Abstract
Anti-arrhythmic properties of n-3 polyunsaturated fatty acids, at least in part mediated by anti-oxidant, anti-inflammatory and anti-fibrotic power, have been widely proved. Effect of fish oil on atrial fibrillation, both in primary and in secondary prevention and after cardiac surgery, are controversial, mostly due to lack of homogeneity between studies but also due to individual variability in response to fatty acids administration. Inclusion of measurement of incorporation of fish oil into cell membranes, appears to be essential in future studies, to assess their antiarrhythmic effect.
Keywords: N-3 polyunsaturated fatty acids, Atrial fibrillation, Upstream therapy, Omega-3 index, Cardiac surgery
Core tip: Individual variability in response to fish oil administration, in terms of eicosapentaenoic and docosahexaenoic acids in corporation into cell membranes, is responsible for controversial results of n-3 poly-unsaturated fatty acids administration in patients suffering atrial fibrillation.
INTRODUCTION
N-3 poly-unsaturated fatty acids (PUFA) anti-arrhythmic effects have been debated for several years, since their electrophysiological properties have been recognized.
Through direct interaction with membrane bound proteins and thanks to incorporation into the phospholipid bilayer, n-3 PUFA are well known to influence ion channels and transmembrane pumps[1] to modulate signal transduction, protein trafficking and ion channels kinetic and to regulate gene expression[2]. N-3 PUFA can also exert anti-inflammatory effects by antagonizing pro-inflammatory prostaglandin formation[2], and exert anti-fibrotic effects[3], as well as cardiac autonomic modulation[4].
In particular, the influence of n-3 PUFA on atrial fibrillation (AF) primary and secondary prevention, including post-operative AF (POAF) has also been the object of numerous clinical studies.
N-3 PUFA in primary and secondary prevention and in POAF
Primary prevention: With regard to primary prevention of AF (Table 1), two studies involving elderly subjects[5,6] and one focusing on patients affected by acute myocardial infarction[7] proved n-3 PUFA to be protective against AF, while other studies[8-12], showed no benefit. The influence of various diet habits, including fish consumption[8,9], can possibly explain different results, as well as different methodologies used for assessment of fish intake and for AF diagnosis. In particular, positive studies, generally included elderly individuals[5-7], suggesting benefit from antifibrotic properties of fish-oil. However, a post-hoc analysis of the randomized controlled trial GISSI-HF[13] showed no effect of long-term PUFA administration on AF development in heart failure patients, thus allowing no conclusions for the role of n-3 PUFA in AF primary prevention.
Table 1.
Clinical studies investigating the effect of n-3 poly-unsaturated fatty acids on primary prevention for atrial fibrillation
| Study design | Population | PUFA administration | PUFA quantification | AF diagnosis | Results |
| Prospective cohort[5] | 4815 individuals; age 72.8 yr; United States | Broiled/backed fish assessment. FU: 12 yr | FFQ | Annual ECG; hospital discharge diagnoses | Lower AF risk of 31% with fish intake ≥ 5 times/wk vs < 1/mo. P = 0.008 |
| Prospective cohort[12] | 2174 subjects; mean age: 52.8 yr; Finland | Serum EPA and DHA and dosage. FU: 17.7 yr | DHA, EPA serum dosage | National computerized hospitalization registry | Lower AF risk of 38% for higher DHA levels. P = 0.02 |
| Prospective cohort[6] | 3326 subjects; age: 74.1 yr; United States | Serum EPA, DHA dosage | DHA, EPA serum dosage | Annual ECG; telephonic contact 2/yr; hospitalizations | Lower AF risk for top vs lowest quartile of PUFA/ DHA levels |
| Population study[7] | 3242 subjects affected by acute myocardial infarction; age: 54.1 yr; Italy | Previous PUFA intake vs not. FU: 360 d | FFQ | AF episodes during hospitalization | Lower risk of AF with fish oil |
| Prospective cohort[8] | 47949 subjects; age: 46 yr; Denmark | Fish-oil intake assessment. FU: 5.7 yr | FFQ | Danish national hospitalization registry | Higher AF risk for top vs lowest quintiles of fish intake |
| Prospective cohort[9] | 5184 subjects; age 67.4 yr; the Netherland | Fish-oil intake assessment. FU: 6.4 yr | FFQ | Two ECGs during FU; clinical data from general practitioners | No AF risk reduction in the highest tertile of fish intake |
| Prospective cohort[10] | 44720 female; age: 63 yr; United States | Fish intake assessment. FU: 6 yr | FFQ | ECG at baseline and at the third and sixth years | No lower AF risk for higher fish intake |
| Prospective cohort[11] | 4526 individuals; age: 62 yr; United States | Fish intake assessment. FU: 4 yr | FFQ | Two ECGs every 4 yr of FU; hospitalizations | No AF risk reduction in the top vs the lowest tertile of fish intake |
| Post-hoc analysis of a RCT (Aleksova)[13] | 5835 systolic heart failure-subjects | N-3 PUFAs 1 g/d vs placebo; FU 3.9 yr | No PUFA dosage | ECG during FU visits | No AF risk reduction with n-3 PUFA |
FU: Follow-up; FFQ: Food frequency questionnaires; AF: Atrial fibrillation; EPA: Eicosapentaenoic acid; DHA: Docosahexaenoic acid; RCT: Randomized controller trial; PUFA: Poly-unsaturated fatty acids.
