Abstract
Iron is essential for the growth and survival of many organisms. Intracellular iron homeostasis must be maintained for cell survival and protection against iron toxicity. The ferric uptake regulator protein (Fur) regulates the high-affinity ferric uptake system in many bacteria. To investigate the function of the fur gene in Xanthomonas vesicatoria (Xv), we generated a fur mutant strain, fur-m, by site-directed mutagenesis. Whereas siderophore production increased in the Xv fur mutant, extracellular polysaccharide production, biofilm formation, swimming ability and quorum sensing signals were all significantly decreased. The fur mutant also had significantly reduced virulence in tomato leaves. The above-mentioned phenotypes significantly recovered when the Xv fur mutation allele was complemented with a wild-type fur gene. Thus, Fur either negatively or positively regulates multiple important physiological functions in Xv.
Introduction
Iron is an essential trace metal element for living organisms because it serves as an enzymatic cofactor and a component of electron transport proteins. Phytopathogenic bacteria use iron as an environmental signal to regulate virulence genes [1]. However, iron can also be toxic when the intracellular concentration exceeds a critical level. This condition can induce detrimental oxidative stress by promoting Fe (II)-mediated forms of reactive oxygen via Fenton reactions [1,2]. Therefore, most bacteria maintain iron homeostasis via the ferric uptake regulator protein (Fur) [3]. The promoter regions of genes negatively regulated by the Fur protein typically have a conserved 19-bp sequence termed the “Fur box” [2,4,5]. During plant-microbe interactions, Fur plays a significant role in maintaining iron balance to reduce the toxicity of reactive oxygen [6]. In addition, Fur has been shown to regulate the expression of key virulence factors in pathogenic bacteria [4]. The Fur protein is a global regulator because it regulates genes related to iron uptake as well as the expression of many genes related to chemotaxis, the tricarboxylic acid cycle, glycolysis, oxidative stress, resistance redox and quorum sensing [2,3,6–8].
Bacterial spot of tomato (Solanum lycopersicum L.) and pepper (Capsicum annuum) is caused by the Gram-negative bacterium, Xanthomonas vesicatoria (Xv). Previous research has identified four phenotypic xanthomonad groups: group A (X. euvesicatoria), group B (Xv), group C (X. perforans) and group D (X. gardneri) [9]. Chen (2010) determined the phenotypic groups of more than 100 Xv strains collected in China [10]. Pathogenic Xv strains attack leaves, stems, fruits, and flowers. Leaf spots are initially small, but when spots are numerous, foliage turns yellow and eventually dies, leading to the defoliation of the plants. This disease has been reported in Europe, Asia, the Americas and other parts of the world [9]. The pepper pathogenic strain has been sequenced and used as a well-established model for studying bacterium-plant interactions [11], including the type III secretion system and several virulence genes [12,13]. However, the function of Fur in Xv has not previously been elucidated. In this communication, we investigated the role of fur in Xv 17, a wild-type Xv strain belonging to group B of xanthomonads that was isolated from tomato fruit in Xinjiang, China [10].
Materials and Methods
Media and bacterial growth conditions
All Xv strains were routinely cultured in solid yeast extract-dextrose-CaCO3 (YDC) medium [14] or liquid Luria-Bertani medium (LB) [15] at 28°C with continuous shaking (180 rpm). Escherichia coli strains were cultured in LB medium at 37°C with continuous shaking (180 rpm). Erwinia carotovora strains were cultured in LB medium at 28°C with continuous shaking (180 rpm). Antibiotics were added to appropriate media at the following concentrations: gentamicin (Gm) 50 μg·ml-1, rifampin (Rif) 50 μg·ml-1, kanamycin (Km) 50 μg·ml-1, chloramphenicol (Cm) 20 μg·ml-1 and ampicillin (Ap) 100 μg·ml-1, respectively. All bacterial strains and plasmids used in this study are listed in Table 1.
Table 1. Bacterial strains and plasmids used in this study.
| Designation | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| Xanthomonas vesicatoria | ||
| 17 | Wild-type, Rif R | This study |
| fur-m | fur mutant strain, containing truncated fur gene and Gm cassette, Rif R, GmR | This study |
| fur-c | fur complementation strain, fur-m containing pMLfur, RifR, KmR, GmR | This study |
| Escherichia coli | ||
| DH5α | Competent cells, Φ80 lacZ | TaKaRa (China) |
| pRK600 | Helper strain in triparental matings, CmR | [18] |
| Agrobacterium tumefaciens | ||
| NTL4 | Quorum-sensing reporter strain, containing traG::lacZ, tra reporter, GmR | [55] |
| Erwinia carotovora subsp. carotovora | ||
| 3 | Indicator strain for quorum sensing | This study |
| Plasmids | ||
| PMD18-T | T-vector, ApR | TaKaRa (China) |
| pK18mob | Cloning and suicide vector with a sacB gene, KmR | [17] |
| pKfur | pK18mob containing a 1572-bp fragment containing fur and its 3’ and 5’ sequences, KmR | This study |
| pKfurGm | pK18 containing wild-type fur with its 209-bp replaced by Gm cassette, KmR, GmR | This study |
| pML123 | Complementation plasmid, Km R, GmR | [16] |
| pMLfur | pML123 containing a 618-bp fragment of fur and its promoter region, KmR, GmR | This study |
aRifR, ApR, KmR, GmR and CmR indicate resistance to rifampicin, ampicillin, kanamycin, gentamicin and chloramphenicol, respectively.
