See Cannon (doi: 10.1093/brain/awv400 ) for a scientific commentary on this article.
Dominant gain-of-function mutations in SCN4A , which encodes the α-subunit of the voltage-gated sodium channel, are a common cause of myotonia and periodic paralysis. Zaharieva et al. now report recessive loss-of-function SCN4A mutations in 11 patents with congenital myopathy. The mutations cause fully non-functional channels or result in reduced channel activity.
Keywords: SCN4A, loss-of-function mutation, foetal hypokinesia, foetal akinesia, congenital myopathy
See Cannon (doi: 10.1093/brain/awv400 ) for a scientific commentary on this article.
Dominant gain-of-function mutations in SCN4A , which encodes the α-subunit of the voltage-gated sodium channel, are a common cause of myotonia and periodic paralysis. Zaharieva et al. now report recessive loss-of-function SCN4A mutations in 11 patents with congenital myopathy. The mutations cause fully non-functional channels or result in reduced channel activity.
Abstract
See Cannon (doi: 10.1093/brain/awv400 ) for a scientific commentary on this article.
Congenital myopathies are a clinically and genetically heterogeneous group of muscle disorders characterized by congenital or early-onset hypotonia and muscle weakness, and specific pathological features on muscle biopsy. The phenotype ranges from foetal akinesia resulting in in utero or neonatal mortality, to milder disorders that are not life-limiting. Over the past decade, more than 20 new congenital myopathy genes have been identified. Most encode proteins involved in muscle contraction; however, mutations in ion channel-encoding genes are increasingly being recognized as a cause of this group of disorders. SCN4A encodes the α-subunit of the skeletal muscle voltage-gated sodium channel (Na v 1.4). This channel is essential for the generation and propagation of the muscle action potential crucial to muscle contraction. Dominant SCN4A gain-of-function mutations are a well-established cause of myotonia and periodic paralysis. Using whole exome sequencing, we identified homozygous or compound heterozygous SCN4A mutations in a cohort of 11 individuals from six unrelated kindreds with congenital myopathy. Affected members developed in utero - or neonatal-onset muscle weakness of variable severity. In seven cases, severe muscle weakness resulted in death during the third trimester or shortly after birth. The remaining four cases had marked congenital or neonatal-onset hypotonia and weakness associated with mild-to-moderate facial and neck weakness, significant neonatal-onset respiratory and swallowing difficulties and childhood-onset spinal deformities. All four surviving cohort members experienced clinical improvement in the first decade of life. Muscle biopsies showed myopathic features including fibre size variability, presence of fibrofatty tissue of varying severity, without specific structural abnormalities. Electrophysiology suggested a myopathic process, without myotonia. In vitro functional assessment in HEK293 cells of the impact of the identified SCN4A mutations showed loss-of-function of the mutant Na v 1.4 channels. All, apart from one, of the mutations either caused fully non-functional channels, or resulted in a reduced channel activity. Each of the affected cases carried at least one full loss-of-function mutation. In five out of six families, a second loss-of-function mutation was present on the trans allele. These functional results provide convincing evidence for the pathogenicity of the identified mutations and suggest that different degrees of loss-of-function in mutant Na v 1.4 channels are associated with attenuation of the skeletal muscle action potential amplitude to a level insufficient to support normal muscle function. The results demonstrate that recessive loss-of-function SCN4A mutations should be considered in patients with a congenital myopathy.
Introduction
Congenital myopathies are a group of clinically and genetically heterogeneous muscle disorders often characterized by specific structural abnormalities in muscle biopsies. The degree of severity varies widely from profound muscle weakness leading to in utero or neonatal lethality, to less severe infant- or childhood-onset weakness, which results in a much milder clinical course ( Wallgren-Pettersson and Laing, 2010 ). Common features are prominent facial muscle weakness with or without ptosis and proximal predominant limb muscle weakness.
Congenital myopathies demonstrate a considerable degree of locus and allelic heterogeneity. Mutations in different genes can cause the same pathology, and mutations in the same gene can lead to different types of muscle pathology. This indicates that disruption of a common pathway, rather than a strict gene-pathology relationship, determines the disease pathogenesis ( Sewry et al. , 2008 ; Nance et al. , 2012 ; Ravenscroft et al. , 2015 ). In the past decade, whole exome sequencing has contributed to a better understanding of the pathogenesis of congenital myopathies, by facilitating the identification of novel disease-causing genes and by broadening our understanding of the range of phenotypes associated with known causative genes.