Post-operative AF: The effect of n-3 PUFA in the context of POAF, that is characterized by inflammation, electrolyte disturbances and hemodynamic instability secondary to cardiac surgery, have also been also widely investigated. An open label study[14] firstly observed a short-term n-3 PUFA administration-related decrease in POAF incidence after coronary artery bypass grafting. Two papers[15,16] also gained benefit from various fish-oil preparations and administration timings (Table 2). A recent randomized-controlled trial (RCT)[17] also observed reduction of POAF with n-3 PUFAs plus vitamins C and E administration in comparison to placebo, in 203 patients scheduled for cardiac surgery. Further studies however, failed to prove both prevention of AF[18,19] and decrease of inflammation[20] from higher serum levels of n-3 PUFA, eicosapentaenoic acid (EPA), or docosahexaenoic acid (DHA), and from higher n3-PUFA atrial content[21,22]. Recently, the multicenter double-blind RCT “OPERA”[23] showed no influence on POAF occurrence, from short-term n3-PUFA administration. The effect was unrelated to patients characteristics, kind of cardiac-surgery, antiarrhythmic drugs, fish intake and serum n-3 PUFA. In a substudy of this trial indeed[24], including 564 subjects receiving short-term PUFA or placebo before surgery, the risk of POAF was unrelated to fish oil concentrations at enrollment and day of surgery. Interestingly, PUFA increase, was characterized by significant inter-individual variableness (0.7%-7.5% after 5 d of supplementation). Finally, Metcalf et al[25], by using combined data from previous RCTs, demonstrated less incidence of POAF among subject within the fourth quintile of red blood cell n-3 DHA, thus suggesting a U-shaped relation between n-3 PUFA intake and POAF. Four recent meta-analyses of the previously presented studies showed in turn, overall protective or neutral effect on POAF from n-3 PUFA[26-29] (Table 3). Of note, none of these meta-analyses has assessed n-3 PUFA treatment duration to surgery as a covariate in a meta-regression analysis (Figure 1).
Table 2.