Construction of the fur mutant
The fur gene in the wild-type strain Xv17 was inactivated by homologous integration as described by Windgassen et al. [16], using the suicide vector pK18mob [17]. Primers for PCR amplification were designed using the free online program Primer 5.0 (Table 2). Each reaction mixture contained 0.5 μl of DNA template, 6.25 μl of 2×PCR Mix (TaKaRa, Bao Biological Engineering (Dalian) Co., Ltd, Dalian, China) and 0.5 μl of each primer for a total reaction volume of 12.5 μl. The PCR conditions were 94°C for 3 min, 25 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 90 s, followed by 72°C for 5 min. The 1,572-bp fragment of Xv17 amplified by the fur1 and fur2 primers contained a 411-bp coding region of the fur gene, as well as the 633- and 528-bp upstream and downstream sequences of the gene (Table 2). After confirmation by sequencing, the fragment was digested by EcoRI and HindIII and cloned into pK18mob to create plasmid pKfur. pKfur was digested with BamHI and NdeI and a 209-bp fragment inside the 411-bp fur gene region was replaced with a Gm gene cassette (855 bp) to create plasmid pKfurGm (Table 1). pKfurGm was introduced from E. coli DH5 into Xv17 by triparental conjugation using pRK600 [18] as a helper plasmid. Transconjugants were screened on YDC supplemented with 10% sucrose and antibiotics (Rif and Gm) and confirmed by PCR using the fur1 and fur2 primers. To confirm the presence of the Gm cassette in the transconjugants, Southern blotting was performed with primers Gm1 and 2 using the marker BM5000 (Biomed, 5,000 bp, 3,000 bp, 2,000 bp, 1,000 bp, 750 bp, 500 bp, 250 bp, 100 bp) as the probe. The confirmed fur mutant strain, fur-m (Table 2), was used for subsequent studies.
Table 2. PCR primers used in this study.
| Primers | Sequence (5’-3’, restriction enzyme sites are underlined | Product of PCR |
|---|---|---|
| fur1 | GAATTCATCGGTCCTGGGAGTC EcoRI | 1572 bp |
| fur2 | AAGCTTCGGCGTGGAAGTGA HindIII | |
| Gm1 | GGATCCGACGCACACCGTGGAAA BamHI | 855 bp |
| Gm2 | CATATGGCGGCGTTGTGACAATTT NdeI | |
| hb1 | AAGCTTTCAGATTGCCCTGGTAG EcoRI | 618 bp |
| hb2 | TCTAGAGGGACACCCAGCTCA HindIII |
Construction of the complemented fur strain
The complementation sequence of the fur gene (618 bp, including the 411-bp fur gene and its promoter region) in Xv17 was amplified using primers hb1 and hb2 (Table 2). The DNA fragment was cloned into pML123 to generate pMLfur (Table 1), which was transferred into the mutant strain fur-m by triparental conjugation. One transconjugant named fur-c was identified through screening on YDC (amended with Rif, Km and Gm (Table 1)). All obtained plasmids and Xv strains were confirmed by PCR and DNA sequencing.
Detection of siderophore production
Siderophore production was measured using chrome azural S (CAS) agar plates and solutions [19]. Bacterial cell suspensions (OD600 = 1.0) were spotted onto a CAS plate and incubated at 28°C for 2 days. Siderophore production was indicated by the presence of a yellow halo around the bacterial colony. To measure siderophore production quantitatively, Xv17, fur-m and fur-c were cultured in LB medium at 28°C, and 0.5 ml of cell suspension of each Xv strain was mixed with 0.5 ml of CAS assay solution every 3 h for 36 h. Two hours after each mixing, the absorbance at 630 nm was measured using a spectrophotometer (Thermo Fisher Scientific). Siderophore production was calculated as follows: [(Ar-As)/Ar]×100%, where Ar and As represent the absorbance of uninoculated and inoculated media, respectively. There were three replicates per treatment and the experiment was repeated three times.
Analyses of biofilm formation
Overnight cultures of Xv17, fur-m and fur-c were adjusted to an OD600 of 1.5. One hundred microliters of each cell suspension was transferred into 10 ml of LB broth in glass test tubes. The liquid cultures were incubated at 28°C for 5 days, and then, the broth was poured out slowly. After drying at 37°C for 1 h, biofilms on the surface of the test tubes were stained with 0.1% methyl violet for 30 min. A ring of violet precipitate developing on the inner wall of the tube indicated biofilm formation. Biofilm formation was also analyzed quantitatively by solubilizing the stained biofilms with 95% ethanol for 1 h and measuring the OD590 of the stained suspension with a spectrophotometer [20].
Detection of quorum sensing (QS) signaling molecules
To assay for the production of QS signaling molecules, A. tumefaciens strain NTL4 (pZLR4) was cultured in 10 ml of AB minimal medium (ABM) [21] at 28°C for 12 h. Six milliliters of the overnight culture and 100 μl of X-gal (20 mg·ml-1) were added to 100 ml of ABM plates (1.2% agar). Two microliters of overnight cultures of strains E. carotovora subsp. carotovora Ecc-3, Xv17, fur-m or fur-c were spotted on each of the ABM plates separately and incubated at 28°C for 12–18 h. A blue halo around colonies indicated the production of QS signal molecules.
Assay for swimming motility
The swimming motility was assayed as described previously [22] with some modifications. Overnight cultures of the Xv strains were adjusted to an OD600 of 0.5 with fresh LB medium. The swimming motility assay was initiated by spotting 2 μl of each cell suspension at the center of 0.3% LB agar plates amended with appropriate antibiotics. The plates were incubated at 28°C, and the halos formed by migrating bacteria were measured after 2 d. The colony diameter of each strain was also measured.
Measurement of extracellular polysaccharide (EPS) production
EPS production was measured as described by Tang et al. [23] with some modifications. Cultures of each Xv strain were grown in LB medium for 4 d until their OD600 reached 2.5. After centrifuging at 10,000 rpm for 10 min, the supernatant was precipitated with ethanol and EPS was collected, dried at 37°C for 3 h and weighed.
