Abstract
The objective of this project was to investigate the potential of Kleptose Linecaps DE17 (KLD) in masking the unpleasant/bitter taste of therapeutic agents by hot melt extrusion (HME). Griseofulvin (GRI) and caffeine anhydrous (CA) were used as a bitter active pharmaceutical ingredient (API) model drugs. Thermogravimetric studies confirmed the stability of GRI, CA, and KLD at the employed extrusion temperatures. The differential scanning calorimetry (DSC) studies revealed a characteristic melting endotherm of GRI at 218–220°C and CA at 230–232°C in the physical mixtures as well as in all extrudates over the period of study, indicating the crystalline nature of drug. HME of KLD was achieved only in the presence of plasticizer. Among the several plasticizers investigated, xylitol showed improved processability of KLD at 15% w/w concentration. Dissolution studies of HME extrudates using simulated salivary medium exhibited ∼threefold less release compared to physical mixture at the end of 5 min (the lesser drug release, better the taste masking efficiency). Furthermore, the results from the sensory evaluation of products in human panel demonstrated strong bitter taste in the case of physical mixture compared to the HME formulation, suggesting the potential of Kleptose Linecaps DE17 as taste masking polymer in melt extruded form.
KEY WORDS: caffeine anhydrous, griseofulvin, hot melt extrusion, human panel studies, Kleptose linecaps DE17, taste masking
INTRODUCTION
Taste masking of bitter active pharmaceutical ingredient (API) is a major challenge especially for pediatric/geriatric formulations. Effective taste masking can be achieved by various reported approaches like fluidized-bed coating, supercritical fluids, complexing agents, pro-drug approach, etc. (1–3). However, there is an imperative need for more robust, cost-effective, and easy to scale-up taste masking technologies. Hot melt extrusion (HME) initially used in the plastic industry has attracted significant interest in the pharmaceutical industry over the last decade as a continuous, one-step process for solid dispersions development with considerable advantages over solvent-based processes such as spray drying and co-precipitation techniques (4,5). Additionally, it can be used to develop various formulations such as controlled release matrices, sustained release matrices, topical films, etc. (6–9). APIs taste masking is usually achieved by the use of taste masking polymers that create solid dispersions in order to prevent bitter drugs from coming in contact with the patient’s taste buds by modifying the drug release from the polymer matrix.
Hot melt extruded dosage forms are complex mixtures of active medicaments, functional excipients, and processing aids. Solid molecular dispersions created by HME are offering several advantages over traditional pharmaceutical processing techniques including the absence of solvents, few processing steps, and continuous operation with improved bioavailability. Several studies have reported that the effective taste masking can be achieved when the drug is molecularly dispersed within the polymer matrix. In addition, the unpleasant taste of the drug can be overcome by forming hydrogen bonding between active substance and polymer matrix (1,2).
Kleptose Linecaps DE17 (dextrose equivalent from 15 to 20 (KLD)) is a spray-dried high amylose pea maltodextrin obtained by partial hydrolyses of pea starch (about 40% amylose) with a molecular weight of ∼12000 Da. The remaining 60% of its composition is composed of glucose, oligosaccharides, and polysaccharides. In water, amylose linear molecule easily forms helical structures (Fig. 1) with an inner hydrophobic cavity. Its flexible and helical design might mask the unpleasant taste of drugs by entrapping them within their coiled amylase chains. It is ideal for pediatric/geriatric formulations, nutraceuticals, and OTC for patient compliance improvement due to its non GMO. Consequently, the main idea of this project was to investigate the potential of KLD in masking the bitter taste of two model drugs; griseofulvin (GRI) and caffeine anhydrous (CA) processed by hot melt extrusion for pediatric/geriatric patients.
Fig. 1.
