Abstract
The purpose of this study is to characterize the properties of Eudragit® FS-based granules prepared using melt extrusion process for colonic drug delivery. 5-Aminosalicylic acid (5-ASA), theophylline, and diclofenac sodium were used as the model compounds. Drug and polymer blends were melt-extruded into thin rods using a single screw extruder. Drugs were found to be dispersed as crystalline particles in the granules. A hammer mill was used to reduce the extrudate into 16–40 mesh granules, which were mixed with lactose and filled into hard gelatin capsules. Three-stage dissolution testing performed using USP paddle method was used to simulate drug release in gastrointestinal tract. In this study, melt extrusion has been demonstrated to be a suitable process to prepare granules for colonic delivery of 5-amino salicylic acid. At 30% drug loading, less than 25% 5-ASA was released from melt-extruded granules of 20–30 mesh in the first two stages (0.1 N hydrochloric acid solution and phosphate buffer pH 6.8) of the dissolution testing. All 5-ASA was released within 4 h when dissolution medium was switched to phosphate buffer pH 7.4. Drug loading, granule size, and microenvironment pH induced by the solubilized drug were identified as the key factors controlling drug release. Granules prepared with melt extrusion demonstrated lower porosity, smaller pore size, and higher physical strength than those prepared with conventional compression process. Eudragit® FS was found to be stable even when processed at 200°C.
KEY WORDS: 5-aminosalicylic acid, colonic drug delivery, Eudragit® FS, granulation, melt extrusion
INTRODUCTION
Oral dosage forms delivering drug to colon are highly desirable to treat a variety of bowel diseases such as colon cancer, ulcerative colitis, and local inflammation (1). The target delivery offers the benefit of the direct treatment at the disease sites and fewer systematic side effects. Colonic delivery was also applied to improve the absorption of drugs that are susceptible to either chemical or enzymatic degradation in upper gastrointestinal tract (2). Colonic delivery has also been used to bypass the first-pass metabolism in order to increase bioavailability (3). There are three primary approaches that have been investigated for the preparation of colon-specific oral dosage forms: activation of the dosage forms by the microflora in the colon (1), time-dependent drug release (4), and pH-dependent drug release (5).
The most common oral colon-targeting dosage forms are based on pH-dependent drug release mechanism. Higher than the pH of any other sections of the gastrointestinal tract, the pH of the terminal ileum and colon is in the range of 7 to 8. Dosage forms that are able to release the drug in high neutral or slightly alkaline environments would be able to deliver the active ingredients to the colon. Asacol®, commercial delayed-release mesalamine tablets targeting colon, was demonstrated by an abdominal X-ray study that the tablets remained intact after oral administration until they reached terminal ileum where the Eudragit® S film coating started to dissolve and the content of the tablets was released (6). Polymeric coating of dosage forms is the most common manufacturing process to prepare pH-triggered colonic drug delivery systems. However, film-coated dosage forms have several disadvantages. Both solvent-based and aqueous coating processes are time and energy consuming. The use of organic solvents in the coating process invokes environmental and health concerns. Dose dumping due to defects in the film coating is another disadvantage associated with film-coated dosage forms.
Matrix granules in which drug are either embedded as particles or dissolved at molecular level in polymer matrix have several advantages over the film-coated dosage forms. Matrix granules could be blended with extragranular excipients and compressed into tablets. Matrix granules are also less susceptible to dose dumping.
The first aim of the current study was to evaluate melt extrusion as a novel process to prepare enteric polymer based and pH-triggered matrix granules for colonic drug delivery. Eudragit® FS was selected as an enteric polymer in this study. The chemical structure of the material is illustrated in Fig. 1. Eudragit® FS has been used for colonic-targeting drug delivery using a film coating process (7). Because of the methacrylic acid group, solubility of Eudragit® FS in aqueous medium is pH-dependent. It is only soluble in an aqueous medium with a pH greater than 7.0. Eudragit® FS is available either as solid granules or as a 30%, w/w, aqueous latex dispersion. A fluidized bed or a pan coating process is commonly used to apply the aqueous dispersion of Eudragit® FS onto beads or tablets for colonic delivery (8). When the dosage forms reached the colon region, the high pH of the intestinal environment triggered the solubilization of film coating and subsequently the release of drug. The granular form of Eudragit® FS was used for extrusion in this study.
Fig. 1.
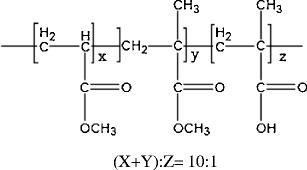
Chemical structure of Eudragit® FS
Melt extrusion was originally developed to process thermoplastic materials in plastics industry. During the extrusion process, thermoplastic materials are converted to a soft mass due to the mechanical stress from rotating screw and the heat from the barrel. The soft mass is then shaped through a die into various extrudates. In pharmaceutical industry, melt extrusion has been used to prepare a variety of dosage forms including molecular dispersions for bioavailability enhancement (9), drug-impregnated devices (10), sustained release dosage forms (11–13), and delayed release dosage forms (14).
Extrusion of enteric polymers to prepare dosage forms for colonic delivery has been reported. Melt extrusion of Eudragit® S100 was used to prepare tablets for colonic delivery (15). Eudragit® FS and S100 has similar pH solubility profiles. However, with a glass transition temperature significantly lower than that of Eudragit® S100, Eudragit® FS is a more suitable carrier for the melt extrusion process. Eudragit® FS as a thermoplastic carrier for colonic dosage forms was only reported by Young and et al. (16). In their study, Eudragit® FS in combination with PEG 8000 at 7:1 ratio were melt-extruded and spheronized to prepare theophylline pellets, which were further compressed into tablets. Our study is the first to investigate the melt-extruded colon-targeting granules in which Eudragit® FS is the sole thermoplastic polymeric carrier. 5-Amino salicylic acid (5-ASA) was used as the model compound in this study. 5-ASA is commonly prescribed to treat ulcerative colitis. To achieve desired therapeutical effect, 5-ASA must be in direct contact with the mucosal membranes of the colon.
The second aim of this study was to characterize the physical properties of melt-extruded Eudragit® FS matrix granules and investigate how these properties impacted drug release. For a given system and drug loading, pore structure such as porosity and tortuosity affects drug release rate (17). Previous studies have demonstrated that the pore structure was dictated by the processing methods (18,19). Melt extrusion process has been shown to result in less porous matrix than granules prepared using conventional compression process (20,21).