Virulence assay on tomato
Overnight cultures of Xv17, fur-m and fur-c were adjusted with fresh LB to an OD600 of 0.5. Thirty milliliters of each inoculum was spray inoculated to six 50-day-old tomato plants of a highly susceptible cultivar (Zhongshu No.4) under greenhouse conditions (25–30°C and 80% relative humidity). The number of leaf spots on the most severely infected leaf of each inoculated plant was counted to compare bacterial spot severity. LB medium was used as a negative control.
Statistical analysis
All experiments in this study were performed at least three times. All values are shown as the mean ± standard deviation (SD). Data were subjected to ANOVA and Duncan’s multiple range tests using the Statistical Package for the Social Sciences (SPSS) software version 17.0. Differences were considered statistically significant at P<0.05.
Results
Confirmation of the Xv fur mutant and complementation strains
The fur gene encodes the Fur protein which is 136 amino acids in length based on the genome sequence of Xv [11] (GenBank accession number AF146022). PCR amplification of the fur mutant strain fur-m with the fur1 and fur2 primers and the subsequent sequencing of the PCR product confirmed that strain fur-m contained a band of 2,218 bp, consisting of a truncated fur gene interrupted by the Gm cassette (data not shown). The presence of the Gm cassette (855 bp) in fur-m was further confirmed by Southern blot and was absent from the wild-type strain Xv17 (data not shown). The fur-m strain was stable after continuous culturing for 20 generations in YDC medium. The fact that the fur complementation strain fur-c was KmR suggested the successful transfer of the plasmid pMLfur into the fur-m strain. The presence of pMLfur in fur-c was further confirmed by PCR using the hd1 and hd2 primers, as two PCR bands were amplified as expected. One band was 618-bp, amplified from pMLfur DNA, and the other was 1,264-bp, amplified from fur-m DNA (data not shown).
Role of fur in Xv siderophore production
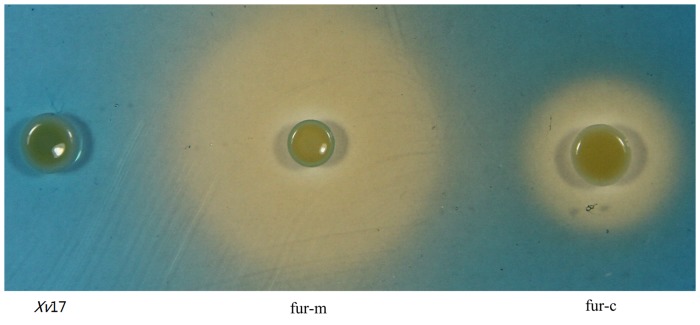
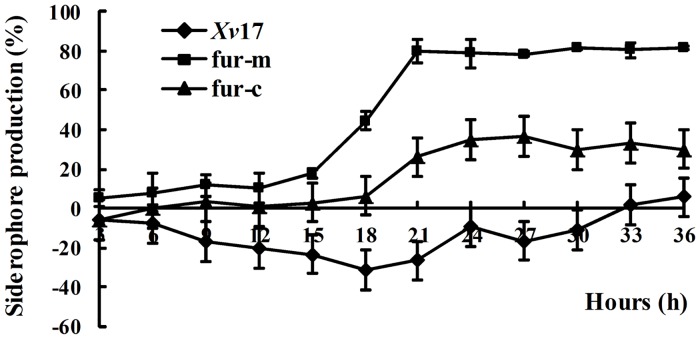
The fur mutant strain fur-m and the fur complemented strain fur-c showed increased siderophore production relative to the wild-type strain Xv17 as indicated by the presence of yellow halos around their colonies (Fig 1). Xv17 lacked a yellow halo, indicating undetectable levels of siderophore production (Fig 1). These results suggested that the increased siderophore production phenotype may be due to the absence of the fur gene. Siderophore production for fur-m and fur-c were slight at 15 h and increased to a maximum level at 21 and 27 h after incubation, respectively (Fig 2). From those times on, the siderophore production remained fairly constant. Maximum siderophore production by fur-m and fur-c was 81.21% at 21 h and 36.44% at 27 h, respectively (Fig 2). However, Xv17 produced little siderophore. Siderophore production in fur-m was significantly higher than that in fur-c throughout the experiment (P<0.05). However, the siderophore production phenotype of fur-m was not completely complemented by an expression vector containing the wild-type fur gene (Fig 2).
Fig 1. Comparison of siderophore production in Xv strains using the Chrome azurol S assay.
Yellow halos around bacterial colonies indicate siderophore production. Xv17: wild-type Xv strain; fur-m: fur mutation strain of Xv 17; and fur-c: fur complementation strain of fur-m.
Fig 2. Quantitative comparison of siderophore production in Xv strains.
Black diamond: wild-type strain Xv17; black square: fur mutation strain fur-m; and black triangle: fur complementation strain fur-c.
