Abstract
Background and Aim
Serum IgG4 concentrations are commonly measured in clinical practice. The aim of this study was to investigate serum IgG4 concentrations in adults and their potential relationship with demographic, lifestyle, metabolic, and allergy-related factors.
Methods
Serum IgG4 concentrations were measured with a commercial assay in 413 individuals (median age 55 years, 45% males) who were randomly selected from a general adult population.
Results
Median IgG4 concentration was 26.8 mg/dL. Five out of the 413 individuals (1.2%) exhibited IgG4 concentrations >135 mg/dL, and 17 out of 411 (4.1%) exhibited an IgG4/total IgG ratio >8%. Serum IgG4 concentrations were significantly higher in males than in females and decreased with age. After adjusting for age and sex, serum IgG4 concentrations were not significantly influenced by alcohol consumption, smoking or common metabolic abnormalities (obesity and the related metabolic syndrome). Serum IgG4 concentrations were not significantly correlated with serum concentrations of proinflammatory cytokines and inflammation markers. Serum IgG4 concentrations were significantly correlated with IgE concentrations. Serum IgG4 concentrations tended to be higher in atopics (individuals with IgE-mediated sensitization to aeroallergens) than in non-atopics, particularly among atopics without respiratory symptoms. Serum IgG4 concentrations were not significantly correlated with total eosinophil blood count. Cases of IgG4-related disease were neither present at baseline nor detected after a median of 11 years of follow-up.
Conclusions
Studies aimed at defining reference IgG4 values should consider partitioning by age and sex. Further studies are needed to confirm the potential influence of atopy status on serum IgG4 concentrations.
Introduction
The fourth subclass of immunoglobulin G (IgG4) is the less abundant serum IgG subclass in humans [1]. The half-life of IgG4 is approximately 21 days [2] and, unlike other IgG subclasses, IgG4 does not bind complement [3]. It is also unique among antibodies in that IgG4 molecules can be asymmetric, i.e., a large fraction of plasma IgG4 molecules have two different antigen-binding sites, resulting in bi-specificity [4,5]. Like IgE, regulation of IgG4 production is dependent on Th2 cells [5].The function of IgG4 is not completely understood. It may play a role in allergic disorders by competing for allergen with IgE bound to mast cells, thus blocking their stimulation [5]. The presence of allergen-specific IgG4, either spontaneously or after allergen immunotherapy, indicates that tolerance-inducing mechanisms have been activated [5].
Serum concentrations of IgG4 have been little studied in general populations. In a study of 172 healthy adults, French and Harrison (1984) found that IgG4 concentrations were higher in males than in females [6]. A similar finding was reported by Aucouturier et al. (1984) in a study of 173 adults [7]. No significant effect of age on IgG4 concentrations was reported in these studies, although a trend to increased concentrations in older individuals was reported [7]. To the best of our knowledge, the potential effect of common factors such as metabolic disorders (obesity and related alterations) and lifestyle factors (alcohol consumption and smoking) has been not investigated. Total serum IgG4 concentrations were not influenced by allergy, a common condition in the general population, in one study [7].
In recent decades, the methods for IgG4 measurement have improved and the interest on IgG4 concentrations has increased due to the emerging concept of IgG4-related disease (IgG4-RD), which integrates a wide number of old diseases from a new perspective [8–11]. Inflammation and fibrosis of one or more organs, with a lymphoplasmacytic infiltrate rich in IgG4-positive plasma cells is the hallmark of IgG4-RD [8–11]. The role of IgG4 in the pathogenesis of IgG4-RD is unclear. It is not known whether IgG4 antibodies are pathogenic or represent a regulatory response to another primary autoimmune or allergic process [8–15]. While raised IgG4 concentrations alone are not diagnostic of IgG4-RD because both sensitivity and specificity are not optimal [11,16–20], they nevertheless constitute one of the classic diagnostic criteria for IgG4-RD [21] and may also be useful for the monitoring of IgG4-RD [17,18].
Determining the distribution of IgG4 concentrations in the general population is important for the interpretation of reference values in clinical practice. Studies on the general population may serve to explore the potential influence of common factors and/or disorders. Accordingly, the aim of this study was to evaluate serum IgG4 concentrations in a general adult population and their potential relationship with demographic, lifestyle, metabolic, and allergy-related factors.
Methods
Study population and design
This study forms part of a survey of the general adult population from A-Estrada, a municipality in north-western Spain. Detailed descriptions of methodology and population characteristics have been reported elsewhere [22]. Briefly, an age-stratified random sample (n = 720) of the adult population (>18 years) of the municipality was drawn from the Health Care Registry, which covers >95% of the population. A total of 469 individuals were studied across the period January 2000-January 2001 [22]. Frozen serum samples for IgG4 determination were available for 413 of these individuals, median age 55 years (range 18–92 years). All participants were Caucasians. A total of 186 (45.0%) were males. The source study (FIS1306/99) was reviewed and approved by the Institutional Review Board of the Complejo Hospitalario Universitario from Santiago de Compostela (Spain). The present study (PGIDIT06PXIB918313) was reviewed and approved by the Clinical Research Ethics Committee from Galicia (Spain). Written informed consent was obtained from each participant in the study, which conformed to the current Helsinki Declaration.
Classification of alcohol consumption and smoking
Alcohol consumption was evaluated in standard drinking units [23], by summing the number of glasses of wine (~10g), bottles of beer (~10g), and units of spirits (~10g) regularly consumed per week. Individuals with an habitual alcohol consumption of 1–140 g/week were defined as light drinkers, those with an alcohol consumption of 141–280 g/week were defined as moderate drinkers, and those with an alcohol consumption >280 g/week were defined as heavy drinkers. The remainder, alcohol abstainers or very occasional alcohol drinkers, were included in the same group. Consumers of at least one cigarette per day were considered smokers. Individuals who had quit smoking during the preceding year were still considered smokers, while those who had quit more than one year prior to the study were considered ex-smokers. Individuals with an habitual tobacco consumption of 1–9 cigarettes/day were defined as light smokers, and those with an habitual tobacco consumption of >9 cigarettes/day were defined as heavy smokers.