The third aim of this study is to investigate the effect of granule size, drug loading levels, and the acidity of drug on the release characteristics of melt-extruded Eudragit® FS granules. To evaluate the effect of drug acidity, drug release of granules containing theophylline, an essentially neutral drug or diclofenac sodium, an alkaline drug was compared against that of granules containing 5-ASA.
The fourth aim of this study is to determine the stability of Eudragit® FS during melt extrusion.
MATERIALS AND METHODS
Materials
5-Amino salicylic acid, theophylline, and diclofenac sodium were purchased from Spectrum Quality Products, Inc. (Gardena, CA). 2-(2-ethoxy ethoxy) ethanol and N-methyl-N-nitroso-p-toluenesulfonamide used for methylation of Eudragit® FS for GPC analysis were purchased from Sigma-Aldrich. All other reagents were of analytical grades and used as such without further modifications. All excipients were gifts from various manufacturers. Eudragit® FS was received as a gift from Evonik (Darmstdat, Germany). Spray-dried lactose was supplied by FMC Corporation (Newark, Delaware).
Melt Extrusion
A 1-1/4 in single screw Brabender® extruder (C. W. Brabender Instrument Inc., S. Hackensack, NJ) was used for hot-melt extrusion in this study. The extruder barrel, with a L/D ratio of 15 to 1, has two heating zones. A single flight screw with a uniform pitch was used in the present study. A 6-mm rod die was used during the melt extrusion process. Eudragit® FS was received as granules and was pulverized using a cryogenic grinder (Mikro Bantam™ grinder, Model CF, Micro Powder Systems, Summit, NJ) into powder finer than 60 mesh. All materials were dried in an oven overnight at 40°C prior to the extrusion to reduce their moisture content. Due to the low glass transition temperature of Eudragit® FS, the hot-melt extrusion process was conducted at a relatively low temperature. The feeding section, melting section, and the die of the extruder were maintained at 65, 75, and 75°C, respectively. The rotation speed of the extruder screw was controlled at 40 rpm. The residence time was approximately 2 min.
Milling and Encapsulation of Melt Extrudate
A Bronco II Grinder (Killion Extruders Inc, Verona, NJ) was used to reduce the particle size of extrudates into coarse granules. The granule size can be controlled by adjusting the rotation speed of the cutting wheel. Melt-extruded granules were blended with spray-dried lactose at 2:1 ratio and filled into size 0 hard gelatin capsules. Lactose was used to prevent agglomeration of melt-extruded granules during the storage. Each capsule contained 300 mg melt-extruded granules and 150 mg lactose.
Methylation of Eudragit® FS
Methylation of Eudragit® FS was needed in order to remove the absorption of Eudragit® FS onto the GPC column. The chemical reactions are illustrated in Figs. 2 and 3. A portion of the Eudragit® FS (approximately 100 mg) was added to 5 mL benzene. The polymer was not soluble in benzene, and a heterogeneous mixture was obtained. The ethyl ether solution of diazomethane prepared above was added to the polymer/benzene mixture. Additional diazomethane solution was added, if necessary, until a yellow solution persisted and a homogeneous solution was obtained. The excessive diazomethane and ether, and residual benzene were removed using a rotary evaporation process. The molecular weight of the methylated polymer was then measured using gel permeation chromatography.
Fig. 2.
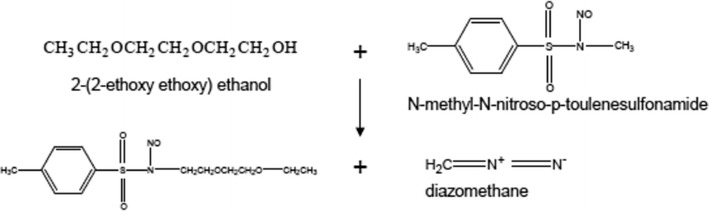
Chemical reaction to synthesize diazomethane
Fig. 3.
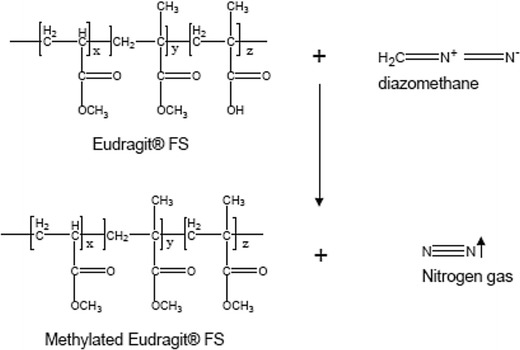
Chemical reaction to synthesize methylated Eudragit® FS
Dissolution Testing
Three-stage dissolution testing was performed according to the USP XXIII paddle method in a Vankel® 6010 dissolution tester connected to a VK 6010 autosampler (Vankel Industries, Inc., Edison, NJ). Nine hundred milliliters of dissolution medium at 37°C was used and the paddle rotation speed was 100 rpm. In the first stage, 0.1 N hydrochloric acid solution was used for 2 h. In the second stage, 50 mM phosphate buffer pH 6.8 was used for 4 h. In the third stage, 50 mM phosphate buffer solution pH 7.4 was used for 6 h. One capsule was placed in each vessel for the dissolution testing. All samples were tested under sink condition except for sodium diclofenac sodium capsules in 0.1 N hydrochloric acid solution.
Dissolution Samples Analysis
The concentration of drug in the dissolution samples was measured using a Beckman DU™-65 UV spectrophotometer. 5-Amino salicylic acid in 0.1 N hydrochloric acid solution was measured at 264 nm, and in phosphate buffer solutions at 262 nm. The analysis wavelength for theophylline is 270 nm. Diclofenac sodium in 0.1 N hydrochloric acid was analyzed at 276 nm and in phosphate buffers at 273 nm.
Thermal Properties Analysis
Differential scanning calorimetry testing was performed using a TA Thermal Analyzer (Model DSC 2920, TA Instruments, New Castle, DE). The temperature was calibrated with an indium standard, melting at 430°K. Sample size was about 5 mg and a temperature ramp of 10°C per minute was used.
Thermogravimetric analysis was applied to determine the thermal degradation temperature of Eudragit® FS. A PerkinElmer 7-series Thermogravimetric Analyzer was used in this study. Nitrogen with ultrahigh purity was used as the purging gas for the furnace chamber. The temperature ramp speed was set at 40°C per minute.