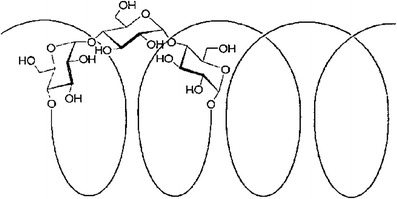
Helical structure of amylose
MATERIALS
Griseofulvin (GRI) micronized was purchased from Spectrum Chemical Corporation (New Brunswick, NJ), and caffeine anhydrous (CA) was obtained from Novel Ingredient Services (West Caldwell, NJ). Kleptose Linecaps DE17 and plasticizers (xylitol, maltitol, sorbitol, and mannitol) were obtained as gift samples from Roquette (Roquette America Inc., Geneva, Illinois). Erythritol was purchased from Sigma-Aldrich (St. Louis, MO). All other solvents used for HPLC were obtained from Fisher Scientific (Hanover Park, Illinois).
METHODS
Thermogravimetric Studies
Thermogravimetric studies were performed on a PerkinElmer Pyris 1 thermogravimetric analyzer (TGA) equipped with Pyris software. Thermal stability during extrusion for KLD, plasticizers (xylitol, erythritol, maltitol, mannitol, and sorbitol), GRI, and CA was evaluated and were heated from 30 to 200°C at a linear heating rate of 20°C/min.
Differential Scanning Calorimetry Studies
The physical characterization of pure GRI, CA, KLD, plasticizers, and extruded formulations was performed by differential scanning calorimetry (DSC), using PerkinElmer Diamond differential scanning calorimeter equipped with Pyris software (Shelton, CT). Approximately, 2–3 mg of the sample was hermetically sealed in a crimped aluminum pan and heated from a temperature range of 30°C to about 50°C higher than the melting point of the drug or the softening temperature of the polymers, at a heating rate of 20°C/min. These studies were performed to determine the crystallinity of the drug in the extruded and physical mixture (PM) formulations.
Preparation of Physical Mixtures/Blends
All the required materials were sieved through USP # 30 mesh, weighed the required quantities, and transferred to twin-shell V-blender (The Patterson-Kelly Co., Inc. East Stroudsburg, PA). The materials were allowed to mix for 10 min at 25 rpm in order to get homogenous mixture.
Preparation of Taste Masked Formulations by HME Technique
Hot Melt Extrusion of KLD
To identify and to develop the optimum HME process conditions for KLD in order to appropriately develop a taste masked formulation, processability of KLD alone was initially assessed. HME of KLD was carried out using co-rotating twin screw extruder (16 mm Prism Euro Lab, Thermo Fisher Scientific) at varying screw speeds of 50–150 rpm over a temperature range of 135–175°C.
Effect of Plasticizers on Extrudability of KLD
In order to increase the extrudability of KLD, different polyols (xylitol, mannitol, sorbitol, erythritol, and maltitol) were screened as plasticizers at 10–20% w/w concentration, except for maltitol being deliberately assessed at 20 and 30% w/w concentration (Table I). The best formulation was identified based on the torque generated (as a process parameter) during the extrusion process and proceed to advance studies.
Table I.
Processing Conditions for HME of KLD and Plasticizer
| Maltodextrin (% w/w) | Plasticizer (%) | Temperature (first zone/later zones) in °C | RPM |
|---|---|---|---|
| Linecaps DE17 (80–90%) | Xylitol (10–20%) | 130–150/140–155 | 50–150 |
| Linecaps DE17 (70–80%) | Maltitol (20–30%) | 130–150/140–155 | 50–150 |
| Linecaps DE17 (80–90%) | Sorbitol (10–20%) | 130–150/140–155 | 50–150 |
| Linecaps DE17 (80–90%) | Mannitol (10–20%) | 130–150/140–155 | 50–150 |
| Linecaps DE17 (80–90%) | Erythritol (10–20%) | 130–150/140–155 | 50–150 |
Hot Melt Extrusion of Taste Masked Formulation of GRI and CA
GRI and CA at 15% w/w drug load were pre-mixed with 70% w/w KLD and xylitol as plasticizers at concentration of 15% w/w using a V-shell blender and further extruded using co-rotating twin screw extruder at screw speeds of 100 rpm at a temperature of 150/155°C (first zone/later zones). Thereafter, melt extrudates were subjected to a low-shear grinding in a mortar and pestle, followed by milling using a coffee grinder for 2–5 min. The obtained particles/granules were screened through USP mesh (#40 pass and #50 retains) and subjected to dissolution and taste evaluation in human panel studies.
