Abstract
A characteristic of neurological disorders is the loss of critical populations of cells that the body is unable to replace, thus there has been much interest in identifying methods of generating clinically relevant numbers of cells to replace those that have been damaged or lost. The process of neural direct conversion, in which cells of one lineage are converted into cells of a neural lineage without first inducing pluripotency, shows great potential, with evidence of the generation of a range of functional neural cell types both in vitro and in vivo, through viral and non-viral delivery of exogenous factors, as well as chemical induction methods. Induced neural cells have been proposed as an attractive alternative to neural cells derived from embryonic or induced pluripotent stem cells, with prospective roles in the investigation of neurological disorders, including neurodegenerative disease modelling, drug screening, and cellular replacement for regenerative medicine applications, however further investigations into improving the efficacy and safety of these methods need to be performed before neural direct conversion becomes a clinically viable option. In this review, we describe the generation of diverse neural cell types via direct conversion of somatic cells, with comparison against stem cell-based approaches, as well as discussion of their potential research and clinical applications.
Keywords: Adult stem cells, Embryonic stem cells, Generation of neural cells, Induced pluripotent stem cells, In vitro differentiation, In vivo differentiation, Clinical applications, Direct conversion, Induced neural cells
Core tip: The process of neural direct conversion, in which cells of one lineage are converted into cells of a neural lineage without first inducing pluripotency, shows great potential for the generation of a range of neural cell types, providing an attractive alternative to neural cells derived from embryonic or induced pluripotent stem cells. In this review, we describe the generation of diverse neural cell types via direct conversion of somatic cells, with comparison against stem cell-based approaches, as well as discussion of their potential research and clinical applications.
INTRODUCTION
While the ability of the mammalian peripheral nervous system to undergo axonal regeneration following injury has been well documented[1-3], the mammalian central nervous system is largely incapable of regeneration and repair[4-6]. A variety of factors are believed to contribute to this lack of recovery, including limited and location restricted neurogenesis, cell death, astrocytic glial scarring, oligodendrocytic myelin inhibition, insufficient growth factor support, and lack of substrates suitable for axonal growth[7-11]. Combined with a lack of effective treatments, these factors lead to the severity of neurological disorders, including spinal cord injury, brain damage, and neurodegenerative diseases such as Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and Alzheimer’s disease, which often result in major disability[12].
Neurological disorders often result from the loss of critical populations of cells that the body is unable to replace[13], thus there has been much interest in identifying methods of generating clinically relevant numbers of functional cells to replace those that have been damaged or lost[14]. Stem cells possess great potential for treatment of neurological disorders, providing a theoretically inexhaustible supply of cells for transplantation[15]. Similarly, the process of neural direct conversion, in which cells of one lineage are converted into cells of a neural lineage without first inducing pluripotency[16], also shows great promise. In this review, we describe the generation of diverse neural cell types via direct conversion of somatic cells, with comparison against stem cell-based approaches, as well as discussion of their potential research and clinical applications.
GENERATION OF NEURAL CELL TYPES THROUGH STEM CELL-BASED APPROACHES
Stem cell-based approaches provide a number of therapeutic advantages, through their ability to offer cellular replacement by transplantation of exogenous stem cells and stem cell-derived neural cell types, or mobilisation and induction of endogenous stem cells to generate new neural cell types, as well as their ability to release neuroprotective and inflammation modulating molecules, creating an enriched environment for minimisation of neurodegeneration[17,18]. Current stem cell-based methods of generating neural cell types utilise embryonic, induced pluripotent, or adult stem cells, with each exhibiting a range of advantages and disadvantages.
Embryonic stem cells
Embryonic stem cells (ESC) are pluripotent, and as such have the capacity to form all tissues in the body[15] (Figure 1), thus they show great promise for the in vitro generation and subsequent study of specific cell lineages[19], with evidence of ectodermal neural progenitor[20,21], neuronal[22,23], astrocytic, and oligodendrocytic[24] cells derived from both mouse and human ESC lines. ESC also have great therapeutic potential, in particular for treatment of neurological disorders[25]. ESC have been shown to differentiate into a range of neural cell types, with noted improvements in function following implantation, with examples in models of Parkinson’s disease[26,27], motor neuron disease[28,29], stroke[30,31], and spinal cord injury[32,33].
Figure 1.
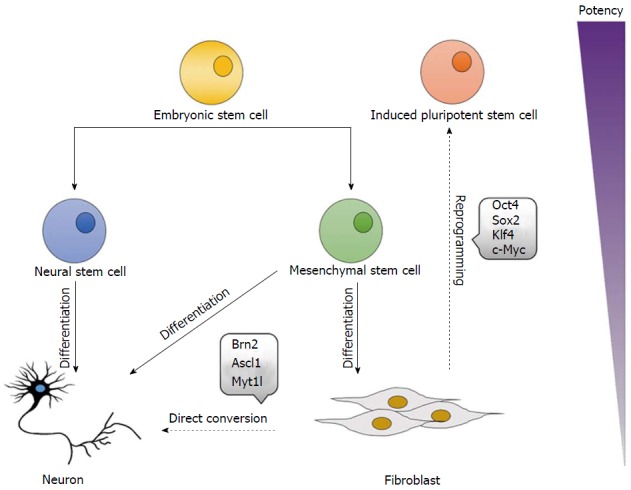
Overview of cellular differentiation, direct conversion, and reprogramming. Embryonic stem cells are pluripotent, and thus capable of differentiating into cells of any lineage. Reprogramming reverses this process, with forced expression of Oct4, Sox2, Klf4, and c-Myc shown to induce pluripotency in fibroblasts, generating induced pluripotent stem cells. Cells are also capable of switching lineages during direct conversion, with forced expression of Brn2, Ascl1, and Myt1l shown to convert fibroblasts into induced neuronal cells.
Despite the research and clinical potential of ESC, their use is surrounded by much debate, due to technical obstacles, as well as legal and ethical issues regarding their isolation[34]. Prior to implantation of ESC-derived differentiated cells, it is necessary to ensure that the implant consists of a pure cell population, due to the risk of teratoma formation or disruption to normal tissue function if undifferentiated ESC or inappropriate cell types are implanted[15]. Another risk includes host rejection of allogeneic ESC-derived differentiated cells, as while immunogenicity can be contained through the use of immunosuppressive drugs, they are associated with numerous side effects that can result in patient susceptibility to infection[15]. Furthermore, the use of ESC is highly controversial due to the fact that they are derived from pre-implantation embryos, with considerable differences in opinion in regards to their ontological and moral status[35].
Induced pluripotent stem cells
Since the seminal discovery that ectopic expression of a set of four pluripotency reprogramming factors (Oct4, Sox2, Klf4, and c-Myc) could induce the generation of pluripotent cells from murine fibroblasts under ESC-like conditions[36] (Figure 1), induced pluripotent stem cells (iPSC) have been proposed as a replacement for ESC, as they not only avoid the use of embryonic material, but can also be patient-derived[37], minimising the potential for immune rejection, and allowing for the production of a variety of somatic cells with the same genetic information as the patient from which the iPSC were derived[38]. iPSC have been utilised in the investigation of a variety of diseases of the central and peripheral nervous systems, including Parkinson’s disease[39], amyotrophic lateral sclerosis[40], schizophrenia[41], and Huntington’s disease[42]. iPSC have also been utilised in toxicology and drug screening studies, with examples of iPSC-derived models of familial dysautonomia[43], Rett syndrome[44], and Alzheimer’s disease[45]. Additionally, a number of studies have investigated the therapeutic potential of iPSC in animal models of neurological disorders, with evidence of locomotor function recovery in an injured mouse spinal cord[46], functional peripheral nerve regeneration in transected rat sciatic nerves[47], and improved motor behaviour in rat models of Parkinson’s disease[48,49]. Significantly, the first therapeutic use of iPSC has been approved for human trials in Japan, with cells from the skin of a patient suffering from age-related macular degeneration reprogrammed into iPSC and subsequently differentiated into retinal pigment epithelium cells, prior to implantation into the eye[50].
Despite the successful therapeutic applications and reduced ethical concerns regarding their use, iPSC are similarly associated with a number of issues, from technical obstacles to safety concerns such as potential tumourigenicity[51]. Technically, the process of reprogramming somatic cells into iPSC can be quite lengthy, taking between 10 d[52] to 8 wk[53], without accounting for extra time required for subsequent differentiation of iPSC into the desired somatic cell type. Reprogramming also occurs at extremely low efficiencies, which can make it difficult to generate sufficient iPSC when working with small cell numbers from the source[54]. There have also been reported differences between iPSC based on their cell source, with gene expression profile studies showing persistent gene expression of donor cell specific markers following reprogramming into iPSC[55,56], and further studies demonstrating not only distinct transcriptional and epigenetic differences, but also that cell source influences their in vitro differentiation potential, suggesting a retained epigenetic memory of their somatic cell of origin, however these differences did appear to be attenuated following continuous passaging[57,58]. Additionally, the reprogramming factors Klf4 and c-Myc are known oncogenes, thus their residual expression has the potential to induce cancer[51], with evidence of tumour formation due to c-Myc reactivation following transplantation of mouse fibroblast-derived iPSC into nude mice[59].