Role of fur in Xv biofilm formation
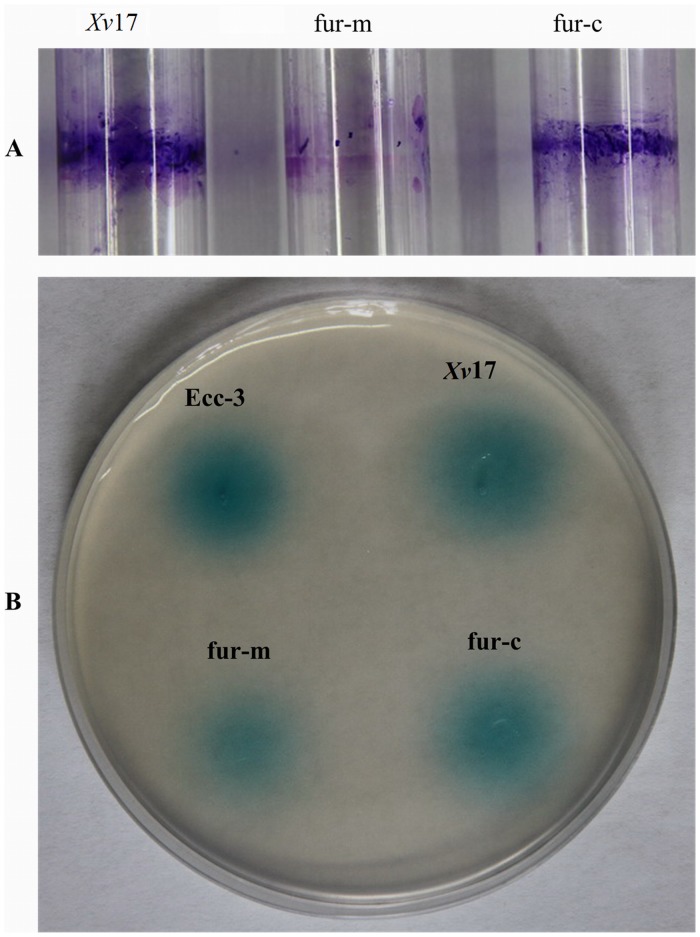
Because biofilm formation may influence the Xv colonization of tomato seed (data not shown), we investigated whether fur plays a role in biofilm formation by Xv. Our qualitative biofilm assay revealed that Xv17 and fur-c formed a ring of biofilm on the surface of glass test tubes, whereas fur-m did not under our conditions (Fig 3A). This observation was confirmed by our quantitative biofilm assay, as the mean absorption value of the biofilm by fur-m (0.058) was significantly lower than that of Xv17 (0.14) and fur-c (0.15) (P<0.05) (Table 3).
Fig 3. Effect of fur on biofilm formation (A) and quorum sensing (QS) signals in Xv (B).
Biofilm formation is indicated by the ring formed on the surface of glass test tubes. QS signal expression was indicated by blue halos 12–18 h after each strain was spotted and after incubation on ABM plates at 28°C. A QS assay was used to detect signaling molecules as a blue halo. Xv17: wild-type Xv strain; fur-m: fur mutation strain of Xv17; and fur-c: fur complementation strain of fur-m. Ecc3: E. carotovora subsp. carotovora strain 3 as a positive control for expression of QS signals.
Table 3. Quantitative measurement of biofilm formation as indicated by absorbance at OD590 in Xv.
| Strain | Absorbance value at OD590 |
|---|---|
| Xv17 | 0.1395±0.0180 a* |
| fur-m | 0.0576±0.0082 b |
| fur-c | 0.1502 ±0.0220 a |
*Values are means of three Experiments with three replicates in each experiment. Values followed by different letters are significantly different (P < 0.05) based on Duncan’s multiple range test.
Involvement of fur in QS
To determine whether the fur gene was involved in Xv QS, we compared Xv17, fur-m and fur-c in terms of their involvement in QS signaling molecule production (Fig 3B). Xv17 and fur-c produced a blue halo that was similar to that of Ecc-3 (positive control), indicating QS signal molecule production, whereas fur-m had a smaller and lighter blue halo (Fig 3B).
Role of fur in swimming motility
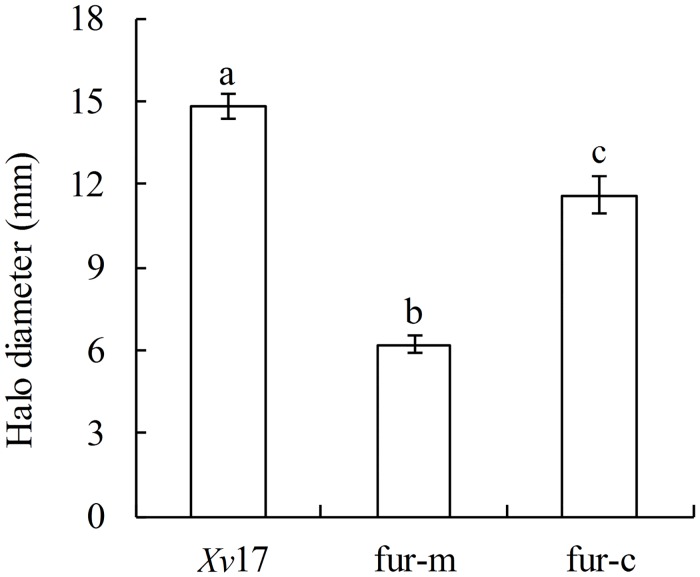
Our assays for swimming motility revealed that halos formed by Xv17 and fur-c expanded to more than 11 mm in two days, whereas those by fur-m did not expand to more than 7 mm. fur-c largely restored the swimming ability of fur-m, but its halo diameter was still significantly smaller than that of Xv17 (P < 0.05). Xv17 (14.83 ± 0.45 mm), fur-m (6.20 ± 0.30 mm) and fur-c (11.60 ± 0.69 mm) differed significantly in their swimming abilities based on the diameters of their halos (P < 0.05) (Fig 4).
Fig 4. Comparison of swimming motility in Xv strains.
Bar heights are the mean diameter of the swimming halos from three replicates ± standard deviation (SD). Different letters above the bars indicate significantly different diameters (P < 0.05) based on Duncan’s multiple range test.