In Vitro Dissolution Studies
Hanson SR-8 dissolution system was used to evaluate the dissolution profile of the extruded formulations. Milled extrudates and physical mixture were studied for in vitro dissolution release in simulated saliva fluid (900 mL) using USP type-I apparatus (Varian Inc., North Carolina) at 37 ± 0.5°C and 75 rpm. Simulated saliva fluid was prepared by dissolving 2.38 g of Na2HPO4, 0.19 g of KH2PO4, and 8.0 g of NaCl in 1 L distilled water adjusted to pH 6.8 (10). Samples (1 mL) were collected at predetermined time points and fresh medium was used to replace the sample volume. The samples were filtered through 0.2 μm nylon syringe filter, centrifuged and then analyzed for GRI and CA concentrations using a Waters HPLC system.
Analytical Method
The HPLC system (Waters Corp, Milford, MA) consisting of model 1525 isocratic chromatographic pump equipped with an auto-sampler (Waters 717 plus), a multi λ fluorescent detector (Waters 2475), a UV detector (Waters 2487), and a reversed phase Zorbax 300SB-CN (4.6 × 150 mm; 3.5 μm particles) column (for GRI) and reversed phase Phenomenex® C18 (4.6 × 150 mm; 5 μm particles) column (for CA) with a security guard cartridge was used.
Analysis of GRI samples were carried out at a excitation wavelength of 300 nm, emission wavelength of 418 nm, and the mobile phase consisted of a mixture of 20 mM aqueous solution of sodium dihydrogen phosphate and acetonitrile (55:45 v/v) with an optimized flow rate of 1 mL/min. Standard solutions of GRI were prepared in mobile phase and calibration curve was plotted against area versus concentration (11).
CA was analyzed using the mobile phase consisting of a mixture of water and methanol at a ratio of 70:30 with an optimized flow rate of 1 mL/min and a detection wavelength of 274 nm. Standard samples were prepared by dissolving the pure CA in mobile phase and calibration curve was plotted against area versus concentration.
Human Panel Gustatory Response (Taste Perception/Palatability) Evaluation
Human panel palatability studies were performed in healthy human volunteers from whom informed consent was obtained previously (ethics committee approval # VIPS/2013/12). The selection criteria being healthy human subjects with age range between 18 and 47 years old, whereas the exclusion criteria being subjects suffering from fever, cold, smokers, mouth sores, and wounds. The healthy volunteers of either sex were first assessed to establish their basic perception level. The surface temperature of the tongue was recorded using an IR thermometer. Firstly, screening of pure drug solution was performed to determine individual threshold and the acuity of bitterness recognition. The individuals who reported a bitter taste for API solution that is about one-fifth of the actual dose of API in the test product (40 mg for CA and 150 mg GRI in the formulations) were recruited for the taste panel. Nine volunteers (five men and four females in the age group of 18–42) were found eligible and trained to asses CA formulations, and six volunteers (three male and three females in the age group of 18–47) were selected for testing GRI formulations. The subjects were then asked to taste the physical mixture and HME formulation extrudates equivalent to 40 mg of CA or 150 mg of GRI. Both the formulations were wetted with water (0.5 mL) and placed on the tongue/buccal cavity for a duration of 30–40 s, and the subjects were requested to score the bitterness on a scale of 0–5 for each product where 0 indicates none and 5 indicates strong bitterness.
Data Analysis
The scores given by all individuals was averaged and expressed as mean ± SD. The mean scores between the physical mixture and HME formulation were compared using t test at 95% confidence level. P < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Physical Characterization
Results from the TGA studies confirmed the stability of CA, GRI, and KLD at the working extrusion temperatures. TGA thermograms of pure API and KLD are shown in Fig. 2. The decrease in the weight was ∼5%, which accounted for moisture content in the polymer, KLD.
Fig. 2.