Definition of metabolic abnormalities
Body mass index (BMI) was calculated as weight (in kg) divided by the square of height (in metres). Following standard criteria, individuals were classified as normal-weight (<25 kg/m2), overweight (25–30 kg/m2), or obese (>30 kg/m2).
Metabolic syndrome was defined according to Adult Treatment Panel III criteria [24], namely: (a) abdominal obesity (waist circumference >102 cm in males or >88 cm in females); (b) hypertriglyceridaemia (fasting serum triglycerides ≥150 mg/dL); (c) low HDL-cholesterol levels (fasting HDL-cholesterol <40 mg/dL in males or <50 mg/dL in females); (d) increased blood pressure (arterial blood pressure ≥130/≥85 mmHg or current anti-hypertensive medication use); and (e) hyperglycaemia (fasting serum glucose ≥110 mg/dl or current anti-diabetic therapy). Individuals who met at least three of these criteria were classified as having metabolic syndrome [24]. Routine laboratory determinations were performed with an Olympus AU-400 analyser (Olympus, Tokyo, Japan).
Atopy traits
Atopy, the genetic tendency to become sensitized and produce IgE antibodies in response to ordinary exposures to allergens via mucosal membranes, is conventionally defined as a positive allergen-specific serum IgE or skin prick test (SPT) to any food or inhalant allergen [25]. As a consequence, these persons can develop typical symptoms of asthma, rhinoconjunctivitis, or eczema [25]. In this study, we systematically investigated allergic sensitization to inhalant allergens (aeroallergens) by means of specific IgE and SPT. Respiratory symptoms were assessed by questionnaire. In addition, the clinical records were reviewed for the presence of food allergies, allergy to drugs and Hymenoptera venoms, and additional hypersensitivity diseases such as contact dermatitis.
Skin prick tests (SPTs)
All subjects underwent SPTs with a panel of relevant aeroallergens in the area. The panel included mites (Dermatophagoides pteronyssinus, Lepydoglyphus destructor, and Tyrophagus putrescentiae), pollens (Lolium perenne, Plantago lanceolata, Betula alba, and Parietaria judaica), moulds (Alternaria alternata, Aspergillus spp., Penicillium notatum, and Cladosporium herbarum), and animal danders (dog and cat) (ALK-Abelló, Spain). Weals >3 mm after 15 minutes were considered positive. Histamine (10 mg/mL) and normal saline were used as positive and negative controls, respectively. A detailed description of the SPT profile in this study population has been reported elsewhere [26]. The presence of at least one positive SPT was deemed to be indicative of allergic sensitization to aeroallergens.
Aeroallergen-specific IgE
The UniCAP-Phadiatop™ test (Thermo Fisher Scientific, formerly Phadia, Uppsala, Sweden) was performed on all participants. This serum test is based on the ImmunoCAP system, and consists of a solid-phase immunoassay for specific IgE using a balanced mixture of relevant aeroallergens causing common allergic respiratory disease. Calculation of results was performed automatically, by reference to the fluorescence response obtained for patient samples compared to the response obtained for the reference serum supplied. The test gives a qualitative result, which is either positive or negative depending on the fluorescence response. When a serum sample gives a fluorescence response higher than or equal to that of the reference serum, the test is interpreted as positive and indicative of allergic sensitization. A previous description of Phadiatop™ positivity in this population and its relationship to positive SPTs has been described elsewhere [27].
Respiratory symptoms
The lifetime history of respiratory symptoms was investigated at baseline in all participants by means of the following questions: (a) “have you ever had a problem with sneezing or a runny or blocked nose when you did not have a cold or the flu?”, and (b) “have you ever had wheezing or whistling in the chest at any time in the past?”, thereby exploring the presence of nasal and bronchial symptoms, respectively. Subjects who answered “yes” to either of these questions were classified as symptomatic, as reported elsewhere [26].
Additional immune diseases
The clinical records of all participants were retrospectively searched for the presence of IgG4-RD at baseline. These records were reviewed again in November 2011 for new-onset immune disease and mortality causes. Two individuals were lost to follow-up. A total of 81 individuals died during the study period. Median follow-up was 137 months (range, 2–142 months).
Serum immunoglobulin assays
Serum samples were stored frozen until use. The IgG4 subclasses were measured by turbidimetry using commercially available kit (The Binding Site Limited, Birmingham, UK) in a SPAPLUS™ analyser (The Binding Site) in strict accordance with the manufacturer's instructions. IgG4 antigen concentration was measured by turbidimetric methods using latex-enhanced antibodies to increase the relative light-scattering signal of the antigen-antibody reaction, increasing the sensitivity to 0.3 mg/dL. Precision studies were performed following CLSI Evaluation of Precision Performance of Clinical Chemistry Approved Guideline (CLSI Document EP5-A) over 21 working days, with two runs per day, obtaining a CV% <5.3 within run. According to the manufacturer, median serum IgG4 concentration in 30 healthy adults was 21.5 mg/dL and the 95th percentile range was 3.9–86.4 mg/dL. A threshold IgG4 concentration of 135 mg/dL is commonly accepted as a criterion for IgG4-related disease [21].
Serum concentrations of total IgG, IgA, and IgM were determined by a commercial nephelometry assay using a BN-II device (Dade Behring, Marburg, Germany), and have been reported elsewhere [28]. The ratio of IgG4 (mg/dL) to total IgG (mg/dL) was calculated and expressed as a percentage. A threshold IgG4/IgG ratio of 8% has been proposed as a criterion for IgG4-related disease [18].
Serum concentrations of total IgE were determined by a commercial chemiluminescent enzyme immunoassay (Immulite, Siemens Medical Solutions, Gwynedd, UK), as reported elsewhere [22]. For some analyses, we calculated the ratio of total IgG4 (mg/dL) to total IgE (IU/mL).