SCN4A encodes the α-subunit of the skeletal muscle voltage-gated sodium channel (Na v 1.4) mainly expressed in skeletal muscle. The tetrodotoxin-sensitive Na v 1.4 channel ( Trimmer et al. , 1989 ; Zhou and Hoffman, 1994 ) is essential for the generation and propagation of the muscle action potential crucial for skeletal muscle contraction. Na v 1.4 is a large protein composed of four homologous domains (DI–DIV) each consisting of six transmembrane segments (S1–S6). Segments S5 and S6 of each domain form the single ion-conducting pore, while S1–S4 form the voltage sensing domains. The four positively charged S4 segments function as voltage sensors regulating the opening and closing of the pore ( Stühmer et al. , 1989 ).
Dominant gain-of-function mutations in SCN4A are a well-established cause of disorders collectively termed sodium channelopathies. The range of phenotypes associated with sodium channelopathies includes episodes of muscle stiffness (potassium-aggravated myotonia), episodes of paralytic attacks associated with reduced serum potassium levels (hypokalaemic periodic paralysis) or combination of attacks of both myotonia and periodic paralysis in the same patient (paramyotonia congenita, hyperkalaemic periodic paralysis) ( Vicart et al. , 2005 ; Ryan et al. , 2007 ; Corrochano et al. , 2014 ). Attacks can be triggered by changes in temperature or ion concentration and can last from minutes to days. In myotonia, the mutated Na v 1.4 causes sarcolemmal hyperexcitability and delayed muscle relaxation after contraction, while in periodic paralysis a prolonged sodium current (hyperkalaemic periodic paralysis) or an aberrant gating pore current through Na v 1.4 (hypokalaemic periodic paralysis type 2) results in membrane depolarization and inexcitability ( Suetterlin et al. , 2014 ). Neonatal hypotonia with variable feeding and respiratory difficulty has also been rarely reported in the sodium channelopathies. This is a transient phenomenon, however, which improves spontaneously ( Matthews et al. , 2008 ).
Recessive loss-of-function SCN4A mutations are rare and have been described in only two patients with congenital myasthenic syndrome. Two heteroallelic missense SCN4A mutations were detected in a patient with neonatal onset of fatigable generalized weakness and recurrent attacks of respiratory and bulbar paralysis ( Tsujino et al. , 2003 ). In the second patient, the onset was ∼12 years of age with episodic generalized weakness lasting for hours and resulting in muscle fatigue later in life ( Arnold et al. , 2015 ). A homozygous missense mutation in SCN4A was identified in this patient, while the heterozygous parents were asymptomatic. In both patients, the mutant Na v 1.4 channels had enhanced fast inactivation, constituting a loss-of-function effect.
Here, we describe homozygous or compound heterozygous SCN4A mutations in 11 individuals from six unrelated families with congenital myopathy. The affected cases had clinical phenotypes ranging from severe foetal hypokinesia resulting in early lethality to ‘classical’ congenital myopathy that improved clinically over time. Functional assessment of the mutant Na v 1.4 channels in HEK293 cells revealed full and partial loss-of-function effects constituting a novel molecular pathomechanism associated with SCN4A mutations.
Materials and methods
Ethical approval
This study was approved by the Health Research Authority, NRES Committee East of England – Hatfield (REC 13/EE/0398), the institutional review boards of BC Children’s Hospital and the University of British Columbia Canada (CW12-0019; H12-00067), the medical ethics committee of the Radboud University Medical Centre (2011/188), Human Research Ethics Committee of the University of Western Australia (RA/4/1/4403).
Informed consent was obtained from all individuals included in this study or from their parents or legal guardians.
Oocytes were isolated from adult female Xenopus laevis following procedures that have been approved by UCL’s Biological Services Management Group and the UK Home Office.
Whole exome sequencing
Whole exome sequencing was performed in all probands from the six families included in this study. Details of the whole exome sequencing procedures are provided in the Supplementary material .