Preparation of Granules Using Conventional Compression Process
A manual hydraulic Carver® press was used to prepare compacts of powder blend. Flat-faced, 6 mm, round tablets tooling were used. The weight of the compacts was 250 mg. Compacts of 16 kP hardness were ground into coarse granules using a motor and pestle.
Pore Structure and Porosity of Granule
A mercury intrusion porosimeter (Porsizer® 9320, Micromeritics Instrument Co., GA) was used to characterize the pore structure of the granules. Prior to the test in a powder penetrometer, all samples were dried in a desiccant chamber for 24 h. The sample was first evacuated below 50 ppa. Mercury was then filled into the penetrometer. Hydraulic pressure was applied on the granules to force mercury into the pores of the granules, and the amount of mercury filled was recorded at each pressure. Since the size of the pores penetrated was a function of the pressure, the pore size and pore size distribution could be readily derived from the amount of mercury filled. Three tests were performed for each sample. The porosimeter was programmed such that the largest pore included in the particle density was 180 pm. The upper limit of the applied pressure was 30,000 psi. The surface tension of the mercury was set at 485.0 dyn/cm and 140° was used as the contact angle between mercury and sample.
The porosity (ϕ) of the granules was calculated from the bulk and true density using the following equation:
The bulk density of the granules was defined as the mass of granules divided by the volume determined by the displacement of mercury at low pressure. The true density is measured using a helium pycnometer.
True Density of Granule
Granules were milled to particles less than 200 mesh. Prior to the test, all samples were dried in a desiccant chamber for 24 h. A helium AccuPyc 1330 pycnometer (Micromeritics Instrument Corporation, Norcross, GA) was used to determine the true density. The sample size was approximately 3–5 g.
Scanning Electron Microscopy
Scanning electron microscopy was used to examine the surface morphology of hot-melt extrudates. All the samples were stored in a dessicator for at least 24 h prior to the test. Samples were attached to brass SEM stages using double-sided carbon tape, and were coated with gold palladium for 60 s under an argon atmosphere using a Pelco® Model 3 Cold Sputter Module (TED Pella, Inc., Tustin, CA) in a high vacuum evaporator equipped with an omni-rotary stage. Samples were examined with a JEOL scanning electron microscope (Model JSM-35C, JEOL USA, Inc., Peabody, MA) at 25 kv.
Molecular Weight and Molecular Weight Distribution of Eudragit® FS
Gel permeation chromatography was used to determine molecular weight of Eudragit® FS prior to and following melt extrusion. The molecular weights of the polymers were determined using a Waters® GPC system (Waters Associates, Inc., Milford, MA), which included a WISP® Model 710B Auto-injector, a Model 510 synchronized HPLC pump, a Temperature Control Module with column heater, and a Model 410 Differential Refractometer.
Ultrastyragel® 100, 1000, and 10,000 columns connected in series were used for the analysis. The temperature of GPC columns and RI detector was set at 32 and 35°C, respectively. Calibration of the unit was achieved using retention time analysis of monodispersed polystyrene molecular weight standards from 600 to 400,000 in molecular weight, and all molecular weights were determined to be relative to these standards. Tetrahydrofuran stabilized with BHT at 0.025% was utilized as the solvent and mobile phase. Eudragit® FS was dissolved in THF at a concentration of 10 mg/mL. Polymer sample solutions were filtered into HPLC vials through a 2 μm nylon filter before analysis. The flow rate of the mobile phase was 0.8 mL/min.
RESULTS AND DISCUSSION
Dissolution Properties of 5-ASA Capsules Containing Melt-Extruded Matrix Granules
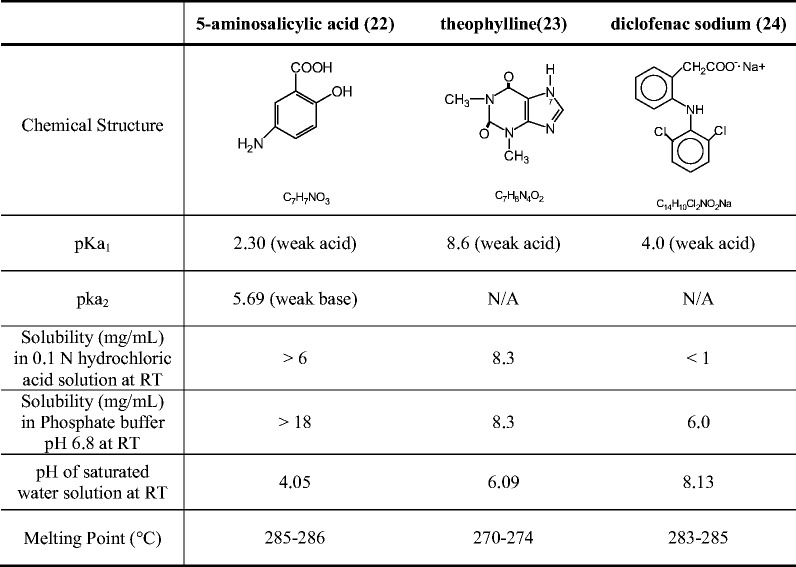
As shown in Table I, 5-ASA is an amphoteric molecule with an acidic carboxylic group and basic amino group. The pKa of the carboxylic acid and amine group is reported to be 2.30 and 5.69, respectively (22). The solubility of 5-ASA increases in aqueous medium is lowest between pH 2 and 5, at which both acidic and basic groups are ionized. The solubility increases at low pH values (pH < 2.0) and high pH values (pH > 5.5) (22).
Table I.
Comparison of the Properties of Model Compounds
As shown in Fig. 4a, 5-ASA was crystalline material with a melting point of 286°C. Eudragit® FS was an amorphous material with a glass transition temperature of 53°C. Since the extrusion was conducted at 75°C and the extruder screw only contained conveying elements, 5-ASA was found to be dispersed as crystalline particles in the extruded granules. Both the glass transition of Eudragit® FS and melting of 5-ASA were observed in the DSC profile (Fig. 4b) of extrudate containing 10% 5-ASA. Dissolution profiles of 5-ASA capsules containing granules of different sizes at 10% drug loading level are presented in Fig. 5. Dissolution characteristics suitable for colonic drug delivery were demonstrated by granules of all three sizes. Less than 35% drug was released during the first 6 h even for the small granules in the size range of 30 to 40 mesh (420 to 595 μm). When dissolution medium was switched to phosphate buffer pH 7.4 to simulate the pH environment in colon, the remaining 5-ASA was completely released within 4 h.
Fig. 4.