Adult stem cells
The numerous limitations associated with the use of ESC and iPSC has led to investigations into alternative sources of stem cells, such as progenitor cells residing within the adult organism[34], with reports of mesenchymal stem cells differentiating into neural-like cells under specific experimental conditions (Figure 1), such as supplementation with a range of chemicals including β-mercaptoethanol, butylated hydroxyanisole, dimethylsulphoxide, isobutylmethylxanthine, dibutyryl cyclic AMP, epidermal growth factor, and brain-derived neurotrophic factor[60-66]. However, assessment of neuronal functionality is varied between studies, with some reporting a lack of action potential generating voltage-gated ion channels in induced neuronal cells[65], and others demonstrating formation of synaptic vesicles, with electrophysiological evidence of functional synaptic transmission[66], thus further investigations are required before the use of adult stem cells can become a viable alternative.
GENERATION OF NEURAL CELL TYPES VIA DIRECT CONVERSION
Lineage restriction was once one of the core principles of developmental biology, with the concept that cells cannot cross germ layer boundaries and are thus restricted in their ability to differentiate into cells of only the germ layer from which they originate[12]. However, these principles have since been challenged, with evidence that forced expression of specific transcription factors could directly convert cells of one lineage into another without first inducing pluripotency, in a process known as direct conversion[67]. Early studies demonstrated the neural direct conversion of astrocytes into neuron-like cells via forced expression of the neurogenic transcription factors Pax6, Ascl1, Ngn2, and Dlx2[68-70], however investigations into neural direct conversion really gained momentum following reports by Vierbuchen et al[16] of the conversion of fibroblasts into neuron-like cells (Figure 1).
The beginnings of neural direct conversion
Based on what had previously been reported in direct conversion studies generating other cell types, Vierbuchen et al[16] hypothesised that multiple transcription factors would be required to induce direct conversion of fibroblasts into neural cells, and as such, they first identified candidate genes that were known to express in neural tissues and play key roles in neural development. Starting from a pool of 19 factors, they elucidated that forced expression of the neuronal transcription factors Brn2, Ascl1, and Myt1l (BAM) could rapidly and efficiently convert mouse embryonic and postnatal fibroblasts into neuron-like cells (termed induced neuronal cells), with a conversion efficiency of 19.5%. Induced neuronal cells demonstrated expression of the pan-neuronal markers beta III tubulin, NeuN, MAP2, and synapsin, as well as the neurotransmitter phenotype markers vGLUT1 and GABA, with the majority of induced neuronal cells described as excitatory, expressing markers of cortical identity. Furthermore, induced neuronal cells exhibited spontaneous action potential generation, ligand-gated ion channels, and the ability to synaptically integrate into pre-existing neural networks, thus indicating that induced cells were of a mature and functional phenotype[16] (Figure 2).
Figure 2.

The process of neural direct conversion, as first described by Vierbuchen et al[16]. Somatic cells (A) are transduced with inducible lentiviral vectors expressing the neuronal transcription factors Brn2, Ascl1, and Myt1l (B), and cultured in a defined neuronal induction media (C). Following culture, induced neuronal cells can be identified by positive beta III tubulin staining (D), prior to further characterisation.
Pang et al[71] subsequently furthered this work, with evidence that human foetal and postnatal fibroblasts could be directly converted into induced neuronal cells via forced expression of BAM in addition to the neuronal transcription factor NeuroD1, following screening of a pool of 20 additional factors. Similarly, induced neuronal cells were predominantly excitatory, demonstrating expression of the pan-neuronal markers beta III tubulin, NeuN, MAP2, NCAM, and synapsin, as well as spontaneous action potential generation, and synaptic integration into pre-existing neural networks. Compared to those derived from mouse fibroblasts[16], human fibroblast-derived induced neuronal cells required longer culture periods to develop synaptic activity, with lower reported conversion efficiencies, ranging from 2%-4%[71].
Generation of induced neuronal cells
Following these initial studies, neural direct conversion was investigated with great interest. Along with the numerous studies demonstrating generation of induced neuronal cells from mouse and human fibroblasts, induced neuronal cells have also been generated from common marmoset fibroblasts using the neuronal transcription factors BAM and NeuroD1, however with a conversion efficiency of < 1%[72]. In addition to fibroblasts, induced neuronal cells have been generated from hepatocytes[73], cord blood-derived stem cells[74,75], pericytes[76], glioma cells[77], adipocyte progenitor cells[78], and astrocytes[79], via forced expression of BAM and variations of BAM, as well as a variety of new transcription factor combinations, as summarised in Table 1.
Table 1.
Summary of studies demonstrating generation of functional induced neuronal cells by direct conversion
| Species | Original cell | Transgenes | Method | End cell | Ref. |
| Mouse | Fibroblast | BAM | iLV | iN | [16] |
| Human | Fibroblast | BAM, NeuroD1 | iLV | iN | [71] |
| Human | Fibroblast | miR-9/9*-124, Ascl1, Myt1l, NeuroD2 | LV | iN | [83] |
| Human | Fibroblast | BAM | iLV | iN | [153] |
| Human | Fibroblast | miR-124, Brn2, Myt1l | iLV | iN | [84] |
| Mouse | Hepatocyte | BAM | iLV | iN | [73] |
| Human | Fibroblast | Ascl1, Ngn2 | iLV | iN | [74] |
| Human | Pericyte | Ascl1, Sox2 | RV | iN | [76] |
| Mouse | Fibroblast | Brn2, Ascl1, Ngn2 | AV | iN | [144] |
| Human | Cord blood cell | Sox2 | RV | iN | [75] |
| Human | Cord blood cell | Sox2, c-Myc | RV | iN | [75] |
| Human | Glioma cell | Brn2, Ascl1, Ngn2 | LV | iN | [77] |
| Mouse | Fibroblast | BAM | NV | iN | [147] |
| Mouse | Adipocyte progenitor cell | BAM | iLV | iN | [78] |
| Mouse | Fibroblast | shR-PTB | LV | iN | [85] |
| Human | Fibroblast | Ascl1, Myt1l, Sox2 | LV | iN | [154] |
| Common marmoset | Fibroblast | BAM, NeuroD1 | iLV | iN | [72] |
| Human | Fibroblast | BAM | iLV | iN | [81] |
| Mouse | Fibroblast | Ascl1 | iLV | iN | [155] |
| Human | Fibroblast | Ascl1 | iLV | iN | [155] |
| Mouse | Astroglia | Ink4a/Arf-/-, Dlx2 | iLV | iN | [79] |
| Mouse | Fibroblast | Ink4a/Arf-/-, BAM | iLV | iN | [79] |
| Mouse | Fibroblast | Brn2, Ascl1, Ngn2, Rarg, Nr5a2 | AV | iN | [80] |
| Human | Fibroblast | Brn2, Ascl1, Ngn2, Rarg, Nr5a2 | AV | iN | [80] |
| Human | Fibroblast | shR-p16-19 | LV | iN | [82] |
| Human | Fibroblast | hTERT | LV | iN | [82] |
BAM: Brn2, Ascl1, and Myt1l; miR: MicroRNA; shR: Small hairpin RNA; PTB: Polypyrimidine-tract-binding protein; iLV: Inducible lentiviral vector; LV: Lentiviral vector; RV: Retroviral vector; AV: Adenoviral vector; NV: Non-viral; iN: Induced neuron.
Conversion efficiencies of various studies ranged from < 0.1% up to 85%, with the more efficient methods incorporating additional factors, such as small molecules inhibiting GSK-3 and SMAD signalling[74], retinoic acid receptor and nuclear receptor signalling[80], delaying transgene activation after transduction[81], and blocking cellular senescence through depletion of p16Ink4a/p19Arf or expression of human telomerase reverse transcriptase[79,82]. Other studies have also investigated the use of microRNA in conjunction with neuronal specific transcription factors, including expression of microRNA-9/9* and microRNA-124[83,84], as well as repression of a single RNA binding polypyrimidine-tract-binding protein, a key target and negative regulator of microRNA-124[85]. microRNA-9/9* and microRNA-124 are known to act on critical target genes that regulate neuronal differentiation and function[83,84], with microRNA-9* and microRNA-124 found to instruct compositional changes of SWI/SNF-like BAF chromatin remodelling complexes in a process that is important for neuronal differentiation and function[83,85]. However, this particular method of neural direct conversion appears less successful than others, with conversion efficiencies in mouse and human fibroblast studies ranging from 1.5%-14%. Throughout all reported studies, induced neuronal cell functionality has been confirmed by electrophysiological analysis. Interestingly, a recent in-depth examination of the electrophysiological profiles of human induced neuronal cells generated by lentiviral vector expression of BAM, Olig2, and Zic1 has revealed that the conversion of fibroblasts to neuron-like cells is incomplete, with passive membrane properties comparable to that of highly immature neurons[86]. However, the induced neuronal cells used in this study were sourced from research that has since been retracted, thus questioning the validity of these results.