Principal clinical studies investigating the effect of n-3 poly-unsaturated fatty acids on post-operative atrial fibrillation
| Study design | Population | PUFA administration | PUFA quantification | AF diagnosis | Results |
| Randomized, open label[14] | 160 CABG pts; age: 66.2 yr; Italy; BB approximately 57%; statins approximately 58% | N-3 PUFA 2 g/d (EPA/DHA: 1:2) ≥ 5 d before CS, until discharge vs not | No PUFA dosage | Continuous 5 d monitoring + daily ECG up to discharge. AF: > 5 min/requiring therapy | Lower AF risk. P = 0.013 |
| Prospective observational[15] | 530 CS pts; age: 66.4 yr; Italy. BB: 53%; statins: 46% | N-3 PUFA 1 g/d (EPA/DHA: 0.9:1.5) 5 d pre-CS vs not | No PUFA dosage | Continuous monitoring during ICU-stay. AF: ≥ 5 min | Lower POAF during ICU stay. P = 0.006 |
| Double blind-RCT[16] | 102 CABG pts; age: 67 yr; Germany | Iv 100 mg fish oil/kg per day during ICU-stay vs soya oil | No PUFA dosage | Continuous monitoring during ICU-stay | Lower AF risk with PUFA. P < 0.05 |
| Prospective cohort[19] | 125 CABG pts; age: approximately 68 yr; Iceland. BB: 77.4%; statins: 84% | N3-PUFA (EPA/DHA: 1.2:1) 2.2 g/d 7 d pre-CABG vs placebo | PUFA dosage basally, before, 3 d after CS | Continuous monitoring during hospital stay. AF: ≥ 5 min | Positive DHA/POAF association (U-curve relationship) |
| Double blind-RCT[23] | 1516 CS pts; age: 64 yr; Italy-United States-Argentina. BB: 76.9%; statins: 57.5% | N3-PUFA (EPA/DHA: 4.6:3.7) 2 g/d 5 d pre-CS up to discharge vs placebo | Serum PUFA dosage basally, before CS | Continuous 5 d monitoring. AF: ≥ 30 s | No lower AF despite 40% higher plasmatic PUFA |
| Double blind-RCT[18] | 243 CS pts; age: 62.7 yr, United States. BB: 79%; statins: 73% | N-3 PUFA 2 g/d vs corn oil | Basal serum PUFA dosage, before, 3 d post CS | Continuous ECG during hospital stay; FU: 1 mo. AF: Episodes requiring treatment | No lower AF; plasma PUFA increase |
| Double blind-RCT[20] | 170 CS pts; age: 67 yr; Iceland. BB approximately 76% | N3-PUFA (EPA/DHA: 1.2:1) 2 g/d 1 wk before and 2 after CS vs olive oil | Serum DHA, EPA dosage basally, pre 3 d post CS | Continuous monitoring during hospital stay. AF: ≥ 5 min | No lower AF; plasma n-3 PUFA increase |
| Double blind-RCT[22] | 200 CS pts; age: 64 yr; Australia, BB: 43%; statins: 73% | N-3 PUFA oil (EPA/DHA: 2.7:1.9) for 3 wk vs placebo | Dosage of serum PUFA basally, pre-CS; atrial PUFA | Continuous 72 h monitoring. AF/flutter ≥ 10 min/requiring treatment | No lower AF risk; increase in serum and atrial PUFA |
| Double blind RCT[21] | 108 CABG pts; age: 64 yr; United Kingdom; BB: 88%; statins: 98% | N-3 PUFA (EPA/DHA: 1.2:1) 2 g/d for approximately 16 d vs olive oil | Dosage of serum PUFA basally, 3 d post CS; atrial PUFA | Continuous 5 d monitoring + daily ECG. AF: > 30 s | No lower AF risk; higher serum and atrial PUFA |
CABG: Coronary artery bypass grafting; pts: Patients; BB: Beta blockers; CS: Cardiac surgery; ICU: Intensive care unit; PUFA: Poly-unsaturated fatty acids; EPA: Eicosapentaenoic acid; DHA: Docosahexaenoic acid; AF: Atrial fibrillation.
Table 3.
Recent metaanalises of studies of n-3 poly-unsaturated fatty acids in post-operative atrial fibrillation
| Ref. | Clinical setting | NO. of studies and of patients | Results |
| Costanzo et al[26] | POAF | 8 RCTs/2687 pts | AF reduction |
| Benedetto et al[27] | POAF | 431 pts | No AF reduction; at meta-regression analysis: Trend toward a benefit from PUFA for administration of EPA/DHA ratio = 1:2 |
| Zhang et al[28] | POAF | 8 RCT/2687 pts | No AF reduction |
| Ali-Hassan-Sayegh et al[29] | POAF | 23 RCTs/4278 pts | AF reduction |
RCTs: Randomized controller trials; pts: Patients; PO: Post-operative; AF: Atrial fibrillation; PUFA: Poly-unsaturated fatty acids; EPA: Eicosapentaenoic acid; DHA: Docosahexaenoic acid.
Figure 1.
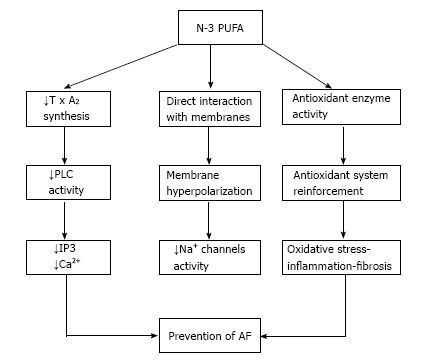
Antiarrhythmic effects of n-3 poly-unsaturated fatty acids. N-3 PUFA: N-3 poly-unsaturated fatty acids; TxA2: Thromboxane A2; PLC: Phospholipase C; IP3: Inositol triphosphate; AF: Atrial fibrillation.
