Abstract
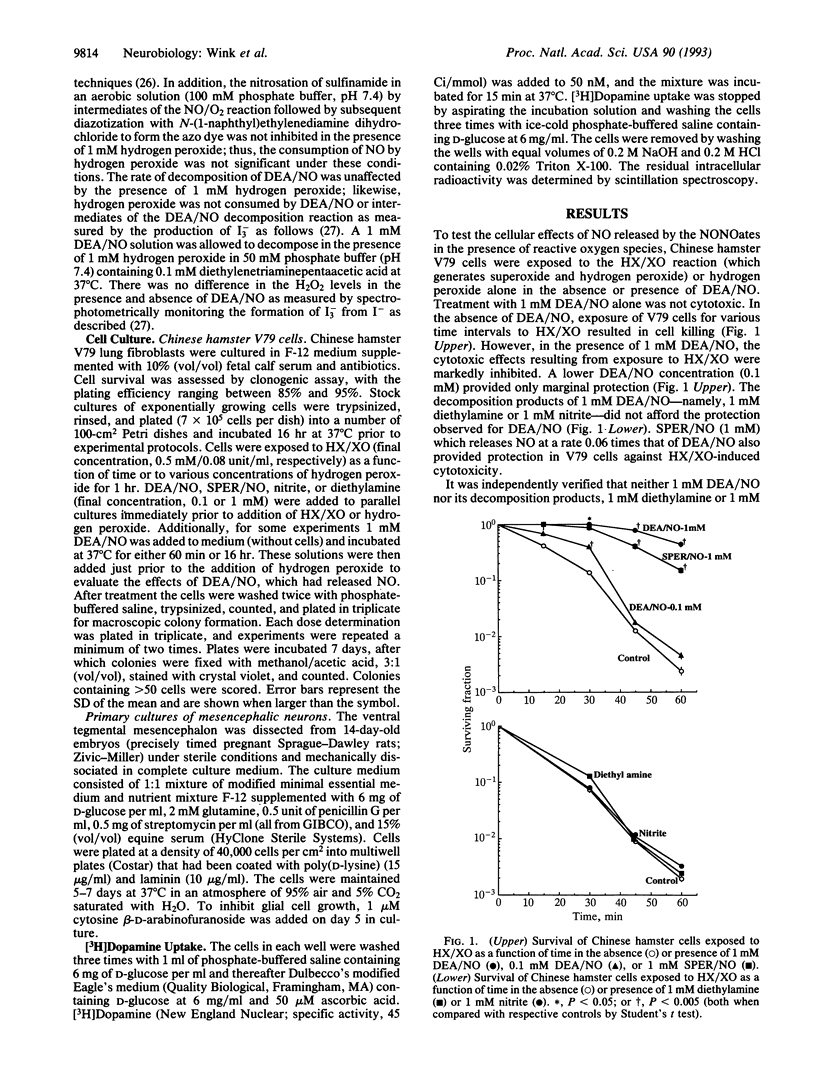
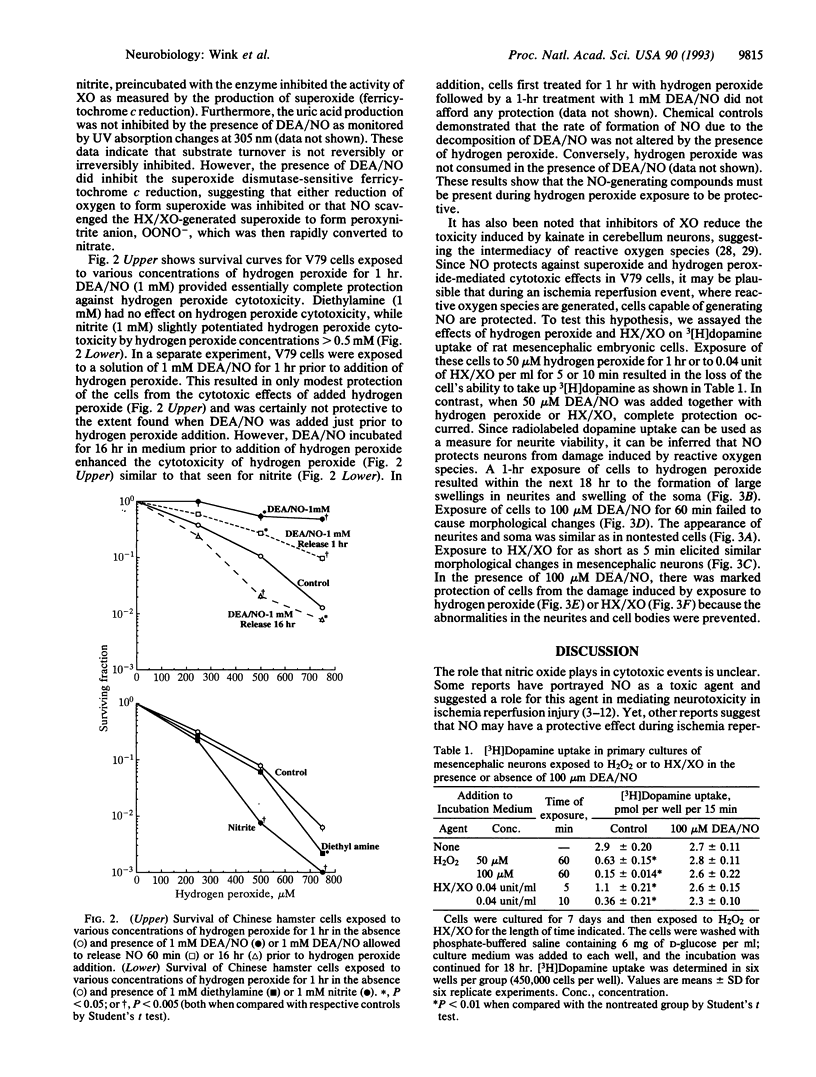
Nitric oxide, NO, which is generated by various components of the immune system, has been presumed to be cytotoxic. However, NO has been proposed to be protective against cellular damage resulting during ischemia reperfusion. Along with NO there is often concomitant formation of superoxide/hydrogen peroxide, and hence a synergistic relationship between the cytotoxic effects of nitric oxide and these active oxygen species is frequently assumed. To study more carefully the potential synergy between NO and active oxygen species in mammalian cell cytotoxicity, we utilized either hypoxanthine/xanthine cell cytotoxicity, we utilized either hypoxanthine/xanthine oxidase (a system that generates superoxide/hydrogen peroxide) or hydrogen peroxide itself. NO generation was accomplished by the use of a class of compounds known as "NONOates," which release NO at ambient temperatures without the requirement of enzyme activation or biotransformation. When Chinese hamster lung fibroblasts (V79 cells) were exposed to hypoxanthine/xanthine oxidase for various times or increasing amounts of hydrogen peroxide, there was a dose-dependent decrease in survival of V79 cells as measured by clonogenic assays. However, in the presence of NO released from (C2H5)2N[N(O)NO]-Na+ (DEA/NO), the cytotoxicity resulting from superoxide or hydrogen peroxide was markedly abrogated. Similarly, primary cultures of rat mesencephalic dopaminergic cells exposed either to hydrogen peroxide or to hypoxanthine/xanthine oxidase resulted in the degradation of the dopamine uptake and release mechanism. As was observed in the case of the V79 cells, the presence of NO essentially abrogated this peroxide-mediated cytotoxic effect on mesencephalic cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S. The double-edged role of nitric oxide in brain function and superoxide-mediated injury. J Dev Physiol. 1991 Jan;15(1):53–59. [PubMed] [Google Scholar]
- Berdichevsky E., Muñoz C., Riveros N., Cartier L., Orrego F. Neuropathological changes in the rat brain cortex in vitro: effects of kainic acid and of ion substitutions. Brain Res. 1987 Oct 13;423(1-2):213–220. doi: 10.1016/0006-8993(87)90842-0. [DOI] [PubMed] [Google Scholar]