Generation of induced neuronal subtypes
Investigations have also expanded into generation of induced neuronal subtypes. Numerous studies have reported the generation of induced dopaminergic neurons, directly converted from both fibroblasts[81,87-91] and astrocytes[92] using transcription factors involved in the specification of dopaminergic neurons, such as Lmx1a, Lmx1b, Nurr1, and FoxA2. Induced dopaminergic neurons were shown to display uptake and production of dopamine and spontaneous pacemaking activity consistent with dopaminergic neurons of the brain, as well as provide symptomatic relief in rat and mouse models of Parkinson’s disease. There have also been reports of the generation of induced motor neurons, directly converted from fibroblasts using BAM in addition to transcription factors that participate in different stages of motor neuron specification[93], as well as the transcription factor Ngn2 supplemented with the small molecules forskolin and dorsomorphin[94]. Induced motor neurons exhibited motor neuron-like features, such as morphology, gene expression, and mature electrophysiological properties, as well as the formation of functional neuromuscular junctions. Similarly, recent studies have also demonstrated the generation of induced medium spiny neurons[95], sensory neurons[96], and astrocytes[97], using transcription factors required for appropriate differentiation of these specific cell types, as summarised in Table 2.
Table 2.
Summary of studies demonstrating generation of functional induced neuronal subtypes by direct conversion
| Species | Original cell | Transgenes | Method | End cell | Ref. |
| Human | Fibroblast | BAM, Lmx1a, FoxA2 | iLV | iDN | [87] |
| Mouse | Fibroblast | Ascl1, Nurr1, Lmx1a | iLV | iDN | [88] |
| Human | Fibroblast | Ascl1, Nurr1, Lmx1a | iLV | iDN | [88] |
| Mouse | Fibroblast | BAM, Lhx3, Hb9, Isl1, Ngn2 | RV | iMN | [93] |
| Human | Fibroblast | BAM, Lhx3, Hb9, Isl1, Ngn2, NeuroD1 | RV | iMN | [93] |
| Mouse | Fibroblast | Ascl1, Pitx3, Lmx1a, Nurr1, FoxA2, EN1 | iLV | iDN | [89] |
| Mouse | Astrocyte | Ascl1, Lmx1b, Nurr1 | iLV | iDN | [92] |
| Mouse | Fibroblast | Ascl1, Lmx1b, Nurr1 | iLV | iDN | [92] |
| Mouse | Cord blood-derived stem cell | Ascl1, Lmx1b, Nurr1 | iLV | iDN | [92] |
| Human | Fibroblast | Ascl1, Ngn2, Sox2, Nurr1, Pitx3 | LV | iDN | [90] |
| Mouse | Fibroblast | Brn2, Ascl1, Lmx1b, Nurr1, Otx2 | RV | iDN | [91] |
| Mouse | Fibroblast | Brn2, Ascl1, Ngn2, Pax6, Hes1, Id1, c-Myc, Klf4 | RV | iNPC → iDN | [91] |
| Human | Fibroblast | Ngn2 | RV | iMN | [94] |
| Human | Fibroblast | BAM, Lmx1a, Lmx1b, FoxA2, Otx2 | iLV | iDN | [81] |
| Human | Fibroblast | Ascl1, Ngn2, Sox2, Nurr1, Pitx3, p53-DN | LV | iDN | [156] |
| Human | Fibroblast | miR-9/9*-124, Myt1l, Bcl11b, Dlx1, Dlx2 | iLV | iMSN | [95] |
| Mouse | Fibroblast | Brn3a, Ngn1/2 | iLV | iSN | [96] |
| Human | Fibroblast | Brn3a, Ngn1/2 | iLV | iSN | [96] |
| Mouse | Fibroblast | Nfia, Nfib, Sox9 | iLV | iA | [97] |
| Human | Fibroblast | Nfia, Nfib, Sox9 | iLV | iA | [97] |
BAM: Brn2, Ascl1, and Myt1l; miR: MicroRNA; shR: Small hairpin RNA; iLV: Inducible lentiviral vector; LV: Lentiviral vector; iN: Induced neuron; iNPC: Induced neural progenitor cell; iDN: Induced dopaminergic neuron; iMN: Induced motor neuron; iMSN: Induced medium spiny neuron; iSN: Induced sensory neuron; iA: Induced astrocyte.
Generation of induced neural stem and progenitor cells
The generation of induced neuronal cells and subtypes is often associated with low conversion efficiencies and yields, resulting in difficulties obtaining sufficient cells for therapeutic applications. This may be in part due to the post-mitotic state of the target cell type (neuron-like cells), with the conversion procedure including a halt in proliferation, thus limiting the ability of these cells to expand once reprogrammed[98-100]. In addition to determining methods of increasing conversion efficiency, studies have expanded into investigating whether similar methods could be utilised for generation of proliferative neural stem and progenitor cells, which are both expandable in vitro and capable of generating multiple neural cell types[101], with initial studies demonstrating the generation of induced neural progenitor[101] and crest[102] cells.
Kim et al[101] first demonstrated the direct conversion of mouse embryonic fibroblasts into induced neural progenitor cells, through transient expression of the iPSC reprogramming factors Oct4, Sox2, Klf4, and c-Myc, followed by incubation in a defined neural reprogramming media. Treatment resulted in the rapid and highly efficient formation of colonies containing cells expressing the rosette neural stem cell marker PLZF and the early neural transcription factor Pax6, without transiting through a pluripotent intermediate stage. Induced cells were both proliferative and functional, capable of differentiating into functional neurons and glial cells[101]. Similarly, Zabierowski et al[102] demonstrated the direct conversion of human melanocytes into induced neural crest cells, driving a cascade of dedifferentiation through forced expression of the intracellular domain of the transmembrane protein Notch1. Induced cells displayed biological attributes consistent with native neural crest cells, including spherical proliferation under stem cell culture conditions, expression of neural crest stem cell-related genes, and differentiation into multiple mesenchymal and neuronal lineages, as well as in vitro and in vivo migration potential[102].
These initial studies led to further investigation of direct conversion into neural stem and progenitor cells, with additional reports of the generation of induced neural crest[103] and progenitor[104] cells, as well as generation of induced neural stem cells[105]. Furthermore, a number of studies also demonstrated the generation of induced neuroblasts[106], and induced oligodendrocyte[107,108] and dopaminergic neuron[109] progenitor cells. While the majority of studies utilised fibroblasts as the starting cell type, there have also been reports of the direct conversion of astrocytes[106,110], Sertoli cells[111], epithelial-like cells in urine[112], cord blood-derived stem cells[113], bone marrow-derived stem cells[114], liver cells, and B lymphocytes[99] into induced neural stem and progenitor cells, as summarised in Table 3.
Table 3.