Verification of the SCN4A c.3145-2A>C splice site mutation
The c.3145-2A>C mutation detected in Family 4 was verified in total RNA extracted from peripheral blood cells from the proband, mother and father. Details of the procedure are provided in the Supplementary material .
Histological analysis
Muscle biopsies were taken from the probands from Families 1, 2, 3 and 4. Post-mortem samples were taken from Patient II.2 Family 4 and from all affected infants from Families 5 and 6. Histological, histochemical and immunohistochemical studies were performed as previously described (Dubowitz et al. , 2013).
MRI
Muscle MRI was performed in the probands from Families 1 and 2. Conventional T 1 - and, in some cases, also T 2 -weighted sequences were used to obtain planes from the lower limbs. Spinal MRI was undertaken in the proband from Family 1, and cerebral MRI in the probands from Families 3 and 4.
Mutagenesis
QuikChange® II (Agilent Technologies) site-directed mutagenesis kit was used to insert mutations in the human SCN4A clone (from Prof. Steve Cannon, University of Texas Southwestern Medical Center, Dallas, USA).
Human embryonic kidney 293 (HEK293) cells were transfected with cDNA for the sodium channel α-subunit and green fluorescent protein (GFP) using Lipofectamine ™ 2000 (Life Technologies).
Whole-cell electrophysiology
Electrophysiology methods are detailed in the Supplementary material . The extracellular solution for HEK293 recordings contained (in mM): 145 NaCl, 4 KCl, 1.8 CaCl 2 , 1 MgCl 2 , and 10 HEPES (pH = 7.35) and the intracellular pipette solution contained (in mM): 145 CsCl, 5 NaCl, 10 EGTA, 10 HEPES (pH = 7.4). The calculated liquid junction potential is −4.4 mV, which was not corrected for. Holding potential was −80 mV. Leak and capacitance subtraction was performed online using a − P /4 procedure.
The voltage protocols are described in the legends and in the Supplementary material . Functional characterization in X. laevis oocytes is described in the Supplementary material .
Statistical analysis
Data analysis, presentation and curve-fitting protocols are described in the Supplementary material . Data are expressed as mean ± standard error of the mean (SEM). Statistical significance was analysed using Student’s t- test and accepted at P < 0.05.
Results
Identification of SCN4A mutations in the congenital myopathy patients
Whole exome sequencing was carried out in the proband from Family 1 as part of the European Commission funded NeurOmics project. Following a filtering procedure ( de Ligt et al. , 2012 ) and assuming a recessive mode of inheritance, we were left with eight high quality variants ( Supplementary Table 3 ). Only the two heterozygous variants in SCN4A were not present in the Exome Aggregation Consortium (ExAC) database and were predicted to be pathogenic and thus were considered as the disease-causing mutations. Segregation analysis showed that the compound heterozygous SCN4A c.311G>A (p.R104H) and c.3403C>T (p.R1135C) mutations detected in the proband had been inherited from the unaffected mother and father, respectively.