Additional laboratory determinations: inflammatory markers
Serum concentrations of proinflammatory cytokines (IL-6, IL-8 and TNF-alpha) were determined by a commercial chemiluminescent enzyme immunoassay (Immulite™), as reported elsewhere [28–30]. Serum concentrations of lipopolysaccharide binding protein (LBP) in this population were measured by the same method, as reported elsewhere [31]. Serum concentrations of the soluble form of CD14 (sCD14), an additional acute phase marker that increases after endotoxin (lipopolysaccharide) exposure, were measured by means of a commercial enzymoimmunoassay (Quantikine, R&D Systems, Minneapolis, USA) [31]. Serum concentrations of tryptase, a marker of mast cell burden and/or activation, were determined with the ImmunoCAP assay (Thermo Fisher Scientific), as reported elsewhere [32]. Blood eosinophil counts were measured in an Olympus Argos/5 Diff (ABX Diagnostics, Hamburg, Germany) automated haematology analyser.
Statistical analyses
All analyses were performed using the Stata software package version 10 (Stata Corp, College Station, TX, USA), the SPSS software package (SPSS Inc, Chicago, IL, USA), and R-package version 3.1.2. We used the Mann-Whitney test, Kruskal-Wallis test and Jonckheere-Terpstra test (for trend analysis) for comparison of quantitative variables, and Spearman's rank test to assess correlation. Multivariate analyses were performed using linear regression, with serum IgG4 levels (log10-transformed to normalise their distribution) as the dependent variable. For that purpose, the only case with undetectable concentrations was attributed an arbitrary value of 0.4 mg/dL. For covariates, age (in years) was introduced into the equation as a quantitative variable, and binary variables entered the equation as ‘1’ (‘present’ or ‘yes’) or ‘0’ (‘absent’ or ‘no’). Variables were forced to enter the equation in all models. To account for stratified sampling, a design-based analysis including compensatory weights was performed for the estimation of IgG4 levels in the overall population. Centile curves for describing the distribution of IgG4 in relation to age and gender were generated using a combined method based on the Cole-Green and Rigby-Stasinopoulos [33,34] algorithm using the GAMLSS package for R. For all analyses, two-tailed P-values lower than 0.05 were considered statistically significant.
Results
Overall distribution of serum IgG4 concentrations in the population
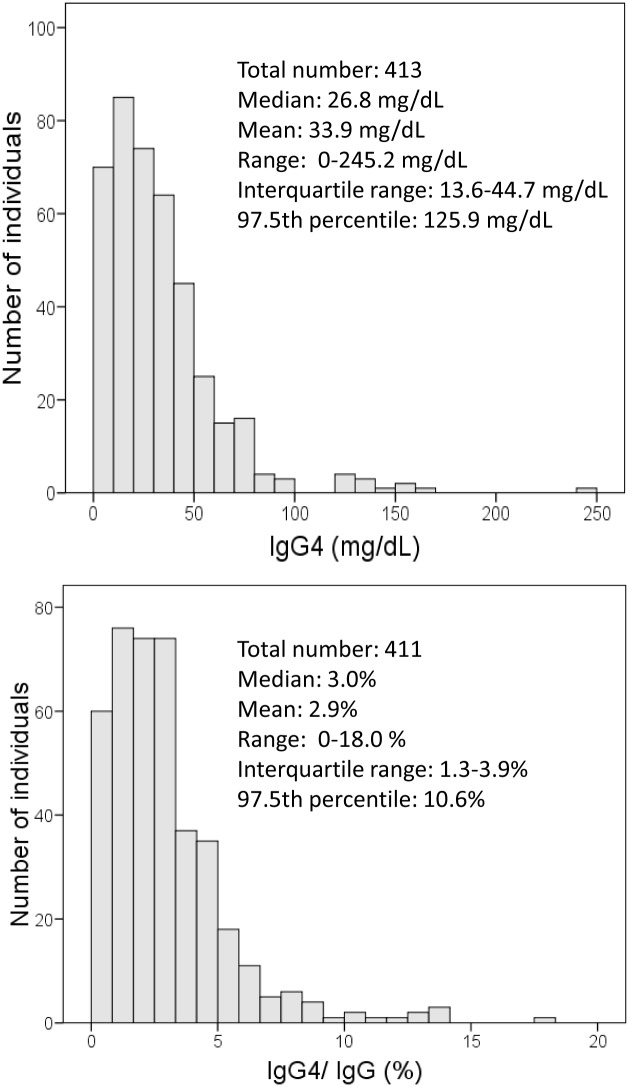
The histograms of serum IgG4 concentrations and IgG4/IgG ratio are represented in Fig 1. The distribution of both IgG4 and the IgG4/IgG ratio was not normal but skewed to the right (Fig 1). Five individuals (1.2%) showed IgG4 concentrations higher than 135 mg/dL, and 17 individuals (4.1%) showed an IgG4/IgG ratio higher than 8%.
Fig 1. Histogram of serum igG4 concentrations and proportion of IgG4 over total IgG.
Estimates (mean, median, and percentiles) were weighted according to the study design.
