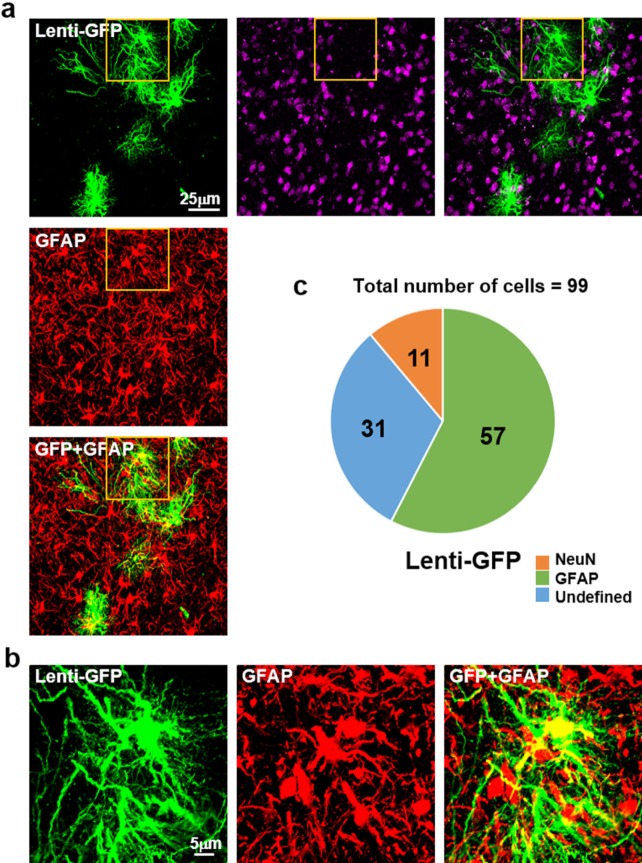
To identify which cells are infected with lentivirus, 25 µl Lenti-GFP diluted with saline was injected into putamen of cynomolgus monkey unilaterally. Brain sections were stained with GFAP and NeuN antibodies and cells with positive staining were counted (Fig. 2a and 2b). Transduction efficiency was measured by counting GFP expressing cells with DAPI. Then we counted how many GFP positive cells were colocalized with GFAP or NeuN. We found that 60% (57/99) of GFP expressing cells were co-localized with GFAP, whereas only 10% (11/99) of GFP expressing cells were NeuN positive (n=99: total number of cells) (Fig. 2c). These results indicated that lentivirus had tropism for GFAP positive astrocytes in putamen of cynomolgus monkey
Fig. 2. Infection by lentivirus. (a) Immunostaining of NeuN (red in horizontal line), GFAP (red in vertical line). Lenti-GFP was injected into the putamen region. The fluorescence is detected by confocal microscope imaging. Scale bar, 25 µm. (b) High magnification images of GFP expressing cell. GFP and GFAP are well colocalized. Scale bar, 5 µm. (c) Cell counting analysis for GFP expressing cells of colocalization with NeuN or GFAP. Approximately 60% of GFP expressing cells are colocalized with GFAP. Analysis is performed with ImageJ (n=99: total number of cells).
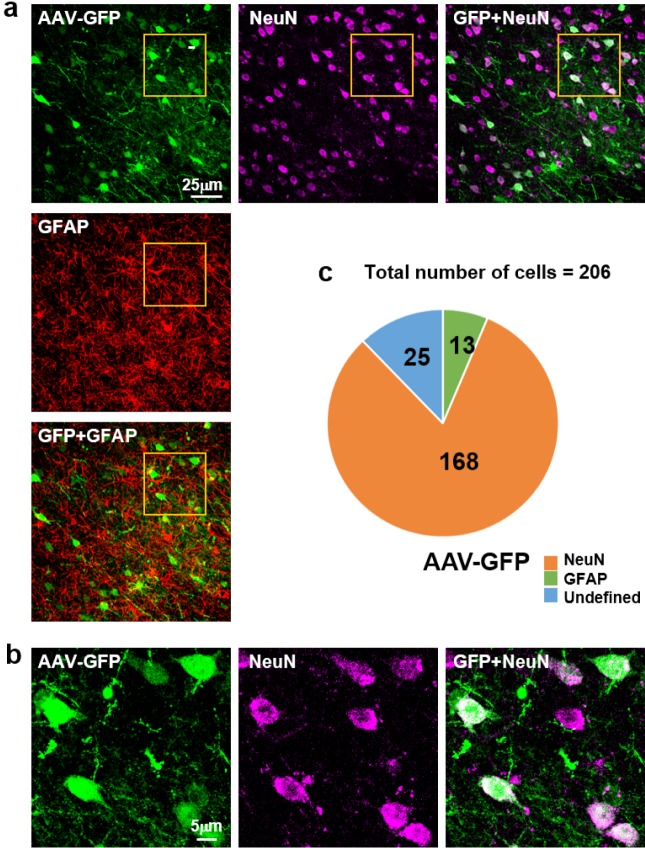
To identify which cells were infected with AAV, 25 µl AAV-GFP diluted with saline was injected into putamen of cynomolgus monkey unilaterally. As it is described already, AAV-DJ has been widely used for the vehicle of the gene delivery because it has a higher transduction efficiency [11]. Therefore, we packaged with AAV-DJ. After being sacrificed, tissues were stained with GFAP and NeuN antibodies and counted (Fig. 3a and 3b). As shown in Fig. 3a, we found that AAV-DJ infected numerous cells. We counted GFP positive cells with DAPI and assessed how many cells are co-localized with GFAP and NeuN. We found that 80% (168/206) of GFP expressing cells were co-localized with GFAP, whereas only 7% (13/206) of GFP positive cells were NeuN positive (n=206: total number of cells) (Fig. 3c). These results indicated that AAV has tropism toward neurons in putamen of cynomolgus monkey brain.