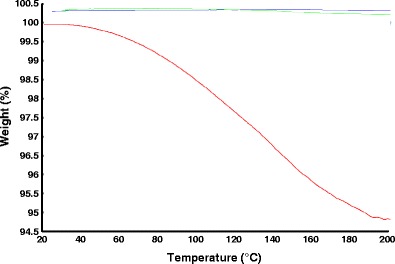
TGA thermograms of KLD ( ), GRI (
), GRI ( ), and CA (
), and CA ( )
)
Figure 3 depicts the overlays of DSC thermograms of pure API (GRI and CA), plasticizer (xylitol), and HME extrudates containing API. The DSC studies revealed a characteristic melting endotherm peak of GRI at 218–220°C and CA at 230–232°C in the case of pure API, physical mixtures as well as in all extrudates over the period of study. DSC studies performed on the physical mixture and extrudates showed no visible signs of shift in the peak, which gave us clear indication of no possible interactions between polymer and drug. Since, the HME processing temperature was below the melting point temperature of pure API, the DSC thermograms of extruded formulations showed a characteristic melting peak, indicating that drug in the extrudates are crystalline in nature but the crystals are coated with the polymer delaying the access to the taste buds. Moreover, the main objective of this project is to mask the bitter taste; effective taste masking can be achieved in liquid phase when drug is molecularly dispersed within polymer helical conformation due to the forming of hydrogen bond. But due to KLD high molecular weight, only a small fraction of the drug can be engulfed within the polymer.
Fig. 3.
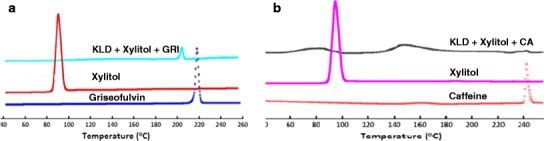
DSC thermograms of a xylitol, griseofulvin, and extrudates; b xylitol, caffeine anhydrous, and extrudates
HME of Kleptose Linecaps DE17
Extrusion of plain KLD was performed and it was found that KLD alone was not extrudable due to formation of solid mass even at higher temperature (∼175°C) (Fig. 4), due to the generation of high torque between the two screws located in between the upper and lower barrel, ending up in immobilizing the machine. Some polymers cannot be extruded without using plasticizers or drug at particular concentration in the formulation, since these polymers produce excessive torque between the screws when extrusion is performed with polymer alone. For example, Repka and co-workers have reported that hydroxypropyl cellulose (HPC) films cannot be extruded without using plasticizers in the formulation (12).
Fig. 4.
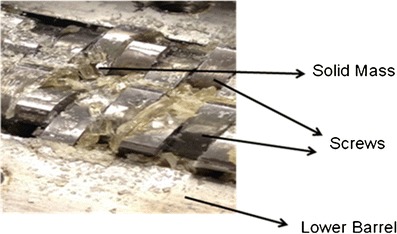
Hot melt extrusion of KLD without plasticizer. Glassy solid mass formed at extrusion temperature
Plasticizers are typically ingredients with low molecular weight, either in liquid or solid state. It is well known that plasticizers have the propensity to decrease the glass transition temperature of amorphous polymers as a function of their concentration by adding to the free volume of polymeric carrier and thereby loosen the local liquid structure of the polymer (13). In principle, this reduction in glass transition temperature during HME results in reduction of thermal degradation of any of the constituents of the formulation and an improved processability. HME of KLD at temperature greater than 175°C was not possible due to observed charring of the polymer. Therefore, the effect of plasticizers was evaluated for process optimization during HME in order to tailor the extrudates properties during hot melt extrusion phase or during post-die processing and to modify the release properties of the final dosage form.
Effect of Plasticizers on HME of KLD
In previous studies of this group (Repka and co-workers), a series of plasticizers like PEG 400, PEG 8000, triethyl citrate, and acetyl tributyl citrate were screened for extruding HPC films. They found that all the studied plasticizers showed good plasticizing effect, except for PEG 400 which initially showed good effect, but on storage the films became unstable (12). Whereas in the present study, the authors are focusing on screening different polyols as plasticizers at concentration of 10–20% w/w, except for maltitol studied at 20 and 30% w/w concentration. The processing temperature for all the formulations was kept constant at 150/155°C and 100 rpm. All the polyols screened as plasticizers when used at lower concentration (10% w/w) and extruded with KLD resulted in foamy extrudates which have a porous (sponge like) structure (Fig. 5). The property of porous structure extrudates has a very significant impact in drug release and milling process resulting in a very high milling efficiency compared to non-porous extrudates. Similar porous nature of the extrudates was observed by other research groups by using super critical CO2 as plasticizer (14,15). In a study by Verreck and co-workers, super critical CO2 was used as plasticizer in extruding Eudragit® E100, PVPVA 64, and ethyl cellulose which reduced the extrusion temperature by 15, 30, and 65°C. It was further observed that the extrudates were foamy in nature which subsequently improved milling (15).