Dissimilarities may be explained by various study designs and populations, AF definitions, cardiac surgery, co-administration of anti-arrhythmic or anti-inflammatory drugs, dietary PUFA intake, EPA/DHA ratios and fish oil-administration modes (i.e., intravenous or through nasogastric tube) and fish-oil administration time courses. Conversely, no effects of n-3 PUFA administration on myocardial infarction and bleeding after cardiac surgery, eventually influencing POAF occurrence, have been demonstrated[27].
Interestingly, all RCTs that failed to demonstrate a beneficial effect, used a formulation containing 1.24 EPA:DHA ratio[18,20,23]. In contrast, Rodorigo et al[17] administered PUFA with an EPA:DHA ratio equal to 0.5.
Secondary prevention: Several studies have finally investigated the effect on n-3 PUFA on relapses of paroxysmal and persistent AF. Two studies[30,31], found fish oil administration (from 1 mo before, to 6 mo after cardioversion) helpful in AF prevention (Table 4). On the other hand, 4 further studies[32-35] failed to prove any effect.
Table 4.
Clinical studies investigating the effect of n-3 poly-unsaturated fatty acids on secondary prevention for atrial fibrillation
| Study design | Population | PUFA administration | PUFA quantification | AF diagnosis | Results |
| Double blind-RCT[30] | 109 pts, age: 70 yr; Italy; heart structural abnormality: 90%; Amiodarone + ACE-i/ARBs: 100% | N-3 PUFA (EPA/DHA 1.2:1) 2 g/d, 1 mo before and 12 after ECV vs olive oil | No PUFA dosage | Weekly ECG for the first 3 wk after ECV and ECG + Holter ECG after 1, 3, 6, 12 mo and at symptoms occurrence | Less AF relapses with PUFA |
| Open-label randomized[31] | 178 pts, Australia. Concomitant amiodarone, sotalol, ACE-i/ARBs | N-3 PUFA (EPA/DHA 1.3:1) 1.8 g/d for approximately 56 d before ECV and 1 year thereafter vs not | Serum dosage of EPA, DHA basally, before ECV | ECG at week 2 and 6 and every 3 mo. AF: ≥ 1 wk | Less AF relapses at 90 d and 1 yr with PUFA, P < 0.001; higher serum EPA, DHA |
| Double blind-RCT[33] | 663 pts; paroxysmal AF: 18%; age: 60.5 yr; United States. No heart abnormality. Amiodarone: 0%, antiarrhythmic drugs: 13%; ACE-i/ARBs: 39% | N-3 PUFA (EPA/DHA 4.6:3.7; load: 8 g/d for 1 wk) 4 g/d for 24 wk vs oil | Serum DHA, EPA dosage basally, after 4 and 24 wk | Biweekly transtelephonic monitoring | No lower symptomatic AF recurrence in the paroxysmal and persistent |
| Prospective[35] | 50 pts; ≥ 2 previous AF episodes; age: 54 yr, Japan. IC antiarrhythmic drugs: 100% | Observational period: no PUFA for 6 mo. Interventional period: EPA 1.8 g/d for 6 mo | Serum EPA, DHA dosage basally and at study end | Daily ECG monitoring and at symptoms occurrence | No lower AF burden and time to first relapse |
| Double blind-RCT[32] | 204 pts, age: 69.3 yr; Italy. LAs 45 mm. First ECV: 59%; IC antiarrhythmic drugs: 29.5%, sotalol: 12.6%, amiodarone: 27.4% | N-3 PUFA (EPA/DHA 1.2:1) 3 g/d ≥ 1 wk before and 2 g/d after ECV for 6 mo vs olive oil | N-3 PUFA serum dosage basally, 6 mo after ECV | Transtelephonic monitoring: 2/first week after ECV and 3/wk for 3 mo + clinical visits after 7 d, 1, 3, 6 mo | No difference in ECV success, AF incidence, time to first relapse. Increase of EPA and DHA |
| Double blind RCT[36] | 337 pts; symptomatic paroxysmal or persistent AF within 6 mo of enrollment | Fish oil (4 g/d) or placebo | Followed, on average, for 271 ± 129 d | Trans-telephonic event recorder, 12-lead ECG or Holter | No lower AF with PUFA |
| Double blind-RCT[37] | 190 pts with paroxysmal or persistent AF | N-3 PUFAs (4 g/d; n = 126) or placebo (n = 64) in a 2:1 ratio | No PUFA dosage | Not specified | No reduction of AF recurrence and inflammation markers |
| Double blind-RCT[34] | 586 pts with symptomatic paroxysmal AF requiring ECV (n = 428), at least 2 episodes of AF in the 6 mo before (n = 55), or both (103) | N-3 PUFA (1 g/d) or placebo for 12 mo | No PUFA dosage | Not specified | No lower AF with PUFA |
RCTs: Randomized controller trials; pts: Patients; PO: Post-operative; AF: Atrial fibrillation; PUFA: Poly-unsaturated fatty acids; EPA: Eicosapentaenoic acid; DHA: Docosahexaenoic acid; ACE-I: Angiotensin converting enzyme inhibitor; ARB: Angiotensin receptor blockers.