Fig. 3. Infection by AAV. (a) Immunostaining of NeuN (red in horizontal line), GFAP (red in vertical line). AAV-GFP was injected into the putamen region. The fluorescence is detected by confocal microscope imaging. Scale bar, 25 µm. (b) High magnification images of GFP expressing cell. GFP and NeuN are well colocalized in neuronal cell body. Scale bar, 5 µm. (c) Cell counting analysis for GFP expressing cells of colocalization with NeuN or GFAP. Approximately 80% of GFP expressing cells are colocalized with NeuN. Analysis is performed with ImageJ (n=206: total number of cells).
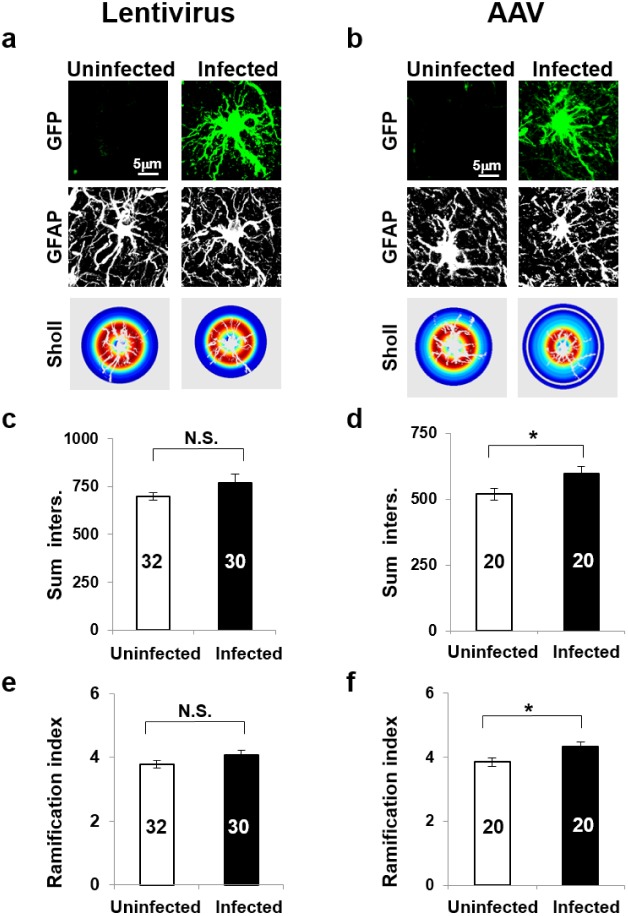
Astrocytes nearby injury site or lesion are morphologically transformed and hypertrophied. It is possible that these hypertrophied reactive astrocytes may not function normally. To assess the degree of reactiveness of infected astrocytes, we performed the Sholl analysis [18] from the infected and uninfected GFAP positive cells (Fig. 4a and 4b). The results of the analysis showed that both the sum of intersections and ramification index, which are indicators of branching of processes, slightly but not significantly increased in lentivirus-infected group (Fig. 4c and 4d), whereas both sum of intersections and ramification index increased significantly in AAV-infected group (Fig. 4e and 4f), compared to corresponding uninfected group. These results demonstrate that the reactiveness induced by lentivirus is less severe than that by AAV. In other words, the astrocytes infected with lentivirus are closer to normal condition than those with AAV.
Fig. 4. Reactiveness of astrocytes induced by virus infection. (a) Immunohistochemistry and sholl analysis images of cell infected by lenti-GFP. (b) Immunohistochemistry and sholl analysis images of cell infected by AAV-GFP. (c) Sum of intersections of uninfected cells and infected cells within region injected lenti-GFP. Analysis was performed by ImageJ. The numbers in each bar are the total number of analyzed cells in each group. Student's t-test (*p>o.o5). (d) Sum of intersections of uninfected cells and infected cells within region injected AAV-GFP. By ImageJ. Student's t-test (*p<o.o5, n=total number of cells in each group). (e) Ramification index of uninfected cells and infected cells within region injected lenti-GFP. By ImageJ. Student's t-test (*p>o.o5, n=total number of cells). (f) Ramification index of uninfected cells and infected cells within region injected AAV-GFP. By ImageJ. Student's t-test (*p<o.o5, n=total number of cells).