Summary of studies demonstrating generation of functional induced neural stem and progenitor cells by direct conversion
| Species | Original cell | Transgenes | Method | End cell | Ref. |
| Mouse | Fibroblast | OSKM | iLV | iNPC | [101] |
| Human | Melanocyte | Notch1 | LV | iNCC | [102] |
| Human | Astrocyte | 4-Oct | LV | iNSC | [110] |
| Human | Astrocyte | Sox2 | LV | iNSC | [110] |
| Human | Astrocyte | Nanog | LV | iNSC | [110] |
| Mouse | Fibroblast | Brn4, Sox2, Klf4, c-Myc, E47 | RV | iNSC | [105] |
| Human | Fibroblast | Oct4, Sox2, Klf4, Zic3 | RV | iNPC | [104] |
| Mouse | Fibroblast | Brn2, Sox2, FoxG1 | iLV | iNPC | [123] |
| Mouse | Fibroblast | OSKM | RV | iNSC | [116] |
| Human | Fibroblast | OSKM | RV | iNSC | [116] |
| Human | Fibroblast | Sox2, Pax6 | NV | iNPC | [98] |
| Mouse | Fibroblast | Sox2 | RV | iNSC | [122] |
| Human | Fibroblast | Sox2 | RV | iNSC | [122] |
| Mouse | Sertoli cell | Ascl1, Ngn2, Hes1, Id1, Pax6, Brn2, Sox2, c-Myc, Klf4 | RV | iNSC | [111] |
| Mouse | Fibroblast | OSKM | RV/iLV | iNSC | [117] |
| Mouse | Fibroblast | Brn2, Nr2e1, Sox2, c-Myc, Bmi1 | RV | iNPC | [157] |
| Human | Fibroblast | OSKM | SV | iNPC | [118] |
| Monkey | Fibroblast | OSKM | SV | iNPC | [118] |
| Mouse | Fibroblast | Sox10, Olig2, Nkx6.2 | LV | iOPC | [107] |
| Human | Urine cells | Oct4, Sox2, Klf4, SV40LT, miR-302-367 | NV | iNPC | [112] |
| Mouse | Fibroblast | Sox10, Olig2, Zfp536 | iLV | iOPC | [108] |
| Rat | Fibroblast | Sox10, Olig2, Zfp536 | iLV | iOPC | [108] |
| Human | Fibroblast | Oct3, Sox2, Klf4, c-Myc | RV | iNPC | [125] |
| Mouse | Fibroblasts | Brn2, Hes1, Hes3, Klf4, c-Myc, Plagl1, Notch1 (NICD), Rfx4 | NV | iNSC | [99] |
| Mouse | Liver cells | Brn2, Hes1, Hes3, Klf4, c-Myc, Plagl1, Notch1 (NICD), Rfx4 | NV | iNSC | [99] |
| Mouse | Blymphocytes | Brn2, Hes1, Hes3, Klf4, c-Myc, Plagl1, Notch1 (NICD), Rfx4 | NV | iNSC | [99] |
| Mouse | Fibroblast | OSKM | iLV | iDNPC | [109] |
| Rat | Bone marrow-derived stem cell | Ngn2 | LV | iNPC | [114] |
| Mouse | Fibroblast | Sox2, Klf4, c-Myc, Brn4 | RV | iNSC | [158] |
| Human | Fibroblast | Sox10 | iLV | iNCC | [103] |
| Human | Fibroblast | 4-Oct | LV | iNPC | [121] |
| Pig | Fibroblast | Oct4, Sox2, Klf4, Lin28, L-Myc | NV | iNPC | [120] |
| Human | Fibroblast | 4-Oct | LV | iNSC | [119] |
| Human | Fibroblast | Oct4, Sox2, Klf4, shR-p53 | NV | iNSC | [119] |
| Human | Fibroblast | Sox2, c-Myc, Brn2 | LV | iNPC | [159] |
| Human | Fibroblast | Sox2, c-Myc, Brn4 | LV | iNPC | [159] |
| Human | Astrocyte | miR-302/367 | LV | iNB | [106] |
| Human | Fibroblast | Sox2, HMGA2 | RV | iNSC | [113] |
| Human | Cord blood-derived stem cell | Sox2, HMGA2 | RV | iNSC | [113] |
OSKM: Oct4, Sox2, Klf4, c-Myc; miR: MicroRNA; shR: Small hairpin RNA; NICD: Notch intracellular domain; HMGA2: High-mobility group A2; iLV: Inducible lentiviral vector; LV: Lentiviral vector; RV: Retroviral vector; SV: Sendai virus vector; NV: Non-viral; iNCC: Induced neural crest cell; iNSC: Induced neural stem cell; iNPC: Induced neural progenitor cell; iOPC: Induced oligodendrocyte progenitor cell; iDNPC: Induced dopaminergic neuronal progenitor cell; iNB: Induced neuroblast.
Generation of induced neural stem and progenitor cells has been achieved using a variety of approaches. One such approach, the cell activation and signalling-directed (CASD) method, combines transient overexpression of pluripotency reprogramming factors and/or small molecules (cell activation) with soluble lineage-specific signals (signalling-directed) to reprogram somatic cells into lineage-specific cell types while bypassing the pluripotent state[115]. CASD induced neural stem and progenitor cells have been generated using a range of pluripotency reprogramming factors and microRNAs, such as Oct4, Sox2, Klf4, c-Myc, SV40LT, and microRNA-302-367, in conjunction with neural stem/progenitor cell permissive culture conditions[101,109,112,116-120]. Other approaches involve overexpression of lineage-specific transcription factors, with examples of induced cells generated using individual factors, such as Oct4[121], Sox2[122], Sox10[103], and Nanog[110], as well as different sets of factors[111,123]. Additionally, a number of studies have also incorporated the use of small molecules such as TGF-β[118], GSK-3[119], MEK[112], ROCK[112], BMP[112], JAK[109], and histone deacetylase[103,120] inhibitors to enhance direct conversion. Making comparisons between different methods of generating neural stem and precursor cells is difficult, particularly as many studies do not report conversion efficiency values, however research has shown that neural direct conversion using lineage-specific factors results in greater chromosomal stability than neural direct conversion using pluripotency reprogramming factors[124], thus suggesting a preference towards this particular method for future clinical applications.
Significantly, Meyer et al[125] also reported the direct conversion of fibroblasts from patients with both familial and sporadic forms of amyotrophic lateral sclerosis (ALS) into induced neural progenitor cells. Induced cells were subsequently differentiated into astrocytes, a key cell type involved in the degeneration of motor neurons in ALS, which demonstrated toxicity toward motor neurons as similarly demonstrated by autopsy spinal cord-derived astrocytes. These findings not only enable personalised modelling of ALS and potentially other neurodegenerative diseases, but could also lead to high-throughput testing of therapeutics for individual patients[125].
Generation of induced neural cells in vivo
Generation of neural cell types through direct conversion has been studied extensively in vitro, with confirmed long-term survival and functional integration following transplantation[126]. As such, investigations have expanded into generation of neural cells through direct conversion in vivo, in which cells are directly converted within their native physiological environment[127]. Preliminary research described the transplantation of fibroblasts and astrocytes transduced with inducible forms of neural reprogramming genes into the adult rat brain, with conversion into induced neuronal and dopaminergic neuronal cells following gene activation in vivo[128]. Further studies demonstrated the direct conversion of endogenous glial cells into induced neural cells, with BAM expression converting resident astrocytes into induced neurons in the mouse striatum[128], Fezf2 expression converting resident embryonic and early postnatal callosal projection neurons into induced corticofugal projection neurons in the mouse neocortex[129], and Sox2 expression converting resident astrocytes into induced neuroblasts in the mouse striatum, with subsequent differentiation into mature and functional neurons[130].
Neural direct conversion in vivo has also been demonstrated in a number of injury models. Ngn2 expression in addition to growth factor exposure has been shown to convert non-neuronal cells into induced neurons in the rat neocortex and striatum following stab wound injury[131]. Similarly, induced neurons have been generated from endogenous NG2 glia by Sox2 expression in the mouse cerebral cortex following stab wound injury[132], as well as from endogenous reactive glial cells by NeuroD1 expression in the mouse cortex following stab wound injury and in an Alzheimer’s disease model[133]. Sox2 expression has also been reported to convert resident astrocytes into induced neuroblasts in an injured mouse spinal cord, with subsequent differentiation into mature and functional neurons[134]. While induced cells were determined to be functional throughout these studies, there was no evidence that they had any significant impact on behavioural recovery following injury, thus further investigation is required to fully elucidate the potential of endogenous cells for neurological repair.
Methods of neural direct conversion
As evident in the summaries of neural direct conversion studies (Tables 1-3), transgene delivery methods vary greatly throughout. Primarily, transgenes are delivered using viral vectors, due to their intrinsic ability to efficiently express their genome in the nucleus of target cells[135], however safety concerns regarding clinical translation have resulted in investigations into non-viral methods of transgene delivery, as well as methods of chemically-induced neural direct conversion.
Integrating viral vector transgene delivery: The majority of neural direct conversion studies to date have utilised retroviral vectors (RV) and lentiviral vectors (LV) for transgene delivery, due to their comparatively higher efficiency and ability to integrate into the target cell genome, thus ensuring sustained transgene expression[136]. However as a result of genomic integration by these vectors, there is an associated risk of spontaneous transgene reactivation, as well as tumour formation due to insertional mutagenesis[59], as previously observed with proto-oncogene activation in four out of ten patients following retrovirus-mediated gene therapy for X-linked severe combined immunodeficiency disorder[137]. LV are often preferable to RV, as while RV require passage through mitosis for transduction, LV do not, and as such are capable of transducing both dividing and non-dividing cells[138]. Additionally, LV are generally considered a safer alternative to RV, as they are designed without the majority of the viral genes, retaining only the cis-acting sequence elements necessary for nuclear export of the RNA, RNA dimerisation, packaging, and reverse transcription[139]. Furthermore, innovations in LV design have led to the creation of self-inactivating LV, knocking out viral long terminal repeat (LTR) enhancer-promoter activity[140], as well as non-integrating lentiviral vectors, with mutations in their integrase or LTRs to inhibit integrase binding[141], thus reducing the risk of integration and vector-related pathologies[139]. LV have also been used in conjunction with drug-based induction systems, in which transgene expression is dependent upon the delivery of a specific drug (e.g., tetracycline, ecdysone, mifepristone), thus allowing for tightly regulated conditional transgene expression, an appealing prospect for a number of potential gene therapy applications[142].