Involvement of fur in Xv EPS production
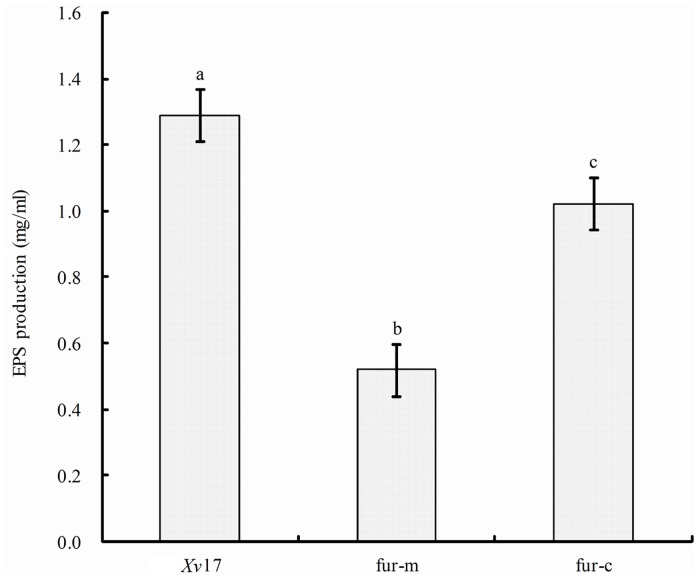
After 4 days of growth in liquid LB medium, fur-m produced 0.52 mg of EPS precipitate per ml of culture compared to 1.28 mg for Xv17 and 1.02 mg for fur-c (Fig 5). fur-m produced significantly less EPS than Xv17 and fur-c (P < 0.05) (Fig 5).
Fig 5. Effect of fur in extracellular EPS production in Xv.
Xv17, fur-m and fur-c were cultured, and their EPS was dried and weighed. Bar heights are the mean EPS weights from three experiments with three replicates in each experiment. Different letters above the bars indicate significantly different EPS weights (P < 0.05) based on Duncan’s multiple range test.
Contributions of fur to Xv virulence
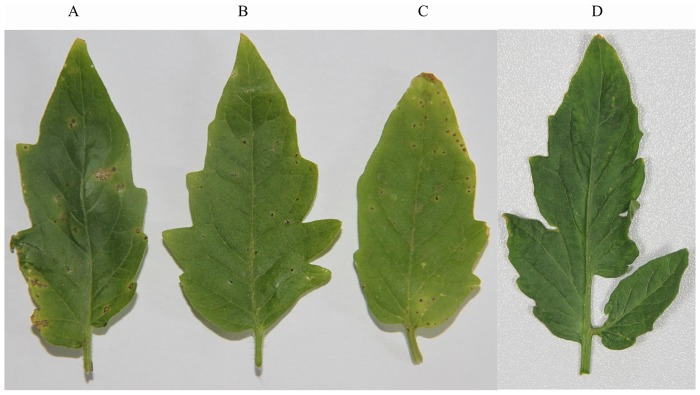
The bacterial spot severity caused by the mutant strain fur-m in tomato leaves was less than that of the wild-type strain Xv17. Xv17 produced blackish-brown spots surrounded by a yellow halo (Fig 6A), and its mean number of leaf spots was 10.33 ± 1.03 (Table 4). In contrast, only black spots appeared on the leaves that were spray-inoculated with the mutant strain fur-m. Moreover, the spots were smaller and did not develop a yellow halo (Fig 6B). The spots of Xv17and fur-c appear to be similar (Fig 6C). fur-m produced fewer spots per leaf (4.67 ± 0.81) than Xv17. The number of spots elicited by the complementation strain fur-c (10.50 ± 1.05) was not significantly different from that of Xv17 (Table 4) (P < 0.05). The difference in virulence between Xv17 and fur-m was significant (P < 0.05).
Fig 6. Effect of fur in Xv virulence on tomato leaves.
Fifty-day-old tomato plants (cv No.4 Zhongshu) were spray inoculated with A) Xv17; B) fur-m; C) fur-c; and D) LB medium (negative control).
Table 4. Virulence of Xv strains on tomato leaves.
| Strain | Mean # of leaf spotx |
|---|---|
| Xv17 | 10.33 ± 1.03 a |
| fur-m | 4.67 ± 0.70 b |
| fur-c | 10.50 ± 1.05 a |
xVirulence was tested on 50-day-old tomato plants (cv No. 4 Zhongshu) that were spray-inoculated with each tested strain separately. Values are the mean numbers of leaf spots per leaf (n = 6). Values followed by different letters are significantly different (P < 0.05) based on Duncan’s multiple range test.
Discussion
Although Fur has been studied in a number of plant pathogenic bacteria including X. oryzae pv. oryzae, X. campestris pv. campestris and Edwardsiella tarda [5–8], its role in Xv has not been previously studied. We investigated the functions of fur in Xv by constructing a fur mutant strain fur-m. Compared with wild-type strain Xv17 which did not produce any siderophore, fur-m produced a significant amount of siderophore, indicating that fur plays an important role in the negative regulation of siderophore synthesis in Xv. In addition, biofilm formation, the production of QS signaling chemicals, swimming motility, EPS production and virulence on tomato were all significantly reduced in fur-m, suggesting that fur in Xv plays an important role, either directly or indirectly, in the regulation of these virulence or virulence-related functions.
Similar to other bacteria, such as X. campestris [24], Pseudomonas aeruginosa [25], and Bacillus subtilis [26], Fur negatively regulates siderophore synthesis in Xv, as evidenced by the presence of yellow halos around the colonies of mutant strain fur-m but the absence of such halos around wild-type strain Xv17. In addition, when the fur mutation was complemented by a wild-type fur gene in a complementation vector (pMLfur), the resulting strain fur-c significantly reduced the siderophore production compared to fur-m, further suggesting that fur is involved in the negative regulation of siderophore synthesis in Xv. Why fur-c still produced a significant amount of siderophore 15–36 h after growth remains unclear (Fig 2), as the fur complemented strains in X. campestris did not produce any visible amount of siderophore [24]. The fur gene may need to be present in cis in Xv genome, not provided in trans in a plasmid, to completely inhibit siderophore synthesis and fully restore the production of EPS and swimming ability in Xv.