Fig. 5.
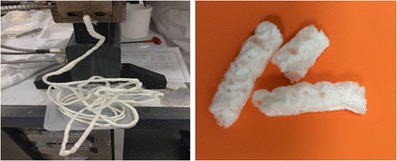
Representative picture showing the foaming of extrudates when plasticizers (xylitol, sorbitol, mannitol, and erythritol) were used at lower concentration (10% w/w)
The torque generated in all the formulations with 10% plasticizer was initially less than 50%, but the torque generated during the process with 10% w/w plasticizer was very high and the extruder stopped toward the end of the feed, which lead to the need to increase the plasticizer concentration to 15 and 20%. Furthermore, the difference in the foamy extrudates to transparent extrudates was observed due to the plasticizer property at that particular concentration. Lower concentration of plasticizer (10%) might not be sufficient for effective plasticizing effect on the polymer. Whereas, increasing the concentration of plasticizer to 20% showed good plasticizing property for the polymer studied. Table II depicts the amount of torque generated with different polyols at different concentrations. Among all the plasticizers investigated, xylitol showed better processability of KLD with minimum torque (Table II). As also shown in Table II, there is no significant difference observed between torque generated at 15 and 20% xylitol concentration. As a result, all further studies were performed with xylitol as plasticizer at 15% w/w concentration.
Table II.
Torque Generated During the HME of KLD with Polyols as Plasticizers
| Type of polyol | Polyol concentration (%) | Torque (%) (Linecaps DE17) | Observation |
|---|---|---|---|
| Xylitol | 10 | 25–30 | Foamy extrudates |
| 20 | 13–15 | Transparent extrudates | |
| Maltitol | 20 | 45–50 | Opaque extrudates |
| 30 | 35–38 | Transparent extrudates | |
| Sorbitol | 10 | 32–38 | Foamy extrudates |
| 20 | 15–20 | Transparent extrudates | |
| Mannitol | 10 | 45–40 | Foamy extrudates |
| 20 | 15–18 | Transparent extrudates | |
| Erythritol | 10 | 35–40 | Foamy extrudates |
| 20 | 20–25 | Transparent extrudates |
HME of GRI and CA Formulations
GRI and CA were chosen as model drugs for evaluating the effectiveness of KLD as taste making polymer. Both drugs were assessed at same drug load (15% w/w) in the formulations. Since the xylitol at 15% has shown the best processing conditions compared to all other polyols with HME of KLD, final formulations with API were extruded with 15% xylitol as plasticizer. The processing conditions for all the formulations were kept constant with temperature at 150/155°C (first zone/later zones) and screw speed of 100 rpm. Both formulations induced low torque at the operating conditions and the resulted extrudates were collected and subjected to milling and screened through USP mesh (#40 pass and #50 retains). The granules, which were retained on the mesh #50, were separated and subjected to in vitro dissolution and in vivo human taste panel studies.
Dissolution Studies
In order to evaluate KLD taste masking efficiency by HME process, the in vitro dissolution profiles of the HME granules loaded with 40 mg equivalent CA or 150 mg equivalent GRI as API were performed and compared to those with physical mixture (at the same API concentrations), and the dissolution profiles are shown in Figs. 6 and 7. According to FIP/AAPS (International Pharmaceutical Federation / American Association Pharmaceutical Sciences) guidelines, dissolution values of early time points (<5 min) can be used to establish the approximate base line of bitter taste (16–18). In the study of Jianchen and co-workers, taste masking of famotidine orally disintegrating tables (ODT) containing microparticles (to mask the bitterness of API) prepared by spray drying technique was evaluated by in vitro dissolution studies and in vivo human taste panel studies. From the dissolution studies, they found that ∼fourfold lesser drug was released in case of ODT with microspheres compared to the ODT without microspheres. In line with these results, the human panel studies resulted with the mean score of 0.33 (on a scale of 0–3, maximum bitterness score being 3) with ODT containing microparticles (taste masked formulation), whereas the ODT without microspheres resulted in the mean score of 1.67 (18).