Non-integrating viral vector transgene delivery: The use of genome integrating RV and LV poses a number of limitations due to the increased risk of gene mutations and insertional mutagenesis, thus studies have investigated transgene delivery via non-integrating adenoviral vectors (AV) and Sendai virus vectors (SV) for safer generation of induced neural cells. Similarly to LV, AV are able to transduce both dividing and non-dividing cells, with transient expression in dividing cells, and long-term expression in non-dividing cells[136]. Importantly, AV demonstrate little to no integration into the target cell genome, instead being maintained episomally as linear or circular DNA molecules[139]. However, AV have been shown to induce several classes of innate immune responses, thus despite minimal genomic integration, AV still have the risk of host immune response to overcome[143]. Furthermore, AV have been associated with a comparatively lower neuronal conversion efficiency than using LV systems[144], and as such it is critical to identify other factors or chemical compounds to obtain neurons with a higher efficiency, as evident in the addition of Rarg and Nr5a2 to the neuronal transcription factor combination of Brn2, Ascl1, and Ngn2, with a demonstrated increase in conversion efficiency from 2.9%[144] to 46.2%[80]. SV are non-integrating viral vectors, capable of transient but strong gene expression in a wide range of dividing and non-dividing cells[145]. Significantly, SV pose no potential pathogenicity towards humans, with temperature-sensitive variants of SV allowing temperature-specific activation/inactivation of gene expression, further alleviating some of the safety concerns associated with their use clinically[146]. SV have been utilised in the generation of highly proliferative induced neural progenitor cells from primate species, with conversion efficiencies ranging from 0.03%-0.19%, and subsequent temperature-mediated removal of viral genomes[118]. Despite the relatively low conversion efficiency, the many favourable safety attributes of SV promotes further investigation into their use in the generation of induced neural cell types.
Non-viral methods of transgene delivery: Neural direct conversion using non-viral transgene delivery methods is becoming an increasingly attractive alternative to viral vector-based methods[136], with a number of studies reporting generation of induced neuronal and neural stem and progenitor cells via non-viral methods. The first example of non-viral neural direct conversion described the generation of induced neuronal cells from mouse embryonic fibroblasts through repeated delivery of plasmids encoding BAM with a bioreducible linear poly(amido amine) polymer, resulting in mature, electrophysiologically functional neuron-like cells with a conversion efficiency of 7.6%[147]. Following the confirmed feasibility of non-viral neural direct conversion, studies expanded into investigating non-viral methods of generating induced neural stem and progenitor cells, capable of differentiating into multiple mature and functional neuronal subtypes. Non-viral delivery of Sox2 and Pax6 by plasmid transfection or protein transduction was initially shown to convert adult human fibroblasts into induced neural progenitor cells, with a conversion efficiency of 0.05%[98]. Following this, non-integrative episomal vectors were utilised for non-viral direct conversion, with induced neural progenitor cells generated from epithelial-like cells in human urine following episomal vector delivery of Oct4, Sox2, Klf4, SV40LT, and microRNA-302-367 in combination with a cocktail of small molecules, with a conversion efficiency of 0.2%[112]. Similarly, episomal vector delivery of Oct4, Sox2, Klf4, Lin28, and L-Myc in combination with histone deacetylase inhibitor treatment has converted pig fibroblasts into induced neural progenitor cells[120], and Oct4, Sox2, Klf4, and small hairpin RNA-p53 with a cocktail of small molecules has converted human fibroblasts into induced neural stem cells[119], however no conversion efficiencies were reported for either study. Interestingly, a secondary system enabling non-viral neural direct conversion has been reported, in which fibroblasts, liver cells, and B lymphocytes were isolated from chimeric mice carrying inducible vectors expressing Brn2, Hes1, Hes3, Klf4, c-Myc, Plagl1, Notch1 (NICD), and Rfx4, with subsequent conversion into induced neural stem cells following transgene induction, however again with no reported conversion efficiencies[99]. Overall, while some non-viral methods achieve conversion efficiencies similar to studies utilising viral vectors[147], others achieve considerably lower conversion efficiencies[98,112] or have not reported them[99,119,120], thus necessitating optimisation of non-viral methods in order for them to become a viable alternative.
Neural direct conversion by chemical induction: An attractive alternative to neural direct conversion via introduction of exogenous genes is chemical induction, with the discovery that iPSC could be generated by small molecules alone[148] prompting investigations into generation of induced neural cell types using similar methods. Initial studies demonstrated the generation of induced neural progenitor cells using a defined chemical cocktail and hypoxic conditions[149]. Induced neural progenitor cells were converted from mouse embryonic fibroblasts, mouse tail tip fibroblasts, and epithelial-like cells in human urine using a two-step induction strategy, with an initial intermediary transition of a chemical cocktail of small molecules inhibiting TGF-β, GSK-3, and histone deacetylation pathways under 5% oxygen, followed by lineage-specific induction in neural expansion media. Chemically induced neural progenitor cells resembled endogenous neural progenitor cells in terms of their proliferation, self-renewability, ability to differentiate into multiple mature and functional neuronal subtypes in vitro and in vivo, and gene expression profile, however induced cells generated from mouse fibroblasts were shown to have retained some fibroblastic epigenetic memory[149]. Similarly, postnatal human fibroblasts have been converted into induced neuronal cells, using a specific cocktail of small molecules consisting of forskolin, and inhibitors of TGF-β, BMP, GSK-3, MEK-ERK, and p53 pathways[150]. Chemically induced neuronal cells displayed a mature neuronal morphology, with positive immunostaining of functional neuronal markers synapsin, vGLUT1, GABA, and tyrosine hydroxylase, however no electrophysiological studies were performed to confirm functionality. Induced cells were generated with a conversion efficiency of > 80%, with efficiency reportedly unaffected by donor age and cellular senescence, thus providing a novel and efficient method of generating transgene-free induced neuronal cells with great clinical potential[150].
Clinical applications of induced neural cell types
Induced neural cell types generated by direct conversion have long been suggested as a source of cells for clinical applications, however their true therapeutic potential has not yet been fully investigated. Studies have recently addressed this gap within the literature, reporting the restorative effects of induced neural stem cells in models of spinal cord injury and Parkinson’s disease. In one study, mouse embryonic fibroblast-derived induced neural stem cells were transplanted into the contused thoracic spinal cord of rats[151]. Following transplantation, induced neural stem cells lost their stem cell identity and differentiated into neurons, astrocytes, and oligodendrocytes, with synaptic formation observed between host and transplanted neurons. Both lesion and cavity size decreased following transplantation of induced cells, with increased myelin production and angiogenesis in the injured area, as well as promotion of axonal regeneration, motor function, and electrophysiological activity. In addition to cellular replacement, transplanted induced cells were shown to exert their therapeutic effect through neuroprotective and immunomodulatory mechanisms, as well as promotion of endogenous regeneration, as evident by decreased expression of apoptotic and inflammatory markers[151]. Similarly, mouse Sertoli cell-derived induced neural stem cells exogenously expressing the dopaminergic neuron-specific factor Lmx1a were transplanted into the striatum of Parkinson’s disease model mice[152]. While transplantation of induced neural stem cells was shown to improve the motor performance of mouse models, with greater tyrosine hydroxylase signal abundance in the lesioned area, only few transplanted cells survived over time, thus suggesting that the therapeutic effects may have occurred in a non-autonomous manner through enhancement of the functions of remaining endogenous cells[152]. Interestingly, induced neural cell types generated via direct conversion with lineage-specific factors have been shown to possess greater chromosomal stability than neural cells derived from pluripotent or adult stem cells[124], further promoting the clinical potential of neural cell types generated via direct conversion.
CONCLUSION
Neurological disorders often result from the loss of critical populations of cells that the body is unable to replace, thus methods of generating clinically relevant numbers of cells to replace those that have been damaged or lost are sought[13,14]. The process of neural direct conversion has been demonstrated to generate a range of functional neural cell types both in vitro and in vivo, through viral and non-viral delivery of exogenous factors, as well as chemical induction methods. Induced neural cells have been proposed as an attractive alternative to neural cells derived from embryonic or induced pluripotent stem cells, with prospective roles in the investigation of neurological disorders, including neurodegenerative disease modelling, drug screening, and cellular replacement for regenerative medicine applications, however further investigations into improving the efficacy and safety of these methods need to be performed before neural direct conversion becomes a clinically viable option.
Footnotes
Supported by The Charles Sturt University Writing Up Award.