Relation of serum IgG4 concentrations to age and sex
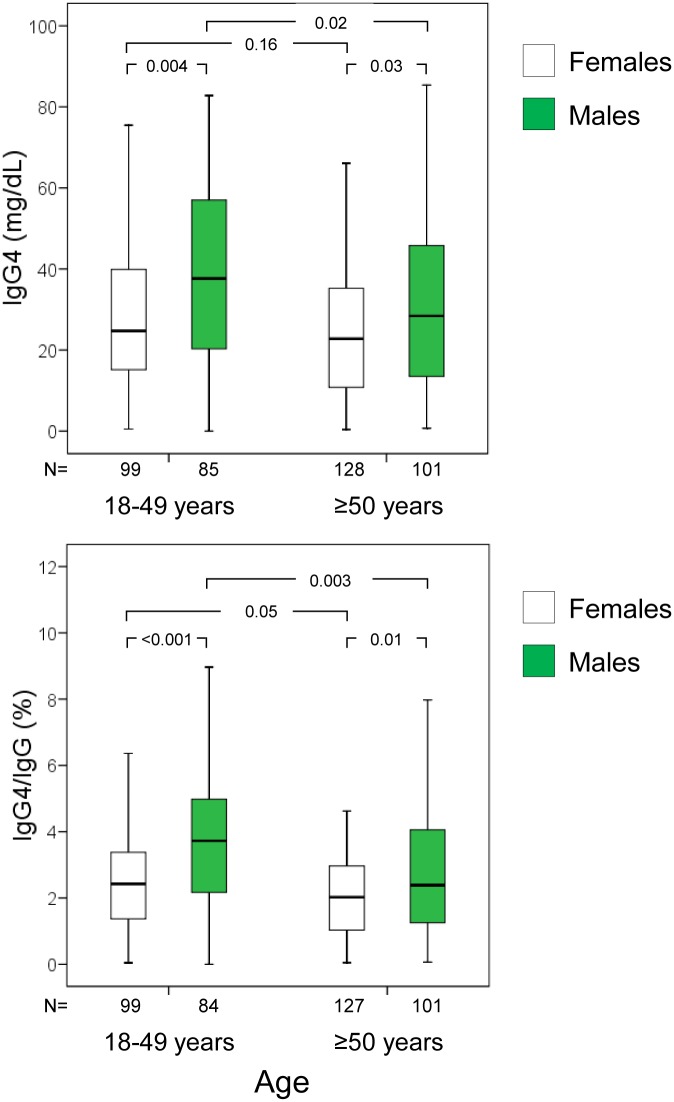
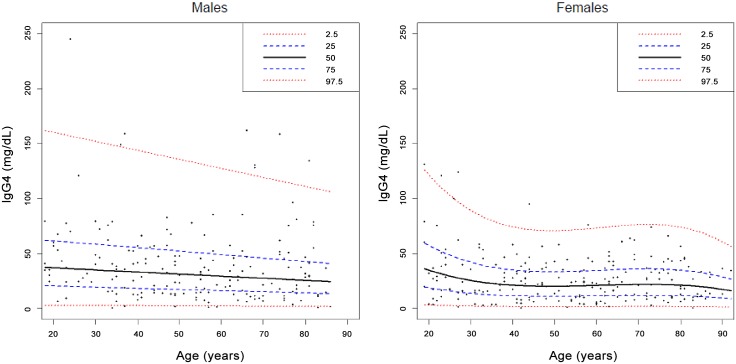
Serum IgG4 concentrations and the IgG4/IgG ratio were higher in males than in females (Table 1), and tended to decrease with age (P for trend 0.009 and <0.001, respectively). After stratification for decades of age, the decline in serum IgG4 and the IgG4/IgG ratio was particularly evident from age 50 years onwards (Table 1) in males and females alike, though it was more evident in males (Fig 2). Median IgG4 difference between individuals younger and older than 50 years was 6.1 mg/dL (95% confidence interval, 0.1–11.9 mg/dL). Median IgG4 difference between males and females was 8.6 mg/dL (95% confidence interval, 3.3–13.6 mg/dL). In the multivariate analyses (linear regression), younger age and male gender were independently associated with serum IgG4 concentrations (Table 2). A detailed analysis of IgG4 centiles in relation to age in males and females is presented in Fig 3.
Table 1. Serum IgG4 concentrations and proportion of IgG4 over total IgG in relation to demographic, lifestyle, metabolic, and atopic factors.
| Factor | No. | IgG4 (mg/dL) | No. | IgG4/IgG (%) |
|---|---|---|---|---|
| Gender | ||||
| Female (reference) | 227 | 23.4 (11.9–38.2) | 226 | 2.1 (1.1–3.2) |
| Male | 186 | 32.0 (14.4–51.6)*** | 185 | 2.9 (1.6–4.6)*** |
| Age (years) | ||||
| 18–30 (ref.) | 61 | 31.5 (16.8–58.5) | 61 | 2.9 (1.6–5.2) |
| >30–40 | 53 | 29.0 (15.7–50.4) | 53 | 3.0 (1.6–4.4) |
| >40–50 | 70 | 28.3 (13.6–43.3) | 69 | 2.6 (1.3–3.7) |
| >50–60 | 61 | 23.7 (11.4–34.9)* | 60 | 2.1 (1.3–3.4)** |
| >60–70 | 67 | 24.5 (11.9–42.5) | 67 | 2.2 (1.1–3.5)* |
| >70–80 | 56 | 26.2 (11.1–42.9) | 56 | 2.2 (1.0–3.3)* |
| >80 | 45 | 20.1 (11.4–37.5)* | 45 | 1.9 (1.1–3.0)** |
| Alcohol consumption | ||||
| Abstainers (ref.) | 193 | 27.7 (14.3–45.2) | 193 | 2.5 (1.4–3.9) |
| Light drinkers | 131 | 23.8 (12.4–41.0) | 129 | 2.2 (1.2–3.6) |
| Moderate drinkers | 52 | 26.3 (12.1–42.1) | 52 | 2.5 (1.3–3.9) |
| Heavy drinkers | 37 | 32.4 (16.0–48.3) | 37 | 2.9 (1.4–4.3) |
| Smoking | ||||
| Never smokers (ref.) | 260 | 24.4 (12.2–40.3) | 259 | 2.1 (1.2–3.3) |
| Ex-smokers | 63 | 26.2 (14.0–46.1) | 63 | 2.5 (1.3–3.9) |
| Light smokers | 20 | 29.6 (13.4–44.3) | 20 | 2.8 (1.1–4.0) |
| Heavy smokers | 70 | 33.2 (20.1–51.7)** | 69 | 3.2 (2.0–4.7)*** |
| Body mass index | ||||
| Normal weight (ref.) | 112 | 29.0 (15.7–44.0) | 112 | 2.8 (1.6–4.1) |
| Overweight | 179 | 25.6 (12.8–45.1) | 177 | 2.4 (1.3–3.9) |
| Obese | 121 | 25.3 (13.1–42.5) | 121 | 2.2 (1.3–3.7) |
| Metabolic syndrome | ||||
| No (ref.) | 313 | 26.9 (13.3–45.4) | 311 | 2.5 (1.3–4.0) |
| Yes | 100 | 25.0 (13.1–39.8) | 100 | 2.2 (1.3–3.5) |
| Specific IgE | ||||
| Negative (ref.) | 308 | 24.9 (12.7–42.0) | 306 | 2.3 (1.2–3.7) |
| Positive | 105 | 33.2 (17.3–47.2)* | 105 | 2.8 (1.5–4.3)* |
| Skin prick tests | ||||
| Negative (ref.) | 302 | 26.2 (12.9–41.7) | 300 | 2.4 (1.2–3.8) |
| Positive | 111 | 28.5 (16.2–46.7) | 111 | 2.6 (1.5–4.4) |
Data are expressed as medians and interquartile (25th-75th percentile) ranges (within parentheses).