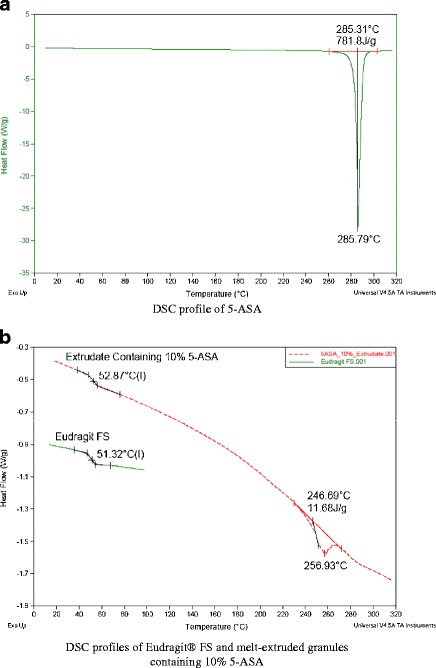
DSC profiles of 5-ASA (a), Eudragit® FS, and melt-extruded granules (b)
Fig. 5.
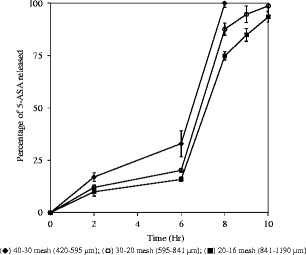
Release profiles of 5-amino salicylic acid capsules containing 300 mg melt-extruded granules of different size (10% drug loading in melt-extruded granules); USP paddle method at 100 rpm; 900 mL medium at 37°C; 0–2 h in 0.1 N HCl; 2–6 h in 50 mM phosphate buffer pH 6.8; 6–10 h in 50 mM phosphate buffer pH 7.4, triplicate samples (black diamond) 40–30 mesh (420–595 pm); (circle) 30–20 mesh (595–841 pm); (black square) 20–16 mesh (841–1190 pm)
Since Eudragit® FS was insoluble in 0.1 N hydrochloric acid solution and phosphate buffer pH 6.8, drug release was controlled by diffusion only during the first 6 h. 5-ASA was solubilized and released via the diffusion through the granule pores permeated with dissolution medium. 5-ASA granules remained the same physically during the first 6 h of dissolution testing. After the pH of the dissolution medium was adjusted to 7.4, a gel layer on the surface of the granules was observed and the particle size of the melt-extruded granules gradually decreased over the time. Eudragit® FS was soluble in phosphate buffer pH 7.4. Therefore, both diffusion of drug through the gel layer and erosion of the Eudragit® FS matrices contributed to the drug release. As show in Fig. 5, the release rate of 5-ASA was dependent on the particle size of the granules. Slower release from the capsules containing larger melt extrudate was due to the decrease in the specific surface area and increase in the average diffusion distance of drug molecules from the granules.
Stability of Dissolution Properties of 5-ASA Capsules Following Storage
5-ASA capsules were packaged in induction-sealed HDPE bottles and stored at 40°C/75% relative humidity and 60°C/without humidity control to investigate the stability in dissolution properties following the storage. Drug release of the capsules was tested at initial time point and following 1 month storage. As shown in Fig. 6, there was no change in the drug release rate following 1-month storage under both conditions.
Fig. 6.
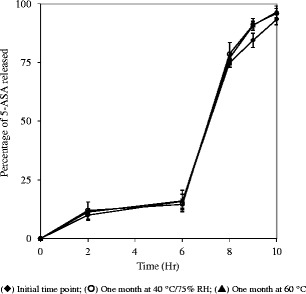
Dissolution profiles of 5-ASA capsules (10% drug loading in melt-extruded granules in 20–16 mesh) at initial time point and following 1 month storage at 40°C/75% RH and 60°C; USP paddle method at 100 rpm; 900 mL medium at 37°C; 0–2 h in 0.1 N HCl; 2–6 h in 50 mM phosphate buffer pH 6.8; 6–10 h in 50 mM phosphate buffer pH 7.4, triplicate samples. Initial time point (black diamond); 1 month at 40°C/75% RH (circle); 1 month at 60°C (black triangle)
Effect of Drug Acidity on Dissolution Properties
Since the solubilization of Eudragit® FS is pH dependent, a study was conducted to investigate the effect of the microenvironment pH created by the drug on the dissolution behavior. 5-ASA, diclofenac sodium, and theophylline were used as the model compounds for this study. A comparison of the chemical properties of 5-ASA, diclofenac sodium, and theophylline is presented in Table I. The pH of the saturated water solution of 5-ASA is 4.1. With imidazole N-H, theophylline is considered a very weak acid. The pH of the saturated water solution of theophylline is 6.1 (23). Diclofenac sodium, sodium salt of a weak acid, is alkaline and the pH of its saturated water solution is 8.1 (24).
Dissolution properties of capsules containing melt-extruded granules of model compounds at 10% drug loading are compared in Fig. 7. In 0.1 N hydrochloric acid solution, the release of diclofenac sodium was the slowest, and less than 5% diclofenac sodium was released within 2 h. The slow release of diclofenac sodium in 0.1 N hydrochloric acid solution was due to the drug’s poor solubility in the dissolution medium. When the dissolution medium was switched to phosphate buffer pH 6.8, less than 10% 5-ASA and theophylline was released in a 4 h time widow. In contrast, diclofenac sodium was completely released and disintegration of the granules was even observed in phosphate buffer pH 6.8 during the dissolution testing.
Fig. 7.
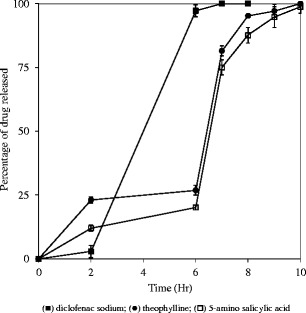
Release profiles of capsules for three different drugs with each capsule containing 300 mg melt-extruded granules (20–30 mesh and 10% drug loading in melt-extruded granules); USP paddle method at 100 rpm; 900 mL medium at 37°C; 0–2 h in 0.1 N HCl; 2–6 h in 50 mM phosphate buffer pH 6.8; 6–10 h in 50 mM phosphate buffer pH 7.4. Triplicate samples (black square) diclofenac sodium; (black circle) theophylline; (white square) 5-amino salicylic acid
It was hypothesized that diclofenac sodium solubilized inside the pores of melt-extruded granules resulted an alkaline microenvironment that induced hydration, swelling, and solubilization of Eudragit® FS even though Eudragit® FS by itself should not be soluble in phosphate buffer pH 6.8. Solubilization of the polymer resulted in an increase in porosity and a decrease in structural integrity of the granules. Release of diclofenac sodium from the melt-extruded granules was therefore accelerated. Alkaline microenvironment induced by the dissolved drug and the consequent increase in drug release rate due to solubilization of enteric polymer has been reported in film-coated dosage forms previously (25).