Fig. 6.
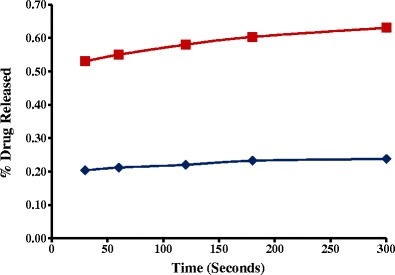
Dissolution profiles in simulated saliva fluid of formulations containing 15% w/w griseofulvin and 15% w/w xylitol as plasticizer: HME ( ) and physical mixture (
) and physical mixture ( )
)
Fig. 7.
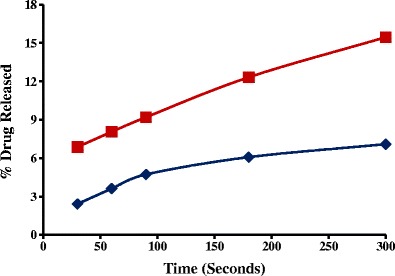
Dissolution profiles in simulated saliva fluid of formulations containing 15% w/w caffeine anhydrous and 15% w/w xylitol as plasticizer: HME ( ) and physical mixture (
) and physical mixture ( )
)
In the present study, GRI-HME granules released ∼threefold less GRI compared to GRI-PM formulation at the end of 5 min dissolution time interval (Fig. 6). Also, similar results were obtained with CA-HME granules, which resulted in ∼2.5 times CA less release compared to PM at the end of 5 min dissolution time interval in simulated saliva fluid as dissolution medium (Fig. 7). The lower the release of drug from the HME granules of both (CA and GRI) formulations the better the taste masking efficiency of KLD and that makes KLD a valuable candidate in the development of taste masked formulation by HME.
In another study by Marie and co-workers, oral taste masked formulations of diclofenac (diclofenac acid (DA), diclofenac sodium (DS), and diclofenac potassium (DP) were assessed by dissolution studies and compared with e-tongue results. The in vitro dissolution results revealed that 15% DA was released after 5 min dissolution time interval, whereas almost 100% was released in case of DS and DP. In line with these results, e-tongue studies also demonstrated least bitterness score with DA compared to other two forms (19). Thus, there is a relationship between the amounts of drug released in dissolution studies and the e-tongue studies. Even with a good correlation between lower drug release in a dissolution test and taste masking effectiveness, the human panel studies are always better and more objective tools to evaluate the formulation efficiency in masking bitterness. Therefore, in order to get the true and objective usefulness of KLD as a taste-masking agent, we focused our further studies on human taste panel evaluation for both APIs (GRI and CA) processed by HME and as a PM.
Human Panel Studies
Initially, the volunteers were asked to taste neat API solutions starting with water (Blank) and with API solutions in the increasing order of API concentration (20–150 mg for GRI and 0.5–40 mg for CA). In this step, the bitterness recognition threshold for all the subjects was assessed (Tables III and IV). After selection of qualified subjects and a washout period of 12–24 h, the individuals were asked to taste the products (physical mixture or hot melt extruded formulation) randomly (blinded) and asked to score the product. The results from the human panel studies demonstrated strong bitter taste with GRI and CA in the case of physical mixture with the average score of 4.08 ± 1.11 and 4.27 ± 0.75, respectively. Whereas, the HME formulation of GRI resulted with a mean score of 1.50 ± 0.63 and CA formulation resulted with a mean score of 1.83 ± 0.96 (Fig. 8). Since there is statistically significant difference (P < 0.05) in the bitterness score between HME formulation and PM, the results of the human panel studies confidently can confirm the effective taste masking of GRI and CA when extruded by HME using KLD as taste masking polymer.
Table III.