A recent study[36] including 337 patients with symptomatic paroxysmal/persistent AF, randomized to receive fish oil (4 g/d) or placebo, showed no difference in time to first AF recurrence, as well as no significant decrease of inflammatory markers at 6 mo. Similarly, another RCT[37], proved no effect from n-3 PUFA on the time to AF relapses, as well as on concentrations of biomarkers of oxidative stress and inflammation and at follow-up. In particular, a large RCT[34] involving 586 patients with symptomatic paroxysmal or persistent AF, randomized to n-3 PUFA (1 g/d) vs placebo for 1 year, also proved no significant differences between the two arms, in terms of symptomatic recurrence of AF.
Contrasting outcomes between studies may be related to differences in PUFA somministration and populations characteristics. Generally, papers including subjects with more evident cardiac disease[30], more often co-administered with amiodarone[30] showed benefit. Of note, some unfavorable papers proved AF relapses to occur mostly within 3 wk, prior to an eventual effect from n-3 PUFA.
DISCUSSION
The effect of n-3 PUFA on AF primary and secondary prevention and after cardiac surgery, remains controversial. A major reason for this uncertainty, is to be found in differences between studies, in particular regarding study designs, patients characteristics, AF definition and types (lone, vagally/adrenergically induced, secondary to structural disease), fish oil-administration modes, formulations and time courses. Moreover, a great variability in n-3 PUFA serum concentrations between subjects, despite similar fish-oil administration, has been recently proved, likely secondary to genetic predisposition in PUFA metabolism.
Noteworthy, however, a recent RCT[38] examined the effects of high (6 g/d) or medium dose (3 g/d) fish oil supplementation, with or without multivitamin, on the inclusion of n-3 and n-6 PUFA within membranes of red blood cells after 16 wk. The authors found all treatments effective in increasing EPA composition of cell membranes in females, but not in males, for whom the higher dose n-3 PUFA plus multivitamin combination was necessary. As a consequence, discrepancies between trials could be partially related to individual capability of n-3 PUFA incorporation, which in turn, could be influenced by sex, age, vitamin and/or drug administration. To counteract the variability in response to fish oil administration, inclusion of blood measures of n-3 PUFA status appears therefore to be essential in future studies.
The “Omega-3 Index” is the percentage of PUFA composed of EPA + DHA in red blood cell membranes[39] may represent a measurement of clinical utility to assess individual response to fish oil intake. Moreover, it may contribute to better understand the pharmacokinetics and pharmacodynamics of PUFA. Considering the results of recent studies showing an U-curve relationship between PUFA concentrations and AF[19,25], the greater protection from AF could be obtained from an individually-targeted approach for fish oil inclusion within membranes.