*P<0.05,
**P<0.01,
***P<0.001 with respect to the reference category (ref).
Fig 2. Boxplots of serum igG4 concentrations and the proportion of IgG4 over total IgG in study participants, stratified by age and sex.
Outliers (values laying more than one and a half times the length of the box [interquartile range] from either end of the box) are not shown but are included in analyses.
Table 2. Multivariate analyses (linear regression) of factors associated with serum IgG4 concentrations.
| Unadjusted | Age- and sex-adjusted | |||
|---|---|---|---|---|
| Factor | B-coefficient (SE) | P-value | B-coefficient (SE) | P-value |
| Gender (0:female; 1: male) | 0.130 (0.041) | 0.002 | 0.129 (0.041) | 0.002 |
| Age (years) | -0.002 (0.001) | 0.024 | -0.002 (0.001) | 0.023 |
| Heavy drinking (0:no; 1: yes) | 0.040 (0.073) | 0.583 | -0.051 (0.077) | 0.504 |
| Current smoking (0:no; 1: yes) | 0.094 (0.050) | 0.061 | 0.021 (0.056) | 0.709 |
| Obesity (0:no; 1: yes) | -0.030 (0.046) | 0.516 | -0.014 (0.045) | 0.754 |
| Metabolic syndrome (0:no; 1: yes) | -0.038 (0.049) | 0.435 | -0.010 (0.049) | 0.845 |
| sIgE (0: negative; 1: positive) | 0.095 (0.048) | 0.046 | 0.067 (0.048) | 0.161 |
| SPT (0: negative; 1: positive) | 0.069 (0.047) | 0.141 | 0.045 (0.048) | 0.347 |
Serum IgG4 concentrations were log10-transformed in order to normalize their distribution. Data are expressed as slope (B-coefficient) and standard error (within parentheses). SPT, skin prick tests, sIgE, specific IgE (Phadiatiop™ test).
Fig 3. Percentiles (2.5, 25, 50, 75, and 97.5th) of serum IgG4 concentrations in relation to age in males and females.
Centiles were calculated using the Cole-Green and Rigby-Stasinopoulos [33,34] algorithm.
Relation of serum IgG4 concentrations to lifestyle variables
Serum IgG4 concentrations and the IgG4/IgG ratio were similar in alcohol abstainers and alcohol drinkers (Table 1). In the univariate analyses, serum IgG4 concentrations and the IgG4/IgG ratio were higher in heavy smokers than in the reference category (never smokers, Table 1). However, the association of smoking with serum IgG4 was greatly attenuated after adjusting for age and sex in the multivariate analyses (Table 2).
Relation of serum IgG4 concentrations to metabolic abnormalities
Serum IgG4 concentrations and IgG4/IgG ratio were not significantly associated with overweight, obesity, the metabolic syndrome (Table 1) or its components (data not shown).
Relation of serum IgG4 concentrations to atopy traits
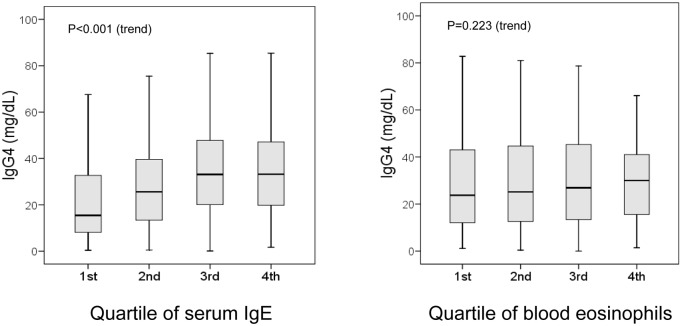
There was a significantly positive correlation between serum IgG4 and serum IgE concentrations (Fig 4). On the contrary, there was no significant correlation between serum IgG4 concentrations and total eosinophil count (Fig 4).
Fig 4. Boxplots of serum IgG4 concentrations in relation to quartiles of total serum IgE and total blood eosinophil count.
Outliers (values laying more than one and a half times the length of the box [interquartile range] from either end of the box) are not shown but are included in analyses. IgG4, IgE, and eosinophil count data are available for all 413 participants.
In the univariate analyses, serum IgG4 concentrations and the IgG4/IgG ratio were higher among individuals who tested positive aeroallergen-specific serum IgE (Phadiatop test) (Table 1). Similar results when individuals were further stratified by SPT results (Table 3). There was no significant association between serum IgG4 concentrations and SPT positivity to aeroallergens (Table 1). The association was more evident when SPT-positive individuals were stratified by presence or absence of respiratory symptoms (Table 3). Asymptomatic, SPT-positive individuals showed the highest IgG4 concentrations, which were higher than those of SPT-negative (Table 3) and symptomatic SPT-positive individuals (P = 0.01). The association between these atopy traits and serum IgG4 concentrations was attenuated after adjusting for age and sex (Table 2). Significant differences were observed among these groups defined by SPT-positivity and respiratory symptoms in terms of the IgG4/IgE ratio (Table 3). The highest IgG4/IgE ratio was observed among SPT-negative individuals and the lowest IgG4/IgE ratio was observed among symptomatic SPT-positive individuals (Table 3). Asymptomatic SPT-positive individuals showed a significantly higher IgG4/IgE ratio than did symptomatic SPT-positive individuals (P = 0.001, Table 3). Differences in the IgG4/IgE ratio among groups, however, were largely dependent on variation of serum IgE concentrations in these groups (Table 3).