To validate the drug-induced microenvironment pH hypothesis, sodium phosphate monobasic, an acidifying agent, was incorporated into the formulation to neutralize the pH effect of diclofenac sodium. As presented in Fig. 8, the burst release of diclofenac sodium in phosphate buffer pH 6.8 was completely suppressed when sodium phosphate monobasic was present in the formulation at levels greater than 5%.
Fig. 8.
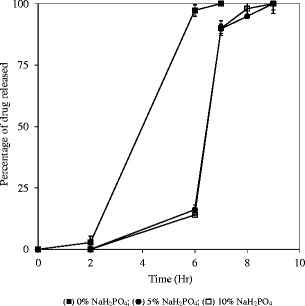
Release profiles of the capsules containing melt-extruded sodium diclofenac granules (20–30 mesh and 10% drug loading in melt-extruded granules) containing different levels of sodium phosphate monobasic; USP paddle method at 100 rpm; 900 mL medium at 37°C; 0–2 h in 0.1 N HCl; 2–6 h in 50 mM phosphate buffer pH 6.8; 610 h in 50 mM phosphate buffer pH 7.4. Triplicate samples (black square) 0% NaH2PO4; (black circle) 5% NaH2PO4; (white square) 10% NaH2PO4
Effect of Drug Loading Levels on Dissolution Properties
Drug release profiles of capsules containing the 5-ASA or theophylline at 10, 20, and 30% levels in melt-extruded granules are shown in Figs. 9 and 10, respectively. The increase in drug content led to higher percentage of drug release during the first 6 h for both drugs. However, opposite trends were observed for the change in the release rate in phosphate buffer pH 7.4. Opposite change in drug release rate with increase in drug loading was observed for theophylline and 5-ASA. With the increase in drug loading, the release rate increased for theophylline while it decreased for 5-ASA. This observation reaffirmed the hypothesis that the micro pH environment created by drug significantly affected the release from the melt-extruded granules in buffer solution pH 7.4. The detailed discussion is presented in the following two paragraphs.
Fig. 9.
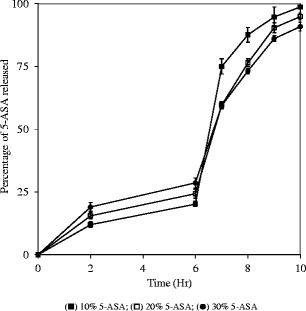
Release profiles of capsules containing 300 mg melt-extruded granules (30–20 mesh) with different 5-ASA loading levels in melt-extruded granules; USP paddle method at 100 rpm; 900 mL medium at 37°C; 0–2 h in 0.1 N HCl; 2–6 h in 50 mM phosphate buffer pH 6.8; 6–10 h in 50 mM phosphate buffer pH 7.4. Triplicate samples (black square) 10% 5-ASA; (white square) 20% 5-ASA; (black circle) 30% 5-ASA
Fig. 10.
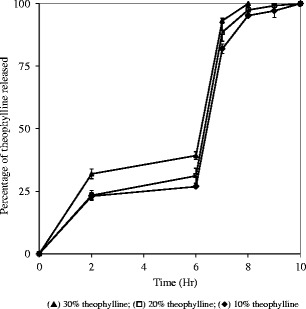
Release profiles of capsules containing 300 mg melt-extruded granules (30–20 mesh) with different theophylline loading levels; USP paddle method at 100 rpm; 900 mL medium at 37°C; 0–2 h in 0.1 N HCl; 2–6 h in 50 mM phosphate buffer pH 6.8; 610 h in 50 mM phosphate buffer pH 7.4. Triplicate samples (black triangle) 30% theophylline; (white square) 20% theophylline; (black diamond) 10% theophylline
Melt-extruded granules were three-dimensional lattices consisting of drug and Eudragit® FS particles. Clusters of interconnecting drug particles were formed in the granule matrices, and only those clusters located on the surface of the matrix were accessible to the dissolution medium. In the case of 5-ASA and theophylline, solubilization of Eudragit® FS did not take place and drug was release via diffusion mechanism only in the 0.1 N HCl solution and phosphate buffer pH 6.8. With an increase in drug loading level, drug clusters became more extensively connected and the tortuosity of the granules also decreased. More drug particles were thus accessible to the dissolution medium resulting in an increase in the drug release rate from the melt-extruded granule (26,27). The drug loading level, at which all drug clusters are interconnected, is called the “drug percolation threshold.” Above this threshold, an infinite cluster is formed and all drug particles are accessible to the dissolution medium. Complete drug release was only expected at drug loading levels equal to or higher than the percolation threshold. It was concluded that the drug percolation threshold was higher than 30% and only finite clusters were formed in the prepared granules. During the initial stage of the dissolution test, drug clusters located on the surface of the granules were readily released into the dissolution medium. As the dissolution process progressed, fewer drug clusters were accessible to the dissolution medium and a plateau was reached.
In phosphate buffer pH 7.4, drug was released via both diffusion and erosion mechanisms since Eudragit® FS was soluble in this medium. With an increase in drug loading, drug release rate was anticipated to increase since there was less retardation effect with lower levels of Eudragit® FS. As shown in Fig. 10, theophylline release rate increased with a higher drug loading in phosphate buffer pH 7.4. However, an opposite drug-loading effect was observed with 5-ASA granules. At a higher loading of 5-ASA, a more acidic microenvironment was created by the solubilized drug within the granules. As a result, the solubilization of the Eudragit® FS was inhibited and slower dissolution rate of 5-ASA was observed at pH 7.4. This result reconfirmed that the microenvironment pH induced by drug was a significant factor in controlling the release rate from melt-extruded Eudragit® FS granules.
Pore Structure of Melt-Extruded Granules and Its Effect on Drug Release
One objective of this study is to compare the pore structure and dissolution properties of melt-extruded granules with those of the granules prepared using conventional compression process. 5-ASA granules (20% drug and 80% Eudragit® FS, 20–30 mesh) were prepared using both processes. Mechanisms for granule formation are different between melt extrusion and conventional compression processes. Fusion bonding is the matrix formation mechanism for melt extrusion process while solid bridging is for compression process (28,29).