Taste Scores Given by Each Individual to Physical Mixture and HME Formulation for Griseofulvin
| Amount (mg) of API in 2 mL water | Volunteers | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| Water (no API) | – | – | – | – | – | – | ||
| 20 | – | 1 | 1 | 0 | – | 1 | Threshold of the subjects is in this range | |
| 40 | 1 | 2 | 3 | 2 | 1 | 2 | ||
| 80 | 2 | 4 | 3 | 5 | 2 | 4 | ||
| 150 | 4 | 4 | 5 | 5 | API equal to that in test formulation | |||
| Product testing (1 g powder/granules) equivalent to 150 mg of GRI | Average | Standard Deviation | ||||||
| Physical mixture | 4.5 | 2 | 4 | 4 | 5 | 5 | 4.08 | 1.11 |
| HME granules | 1 | 2 | 2 | 1.5 | 2 | 0.5 | 1.5 | 0.63 |
Table IV.
Taste Scores Given by Each Individual to Physical Mixture and HME Formulation for Caffeine Anhydrous
| Amount (mg) of API in 2 ml water | Volunteers | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||
| Water (no API) | – | – | – | – | – | – | – | – | – | ||
| 0.5 | 1 | – | – | – | – | 1 | 1 | – | 1 | Threshold of the subjects is in this range | |
| 1 | 2 | – | 1 | – | 1 | 4 | 3 | 1 | 2 | ||
| 5 | 3 | 1 | 4 | 1 | 3 | 5 | 4 | 4 | 3 | ||
| 10 | 4 | 2 | 4.5 | 5 | 5 | 5 | 5 | 4 | |||
| 40 | 5 | 4 | 5 | 5 | API equal to that in test formulation | ||||||
| Product testing (250 mg powder/granules) equivalent to 40 mg of CA | Average | Standard Deviation | |||||||||
| Physical mixture | 5 | 3.5 | 4 | 5 | 3 | 5 | 4 | 4 | 5 | 4.28 | 0.75 |
| HME granules | 1 | 2 | 2 | 3 | 2 | 0.5 | 2.5 | 3 | 0.5 | 1.83 | 0.97 |
Fig. 8.
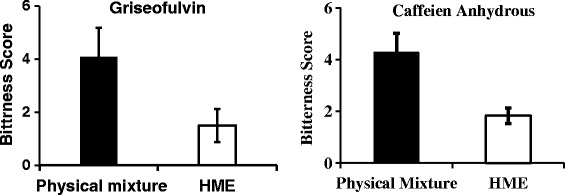
Average scores given by volunteers for griseofulvin and caffeine anhydrous drug in physical mixture and HME formulations
CONCLUSIONS
Hot melt extrusion of KLD with xylitol as a plasticizer demonstrated very good extrudability compared to all other plasticizers screened in the project. Furthermore, taste masked formulation of GRI and CA was extruded at 15% w/w drug load with 15% w/w xylitol and 70% w/w KLD. Lower release (good taste masking) of GRI and CA from the in vitro dissolution studies and results from the human panel studies suggest the potential use of Kleptose Linecaps DE17 in development of taste masked formulation by HME.
Acknowledgments
The authors acknowledge the team of scientists at Institute for Drug Delivery and Biomedical Research, Bangalore for valuable input and technical support for sensory evaluation of the products.
Conflict of Interest
Authors report no conflict of interest.