Table 3. Concentrations of serum IgG4, total serum IgE, and their ratio in relation to atopy traits.
| Factor | No. | IgG4 (mg/dL) | IgE (kU/L) | IgG4/IgE ratio |
|---|---|---|---|---|
| SPT/sIgE positivity | ||||
| SPT-negative, sIgE negative (ref) | 273 | 26.0 (12.8–42.1) | 34.9 (14.8–71.9) | 0.69 (0.30–1.36) |
| SPT-negative, sIgE positive | 29 | 33.2 (14.1–42.7) | 114 (66.5–452)** | 0.19 (0.11–0.34)** |
| SPT-positive, sIgE negative | 35 | 22.0 (10.9–43.1) | 89.6 (42.6–156)** | 0.31 (0.08–0.57)** |
| SPT-positive, sIgE positive | 76 | 33.2 (18.3–47.7)* | 213 (125–463)** | 0.10 (0.04–0.37)** |
| SPT/respiratory symptoms | ||||
| SPT-negative, asymptomatic (ref) | 172 | 27.6 (13.2–45.9) | 37.9 (17.1–86.0) | 0.61 (025–1.36) |
| SPT-negative, symptomatic | 130 | 24.0 (12.8–36.8) | 42.3 (15.5–84.5) | 0.56 (0.28–1.15) |
| SPT-positive, asymptomatic | 41 | 38.4 (21.7–56.6)* | 134 (53.6–252)** | 0.31 (0.10–0.67)** |
| SPT-positive, symptomatic | 70 | 24.2 (12.6–42.8) | 194 (109–470)** | 0.10 (0.04–0.35)** |
Data are expressed as medians and interquartile (25th-75th percentile) ranges (within parentheses).
*P<0.05,
**P<0.001 with respect to the reference category.
Ref, reference category; SPT, skin prick test; sIgE specific IgE (Phadiatop™ test).
In addition to respiratory allergy, hypersensitivity diseases in the series included two individuals with contact dermatitis, two individuals with Hymenoptera venom allergy, and one individual with beta-lactam allergy. Serum IgG4 concentrations in these 5 cases were similar to those from the overall sample (median 24.1 mg/dL, and range 10.0–32.4 mg/dL).
Additional immune diseases
No cases of IgG4-RD were present in the clinical records at baseline. Likewise, no cases indicative of IgG4-RD were detected during follow-up. New-onset diseases of potential immune basis included thyroid disease (n = 9), psoriasis (n = 4), rheumatoid arthritis (n = 3), ulcerative colitis (n = 2), Still´s disease (n = 1), Raynaud´s phenomenon (n = 1), autoimmune thrombopenia (n = 1), sarcoidosis (n = 1), small-vessel vasculitis (n = 1), and chronic autoimmune hepatitis (n = 1). There were no differences in baseline IgG4 concentrations between individuals who developed immune disease and those who did not (data not shown); or between individuals who died during follow-up and those who did not (data not shown).
Relation of serum IgG4 concentrations to other immunoglobulins and inflammatory markers
Serum IgG4 concentrations were significantly correlated with those of IgG and IgA, though correlation was weak, particularly for IgA (Table 2). There was no significant correlation between serum IgG4 concentrations and serum levels of proinflammatory cytokines (IL-6 and TNF-alpha), acute phase reactants (LBP and sCD14), and mast cell tryptase (Table 4). There was a weakly significant, negative correlation between IL-8 and IgG4 concentrations (Table 4). Serum IgG4 concentrations were not significantly correlated with markers of liver or kidney dysfunction (data not shown).
Table 4. Correlation of serum concentrations of IgG4 and concentrations of immunoglobulins (total IgG, IgA, IgM) and inflammatory markers (mast cell tryptase, soluble CD14 [sCD14], lipopolysaccharide-binding protein [LBP], and proinflammatory cytokines [IL-6, IL-8, and TNF-alpha]).
| IgG (mg/dL) | IgA (mg/dL) | IgM (mg/dL) | Tryptase (μg/L) | IL-6 (pg/mL) | IL-8 (pg/mL) | TNF-α (pg/mL) | sCD14 (μg/mL) | LBP (μg/mL) | |
|---|---|---|---|---|---|---|---|---|---|
| Coefficient of correlation with IgG4 (mg/dL) | 0.250 | 0.108 | -0.085 | -0.060 | 0.016 | -0.118 | -0.070 | -0.035 | -0.081 |
| 95% confidence interval for the coefficient | 0.154; 0.343 | 0.003; 0.214 | -0.176; 0.018 | -0149; 0.039 | -0.085; 0.114 | -0.215; -0.016 | -0.174; 0.027 | -0.129; 0.068 | -0.172; 0.011 |
| No. | 411 | 411 | 411 | 413 | 411 | 411 | 411 | 413 | 413 |
| P-value | <0.001 | 0.03 | 0.09 | 0.22 | 0.75 | 0.01 | 0.16 | 0.48 | 0.10 |
Discussion
This study shows that serum concentrations of IgG4 in the adult population vary with age and sex, and are higher in males and younger individuals. These differences are independent of both lifestyle (alcohol and smoking) and common metabolic abnormalities (obesity and related disorders). According to our results, median IgG4 values may be 36% higher in males than in females. This is in contrast with total IgG concentrations, which tended to be slightly higher in females than in males in the same population [28]. Sex differences in immunoglobulin concentrations, specifically higher IgM levels in females, have been attributed to hormonal effects on B lymphocytes [35,36]. Serum IgG4 concentrations decreased with age, e.g., median IgG4 values were 33% lower in elderly individuals than in those aged 18–31 years. This is also in contrast with total IgG concentrations, which increased with ageing in the same population [28]. Previous studies also reported higher IgG4 concentrations in males [6,7]. However, decreasing IgG4 concentrations with age have been not reported previously. Moreover, a non-significant trend to increasing IgG4 concentrations with ageing was reported in some studies [7], which included fewer individuals from a narrower age range than the present study. In our experience, the decrease in IgG4 concentrations with age was significant and independent of covariates. The ratio of serum IgG4 to total IgG (IgG4/IgG) showed a similar relationship with age and sex to that shown by IgG4 alone. The relationship of the IgG4/IgG ratio with these demographic variables was even more evident than that of IgG4 alone, probably because IgG4 and total IgG tend to display opposite associations with age and sex. Such sex-and-age differences may be particularly important when interpreting IgG4 concentrations in diseases that predominate in specific demographic subsets, such as IgG4-RD, which predominates in males and in individuals older than 60 years [11,12,20]. Accordingly, future studies conducted to establish IgG4 reference values should take age and sex changes into account.