- Buisson A., Plotkine M., Boulu R. G. The neuroprotective effect of a nitric oxide inhibitor in a rat model of focal cerebral ischaemia. Br J Pharmacol. 1992 Aug;106(4):766–767. doi: 10.1111/j.1476-5381.1992.tb14410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkitt M. J., Gilbert B. C. Model studies of the iron-catalysed Haber-Weiss cycle and the ascorbate-driven Fenton reaction. Free Radic Res Commun. 1990;10(4-5):265–280. doi: 10.3109/10715769009149895. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Dawson V. L., Snyder S. H. A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol. 1992 Sep;32(3):297–311. doi: 10.1002/ana.410320302. [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast D. J., Lynch R. C., Leu R. W. Nitric oxide production by tumor targets in response to TNF: paradoxical correlation with susceptibility to TNF-mediated cytotoxicity without direct involvement in the cytotoxic mechanism. J Leukoc Biol. 1992 Sep;52(3):255–261. doi: 10.1002/jlb.52.3.255. [DOI] [PubMed] [Google Scholar]
- Galea E., Feinstein D. L., Reis D. J. Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10945–10949. doi: 10.1073/pnas.89.22.10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally J. A., Montague P. R., Reeke G. N., Jr, Edelman G. M. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci U S A. 1990 May;87(9):3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambassi F., Pistelli A., Di Bello M. G., Lupini M., Mannaioni P. F., Masini E. Ischemia-reperfusion injury and histamine release in isolated perfused guinea-pig heart: effects of nitric oxide generators. Pharmacol Res. 1992 Feb-Mar;25 (Suppl 1):11–12. doi: 10.1016/1043-6618(92)90516-e. [DOI] [PubMed] [Google Scholar]
- Gelvan D., Saltman P., Powell S. R. Cardiac reperfusion damage prevented by a nitroxide free radical. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4680–4684. doi: 10.1073/pnas.88.11.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanbauer I., Wink D., Osawa Y., Edelman G. M., Gally J. A. Role of nitric oxide in NMDA-evoked release of [3H]-dopamine from striatal slices. Neuroreport. 1992 May;3(5):409–412. doi: 10.1097/00001756-199205000-00008. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Chen J., Tsai M., Martin J. C., Smith C. D., Beckman J. S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992 Nov 1;298(2):431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Schini V. B., Farhood A. Role of nitric oxide in the oxidant stress during ischemia/reperfusion injury of the liver. Life Sci. 1992;50(23):1797–1804. doi: 10.1016/0024-3205(92)90064-v. [DOI] [PubMed] [Google Scholar]