During melt extrusion, Eudragit® FS was transformed into a rubbery state and fused together under the intensive shear of the rotating screw. 5-ASA drug particles were encircled by the Eudragit® FS melt. The formulation was compressed and densified in metering section and die. After the formulation exited the die and solidified at the ambient conditions, the 5-ASA/Eudragit® FS matrix was held together by the solidified Eudragit® FS melt.
In compression process, the blend of 5-ASA and Eudragit® FS was densified using mechanical force into compacts, which were grounded into granules in a downstream process. Initially, the compression pressure forced the drug and polymer particles to reposition in the powder bed. Further increase in the pressure resulted in brittle fracture and viscoelastic deformation of the particles, and densification of the formulation. When the distance between the surfaces of two particles was less than 50 nm, a strong attractive force and the solid bridging occurred at the points of contact. Following the release of the compression force, the 5-ASA/Eudragit® FS matrix was held together by the solid bridges.
Dissolution profiles of 5-ASA granules prepared using two different processes are presented in Fig. 11. The granules prepare using melt granulation process demonstrated pH-dependent release profiles suitable for colonic drug delivery, and the granules remained intact in the first two stages, 2 h in 0.1 N hydrochloric acid and 4 h in phosphate buffer pH 6.8. In contrast, a burse release of greater than 90% in the first 2 h and disintegration of the granules was observed with the granules prepared using compression process. Since Eudragit® FS was not soluble in 0.1 N hydrochloric acid, 5-ASA was released by diffusion only during the first 2 h. The diffusion from a matrix is controlled by the pore characteristics: porosity and tortuosity. Porosity defines the percent volume of the pore channel in the matrix. Diffusion of drug through the pores channels filled with the dissolution media is much faster than the diffusion through the polymer phase. Therefore, a higher porosity would result in faster dissolution rate. Tortuosity defines the ratio between the length of the channel connecting two points and the distance between two points. A higher tortuosity would result in longer the diffusion channels and a lower drug release rate (30). It was hypothesized that slower drug release from the melt-extruded granules was due to their less porous structure.
Fig. 11.
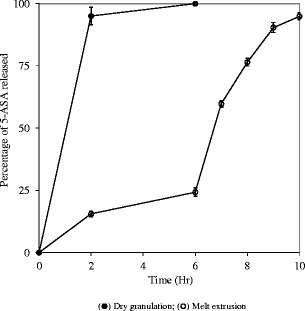
Dissolution profiles of 5-ASA granules (20% drug and 80% Eudragit® FS, 30–20 mesh) prepared using different processes; USP paddle method at 100 rpm; 900 mL medium at 37°C; 0–2 h in 0.1 N HCl; 2–6 h in 50 mM phosphate buffer pH 6.8; 610 h in 50 mM phosphate buffer pH 7.4. Triplicate samples (black circle) dry granulation; (white circle) melt extrusion
The pore structure of 5-ASA granules prepared by both processes was characterized using scanning electron microscope and mercury intrusion pycnometer. As shown in the SEM images (Fig. 12), the compressed 5-ASA granules were porous with the surfaces of individual particles being discernible, while the melt-extruded granules demonstrated smooth and homogeneous surface. The pore characteristics and porosity of 5-ASA granules are summarized in Table II. The medium pore diameter of the compressed granules was found to be twice as large as that of the melt-extruded granules. The porosity of the compressed granules was 50% greater than melt-extruded granules. It was therefore concluded from the granule pore structure analysis that the burst release from the compressed granules was resulted from their higher porosity, larger pore size, and lower tortuosity. For melt-extruded granules, fast initial release followed with a plateau phase indicated that most of the drug particles was totally enclosed and isolated by the surrounding Eudragit® FS.
Fig. 12.

Scanning electron microscope images of 5-ASA granules (20% drug and 80% Eudragit® FS) prepared using two different processes
Table II.
Pore Structure Characteristics of 5-ASA Granules (20% Drug and 80% Eudragit® FS, 20–30 mesh) Prepared with Different Processes
| Granules prepared by melt extrusion method | Granules prepared by compression method | |
|---|---|---|
| Medium pore diametera (pm) | 0.0186 ± 0.0003 | 0.0420 ± 0.0004 |
| Particle density (g/mL) | 1.2762 ± 0.0325 | 1.0936 ± 0.02855 |
| Porosity (%) | 4.2975 ± 0.0621 | 5.4163 ± 0.0872 |
aMedium pore diameter was calculated based on pore volume. Three samples were tested for each type of granules
Solid bridges formed during compression were much weaker than the fusion bonds produced in melt extrusion process, resulting in Eudragit® FS granules with a lower mechanical strength. Much more significant disintegration of compressed granules during the dissolution testing was attributed to the low mechanical strength of the granules.
The same dissolution behaviors were observed with drug-free Eudragit® FS granules (20–30 mesh) prepared with two different processes. The contents in the dissolution vessel were filtered through a 0.2 μm Nylon filter to collect the undissolved polymer at specific time points. The material collected was rinse with purified water and lyophilized to determine the weight of the undissolved polymer and to calculate the polymer dissolution rate. Granules prepared using melt extrusion process remained intact through the first two stages of dissolution (2 h in 0.1 N hydrochloric acid and 4 h in phosphate buffer pH 6.8) while disintegration of compressed granules was observed. As shown in Fig. 13, the compressed Eudragit® FS granules were all solubilized while only 50% of the melt-extruded Eudragit® FS granules were dissolved following 2 h in phosphate buffer pH 7.4.
Fig. 13.
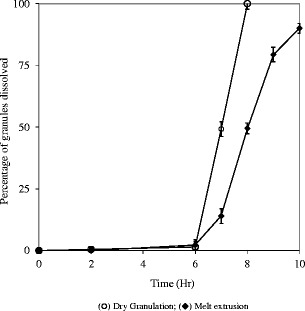
Solubilization profiles of drug-free Eudragit® FS granules (20–30 mesh) prepared using different processes; USP paddle method at 100 rpm; 900 mL medium at 37°C; 0–2 h in 0.1 N HCl; 2–6 h in 50 mM phosphate buffer pH 6.8; 6–10 h in 50 mM phosphate buffer pH 7.4. Triplicate samples (white circle) dry granulation; (black diamond) melt extrusion
Stability of Eudragit® FS During Melt Extrusion
Eudragit® FS was subjected to both the shear stress and thermal stress during melt extrusion process. Shear stress could result in random polymer chain scission. The thermal stress could lead to chemical degradation such as depolymerization, hydrolysis, and oxidation (31).