Serum IgG4 concentrations were not significantly influenced by common lifestyle factors, such as alcohol consumption and smoking. This is in contrast with serum IgA and total IgG concentrations, which are respectively increased and decreased in alcohol consumers [28]. Likewise, there was no significant association between common metabolic abnormalities (obesity and the related metabolic syndrome) and IgG4 concentrations. Both obesity and metabolic syndrome are low-grade inflammatory disorders [37] that were found to be associated with increased IL-6 and IgA values in previous studies in the same population [28]. In our experience, serum IgG4 concentrations were not correlated with most markers of inflammation (with the exception of a weakly negative correlation with serum IL-8). Likewise, serum IgG4 concentrations seemed unaffected by mild liver or kidney dysfunction (data not shown).
There were some intriguing associations between serum IgG4 concentrations and allergy traits. Serum IgG4 and IgE concentrations were significantly correlated. This is consistent with a common Th2-driven stimulus for both IgE and IgG4 [4]. Furthermore, it is consistent with the frequent finding of increased serum IgE and additional Th2-driven phenomena in patients with IgG4-RD [10,11]. Serum IgG4 concentrations tended to be higher among individuals with atopy (as defined by sensitization to aeroallergens), particularly among those who were asymptomatic (i.e., with neither nasal nor bronchial symptoms) as opposed to those who were symptomatic. Moreover, symptomatic atopic individuals registered the lowest ratios of total serum IgG4 to total serum IgE (IgG4/IgE), although this was largely a consequence of increased total IgE in atopic individuals. Taken together, these results are consistent with a tolerogenic effect of IgG4 for allergic respiratory disease [4]. These results should be interpreted with caution, however, because statistical significance was attenuated after adjustment for age and sex. Furthermore, the increased IgG4/IgE ratio that is used as a clinical marker to detect the efficacy of tolerance-inducing therapies in patients with allergy [38–39] refers to specific rather than total IgG4 and IgE. Finally, the study included a systematic evaluation of IgE-mediated sensitization to aeroallergens, but the potential sensitization to different allergens was only assessed by review of clinical records. Serum IgG4 was significantly correlated neither with blood eosinophil counts nor with serum tryptase, a marker of mast cell burden or mast cell activation [32].
The strengths of the study include its: population-based design, random sampling and wide age range of participants; use of a reliable, standard method for IgG4 determination; long-term follow-up; and attempt to reduce confounding by controlling for a number of common factors. To the best of our knowledge, there have been no previous studies of a similar nature conducted on a general population. With regard to external validity, it should be noted that all participants were Caucasians and that many cases of IgG4-RD (autoimmune pancreatitis, in particular) have been reported in Asian populations [14,15,18,20]. To the best of our knowledge, no ethnic-related differences in IgG4 concentrations have been described, though this cannot be excluded.
The attention paid to serum IgG4 concentrations has increased in recent years due to the emerging concept of IgG4-RD. The present study was not conducted to investigate the diagnostic accuracy of serum IgG4 for the diagnosis of IgG4-RD. Furthermore, the study was not powered to detect the incidence of such a rare disorder [40]. The diagnosis of IgG4-RD usually requires a tissue biopsy [10,11,19,41–43]. Elevated concentrations of IgG4 are consistent with, but not diagnostic of, IgG4-RD [10,11,19,41–43]. It is recommended that patients suspected of having an IgG4-RD have their serum IgG4 concentration measured. The results of the present study may serve to better interpret serum IgG4 concentrations in clinical practice. In summary, serum concentrations of IgG4 may be influenced by age and sex, and are not significantly influenced by common factors such as smoking, alcohol consumption, obesity and metabolic syndrome. Further studies are needed to confirm the potential influence of atopy status on serum IgG4 concentrations. Future studies aimed at defining reference IgG4 values should consider partitioning by age and sex.
Supporting Information
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by grants from the Instituto de Salud Carlos III, Spanish Ministry of Health (PI13/02594 and Red de Investigación en Actividades Preventivas y Promoción de la Salud, redIAPP RD12/0005/0007), Fondo Europeo de Desarrollo Regional (FEDER), and the Consellería de Innovación e Industria, Xunta de Galicia (PGIDIT06PXIB918313). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.French M. Serum IgG subclasses in normal adults. Monogr Allergy 1986;19:100–107. [PubMed] [Google Scholar]
- 2.Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J Clin Invest 1970;49:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton DR, Gregory L, Jefferis R. Aspects of the molecular structure of IgG subclasses. Monogr Allergy 1986;19:7–35. [PubMed] [Google Scholar]
- 4.Schuurman J, Van Ree R, Perdok GJ, Van Doorn HR, Tan KY, Aalberse RC. Normal human immunoglobulin G4 is bispecific: it has two different antigen-combining sites. Immunology 1999;97:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy 2009;39:469–477. 10.1111/j.1365-2222.2009.03207.x [DOI] [PubMed] [Google Scholar]
- 6.French MA, Harrison G. Serum IgG subclass concentrations in healthy adults: a study using monoclonal antisera. Clin Exp Immunol 1984;56:473–475. [PMC free article] [PubMed] [Google Scholar]
- 7.Aucouturier P, Danon F, Daveau M, Guillou B, Sabbah A, Besson J, et al. Measurement of serum IgG4 levels by a competitive immunoenzymatic assay with monoclonal antibodies. J Immunol Methods 1984;74:151–162. [DOI] [PubMed] [Google Scholar]
- 8.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001;344:732–738. [DOI] [PubMed] [Google Scholar]
- 9.Fragoulis GE, Moutsopoulos HM. IgG4 syndrome: old disease, new perspective. J Rheumatol 2010;37:1369–70. 10.3899/jrheum.100383 [DOI] [PubMed] [Google Scholar]