Conflict-of-interest statement: The authors declare that there are no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 1, 2015
First decision: November 3, 2015
Article in press: January 4, 2016
P- Reviewer: Liu L, Politi LE, Zou ZM S- Editor: Qiu S L- Editor: A E- Editor: Li D
References
- 1.Zochodne DW. The microenvironment of injured and regenerating peripheral nerves. Muscle Nerve Suppl. 2000;9:S33–S38. doi: 10.1002/1097-4598(2000)999:9<::aid-mus7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller’s observations to neuroinflammation. J Peripher Nerv Syst. 2002;7:13–27. doi: 10.1046/j.1529-8027.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- 3.Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- 4.Aguayo AJ. Axonal regeneration from injured neurons in the adult mammalian central nervous system. In: Cotman CW, editor. Synaptic Plasticity. New York: The Guilford Press; 1985. pp. 457–484. [Google Scholar]
- 5.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 6.Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 7.Dezawa M, Kawana K, Negishi H, Adachi-Usami E. Glial cells in degenerating and regenerating optic nerve of the adult rat. Brain Res Bull. 1999;48:573–579. doi: 10.1016/s0361-9230(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz M, Moalem G, Leibowitz-Amit R, Cohen IR. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 1999;22:295–299. doi: 10.1016/s0166-2236(99)01405-8. [DOI] [PubMed] [Google Scholar]
- 9.Lacroix S, Tuszynski MH. Neurotrophic factors and gene therapy in spinal cord injury. Neurorehabil Neural Repair. 2000;14:265–275. doi: 10.1177/154596830001400403. [DOI] [PubMed] [Google Scholar]
- 10.Asher RA, Morgenstern DA, Moon LD, Fawcett JW. Chondroitin sulphate proteoglycans: inhibitory components of the glial scar. Prog Brain Res. 2001;132:611–619. doi: 10.1016/S0079-6123(01)32106-4. [DOI] [PubMed] [Google Scholar]
- 11.Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001;11:89–94. doi: 10.1016/s0959-4388(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 12.Hess DC, Borlongan CV. Stem cells and neurological diseases. Cell Prolif. 2008;41 Suppl 1:94–114. doi: 10.1111/j.1365-2184.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- 15.Rippon HJ, Bishop AE. Embryonic stem cells. Cell Prolif. 2004;37:23–34. doi: 10.1111/j.1365-2184.2004.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011;70:353–361. doi: 10.1002/ana.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop AE, Buttery LD, Polak JM. Embryonic stem cells. J Pathol. 2002;197:424–429. doi: 10.1002/path.1154. [DOI] [PubMed] [Google Scholar]
- 20.Uemura M, Refaat MM, Shinoyama M, Hayashi H, Hashimoto N, Takahashi J. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J Neurosci Res. 2010;88:542–551. doi: 10.1002/jnr.22223. [DOI] [PubMed] [Google Scholar]
- 21.Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- 23.Strübing C, Ahnert-Hilger G, Shan J, Wiedenmann B, Hescheler J, Wobus AM. Differentiation of pluripotent embryonic stem cells into the neuronal lineage in vitro gives rise to mature inhibitory and excitatory neurons. Mech Dev. 1995;53:275–287. doi: 10.1016/0925-4773(95)00446-8. [DOI] [PubMed] [Google Scholar]
- 24.Fraichard A, Chassande O, Bilbaut G, Dehay C, Savatier P, Samarut J. In vitro differentiation of embryonic stem cells into glial cells and functional neurons. J Cell Sci. 1995;108(Pt 10):3181–3188. doi: 10.1242/jcs.108.10.3181. [DOI] [PubMed] [Google Scholar]
- 25.Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell Res Ther. 2010;1:37. doi: 10.1186/scrt37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Gómez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS, Musachio JL, Chin FT, Toyama H, Seidel J, et al. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25:918–928. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115:102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper JM, Krishnan C, Darman JS, Deshpande DM, Peck S, Shats I, Backovic S, Rothstein JD, Kerr DA. Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proc Natl Acad Sci USA. 2004;101:7123–7128. doi: 10.1073/pnas.0401103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 30.Daadi MM, Maag AL, Steinberg GK. Adherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke model. PLoS One. 2008;3:e1644. doi: 10.1371/journal.pone.0001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daadi MM, Li Z, Arac A, Grueter BA, Sofilos M, Malenka RC, Wu JC, Steinberg GK. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009;17:1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 33.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Wert G, Mummery C. Human embryonic stem cells: research, ethics and policy. Hum Reprod. 2003;18:672–682. doi: 10.1093/humrep/deg143. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Hirschi KK, Li S, Roy K. Induced pluripotent stem cells for regenerative medicine. Annu Rev Biomed Eng. 2014;16:277–294. doi: 10.1146/annurev-bioeng-071813-105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 39.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 41.Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, Viale A, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yahata N, Asai M, Kitaoka S, Takahashi K, Asaka I, Hioki H, Kaneko T, Maruyama K, Saido TC, Nakahata T, et al. Anti-Aβ drug screening platform using human iPS cell-derived neurons for the treatment of Alzheimer’s disease. PLoS One. 2011;6:e25788. doi: 10.1371/journal.pone.0025788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, Tomisato S, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci USA. 2010;107:12704–12709. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang A, Tang Z, Park IH, Zhu Y, Patel S, Daley GQ, Li S. Induced pluripotent stem cells for neural tissue engineering. Biomaterials. 2011;32:5023–5032. doi: 10.1016/j.biomaterials.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doi D, Samata B, Katsukawa M, Kikuchi T, Morizane A, Ono Y, Sekiguchi K, Nakagawa M, Parmar M, Takahashi J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Reports. 2014;2:337–350. doi: 10.1016/j.stemcr.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoag H. Invasive-species control: Bounty hunters. Nature. 2014;513:294–295. doi: 10.1038/513294a. [DOI] [PubMed] [Google Scholar]
- 51.Sun N, Longaker MT, Wu JC. Human iPS cell-based therapy: considerations before clinical applications. Cell Cycle. 2010;9:880–885. doi: 10.4161/cc.9.5.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Utikal J, Maherali N, Kulalert W, Hochedlinger K. Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. J Cell Sci. 2009;122:3502–3510. doi: 10.1242/jcs.054783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim JB, Greber B, Araúzo-Bravo MJ, Meyer J, Park KI, Zaehres H, Schöler HR. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461:649–643. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 54.Liu SP, Fu RH, Huang YC, Chen SY, Chien YJ, Hsu CY, Tsai CH, Shyu WC, Lin SZ. Induced pluripotent stem (iPS) cell research overview. Cell Transplant. 2011;20:15–19. doi: 10.3727/096368910X532828. [DOI] [PubMed] [Google Scholar]
- 55.Marchetto MC, Yeo GW, Kainohana O, Marsala M, Gage FH, Muotri AR. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS One. 2009;4:e7076. doi: 10.1371/journal.pone.0007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghosh Z, Wilson KD, Wu Y, Hu S, Quertermous T, Wu JC. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS One. 2010;5:e8975. doi: 10.1371/journal.pone.0008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 60.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 61.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 62.Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282:148–152. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- 63.Bossolasco P, Cova L, Calzarossa C, Rimoldi SG, Borsotti C, Deliliers GL, Silani V, Soligo D, Polli E. Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp Neurol. 2005;193:312–325. doi: 10.1016/j.expneurol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 64.Guo L, Yin F, Meng HQ, Ling L, Hu-He TN, Li P, Zhang CX, Yu S, Duan DS, Fan HX. Differentiation of mesenchymal stem cells into dopaminergic neuron-like cells in vitro. Biomed Environ Sci. 2005;18:36–42. [PubMed] [Google Scholar]
- 65.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho KJ, Trzaska KA, Greco SJ, McArdle J, Wang FS, Ye JH, Rameshwar P. Neurons derived from human mesenchymal stem cells show synaptic transmission and can be induced to produce the neurotransmitter substance P by interleukin-1 alpha. Stem Cells. 2005;23:383–391. doi: 10.1634/stemcells.2004-0251. [DOI] [PubMed] [Google Scholar]