Bacterial biofilms are considered to be resistant to environmental stress on the plant surface [27–29] and to offer protection from the antimicrobial compounds secreted by plants [27,30]. When fur was mutated, the mutant strain produced little biofilm relative to the wild-type and the fur complementation strains, suggesting that fur is important for biofilm formation in Xv.
Quorum sensing regulates a variety of physiological functions in bacteria, including motility, conjugation, competence, sporulation, secretion, antibiotic production, virulence and biofilm formation [29, 31]. Numerous Xanthomonas species have evolved QS systems for genetic regulation at the community level [32]. fur has been reported to control the expression of the psyR and psyI genes involved in QS in Pseudomonas syringae pv. tabaci [2]. Previous studies have demonstrated that N-acyl homoserine lactone (N-AHL) [33], LuxR and LuxI [34], lasI/rhl I [35], qscR [36] and aiiA [37] are all involved in QS and contribute to virulence on their host plants. Our results indicated the reduced production of QS signal molecules in fur-m, suggesting either a direct or indirect relationship between fur and QS.
Motility is considered to be an important epiphytic fitness trait, enabling bacterial cells to locate resources and access sites that allow them to avoid environmental stresses [38]. Motility regulation is associated with N-AHL-dependent genes (QS system) in P. syringae [33] and the GacS/GacA system in P. aeruginosa [39]. GacS/GacA-dependent gene regulation can be considered as part of the QS machinery [40]. Our results suggest that fur may be at least partially involved in regulating Xv motility because the swimming motility was significantly reduced for the Xv fur mutant strain. Although fur was related to QS and swimming, it was not clear whether swimming motility was regulated by the QS and Gac systems in Xv. Moreover, bacterial motility is a critical virulence factor because it facilitates pathogen entry into plant tissues [41]. EPS produced by pathogens are thought to be involved in adhesion, biofilm maintenance [42], heavy metal stress tolerance [43] and plant disease symptom development [44]. Many genes are known to regulate bacterial EPS production in vivo, including zur of X. oryzae pv. oryzae [45]; hrp in Xv [46]; the che, flg, flh and fli genes in E. coli K-12 strains [47]; and the luxR homologue in Vibrio alginolyticus [48]. Moreover, the deletion of these genes often results in the reduction of bacterial virulence. Deletion of the Xv fur gene significantly compromised EPS production. However, bioinformatic analysis indicates that the promoter regions of the above genes related to EPS production do not contain the Fur box to which Fur binds (data not shown). Thus, the mechanism by which Fur regulates EPS production remains the subject of future studies.
Xv is a serious threat to tomato and pepper production. fur has been found to be important for the virulence and pathogenicity of a number of pathogenic bacteria in animal and plant hosts [2,5,24,49,50]. The virulence of the Xv fur mutant strain was significantly reduced in our study, suggesting that fur contributes to Xv virulence on tomato. A previous study has shown that the rapid accumulation of reactive oxygen species in the plant is closely related to plant disease [51]. Bacteria could take advantage of the generation of active oxygen species to kill the host cells during invasion and colonization [52,53]. The X. campestris fur mutant was vulnerable to oxidative stress, which may at least partially account for the attenuated virulence phenotype of the fur mutant [54]. It is not surprising that our fur mutant was reduced in virulence, as in our study, many of the virulence or virulence-related factors, such as QS, biofilm formation, motility and EPS production, were all significantly reduced when fur was mutated. Our results suggest that in addition to siderophore production, fur also contributes significantly to other important physiological functions that directly or indirectly lead to the virulence of Xv in its host plants. Future research is needed to determine whether QS and the virulence factors, such as EPS production, biofilm formation and motility, are regulated directly by Fur or indirectly due to increased iron levels in fur mutant strains.
Acknowledgments
We are grateful to Liqun Zhang from China Agriculture University for providing plasmid pRK600 and the quorum-sensing reporter strain A. tumefaciens NTL4 for our study. We also thank Ron Walcott at the Department of Plant Pathology, University of Georgia, USA and John Hartung at USDA/ARS in Beltsville, MD, USA for their editing and critical review of our manuscript. We thank Youpu Cheng and Hong Long at the College of Horticulture and Landscape, Tianjin Agricultural University, China for modifying the figures. Finally, we thank American Journal Experts (AJE) for providing professional modification.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by the earmarked fund for Modern Agro-industry Technology Research System (CARS-26) and Special Fund for Agro-scientific Research in the Public Interest (201003066). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bender KS, Yen H-B, Hemme CL, Yang Z, He Z, He Q, et al. Analysis of a ferric uptake regulator (fur) mutant of Desulfovibrio vulgaris Hildenborough. Appl Environ Microbiol. 2007;73: 5389–5400. 10.1128/AEM.00276-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cha JY, Lee JS, Oh J, Choi JW, Baik HS. Functional analysis of the role of fur in the virulence of Pseudomonas syringae pv. tabaci11528: fur controls expression of genes involved in quorum-sensing. Biochem Biophys Res Commun. 2008;366: 281–287. 10.1016/j.bbrc.2007.11.021 [DOI] [PubMed] [Google Scholar]