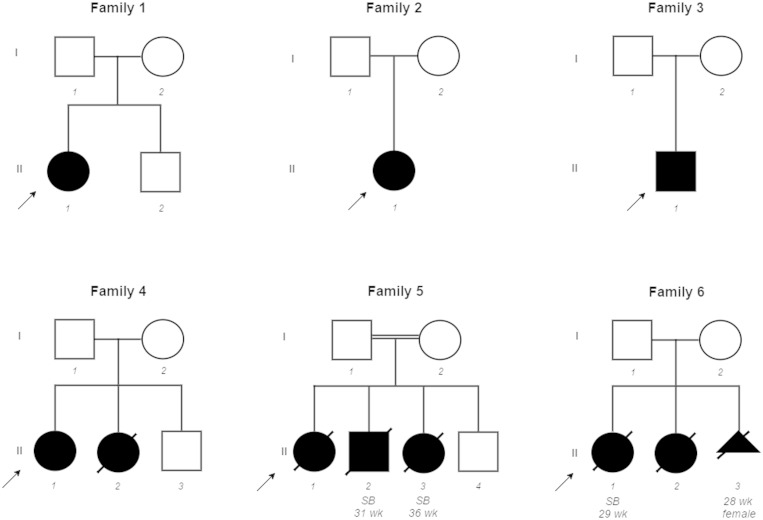
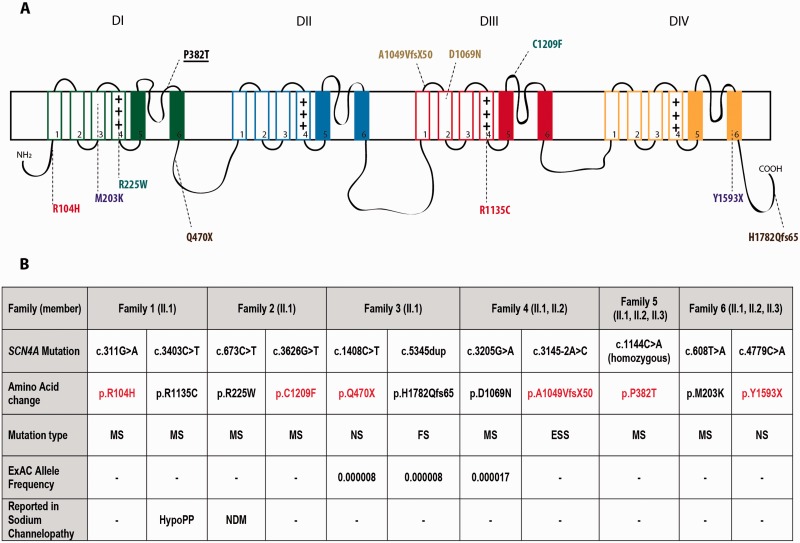
Through an international collaborative effort we identified 10 additional cases with congenital myopathy from five unrelated families ( Fig. 1 ). The affected cases carried homozygous or compound heterozygous SCN4A variants that were predicted to be pathogenic. All 11 SCN4A mutations identified in the cohort members are described in Fig. 2 . The SCN4A mutations were validated by Sanger sequencing and segregation consistent with an autosomal recessive pattern of inheritance was confirmed in all six families. The location of the mutations relative to the domains of the Na v 1.4 protein is presented in Fig. 2 A.
Figure 1.
Pedigrees of all families with SCN4A mutations described in this study. Affected individuals are represented with shaded symbols, probands are indicated with an arrow, age at stillbirth (SB) or pregnancy termination are shown beneath symbols.
Figure 2.
SCN4A mutations in congenital myopathy patients. Location of the mutations mapped onto a secondary structure of Na v 1.4 channel ( A ). Compound heterozygous mutations identified in the affected individuals from one family are presented with the same colour. The homozygous mutation in Family 5 is shown underlined. Position, amino acid change, mutation type, frequency in ExAC database and association with sodium channelopathy of the reported mutations ( B ). Full loss-of-function mutations are presented in red. MS = missense mutation; NS = nonsense mutation; FS = frameshift mutation; ESS = essential splice site mutation; HypoPP = hypokalaemic periodic paralysis; NDM = non-dystrophic myotonia; - = not available.
Consistent with mutations responsible for a rare early-onset recessive disorder, eight of the variants had not been reported previously in ExAC, and three mutations (p.Q470X, p.H1782Qfs65 and p.D1069N) were present at very low frequency (<0.00001) ( Fig. 2 B).
Of the 11 SCN4A mutations identified, nine had not been previously reported, p.R1135C has been described in a homozygous state in a patient with hypokalaemic periodic paralysis ( Groome et al. , 2014 ) and p.R225W was reported in a heterozygous state in a patient with mild non-dystrophic myotonia ( Lee et al. , 2009 ).
Clinical features of cohort members
We detected a continuum of clinical severity ranging from severe foetal hypokinesia resulting in early lethality in seven cases, to a milder congenital myopathy phenotype in four surviving individuals. Phenotypic features of the cohort are summarized in Table 1 and clinical data are detailed in Supplementary Table 1 . Clinical and muscle MRI images are shown in Fig. 3 . The most salient clinical features are described below.
Table 1.