- 67.Graf T. Historical origins of transdifferentiation and reprogramming. Cell Stem Cell. 2011;9:504–516. doi: 10.1016/j.stem.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 68.Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Götz M. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 69.Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, Götz M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heinrich C, Blum R, Gascón S, Masserdotti G, Tripathi P, Sánchez R, Tiedt S, Schroeder T, Götz M, Berninger B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Z, Kohda K, Ibata K, Kohyama J, Akamatsu W, Yuzaki M, Okano HJ, Sasaki E, Okano H. Reprogramming non-human primate somatic cells into functional neuronal cells by defined factors. Mol Brain. 2014;7:24. doi: 10.1186/1756-6606-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, Südhof TC, Wernig M. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9:374–382. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ladewig J, Mertens J, Kesavan J, Doerr J, Poppe D, Glaue F, Herms S, Wernet P, Kögler G, Müller FJ, et al. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods. 2012;9:575–578. doi: 10.1038/nmeth.1972. [DOI] [PubMed] [Google Scholar]
- 75.Giorgetti A, Marchetto MC, Li M, Yu D, Fazzina R, Mu Y, Adamo A, Paramonov I, Cardoso JC, Monasterio MB, et al. Cord blood-derived neuronal cells by ectopic expression of Sox2 and c-Myc. Proc Natl Acad Sci USA. 2012;109:12556–12561. doi: 10.1073/pnas.1209523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karow M, Sánchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascón S, Khan MA, Lie DC, Dellavalle A, et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 77.Zhao J, He H, Zhou K, Ren Y, Shi Z, Wu Z, Wang Y, Lu Y, Jiao J. Neuronal transcription factors induce conversion of human glioma cells to neurons and inhibit tumorigenesis. PLoS One. 2012;7:e41506. doi: 10.1371/journal.pone.0041506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Y, Jiao J, Gao R, Yao H, Sun XF, Gao S. Direct conversion of adipocyte progenitors into functional neurons. Cell Reprogram. 2013;15:484–489. doi: 10.1089/cell.2013.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Price JD, Park KY, Chen J, Salinas RD, Cho MJ, Kriegstein AR, Lim DA. The Ink4a/Arf locus is a barrier to direct neuronal transdifferentiation. J Neurosci. 2014;34:12560–12567. doi: 10.1523/JNEUROSCI.3159-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi Z, Shen T, Liu Y, Huang Y, Jiao J. Retinoic acid receptor γ (Rarg) and nuclear receptor subfamily 5, group A, member 2 (Nr5a2) promote conversion of fibroblasts to functional neurons. J Biol Chem. 2014;289:6415–6428. doi: 10.1074/jbc.M113.515601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pereira M, Pfisterer U, Rylander D, Torper O, Lau S, Lundblad M, Grealish S, Parmar M. Highly efficient generation of induced neurons from human fibroblasts that survive transplantation into the adult rat brain. Sci Rep. 2014;4:6330. doi: 10.1038/srep06330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun CK, Zhou D, Zhang Z, He L, Zhang F, Wang X, Yuan J, Chen Q, Wu LG, Yang Q. Senescence impairs direct conversion of human somatic cells to neurons. Nat Commun. 2014;5:4112. doi: 10.1038/ncomms5112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xue Y, Ouyang K, Huang J, Zhou Y, Ouyang H, Li H, Wang G, Wu Q, Wei C, Bi Y, et al. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell. 2013;152:82–96. doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koppensteiner P, Boehm S, Arancio O. Electrophysiological profiles of induced neurons converted directly from adult human fibroblasts indicate incomplete neuronal conversion. Cell Reprogram. 2014;16:439–446. doi: 10.1089/cell.2014.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Björklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci USA. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 89.Kim J, Su SC, Wang H, Cheng AW, Cassady JP, Lodato MA, Lengner CJ, Chung CY, Dawlaty MM, Tsai LH, et al. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell. 2011;9:413–419. doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu X, Li F, Stubblefield EA, Blanchard B, Richards TL, Larson GA, He Y, Huang Q, Tan AC, Zhang D, et al. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell Res. 2012;22:321–332. doi: 10.1038/cr.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sheng C, Zheng Q, Wu J, Xu Z, Sang L, Wang L, Guo C, Zhu W, Tong M, Liu L, et al. Generation of dopaminergic neurons directly from mouse fibroblasts and fibroblast-derived neural progenitors. Cell Res. 2012;22:769–772. doi: 10.1038/cr.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Addis RC, Hsu FC, Wright RL, Dichter MA, Coulter DA, Gearhart JD. Efficient conversion of astrocytes to functional midbrain dopaminergic neurons using a single polycistronic vector. PLoS One. 2011;6:e28719. doi: 10.1371/journal.pone.0028719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu ML, Zang T, Zou Y, Chang JC, Gibson JR, Huber KM, Zhang CL. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat Commun. 2013;4:2183. doi: 10.1038/ncomms3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Victor MB, Richner M, Hermanstyne TO, Ransdell JL, Sobieski C, Deng PY, Klyachko VA, Nerbonne JM, Yoo AS. Generation of human striatal neurons by microRNA-dependent direct conversion of fibroblasts. Neuron. 2014;84:311–323. doi: 10.1016/j.neuron.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blanchard JW, Eade KT, Szűcs A, Lo Sardo V, Tsunemoto RK, Williams D, Sanna PP, Baldwin KK. Selective conversion of fibroblasts into peripheral sensory neurons. Nat Neurosci. 2015;18:25–35. doi: 10.1038/nn.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caiazzo M, Giannelli S, Valente P, Lignani G, Carissimo A, Sessa A, Colasante G, Bartolomeo R, Massimino L, Ferroni S, et al. Direct conversion of fibroblasts into functional astrocytes by defined transcription factors. Stem Cell Reports. 2015;4:25–36. doi: 10.1016/j.stemcr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maucksch C, Firmin E, Butler-Munro C, Montgomery J, Dottori M, Connor B. Non-Viral Generation of Neural Precursor-like Cells from Adult Human Fibroblasts. J Stem Cells Regen Med. 2012;8:162–170. doi: 10.46582/jsrm.0803009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cassady JP, D’Alessio AC, Sarkar S, Dani VS, Fan ZP, Ganz K, Roessler R, Sur M, Young RA, Jaenisch R. Direct lineage conversion of adult mouse liver cells and B lymphocytes to neural stem cells. Stem Cell Reports. 2014;3:948–956. doi: 10.1016/j.stemcr.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thoma EC, Merkl C, Heckel T, Haab R, Knoflach F, Nowaczyk C, Flint N, Jagasia R, Jensen ZS, Truong HH, et al. Chemical conversion of human fibroblasts into functional Schwann cells. Stem Cell Reports. 2014;3:539–547. doi: 10.1016/j.stemcr.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci USA. 2011;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zabierowski SE, Baubet V, Himes B, Li L, Fukunaga-Kalabis M, Patel S, McDaid R, Guerra M, Gimotty P, Dahmane N, et al. Direct reprogramming of melanocytes to neural crest stem-like cells by one defined factor. Stem Cells. 2011;29:1752–1762. doi: 10.1002/stem.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim YJ, Lim H, Li Z, Oh Y, Kovlyagina I, Choi IY, Dong X, Lee G. Generation of multipotent induced neural crest by direct reprogramming of human postnatal fibroblasts with a single transcription factor. Cell Stem Cell. 2014;15:497–506. doi: 10.1016/j.stem.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 104.Kumar A, Declercq J, Eggermont K, Agirre X, Prosper F, Verfaillie CM. Zic3 induces conversion of human fibroblasts to stable neural progenitor-like cells. J Mol Cell Biol. 2012;4:252–255. doi: 10.1093/jmcb/mjs015. [DOI] [PubMed] [Google Scholar]
- 105.Han DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 106.Ghasemi-Kasman M, Hajikaram M, Baharvand H, Javan M. MicroRNA-Mediated In Vitro and In Vivo Direct Conversion of Astrocytes to Neuroblasts. PLoS One. 2015;10:e0127878. doi: 10.1371/journal.pone.0127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Najm FJ, Lager AM, Zaremba A, Wyatt K, Caprariello AV, Factor DC, Karl RT, Maeda T, Miller RH, Tesar PJ. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat Biotechnol. 2013;31:426–433. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang N, Zuchero JB, Ahlenius H, Marro S, Ng YH, Vierbuchen T, Hawkins JS, Geissler R, Barres BA, Wernig M. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol. 2013;31:434–439. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim HS, Kim J, Jo Y, Jeon D, Cho YS. Direct lineage reprogramming of mouse fibroblasts to functional midbrain dopaminergic neuronal progenitors. Stem Cell Res. 2014;12:60–68. doi: 10.1016/j.scr.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 110.Corti S, Nizzardo M, Simone C, Falcone M, Donadoni C, Salani S, Rizzo F, Nardini M, Riboldi G, Magri F, et al. Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp Cell Res. 2012;318:1528–1541. doi: 10.1016/j.yexcr.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sheng C, Zheng Q, Wu J, Xu Z, Wang L, Li W, Zhang H, Zhao XY, Liu L, Wang Z, et al. Direct reprogramming of Sertoli cells into multipotent neural stem cells by defined factors. Cell Res. 2012;22:208–218. doi: 10.1038/cr.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang L, Wang L, Huang W, Su H, Xue Y, Su Z, Liao B, Wang H, Bao X, Qin D, et al. Generation of integration-free neural progenitor cells from cells in human urine. Nat Methods. 2013;10:84–89. doi: 10.1038/nmeth.2283. [DOI] [PubMed] [Google Scholar]