- 10.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 2012;366:539–51. 10.1056/NEJMra1104650 [DOI] [PubMed] [Google Scholar]
- 11.Della-Torre E, Lanzillotta M, Doglioni C. Immunology of IgG4-related disease. Clin Exp Immunol 2015;181:191–206. 10.1111/cei.12641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Della-Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-related disease. Allergy 2014;69:269–272. 10.1111/all.12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattoo H, Della-Torre E, Mahajan VS, Stone JH, Pillai S. Circulating Th2 memory cells in IgG4-related disease are restricted to a defined subset of subjects with atopy. Allergy 2014;69:399–402. 10.1111/all.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zen Y, Fujii T, Harada K, Kawano M, Yamada K, Takahira M, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 2007;45:1538–1546. [DOI] [PubMed] [Google Scholar]
- 15.Masaki Y, Dong L, Kurose N, Kitagawa K, Morikawa Y, Yamamoto M, et al. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis 2009;68:1310–1315. 10.1136/ard.2008.089169 [DOI] [PubMed] [Google Scholar]
- 16.Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol 2007;102:1646–1653. [DOI] [PubMed] [Google Scholar]
- 17.Tabata T, Kamisawa T, Takuma K, Egawa N, Setoguchi K, Tsuruta K, et al. Serial changes of elevated serum IgG4 levels in IgG4-related systemic disease. Intern Med 2011;50:69–75. [DOI] [PubMed] [Google Scholar]
- 18.Masaki Y, Kurose N, Yamamoto M, Takahashi H, Saeki T, Azumi A, et al. Cutoff values of serum IgG4 and histopathological IgG4+ plasma cells for diagnosis of patients with IgG4-related disease. Int J Rheumatol 2012:580814 10.1155/2012/580814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 2015;74:14–18. 10.1136/annrheumdis-2013-204907 [DOI] [PubMed] [Google Scholar]
- 20.Inoue D, Yoshida K, Yoneda N, Ozaki K, Matsubara T, Nagai K, et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore) 2015;94:e680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 2012;22:21–30. 10.1007/s10165-011-0571-z [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Quintela A, Gude F, Boquete O, Rey J, Meijide LM, Suarez F, et al. Association of alcohol consumption with total serum immunoglobulin E levels and allergic sensitization in an adult population-based survey. Clin Exp Allergy 2003;33:199–205. [DOI] [PubMed] [Google Scholar]
- 23.Gual A, Martos AR, Lligona A, Llopis JJ. Does the concept of a standard drink apply to viticultural societies? Alcohol Alcohol 1999;34:153–160. [DOI] [PubMed] [Google Scholar]
- 24.Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 25.Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004;113:832–836. [DOI] [PubMed] [Google Scholar]
- 26.Vidal C, Boquete O, Gude F, Rey J, Meijide LM, Fernandez-Merino MC, et al. High prevalence of storage mite sensitization in a general adult population. Allergy 2004;59:401–405. [DOI] [PubMed] [Google Scholar]
- 27.Vidal C, Gude F, Boquete O, Fernández-Merino MC, Meijide LM, Rey J, et al. Evaluation of the Phadiatop test in the diagnosis of allergic sensitization in a general adult population. J Investig Allergol Clin Immunol 2005;15:124–130. [PubMed] [Google Scholar]
- 28.Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol 2008;151:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Quintela A, Campos J, Loidi L, Quinteiro C, Perez LF, Gude F. Serum TNF-alpha levels in relation to alcohol consumption and common TNF gene polymorphisms. Alcohol 2008;42:513–518. 10.1016/j.alcohol.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Quintela A, Campos J, Gude F, Perez LF, Tomé S. Serum concentrations of interleukin-8 in relation to different levels of alcohol consumption. Cytokine 2007;38:54–60. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Quintela A, Alonso M, Campos J, Vizcaino L, Loidi L, Gude F. Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: the role of obesity. PLoS One 2013;8:e54600 10.1371/journal.pone.0054600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Quintela A, Vizcaino L, Gude F, Rey J, Meijide L, Fernandez-Merino C, et al. Factors influencing serum total tryptase concentrations in a general adult population. Clin Chem Lab Med 2010;48:701–706. 10.1515/CCLM.2010.124 [DOI] [PubMed] [Google Scholar]
- 33.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992;11:1305–1319. [DOI] [PubMed] [Google Scholar]
- 34.Rigby RA, Stasinopoulos DM. Using the Box-Cox t distribution in GAMLSS to model skewness and kurtosis. Statistical Modelling 2006;6:209–229. [Google Scholar]
- 35.Kanda N, Tamaki K (1999) Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol 1999;103:282–288. [DOI] [PubMed] [Google Scholar]
- 36.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response. Hum Reprod Update 2005;11:411–423. [DOI] [PubMed] [Google Scholar]
- 37.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 38.Alvarado MI, Jimeno L, De La Torre F, Boissy P, Rivas B, Lazaro MJ, et al. Profilin as a severe food allergen in allergic patients overexposed to grass pollen. Allergy 2014;69:1610–1616. 10.1111/all.12509 [DOI] [PubMed] [Google Scholar]
- 39.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 2015;372:803–813. 10.1056/NEJMoa1414850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchida K, Masamune A, Shimosegawa T, Okazaki K. Prevalence of IgG4-related disease in Japan based on nationwide survey in 2009. Int J Rheumatol 2012:358371 10.1155/2012/358371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stone JH, Brito-Zerón P, Bosch X, Ramos-Casals M. Diagnostic approach to the complexity of IgG4-related disease. Mayo Clin Proc 2015;90:927–939. 10.1016/j.mayocp.2015.03.020 [DOI] [PubMed] [Google Scholar]
- 42.Islam AD, Selmi C, Datta-Mitra A, Sonu R, Chen M, Gershwin ME, et al. The changing faces of IgG4-related disease: clinical manifestations and pathogenesis. Autoimmun Rev 2015;14:914–922. 10.1016/j.autrev.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 43.Khosroshahi A, Wallace ZS, Crowe JL, Akamizu T, Azumi A, Carruthers MN, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol 2015;67:1688–1699. 10.1002/art.39132 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.