CONCLUSION
The complexity of the biological interactions of n-3 PUFA, their incorporation into cell membranes and the variability of clinical contexts, likely justify why PUFA administration does not automatically lead to AF reduction. RCTs focusing on clinical contexts of AF, and characterized by more accurate follow-ups and definitions of PUFA incorporation into red blood cells (or hopefully, in atrial tissue in the setting of cardiac surgery), are required. The RCT NCT00692718, will hopefully add information regarding fish oil effect on AF prevention in the context of HF and/or AMI.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 3, 2015
First decision: September 26, 2015
Article in press: January 4, 2016
P- Reviewer: Bonanno C, Chen SJ, Ho KM, Letsas K S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
References
- 1.Ander BP, Dupasquier CM, Prociuk MA, Pierce GN. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp Clin Cardiol. 2003;8:164–172. [PMC free article] [PubMed] [Google Scholar]
- 2.Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, Hostetler HA, Vespa A, Landrock D, Landrock KK, et al. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids. 2008;43:1–17. doi: 10.1007/s11745-007-3111-z. [DOI] [PubMed] [Google Scholar]
- 3.da Cunha DN, Hamlin RL, Billman GE, Carnes CA. n-3 (omega-3) polyunsaturated fatty acids prevent acute atrial electrophysiological remodeling. Br J Pharmacol. 2007;150:281–285. doi: 10.1038/sj.bjp.0706977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117:1130–1137. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, Lefkowitz D, Siscovick DS. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu JH, Lemaitre RN, King IB, Song X, Sacks FM, Rimm EB, Heckbert SR, Siscovick DS, Mozaffarian D. Association of plasma phospholipid long-chain ω-3 fatty acids with incident atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2012;125:1084–1093. doi: 10.1161/CIRCULATIONAHA.111.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macchia A, Monte S, Pellegrini F, Romero M, Ferrante D, Doval H, D’Ettorre A, Maggioni AP, Tognoni G. Omega-3 fatty acid supplementation reduces one-year risk of atrial fibrillation in patients hospitalized with myocardial infarction. Eur J Clin Pharmacol. 2008;64:627–634. doi: 10.1007/s00228-008-0464-z. [DOI] [PubMed] [Google Scholar]
- 8.Frost L, Vestergaard P. n-3 Fatty acids consumed from fish and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81:50–54. doi: 10.1093/ajcn/81.1.50. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer IA, Heeringa J, Geleijnse JM, Zock PL, Witteman JC. Intake of very long-chain n-3 fatty acids from fish and incidence of atrial fibrillation. The Rotterdam Study. Am Heart J. 2006;151:857–862. doi: 10.1016/j.ahj.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 10.Berry JD, Prineas RJ, van Horn L, Passman R, Larson J, Goldberger J, Snetselaar L, Tinker L, Liu K, Lloyd-Jones DM. Dietary fish intake and incident atrial fibrillation (from the Women’s Health Initiative) Am J Cardiol. 2010;105:844–848. doi: 10.1016/j.amjcard.2009.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen J, Johnson VM, Sullivan LM, Jacques PF, Magnani JW, Lubitz SA, Pandey S, Levy D, Vasan RS, Quatromoni PA, et al. Dietary factors and incident atrial fibrillation: the Framingham Heart Study. Am J Clin Nutr. 2011;93:261–266. doi: 10.3945/ajcn.110.001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virtanen JK, Mursu J, Voutilainen S, Tuomainen TP. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation. 2009;120:2315–2321. doi: 10.1161/CIRCULATIONAHA.109.852657. [DOI] [PubMed] [Google Scholar]
- 13.Aleksova A, Masson S, Maggioni AP, Lucci D, Fabbri G, Beretta L, Mos L, Paino AM, Nicolosi GL, Marchioli R, et al. n-3 polyunsaturated fatty acids and atrial fibrillation in patients with chronic heart failure: the GISSI-HF trial. Eur J Heart Fail. 2013;15:1289–1295. doi: 10.1093/eurjhf/hft103. [DOI] [PubMed] [Google Scholar]