- 3.Hantke K. Iron and metal regulation in bacteria. Curr Opin Microbiol. 2001;4: 172–177. 10.1016/S1369-5274(00)00184-3 [DOI] [PubMed] [Google Scholar]
- 4.Pennella MA, Giedroc DP. Structural determinants of metal selectivity in prokaryotic metal-responsive transcriptional regulators. Biometals. 2005;18: 413–428. 10.1007/s10534-005-3716-8 [DOI] [PubMed] [Google Scholar]
- 5.Wang F, Cheng S, Sun K, Sun L. Molecular analysis of the fur (ferric uptake regulator) gene of a pathogenic Edwardsiella tarda strain. J Microbiol. 2008;46: 350–355. 10.1007/s12275-008-0038-x [DOI] [PubMed] [Google Scholar]
- 6.Andrews SC, Robinson AK, Rodríguez-quiñones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27: 215–237. 10.1016/S0168-6445(03)00055-X [DOI] [PubMed] [Google Scholar]
- 7.Massé E, Escorcia FE, Gottesman S. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev. 2003;17: 2374–2383. 10.1101/gad.1127103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SJ, Gunsalus RP. Oxygen, iron, carbon, and superoxide control of the fumarase fumA and fumC genes of Escherichia coli: role of the arcA, fnr, and soxR gene products. J Bacteriol. 1995;177: 6255–6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones JB, Lacy GH, Bouzar H, Stall RE, Schaad NW. Reclassification of the Xanthomonads associated with bacterial spot disease of tomato and pepper. Syst Appl Microbiol. 2004;27: 755–762. 10.1078/0723202042369884 [DOI] [PubMed] [Google Scholar]
- 10.Chen X. Species and physiological races identification of bacterial spot pathogen on tomato and pepper in China. Bejing: Chinese Academy of Agricultural Sciences; 2010. [Google Scholar]
- 11.Thieme F, Koebnik R, Bekel T, Berger C, Boch J, Büttner D, et al. Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. Vesicatoria revealed by the complete genome sequence. J Bacteriol. 2005;187: 7254–7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Büttner D, Bonas U. Getting across-bacterial type III effector proteins on their way to the plant cell. EMBO J. 2002;21: 5313–5322. 10.1093/emboj/cdf536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Büttner D, Noël L, Thieme F, Bonas U. Genomic approaches in Xanthomonas campestris pv. Vesicatoria allow fishing for virulence genes. J Biotehnol. 2003;106: 203–214. [DOI] [PubMed] [Google Scholar]
- 14.Miller JH. Experiments in molecular genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 15.MacLean AM, MacPherson G, Aneja P, Finan TM. Characterization of the β-ketoadipate pathway in Sinorhizobium meliloti. Appl Environ Mirobiol. 2006;72: 5403–5413. 10.1128/AEM.00580-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Windgassen M, Urban A, Jaeger K. Rapid gene inactivation in Pseudomonas aeruginosa. FEMS Microbiol Lett. 2000;193: 201–205. 10.1111/j.1574-6968.2000.tb09424.x [DOI] [PubMed] [Google Scholar]
- 17.Labes M, Pühler A, Simon R. A new family of RSF1010-derived expression and lac-fusion broad-host-range vectors for Gram-negative bacteria. Gene. 1990;89: 37–46. 10.1016/0378-1119(90)90203-4 [DOI] [PubMed] [Google Scholar]
- 18.Cha C, Gao P, Chen Y, Shaw PD, Farrand SK. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. MPMI. 1998;11: 1119–1129. 10.1094/MPMI.1998.11.11.1119 [DOI] [PubMed] [Google Scholar]
- 19.Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160: 47–56. 10.1016/0003-2697(87)90612-9 [DOI] [PubMed] [Google Scholar]
- 20.Kaplan JB, Ragunath C, Velliyagounder K, Fine DH, Ramasubbu N. Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother. 2004;48: 2633–2636. 10.1128/AAC.48.7.2633-2636.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chilton M-, Currier TC, Farrand SK, Bendich AJ, Gordon MP, Nester EW. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci U S A. 1974;71: 3672–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young GM, Smith MJ, Minnich SA, Miller VL. The Yersinia enterocolitica motility master regulatory operon, flhDC, is required for flagellin production, swimming motility, and swarming motility. J Bacteriol. 1999;181: 2823–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang JL, Liu YN, Barber CE, Dow JM, Wootton JC, Daniels MJ. Genetic and molecular analysis of a cluster of rpf genes involved in positive regulation of synthesis of extracellular enzymes and polysaccharide in Xanthomonas campestris pathovar campestris. Mol Gen Genet. 1991; 226: 409–417. [DOI] [PubMed] [Google Scholar]
- 24.Jittawuttipoka T, Sallabhan R, Vattanaviboon P, Fuangthong M, Mongkolsuk S. Mutations of ferric uptake regulator (fur) impair iron homeostasis, growth, oxidative stress survival, and virulence of Xanthomonas campestris pv. Campestris. Arch Microbiol. 2010;192: 331–339. 10.1007/s00203-010-0558-8 [DOI] [PubMed] [Google Scholar]
- 25.Ochsner UA, Vasil AI, Vasil ML. Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophores and exotoxin a expression: purification and activity on iron-regulated promoters. J Bacteriol. 1995;10: 7194–7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ollinger J, Song KB, Antelmann H, Hecker M, Helmann JD. Role of the fur regulon in iron transport in bacillus subtils. J Bacteriol. 2006;3: 3664–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahar O, Goffer T, Burdman S. Type IV pili are required for virulence, twitching motility, and biofilm formation of Acidovorax avenae subsp. Citrulli. Mol Plant Microbe Interact. 2009;22: 909–920. 10.1094/MPMI-22-8-0909 [DOI] [PubMed] [Google Scholar]
- 28.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358: 135–138. 10.1016/S0140-6736(01)05321-1 [DOI] [PubMed] [Google Scholar]