Eudragit® FS is most susceptible to hydrolysis of ester bonds. It is recommended to store the latex dispersion of Eudragit® FS between 5 and 10°C to minimize the hydrolysis. Prior to the extrusion, Eudragit® FS was dried overnight in a vacuum chamber to reduce the moisture content of the polymer in order to minimize any hydrolytic degradation during melt extrusion.
As shown in Fig. 14, the thermal depolymerization temperature of Eudragit® FS was determined to be 300°C by thermal gravimetrical analysis. Therefore, the most likely degradations of Eudragit® FS during melt extrusion are shear stress induced polymer chain scission and hydrolysis. Both of these two degradations would lead to decrease in the polymer molecular weight.
Fig. 14.
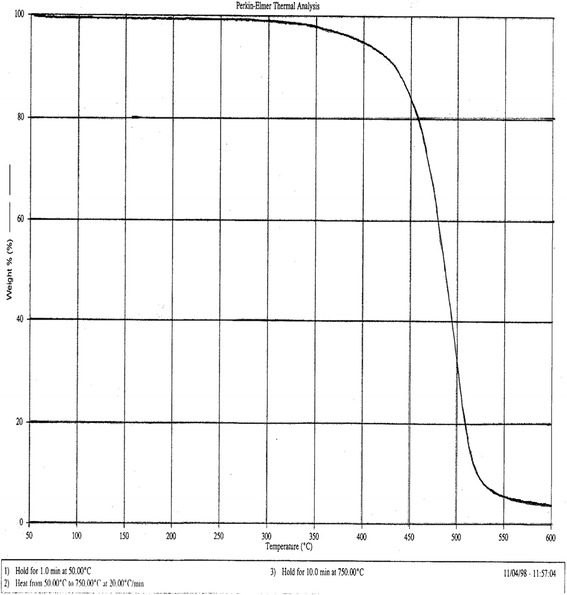
Thermogravimetric profile of Eudragit® FS
The number average molecular weight (Mn), weight average molecular weight (Mw), and polydispersity of Eudragit® FS before and after melt extrusion were analyzed via gel permeation chromatography method. The molecular weight of the polymer was determined relative to monodispersed polystyrene standards. Carboxylic groups of methacrylic acid units were esterified to eliminate the absorption of the polymer to the GPC columns. As shown in Table III, Eudragit® FS demonstrated good stability during melt extrusion process. The average molecular weights of Eudragit® FS remained unchanged even when the material was processed at a barrel temperature of 200°C and screw speed of 40 rpm.
Table III.
Molecular Weight of Eudragit® FS Prior to and Following Melt Extrusion
| Processing condition | Number average molecular weight (Mw) | Weight average molecular weight (Mn) | Polydispersity | |
|---|---|---|---|---|
| Barrel temp (°C) | Screw speed (rpm) | |||
| Unprocessed | N/A | 62,951 | 216,502 | 3.4 |
| 100 | 40 | 63,578 | 213,567 | 3.4 |
| 150 | 40 | 64,583 | 214,046 | 3.3 |
| 200 | 40 | 64,225 | 219,568 | 3.4 |
CONCLUSIONS
This study has demonstrated that Eudragit® FS-based granules for colonic drug delivery could be prepared using melt extrusion process. In comparison with the granules prepared using conventional compression process, melt-extruded granules had smaller pore size and lower porosity that contributed to better control in drug release. Drug was released via diffusion mechanism when Eudragit® FS was not soluble in medium with pH lower than 7.0. Both diffusion of drug and erosion of polymer matrix contributed to drug release when Eudragit® FS became soluble in the dissolution medium with pH greater than 7.0.
Granule size, drug acidity, and drug loading were identified as three critical parameters controlling drug release rate from the melt-extruded granules. With an increase in the size of the granules, the undesired drug release in the first two stages (0.1 N hydrochloric acid solution and phosphate buffer pH 6.8) became less and drug release in the third stage (phosphate buffer pH 7.4) became slower. Since the solubilization of Eudragit® FS was affected by the microenvironment pH induced by solubilized drug, the acidity of drug was found to be a critical factor in controlling the release of drugs from melt-extruded granules. In general, a higher drug loading resulted in faster drug release since smaller amount of polymer was present to retard the drug release. However, the opposite effect was observed with acidic drug, which slowed down the solubilization of Eudragit® FS by lowering the pH of the microenvironment.
References
- 1.Amrutkar JR, Gattani SG. Chitosan-chondroitin sulfate based matrix tablets for colon specific delivery of indomethacin. AAPS PharmSciTech. 2009;10(2):670–7. doi: 10.1208/s12249-009-9253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maroni A, Zema L, Del Curto MD, Foppoli A, Gazzaniga A. Oral colon delivery of insulin with the aid of functional adjuvants. Adv Drug Deliv Rev. 2012;64(6):540–56. doi: 10.1016/j.addr.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Gupta SK, Sathyan G. Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate-release oxybutynin. J Clin Pharmacol. 1999;39(3):289–96. [PubMed] [Google Scholar]
- 4.Gupta VK, Assmus MW, Beckert TE, Price JC. A novel pH- and time-based multi-unit potential colonic drug delivery system. II. Optimization of multiple response variables. Int J Pharm. 2001;213(1–2):93–102. doi: 10.1016/S0378-5173(00)00650-5. [DOI] [PubMed] [Google Scholar]
- 5.Khan MZ, Prebeg Z, Kurjakovic N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation of drug release using Eudragit L100-55 and Eudragit S100 combinations. J Control Release: Off J Control Release Soc. 1999;58(2):215–22. doi: 10.1016/S0168-3659(98)00151-5. [DOI] [PubMed] [Google Scholar]
- 6.Sinha A, Ball D, Connor AL, Nightingale J, Wilding IR. Intestinal performance of two mesalamine formulations in patients with active ulcerative colitis as assessed by gamma scintigraphy. Pract Gastroenterol. 2003;27(10):56–69. [Google Scholar]
- 7.Dvorracckova K, Rabiskova M, Muselik J, Gajdziok J, Bajerova M. Coated hard capsules as pH-dependent drug transport systems to ileo-colonic compartment. Drug Dev Ind Pharm. 2011;37(10):1131–40. doi: 10.3109/03639045.2011.561350. [DOI] [PubMed] [Google Scholar]
- 8.Kulthe SS, Bahekar JK, Godhani CC, Choudhari YM, Inamdar NN, Mourya VK. Modulated release of 5-fluorouracil from pH-sensitive and colon targeted pellets: an industrially feasible approach. Drug Dev Ind Pharm. 2013;39(1):138–45. doi: 10.3109/03639045.2012.660951. [DOI] [PubMed] [Google Scholar]