- 14.Calò L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, Meo A, Pandozi C, Staibano M, Santini M. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–1728. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- 15.Mariscalco G, Sarzi Braga S, Banach M, Borsani P, Bruno VD, Napoleone M, Vitale C, Piffaretti G, Pedretti RF, Sala A. Preoperative n-3 polyunsatured fatty acids are associated with a decrease in the incidence of early atrial fibrillation following cardiac surgery. Angiology. 2010;61:643–650. doi: 10.1177/0003319710370962. [DOI] [PubMed] [Google Scholar]
- 16.Heidt MC, Vician M, Stracke SK, Stadlbauer T, Grebe MT, Boening A, Vogt PR, Erdogan A. Beneficial effects of intravenously administered N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a prospective randomized study. Thorac Cardiovasc Surg. 2009;57:276–280. doi: 10.1055/s-0029-1185301. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigo R, Korantzopoulos P, Cereceda M, Asenjo R, Zamorano J, Villalabeitia E, Baeza C, Aguayo R, Castillo R, Carrasco R, et al. A randomized controlled trial to prevent post-operative atrial fibrillation by antioxidant reinforcement. J Am Coll Cardiol. 2013;62:1457–1465. doi: 10.1016/j.jacc.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Sandesara CM, Chung MK, Van Wagoner DR, Barringer TA, Allen K, Ismail HM, Zimmerman B, Olshansky B. A Randomized, Placebo-Controlled Trial of Omega-3 Fatty Acids for Inhibition of Supraventricular Arrhythmias After Cardiac Surgery: The FISH Trial. J Am Heart Assoc. 2012;1:e000547. doi: 10.1161/JAHA.111.000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skuladottir GV, Heidarsdottir R, Arnar DO, Torfason B, Edvardsson V, Gottskalksson G, Palsson R, Indridason OS. Plasma n-3 and n-6 fatty acids and the incidence of atrial fibrillation following coronary artery bypass graft surgery. Eur J Clin Invest. 2011;41:995–1003. doi: 10.1111/j.1365-2362.2011.02497.x. [DOI] [PubMed] [Google Scholar]
- 20.Heidarsdottir R, Arnar DO, Skuladottir GV, Torfason B, Edvardsson V, Gottskalksson G, Palsson R, Indridason OS. Does treatment with n-3 polyunsaturated fatty acids prevent atrial fibrillation after open heart surgery? Europace. 2010;12:356–363. doi: 10.1093/europace/eup429. [DOI] [PubMed] [Google Scholar]
- 21.Saravanan P, Bridgewater B, West AL, O’Neill SC, Calder PC, Davidson NC. Omega-3 fatty acid supplementation does not reduce risk of atrial fibrillation after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. Circ Arrhythm Electrophysiol. 2010;3:46–53. doi: 10.1161/CIRCEP.109.899633. [DOI] [PubMed] [Google Scholar]
- 22.Farquharson AL, Metcalf RG, Sanders P, Stuklis R, Edwards JR, Gibson RA, Cleland LG, Sullivan TR, James MJ, Young GD. Effect of dietary fish oil on atrial fibrillation after cardiac surgery. Am J Cardiol. 2011;108:851–856. doi: 10.1016/j.amjcard.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Marchioli R, Macchia A, Silletta MG, Ferrazzi P, Gardner TJ, Latini R, Libby P, Lombardi F, O’Gara PT, et al. Fish oil and postoperative atrial fibrillation: the Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) randomized trial. JAMA. 2012;308:2001–2011. doi: 10.1001/jama.2012.28733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu JH, Marchioli R, Silletta MG, Macchia A, Song X, Siscovick DS, Harris WS, Masson S, Latini R, Albert C, et al. Plasma phospholipid omega-3 fatty acids and incidence of postoperative atrial fibrillation in the OPERA trial. J Am Heart Assoc. 2013;2:e000397. doi: 10.1161/JAHA.113.000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metcalf RG, Skuladottir GV, Indridason OS, Sullivan TR, Bjorgvinsdottir L, Sanders P, Arnar DO, Gibson RA, Heidarsdottir R, Cleland LG, et al. U-shaped relationship between tissue docosahexaenoic acid and atrial fibrillation following cardiac surgery. Eur J Clin Nutr. 2014;68:114–118. doi: 10.1038/ejcn.2013.215. [DOI] [PubMed] [Google Scholar]