- 29.Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55: 165–199. 10.1146/annurev.micro.55.1.165 [DOI] [PubMed] [Google Scholar]
- 30.Pujol C, Eugene E, Marceau M, Nassif X. The meningococcal PilT protein is required for induction of intimate attachment to epithelial cells following pilus-mediated adhesion. Proc Natl Acad Sci USA. 1999;96: 4017–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen MS, Chai Y, Britigan BE, McKenna W, Adams J, Svendsen T, et al. Role of extracellular iron in the action of the quinone antibiotic streptonigrin: mechanisms of killing and resistance of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1987;31: 1507–1513. 0066-4804/87/101507-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Y, Zhang L. Quorum sensing and virulence regulation in Xanthomonas campestris. FEMS Microbiol Rev. 2008;32: 842–857. 10.1111/j.1574-6976.2008.00120.x [DOI] [PubMed] [Google Scholar]
- 33.Quiñones B, Dulla G, Lindow SE. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol Plant Microbe Interact. 2005;18: 682–693. 10.1094/MPMI-18-0682 [DOI] [PubMed] [Google Scholar]
- 34.Latifi A, Winson MK, Foglino M, Bycroft BW, Stewart GSAB, Lazdunski A, et al. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol Microbiol. 1995;199: 333–343. [DOI] [PubMed] [Google Scholar]
- 35.Rumbaugh KP, Griswold JA, Iglewski BH, Hamood AN. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect Immun. 1999;67: 5854–5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chugani SA, Whiteley M, Lee KM, D’Argenio D, Manoil C, Greenberg EP. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2001;98: 2752–2757. 10.1073/pnas.051624298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong YH, Xu JL, Li XZ, Zhang LH. -, Aii A. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc Natl Acad Sci U S A. 2000;97: 3526–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindow SE, Andersen G, Beattie GA. Characteristics of insertional mutants of Pseudomonas syringae with reduced epiphytic fitness. Appl Environ Microbiol. 1993;59: 1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kay E, Humair B, Dénervaud V, Riedel K, Spahr S, Eberl L, et al. Two GacA-dependent small RNAs modulate the quorum-sensing response in Pseudomonas aeruginosa. J Bacteriol. 2006;188: 6026–6033. 10.1128/JB.00409-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kay E, Reimmann C, Haas D. Small RNAs in bacterial cell-cell communication. Microbe-American Society for Microbiology. 2006;1: 63. [Google Scholar]
- 41.Ichinose Y, Taguchi F, Mukaihara T. Pathogenicity and virulence factors of Pseudomonas syringae. J Gen Plant Pathol. 2013;79: 285–296. 10.1007/s10327-013-0452-8 [DOI] [Google Scholar]
- 42.Marcotte L, Kegelaer G, Sandt C, Barbeau J, Lafleur M. An alternative infrared spectroscopy assay for the quantification of polysaccharides in bacterial samples. Anal Biochem. 2007;361: 7–14. [DOI] [PubMed] [Google Scholar]
- 43.Kazy SK, Sar P, Singh SP, Sen AK, D’Souza SF. Extracellular polysaccharides of a copper-sensitive and a copper-resistant Pseudomonas aeruginosa strain: synthesis, chemical nature and copper binding. World J Microbiol Biotechnol. 2002;18: 583–588. 10.1023/A:1016354713289 [DOI] [Google Scholar]
- 44.Denny TP. Involvement of bacterial polysaccharides in plant pathogenesis. Annu Rev Phytopathol. 1995;33: 173–197. 10.1146/annurev.py.33.090195.001133 [DOI] [PubMed] [Google Scholar]
- 45.Yang W, Liu Y, Chen L, Gao T, Hu B, Zhang D, et al. Zinc uptake regulator (zur) gene involved in zinc homeostasis and virulence of Xanthomonas oryzae pv. Oryzae in rice. Curr Microbiol. 2007;54: 307–314. 10.1007/s00284-006-0485-8 [DOI] [PubMed] [Google Scholar]
- 46.Brown I, Mansfield J, Bonas U. The hrp genes in Xanthomonas campestris pv. Vesicatoria determine ability to suppress papilla deposition in pepper mesophyll cells. Mol Plant Microbe Interact. 1995;8: 825–836. [Google Scholar]
- 47.Inoue T, Shingaki R, Hirose S, Waki K, Mori H, Fukui K. Genome-wide screening of genes required for swarming motility in Escherichia coli K-12. J Bacteriol. 2007;189: 950–9570.1128/JB.01294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rui H, Liu Q, Ma Y, Wang Q, Zhang Y. Roles of LuxR in regulating extracellular alkaline serine protease A, extracellular polysaccharide and mobility of Vibrio alginolyticus. FEMS Microbiol Lett. 2008;285: 155–162. 10.1111/j.1574-6968.2008.01185.x [DOI] [PubMed] [Google Scholar]
- 49.Subramoni S, Sonti RV. Growth deficiency of a Xanthomonas oryzae pv. Oryzae fur mutant in rice leaves is rescued by ascorbic acid supplementation. Mol Plant Microbe Interact. 2005;18: 644–651. 10.1094/MPMI-18-0644 [DOI] [PubMed] [Google Scholar]
- 50.Harvie DR, Vílchez S, Steggles JR, Ellar DJ. Bacillus cereus fur regulates iron metabolism and is required for full virulence. Microbiology 2005;151: 569–577. 10.1099/mic.0.27744-0 [DOI] [PubMed] [Google Scholar]
- 51.Venisse J-, Gullner G, Brisset MN. Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiol. 2001;125: 2164–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torres VJ, Attia AS, Mason WJ, Hood MI, Corbin BD, Beasley FC, et al. Staphylococcus aureus fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia. Infect Immun. 2010;78: 1618–1628. 10.1128/IAI.01423-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48: 251–275. [DOI] [PubMed] [Google Scholar]
- 54.Liu HQ. Function analysis of fur gene in Xanthomonas campestris pv. Vesicatoria and role of interaction of plants and bacteria. Shanxi, China: Shanxi Agriculture University; 2004. [Google Scholar]
- 55.Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of corynebacterium glutamicum. Gene. 1994;145: 69–73. 10.1016/0378-1119(94)90324-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.