- Johnson G., 3rd, Tsao P. S., Lefer A. M. Cardioprotective effects of authentic nitric oxide in myocardial ischemia with reperfusion. Crit Care Med. 1991 Feb;19(2):244–252. doi: 10.1097/00003246-199102000-00021. [DOI] [PubMed] [Google Scholar]
- Kanner J., Harel S., Granit R. Nitric oxide as an antioxidant. Arch Biochem Biophys. 1991 Aug 15;289(1):130–136. doi: 10.1016/0003-9861(91)90452-o. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L., Costa E., Wroblewski J. T. Sodium nitroprusside inhibits N-methyl-D-aspartate-evoked calcium influx via a nitric oxide- and cGMP-independent mechanism. Mol Pharmacol. 1992 Apr;41(4):779–784. [PubMed] [Google Scholar]
- Linz W., Wiemer G., Schölkens B. A. ACE-inhibition induces NO-formation in cultured bovine endothelial cells and protects isolated ischemic rat hearts. J Mol Cell Cardiol. 1992 Aug;24(8):909–919. doi: 10.1016/0022-2828(92)91103-c. [DOI] [PubMed] [Google Scholar]
- Maragos C. M., Morley D., Wink D. A., Dunams T. M., Saavedra J. E., Hoffman A., Bove A. A., Isaac L., Hrabie J. A., Keefer L. K. Complexes of .NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J Med Chem. 1991 Nov;34(11):3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide: biosynthesis and biological significance. Trends Biochem Sci. 1989 Dec;14(12):488–492. doi: 10.1016/0968-0004(89)90181-3. [DOI] [PubMed] [Google Scholar]
- Masini E., Bianchi S., Mugnai L., Gambassi F., Lupini M., Pistelli A., Mannaioni P. F. The effect of nitric oxide generators on ischemia reperfusion injury and histamine release in isolated perfused guinea-pig heart. Agents Actions. 1991 May;33(1-2):53–56. doi: 10.1007/BF01993125. [DOI] [PubMed] [Google Scholar]
- Matheis G., Sherman M. P., Buckberg G. D., Haybron D. M., Young H. H., Ignarro L. J. Role of L-arginine-nitric oxide pathway in myocardial reoxygenation injury. Am J Physiol. 1992 Feb;262(2 Pt 2):H616–H620. doi: 10.1152/ajpheart.1992.262.2.H616. [DOI] [PubMed] [Google Scholar]
- Mitchell J. B., Samuni A., Krishna M. C., DeGraff W. G., Ahn M. S., Samuni U., Russo A. Biologically active metal-independent superoxide dismutase mimics. Biochemistry. 1990 Mar 20;29(11):2802–2807. doi: 10.1021/bi00463a024. [DOI] [PubMed] [Google Scholar]