- 26.Costanzo S, di Niro V, Di Castelnuovo A, Gianfagna F, Donati MB, de Gaetano G, Iacoviello L. Prevention of postoperative atrial fibrillation in open heart surgery patients by preoperative supplementation of n-3 polyunsaturated fatty acids: an updated meta-analysis. J Thorac Cardiovasc Surg. 2013;146:906–911. doi: 10.1016/j.jtcvs.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Benedetto U, Angeloni E, Melina G, Danesi TH, Di Bartolomeo R, Lechiancole A, Refice S, Roscitano A, Comito C, Sinatra R. n-3 Polyunsaturated fatty acids for the prevention of postoperative atrial fibrillation: a meta-analysis of randomized controlled trials. J Cardiovasc Med (Hagerstown) 2013;14:104–109. doi: 10.2459/JCM.0b013e32834a13c1. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Zhen Y, Tao A, Bao Z, Zhang G. Polyunsaturated fatty acids for the prevention of atrial fibrillation after cardiac surgery: an updated meta-analysis of randomized controlled trials. J Cardiol. 2014;63:53–59. doi: 10.1016/j.jjcc.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Ali-Hassan-Sayegh S, Mirhosseini SJ, Rezaeisadrabadi M, Dehghan HR, Sedaghat-Hamedani F, Kayvanpour E, Popov AF, Liakopoulos OJ. Antioxidant supplementations for prevention of atrial fibrillation after cardiac surgery: an updated comprehensive systematic review and meta-analysis of 23 randomized controlled trials. Interact Cardiovasc Thorac Surg. 2014;18:646–654. doi: 10.1093/icvts/ivu020. [DOI] [PubMed] [Google Scholar]
- 30.Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, Gheorghiade M, Dei Cas L. n-3 polyunsaturated fatty acids in the prevention of atrial fibrillation recurrences after electrical cardioversion: a prospective, randomized study. Circulation. 2011;124:1100–1106. doi: 10.1161/CIRCULATIONAHA.111.022194. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Sutherland F, Morton JB, Lee G, Morgan J, Wong J, Eccleston DE, Voukelatos J, Garg ML, Sparks PB. Long-term omega-3 polyunsaturated fatty acid supplementation reduces the recurrence of persistent atrial fibrillation after electrical cardioversion. Heart Rhythm. 2012;9:483–491. doi: 10.1016/j.hrthm.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 32.Bianconi L, Calò L, Mennuni M, Santini L, Morosetti P, Azzolini P, Barbato G, Biscione F, Romano P, Santini M. n-3 polyunsaturated fatty acids for the prevention of arrhythmia recurrence after electrical cardioversion of chronic persistent atrial fibrillation: a randomized, double-blind, multicentre study. Europace. 2011;13:174–181. doi: 10.1093/europace/euq386. [DOI] [PubMed] [Google Scholar]
- 33.Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304:2363–2372. doi: 10.1001/jama.2010.1735. [DOI] [PubMed] [Google Scholar]
- 34.Macchia A, Grancelli H, Varini S, Nul D, Laffaye N, Mariani J, Ferrante D, Badra R, Figal J, Ramos S, et al. Omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: results of the FORWARD (Randomized Trial to Assess Efficacy of PUFA for the Maintenance of Sinus Rhythm in Persistent Atrial Fibrillation) trial. J Am Coll Cardiol. 2013;61:463–468. doi: 10.1016/j.jacc.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe E, Sobue Y, Sano K, Okuda K, Yamamoto M, Ozaki Y. Eicosapentaenoic acid for the prevention of recurrent atrial fibrillation. Ann Noninvasive Electrocardiol. 2011;16:373–378. doi: 10.1111/j.1542-474X.2011.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, Jones P, Ramprasath VR, O’Hara G, Kopecky S, et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. J Am Coll Cardiol. 2014;64:1441–1448. doi: 10.1016/j.jacc.2014.07.956. [DOI] [PubMed] [Google Scholar]
- 37.Darghosian L, Free M, Li J, Gebretsadik T, Bian A, Shintani A, McBride BF, Solus J, Milne G, Crossley GH, et al. Effect of omega-three polyunsaturated fatty acids on inflammation, oxidative stress, and recurrence of atrial fibrillation. Am J Cardiol. 2015;115:196–201. doi: 10.1016/j.amjcard.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pipingas A, Cockerell R, Grima N, Sinclair A, Stough C, Scholey A, Myers S, Croft K, Sali A, Pase MP. Randomized controlled trial examining the effects of fish oil and multivitamin supplementation on the incorporation of n-3 and n-6 fatty acids into red blood cells. Nutrients. 2014;6:1956–1970. doi: 10.3390/nu6051956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Superko HR, Superko AR, Lundberg GP, Margolis B, Garrett BC, Nasir K, Agatston AS. Omega-3 Fatty Acid Blood Levels Clinical Significance Update. Curr Cardiovasc Risk Rep. 2014;8:407. doi: 10.1007/s12170-014-0407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