- 113.Yu KR, Shin JH, Kim JJ, Koog MG, Lee JY, Choi SW, Kim HS, Seo Y, Lee S, Shin TH, et al. Rapid and Efficient Direct Conversion of Human Adult Somatic Cells into Neural Stem Cells by HMGA2/let-7b. Cell Rep. 2015;10:441–452. doi: 10.1016/j.celrep.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 114.Cheng F, Lu XC, Hao HY, Dai XL, Qian TD, Huang BS, Tang LJ, Yu W, Li LX. Neurogenin 2 converts mesenchymal stem cells into a neural precursor fate and improves functional recovery after experimental stroke. Cell Physiol Biochem. 2014;33:847–858. doi: 10.1159/000358657. [DOI] [PubMed] [Google Scholar]
- 115.Zhu S, Wang H, Ding S. Reprogramming fibroblasts toward cardiomyocytes, neural stem cells and hepatocytes by cell activation and signaling-directed lineage conversion. Nat Protoc. 2015;10:959–973. doi: 10.1038/nprot.2015.059. [DOI] [PubMed] [Google Scholar]
- 116.Matsui T, Takano M, Yoshida K, Ono S, Fujisaki C, Matsuzaki Y, Toyama Y, Nakamura M, Okano H, Akamatsu W. Neural stem cells directly differentiated from partially reprogrammed fibroblasts rapidly acquire gliogenic competency. Stem Cells. 2012;30:1109–1119. doi: 10.1002/stem.1091. [DOI] [PubMed] [Google Scholar]
- 117.Thier M, Wörsdörfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nöthen MM, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 118.Lu J, Liu H, Huang CT, Chen H, Du Z, Liu Y, Sherafat MA, Zhang SC. Generation of integration-free and region-specific neural progenitors from primate fibroblasts. Cell Rep. 2013;3:1580–1591. doi: 10.1016/j.celrep.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu S, Ambasudhan R, Sun W, Kim HJ, Talantova M, Wang X, Zhang M, Zhang Y, Laurent T, Parker J, et al. Small molecules enable OCT4-mediated direct reprogramming into expandable human neural stem cells. Cell Res. 2014;24:126–129. doi: 10.1038/cr.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu XL, Yang JP, Fu LN, Ren RT, Yi F, Suzuki K, Liu K, Ding ZC, Qu J, Zhang WQ, et al. Direct reprogramming of porcine fibroblasts to neural progenitor cells. Protein Cell. 2014;5:4–7. doi: 10.1007/s13238-013-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mitchell RR, Szabo E, Benoit YD, Case DT, Mechael R, Alamilla J, Lee JH, Fiebig-Comyn A, Gillespie DC, Bhatia M. Activation of neural cell fate programs toward direct conversion of adult human fibroblasts into tri-potent neural progenitors using OCT-4. Stem Cells Dev. 2014;23:1937–1946. doi: 10.1089/scd.2014.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lujan E, Chanda S, Ahlenius H, Südhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci USA. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weissbein U, Ben-David U, Benvenisty N. Virtual karyotyping reveals greater chromosomal stability in neural cells derived by transdifferentiation than those from stem cells. Cell Stem Cell. 2014;15:687–691. doi: 10.1016/j.stem.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 125.Meyer K, Ferraiuolo L, Miranda CJ, Likhite S, McElroy S, Renusch S, Ditsworth D, Lagier-Tourenne C, Smith RA, Ravits J, et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc Natl Acad Sci USA. 2014;111:829–832. doi: 10.1073/pnas.1314085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hemmer K, Zhang M, van Wüllen T, Sakalem M, Tapia N, Baumuratov A, Kaltschmidt C, Kaltschmidt B, Schöler HR, Zhang W, et al. Induced neural stem cells achieve long-term survival and functional integration in the adult mouse brain. Stem Cell Reports. 2014;3:423–431. doi: 10.1016/j.stemcr.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fu L, Zhu X, Yi F, Liu GH, Izpisua Belmonte JC. Regenerative medicine: transdifferentiation in vivo. Cell Res. 2014;24:141–142. doi: 10.1038/cr.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Björklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci USA. 2013;110:7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rouaux C, Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nat Cell Biol. 2013;15:214–221. doi: 10.1038/ncb2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grande A, Sumiyoshi K, López-Juárez A, Howard J, Sakthivel B, Aronow B, Campbell K, Nakafuku M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heinrich C, Bergami M, Gascón S, Lepier A, Viganò F, Dimou L, Sutor B, Berninger B, Götz M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Reports. 2014;3:1000–1014. doi: 10.1016/j.stemcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dropulić B. Lentiviral vectors: their molecular design, safety, and use in laboratory and preclinical research. Hum Gene Ther. 2011;22:649–657. doi: 10.1089/hum.2011.058. [DOI] [PubMed] [Google Scholar]
- 136.Wyse RD, Dunbar GL, Rossignol J. Use of genetically modified mesenchymal stem cells to treat neurodegenerative diseases. Int J Mol Sci. 2014;15:1719–1745. doi: 10.3390/ijms15021719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lentz TB, Gray SJ, Samulski RJ. Viral vectors for gene delivery to the central nervous system. Neurobiol Dis. 2012;48:179–188. doi: 10.1016/j.nbd.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miyoshi H, Blömer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Philippe S, Sarkis C, Barkats M, Mammeri H, Ladroue C, Petit C, Mallet J, Serguera C. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proc Natl Acad Sci USA. 2006;103:17684–17689. doi: 10.1073/pnas.0606197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Escors D, Breckpot K. Lentiviral vectors in gene therapy: their current status and future potential. Arch Immunol Ther Exp (Warsz) 2010;58:107–119. doi: 10.1007/s00005-010-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 144.Meng F, Chen S, Miao Q, Zhou K, Lao Q, Zhang X, Guo W, Jiao J. Induction of fibroblasts to neurons through adenoviral gene delivery. Cell Res. 2012;22:436–440. doi: 10.1038/cr.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Griesenbach U, Inoue M, Hasegawa M, Alton EW. Sendai virus for gene therapy and vaccination. Curr Opin Mol Ther. 2005;7:346–352. [PubMed] [Google Scholar]
- 146.Iida A, Inoue M. Concept and technology underlying Sendai virus (SeV) vector development. In: Nagai Y, editor. Sendai Virus Vector. Japan: Springer; 2013. pp. 69–89. [Google Scholar]
- 147.Adler AF, Grigsby CL, Kulangara K, Wang H, Yasuda R, Leong KW. Nonviral direct conversion of primary mouse embryonic fibroblasts to neuronal cells. Mol Ther Nucleic Acids. 2012;1:e32. doi: 10.1038/mtna.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 149.Cheng L, Hu W, Qiu B, Zhao J, Yu Y, Guan W, Wang M, Yang W, Pei G. Generation of neural progenitor cells by chemical cocktails and hypoxia. Cell Res. 2014;24:665–679. doi: 10.1038/cr.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dai P, Harada Y, Takamatsu T. Highly efficient direct conversion of human fibroblasts to neuronal cells by chemical compounds. J Clin Biochem Nutr. 2015;56:166–170. doi: 10.3164/jcbn.15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hong JY, Lee SH, Lee SC, Kim JW, Kim KP, Kim SM, Tapia N, Lim KT, Kim J, Ahn HS, et al. Therapeutic potential of induced neural stem cells for spinal cord injury. J Biol Chem. 2014;289:32512–32525. doi: 10.1074/jbc.M114.588871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu J, Sheng C, Liu Z, Jia W, Wang B, Li M, Fu L, Ren Z, An J, Sang L, et al. Lmx1a enhances the effect of iNSCs in a PD model. Stem Cell Res. 2015;14:1–9. doi: 10.1016/j.scr.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 153.Pfisterer U, Wood J, Nihlberg K, Hallgren O, Bjermer L, Westergren-Thorsson G, Lindvall O, Parmar M. Efficient induction of functional neurons from adult human fibroblasts. Cell Cycle. 2011;10:3311–3316. doi: 10.4161/cc.10.19.17584. [DOI] [PubMed] [Google Scholar]
- 154.Wang P, Zhang HL, Li W, Sha H, Xu C, Yao L, Tang Q, Tang H, Chen L, Zhu J. Generation of patient-specific induced neuronal cells using a direct reprogramming strategy. Stem Cells Dev. 2014;23:16–23. doi: 10.1089/scd.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chanda S, Ang CE, Davila J, Pak C, Mall M, Lee QY, Ahlenius H, Jung SW, Südhof TC, Wernig M. Generation of induced neuronal cells by the single reprogramming factor ASCL1. Stem Cell Reports. 2014;3:282–296. doi: 10.1016/j.stemcr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu X, Huang Q, Li F, Li CY. Enhancing the efficiency of direct reprogramming of human primary fibroblasts into dopaminergic neuron-like cells through p53 suppression. Sci China Life Sci. 2014;57:867–875. doi: 10.1007/s11427-014-4730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tian C, Ambroz RJ, Sun L, Wang Y, Ma K, Chen Q, Zhu B, Zheng JC. Direct conversion of dermal fibroblasts into neural progenitor cells by a novel cocktail of defined factors. Curr Mol Med. 2012;12:126–137. doi: 10.2174/156652412798889018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim SM, Flaßkamp H, Hermann A, Araúzo-Bravo MJ, Lee SC, Lee SH, Seo EH, Lee SH, Storch A, Lee HT, et al. Direct conversion of mouse fibroblasts into induced neural stem cells. Nat Protoc. 2014;9:871–881. doi: 10.1038/nprot.2014.056. [DOI] [PubMed] [Google Scholar]
- 159.Zou Q, Yan Q, Zhong J, Wang K, Sun H, Yi X, Lai L. Direct conversion of human fibroblasts into neuronal restricted progenitors. J Biol Chem. 2014;289:5250–5260. doi: 10.1074/jbc.M113.516112. [DOI] [PMC free article] [PubMed] [Google Scholar]