Abbreviations
- KLD
Kleptose linecaps DE17
- HME
Hot melt extrusion
- GRI
Griseofulvin
- CA
Caffeine anhydrous
- PM
Physical mixture
- API
Active pharmaceutical ingredient
- DSC
Differential scanning calorimetry
- TGA
Thermogravimetric analysis
References
- 1.Douroumis D. Practical approaches of taste masking technologies in oral solid forms. Expert Opin Drug Deliv. 2007;4(4):417–26. doi: 10.1517/17425247.4.4.417. [DOI] [PubMed] [Google Scholar]
- 2.Douroumis D. Orally disintegrating dosage forms and taste-masking technologies; 2010. Expert Opin Drug Deliv. 2011;8(5):665–75. doi: 10.1517/17425247.2011.566553. [DOI] [PubMed] [Google Scholar]
- 3.Woertz K, Tissen C, Kleinebudde P, Breitkreutz J. Rational development of taste masked oral liquids guided by an electronic tongue. Int J Pharm. 2010;400(1–2):114–23. doi: 10.1016/j.ijpharm.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F. Pharmaceutical applications of hot-melt extrusion: Part II. Drug Deve Ind Pharm. 2007;33(10):1043–57. doi: 10.1080/03639040701525627. [DOI] [PubMed] [Google Scholar]
- 5.Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK. Pharmaceutical applications of hot-melt extrusion: part I. Drug Deve Ind Pharm. 2007;33(9):909–26. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]
- 6.Almeida A, Possemiers S, Boone MN, De Beer T, Quinten T, Van Hoorebeke L. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. Eur J Pharm Biopharm. 2011;77(2):297–305. doi: 10.1016/j.ejpb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Verhoeven E, De Beer TR, Van den Mooter G, Remon JP, Vervaet C. Influence of formulation and process parameters on the release characteristics of ethylcellulose sustained-release mini-matrices produced by hot-melt extrusion. Eur J Pharm Biopharm. 2008;69(1):312–9. doi: 10.1016/j.ejpb.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Verhoeven E, De Beer TR, Schacht E, Van den Mooter G, Remon JP, Vervaet C. Influence of polyethylene glycol/polyethylene oxide on the release characteristics of sustained-release ethylcellulose mini-matrices produced by hot-melt extrusion: in vitro and in vivo evaluations. Eur J Pharm Biopharm. 2009;72(2):463–70. doi: 10.1016/j.ejpb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Zhang F, McGinity JW. Properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot-melt extrusion. Eur J Pharm Biopharm. 2001;52(2):181–90. doi: 10.1016/S0939-6411(01)00162-X. [DOI] [PubMed] [Google Scholar]
- 10.Azarmi S, Roa W, Lobenberg R. Current perspectives in dissolution testing of conventional and novel dosage forms. Int J Pharm. 2007;328(1):12–21. doi: 10.1016/j.ijpharm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Wie B, Liang D, Bates TR. Development and validation of a HPLC method to determine griseofulvin in rat plasma: application to pharmacokinetic studies. Anal Chem Insights. 2008;3:103–9. doi: 10.4137/aci.s953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Repka MA, Gerding TG, Repka SL, McGinity JW. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot melt extrusion. Drug Dev Ind Pharm. 1999;25(5):625–33. doi: 10.1081/DDC-100102218. [DOI] [PubMed] [Google Scholar]
- 13.Greet V. The influence of plasticizers in hot-melt extrusion: Pharmaceutical applications. First Edition, edited by Dennis. 2012.
- 14.Verreck G, Decorte A, Heymans K, Adriaensen J, Liu D, Tomasko D. Hot stage extrusion of p-amino salicylic acid with EC using CO2 as a temporary plasticizer. Int J Pharm. 2006;327(1–2):45–50. doi: 10.1016/j.ijpharm.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Verreck G, Decorte A, Heymans K, Adriaensen J, Cleeren D, Jacobs A. The effect of pressurized carbon dioxide as a temporary plasticizer and foaming agent on the hot stage extrusion process and extrudate properties of solid dispersions of itraconazole with PVP-VA 64. Eur J Pharm Biopharm. 2005;26(3–4):349–58. doi: 10.1016/j.ejps.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Siewert M, Dressman J, Brown CK, Shah VP. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech. 2003;4(1):E7. doi: 10.1208/pt040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand V, Kataria M, Kukkar V, Saharan V, Choudhury PK. The latest trends in the taste assessment of pharmaceuticals. Drug Discov. 2007;12(5–6):257–65. doi: 10.1016/j.drudis.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Bovet LL, Zhao K. Taste masking microspheres for orally disintegrating tablets. Int J Pharm. 2008;359(1–2):63–9. doi: 10.1016/j.ijpharm.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Guhmann M, Preis M, Gerber F, Pollinger N, Breitkreutz J, Weitschies W. Development of oral taste masked diclofenac formulations using a taste sensing system. Int J Pharm. 2012;438(1–2):81–90. doi: 10.1016/j.ijpharm.2012.08.047. [DOI] [PubMed] [Google Scholar]