- Moncada C., Lekieffre D., Arvin B., Meldrum B. Effect of NO synthase inhibition on NMDA- and ischaemia-induced hippocampal lesions. Neuroreport. 1992 Jun;3(6):530–532. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morikawa E., Huang Z., Moskowitz M. A. L-arginine decreases infarct size caused by middle cerebral arterial occlusion in SHR. Am J Physiol. 1992 Nov;263(5 Pt 2):H1632–H1635. doi: 10.1152/ajpheart.1992.263.5.H1632. [DOI] [PubMed] [Google Scholar]
- Morikawa E., Rosenblatt S., Moskowitz M. A. L-arginine dilates rat pial arterioles by nitric oxide-dependent mechanisms and increases blood flow during focal cerebral ischaemia. Br J Pharmacol. 1992 Dec;107(4):905–907. doi: 10.1111/j.1476-5381.1992.tb13382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuji T., Matsui T., Koide T., Asano T. Blockade of nitric oxide formation by N omega-nitro-L-arginine mitigates ischemic brain edema and subsequent cerebral infarction in rats. Neurosci Lett. 1992 Dec 7;147(2):159–162. doi: 10.1016/0304-3940(92)90584-t. [DOI] [PubMed] [Google Scholar]
- Nowicki J. P., Duval D., Poignet H., Scatton B. Nitric oxide mediates neuronal death after focal cerebral ischemia in the mouse. Eur J Pharmacol. 1991 Nov 12;204(3):339–340. doi: 10.1016/0014-2999(91)90862-k. [DOI] [PubMed] [Google Scholar]
- Saran M., Michel C., Bors W. Reaction of NO with O2-. implications for the action of endothelium-derived relaxing factor (EDRF). Free Radic Res Commun. 1990;10(4-5):221–226. doi: 10.3109/10715769009149890. [DOI] [PubMed] [Google Scholar]
- Siegfried M. R., Erhardt J., Rider T., Ma X. L., Lefer A. M. Cardioprotection and attenuation of endothelial dysfunction by organic nitric oxide donors in myocardial ischemia-reperfusion. J Pharmacol Exp Ther. 1992 Feb;260(2):668–675. [PubMed] [Google Scholar]
- Weissman B. A., Kadar T., Brandeis R., Shapira S. NG-nitro-L-arginine enhances neuronal death following transient forebrain ischemia in gerbils. Neurosci Lett. 1992 Nov 9;146(2):139–142. doi: 10.1016/0304-3940(92)90062-c. [DOI] [PubMed] [Google Scholar]
- Wink D. A., Darbyshire J. F., Nims R. W., Saavedra J. E., Ford P. C. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1993 Jan-Feb;6(1):23–27. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- Woditsch I., Schrör K. Prostacyclin rather than endogenous nitric oxide is a tissue protective factor in myocardial ischemia. Am J Physiol. 1992 Nov;263(5 Pt 2):H1390–H1396. doi: 10.1152/ajpheart.1992.263.5.H1390. [DOI] [PubMed] [Google Scholar]
- Zhu L., Gunn C., Beckman J. S. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992 Nov 1;298(2):452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]