- 9.Friesen D, Shanker R, Crew M, Smithey D. Hydroxypropyl methylcellulose based spray- dried dispersions: an overview. Mol Pharm. 2008;5(6):1003–19. doi: 10.1021/mp8000793. [DOI] [PubMed] [Google Scholar]
- 10.Laarhoven JAH, Kruft MAB, Vromans H. Effect of supersaturation and crystallization phenomena on the release properties of a controlled release device based on EVA copolymer. J Control Release. 2002;82:309–19. doi: 10.1016/S0168-3659(02)00139-6. [DOI] [PubMed] [Google Scholar]
- 11.Follonier N, Doelker E, Cole E. Evaluation of hot-melt extrusion as a new technique for the production of polymer-based pellets for sustained-release capsules containing high loading of freely water soluble drugs. Drug Dev Ind Pharm. 1994;20(8):1323–39. doi: 10.3109/03639049409038373. [DOI] [Google Scholar]
- 12.Hasa D, Perissutti B, Grassi M, Zacchigna M, Pagotto M, Lenaz D, et al. Melt extruded helical waxy matrices as a new sustained drug delivery system. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharm Verfahrenstechnik eV. 2011;79(3):592–600. doi: 10.1016/j.ejpb.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, McGinity JW. Properties of sustained release tablets prepared by hot-melt extrusion. Pharm Dev Technol. 1999;4(2):241–50. doi: 10.1081/PDT-100101358. [DOI] [PubMed] [Google Scholar]
- 14.Yang R, Wang Y, Zheng X, Meng J, Tang X, Zhang X. Preparation and evaluation of ketoprofen hot-melt extruded enteric and sustained-release tablets. Drug Dev Ind Pharm. 2008;34(1):83–9. doi: 10.1080/03639040701580572. [DOI] [PubMed] [Google Scholar]
- 15.Schilling SU, Lirola HL, Shah NH, Malick AW, McGinity JW. Influence of plasticizer type and level on the properties of Eudragit S100 matrix pellets prepared by hot-melt extrusion. J Microencapsul. 2010;27(6):521–32. doi: 10.3109/02652048.2010.484105. [DOI] [PubMed] [Google Scholar]
- 16.Young C, Dietzsch C, McGinity JW. Compression of controlled-release pellets produced by hot-melt extrusion and spheronization process. Pharm Dev Technol. 2005;10(1):133–9. doi: 10.1081/PDT-49695. [DOI] [PubMed] [Google Scholar]
- 17.Carli F, Motta A. Particle size and surface area distributions of pharmaceutical powders by microcomputerized mercury porosimetry. J Pharm Sci. 1984;73(2):197–203. doi: 10.1002/jps.2600730213. [DOI] [PubMed] [Google Scholar]
- 18.Ertel KD, Zoglio MA, Ritschel WA, Carstensen JT. Physical aspects of wet granulation 4. Effect of kneading time on dissolution rate and tablet properties. Drug Dev Ind Pharm. 1990;16(6):963–81. doi: 10.3109/03639049009114922. [DOI] [Google Scholar]
- 19.Zoglio MA, Carstensen JT. Physical aspects of wet grannulation 3. Effect of wet granulation on granule porosity. Drug Dev Ind Pharm. 1983;9(8):1417–34. doi: 10.3109/03639048309052385. [DOI] [Google Scholar]
- 20.Rubio MR, Ghaly ES. In-vitro release of acetaminophen from sodium alginate controlled-release pellets. Drug Dev Ind Pharm. 1994;20(7):1239–51. doi: 10.3109/03639049409038364. [DOI] [Google Scholar]
- 21.Crowley MM, Schroeder B, Fredersdorf A, Obara S, Talarico M, Kucera S, et al. Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion. Int J Pharm. 2004;269:509–22. doi: 10.1016/j.ijpharm.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 22.French DL, Mauger JW. Evaluation of the physicochemical properties and dissolution characteristics of mesalamine: relevance to controlled intestinal drug delivery. Pharm Res. 1993;10(9):1285–90. doi: 10.1023/A:1018909527659. [DOI] [PubMed] [Google Scholar]
- 23.Cohen JL. Analytical profiles of drug substance. New York: Academic Press, Inc.; 1975. [Google Scholar]
- 24.Adeyeye CM. Analytical profiles of drug substances. New York: Academic Press, Inc.; 1990. [Google Scholar]
- 25.Bruce LD, Koleng JJ, McGinity JW. The influence of polymeric subcoats and pellet formulation on the release of chlorpheniramine maleate from enteric coated pellets. Drug Dev Ind Pharm. 2003;29(8):909–24. doi: 10.1081/DDC-120024187. [DOI] [PubMed] [Google Scholar]
- 26.Caraballo I, Millan M, Rabasco AM. Relationship between drug percolation threshold and particle size in matrix tablets. Pharm Res. 1996;13(3):387–90. doi: 10.1023/A:1016088424993. [DOI] [PubMed] [Google Scholar]
- 27.Elarini SK, Leuenberger H. Modeling of drug-release from polymer matrices—effect of drug loading. Int J Pharm. 1995;121(2):141–8. doi: 10.1016/0378-5173(94)00418-5. [DOI] [Google Scholar]
- 28.Nystrom C, Alderborn G, Duberg M, Karehill P-G. Bonding of surface area and bonding mechanism—two important factors for the understanding of powder compactability. Drug Dev Ind Pharm. 1993;19(17–18):2143–96. doi: 10.3109/03639049309047189. [DOI] [Google Scholar]
- 29.DiNunzio JC, Martin C, Zhang F. Melt extrusion: shaping drug delivery in the 21st century. Pharm Technol 2010; (Special Issue):30–7.
- 30.Frenning G. Modelling drug release from inert matrix systems: from moving-boundary to continuous-field descriptions. Int J Pharm. 2011;418(1):88–99. doi: 10.1016/j.ijpharm.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 31.Capone C, Landro LD, Inzoli F, Penco M. Thermal and mechanical degradation during polymer extrusion processing. Polym Eng Sci. 2007;47(11):1813–9. doi: 10.1002/pen.20882. [DOI] [Google Scholar]