DISCUSSION
From the results of current study, we conclude that lentivirus is well-suited for infecting astrocytes whereas AAV can be more appropriate for infecting neurons in cynomolgus monkey brain. In addition, we have demonstrated that AAV-DJ is an effective tool for gene delivery to the monkey brain. This conclusion is based on three observations: 1) AAV-DJ vector showed the high transduction effectivity even in the cynomolgus monkey brain. 2) We found a preferred cellular tropism of lentivirus for astrocytes and AAV for neurons, 3) infected astrocytes showed less hypertrophy by lentivirus than AAV. The reactivity of astrocytes by virus infection is not an uncommon phenomenon. However, in this study we found that infection of lentivirus causes minimal reactiveness in astrocytes. This is an important factor to consider as neuroinflammation is directly linked to reactivity of glial cells including astrocytes. Therefore, to minimize neuroinflammation during virus-mediated gene-delivery, lentivirus should be considered in monkey brain. The origin of the cellular tropism of certain viruses is not clear. Future studies are needed to determine the molecular mechanism of cellular tropism of various viruses and the cause of reactivity by viral infection in astrocytes.
Ac knowledgement
This study was supported by Creative Research Initiative Program, Korean National Research Foundation (2015R1A3A2066619), KIST Institutional Grant (2E26662), and KU-KIST Graduate School of Science and Technology program (R1435281).
References
- 1.Lundstrom K. Latest development in viral vectors for gene therapy. Trends Biotechnol. 2003;21:117–122. doi: 10.1016/S0167-7799(02)00042-2. [DOI] [PubMed] [Google Scholar]
- 2.Eleftheriadou I, Mazarakis ND. Lentiviral vectors for gene delivery to the nervous system. Gene delivery and therapy for neurological disorders. Neuromethods. 2015;98:23–66. [Google Scholar]
- 3.Lim ST, Airavaara M, Harvey BK. Viral vectors for neurotrophic factor delivery: a gene therapy approach for neurodegenerative diseases of the CNS. Pharmacol Res. 2010;61:14–26. doi: 10.1016/j.phrs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manfredsson FP, Mandel RJ. Development of gene therapy for neurological disorders. Discov Med. 2010;9:204–211. [PubMed] [Google Scholar]
- 5.Papale A, Cerovic M, Brambilla R. Viral vector approaches to modify gene expression in the brain. J Neurosci Methods. 2009;185:1–14. doi: 10.1016/j.jneumeth.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Schneider B, Zufferey R, Aebischer P. Viral vectors, animal models and new therapies for Parkinson's disease. Parkinsonism Relat Disord. 2008;14(Suppl 2):S169–S171. doi: 10.1016/j.parkreldis.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Tsuda Y, Parkins CJ, Caposio P, Feldmann F, Botto S, Ball S, Messaoudi I, Cicin-Sain L, Feldmann H, Jarvis MA. A cytomegalovirus-based vaccine provides long-lasting protection against lethal Ebola virus challenge after a single dose. Vaccine. 2015;33:2261–2266. doi: 10.1016/j.vaccine.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charbel Issa P, De Silva SR, Lipinski DM, Singh MS, Mouravlev A, You Q, Barnard AR, Hankins MW, During MJ, Maclaren RE. Assessment of tropism and effectiveness of new primate-derived hybrid recombinant AAV serotypes in the mouse and primate retina. PLoS One. 2013;8:e60361. doi: 10.1371/journal.pone.0060361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markakis EA, Vives KP, Bober J, Leichtle S, Leranth C, Beecham J, Elsworth JD, Roth RH, Samulski RJ, Redmond DE., Jr Comparative transduction efficiency of AAV vector serotypes 1-6 in the substantia nigra and striatum of the primate brain. Mol Ther. 2010;18:588–593. doi: 10.1038/mt.2009.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, Kay MA. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adenoassociated viruses. J Virol. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakhan R, Baylink DJ, Lau KH, Tang X, Sheng MH, Rundle CH, Qin X. Local administration of AAV-DJ pseudoserotype expressing COX2 provided early onset of transgene expression and promoted bone fracture healing in mice. Gene Ther. 2015;22:721–728. doi: 10.1038/gt.2015.40. [DOI] [PubMed] [Google Scholar]
- 12.Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 13.Norenberg MD. Astrocyte responses to CNS injury. J Neuropathol Exp Neurol. 1994;53:213–220. doi: 10.1097/00005072-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 15.Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, Jaenisch R, Jacks T. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci U S A. 2004;101:10380–10385. doi: 10.1073/pnas.0403954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokal RR, Rohlf FJ. Taxonomic congruence in the Leptopodomorpha re-examined. Syst Zool. 1981;30:309–325. [Google Scholar]
- 18.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]