Clinical characteristics of congenital myopathy patients with homozygous or compound heterozygous SCN4A mutations
| Family | In utero and congenital features in surviving cohort members | Muscle involvement and progression over time in surviving cohort members | Additional features in surviving cohort members |
|---|---|---|---|
| Age | |||
| Gender | |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||
|
In utero and congenital features in deceased cohort members | External examination findings in deceased cohort members | Internal examination findings in deceased cohort members |
|
| |||
|
|
|
Autopsy not performed |
|
|
|
|
|
In utero and congenital features in deceased cohort members | External examination findings in deceased cohort members | Internal examination findings in deceased cohort members |
|
| |||
|
|
|
Lungs: pulmonary hypoplasia |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lungs: pulmonary hypoplasia |
‘X’/40 = ‘X’ weeks gestation. For example 31/40 = 31 weeks gestation; BiPAP = bilevel positive airway pressure; CPAP = continuous positive airways pressure; d = days; DLL = distal lower limb; FVC = forced vital capacity; L = limited data available; NG = nasogastric; PEG = percutaneous endoscopic gastrostomy; OL = onset of labour; PLL = proximal lower limb; PUL = proximal upper limb; DUL = distal upper limb; ROM = range of motion; SROM = spontaneous rupture of membranes; EV = equinovarus; y = years; m = months.
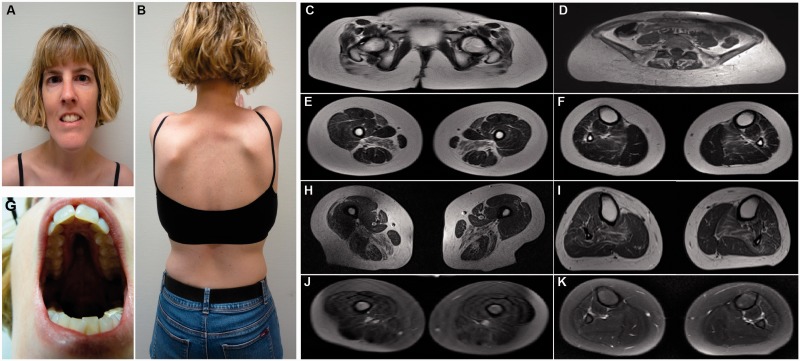
Figure 3.
Clinical features in SCN4A congenital myopathy patients. Clinical images of the proband from Family 2 show mild facial weakness, elongated face ( A ), high arched palate ( G ) and bilateral scapular winging ( B ). T 1 -weighted muscle MRI images in the affected case from Family 1 (at age 6 years) ( C , E and F ) and in the proband from Family 2 (at age 35 years) ( D , H and I ) showed severe involvement of the gluteal muscles ( C and D ), bilateral, symmetric involvement of sartorius and adductor magnus in the upper leg ( E and H ) and involvement of soleus in the lower leg ( F and I ). T 2 -weighed muscle MRI images in the affected case from Family 1 demonstrated no oedema ( J and K ).
Clinical features of severely affected cohort members with foetal hypokinesia
In utero history
The seven most severely affected cohort members developed marked foetal hypokinesia from 19 to 32 weeks gestation. All seven also developed in utero -onset limb contractures and/or talipes, which were first detected at 19 to 31 weeks . Talipes was the most common limb deformity (seen on ultrasound in 5/7 severely affected infants; first detected between 19 to 27 weeks). Additional lower limb joint contractures were noted prior to delivery in five of the infants. In utero -onset upper limb and/or finger contractures were detected on ultrasound in four cases, sometimes in combination with lower limb contractures (three cases). Almost all severely affected cohort members also developed features suggestive of hydrops (present in 6/7; evident from 20 to 31 weeks). The mothers of all seven severely affected cohort members developed polyhydramnios (onset 20 to 31 weeks), which typically developed in parallel with hypokinesia and foetal limb contractures. Decompression procedures to reduce liquor volume were required in two cases.
Prematurity and early death
One severely affected infant was born at term but died at 5 h of age from respiratory complications. The other six severely affected infants died at or before 36 weeks gestation: two infants died spontaneously prior to delivery (at 29 and 31 weeks gestation), one died during delivery (at 36 weeks), two died within 8 h of their premature delivery due to severe respiratory insufficiency (at 32 and 33 weeks), and one pregnancy was terminated at 28 weeks gestation due to recurrence of features seen in two severely affected pregnancies.
All seven cohort members who did not survive beyond the first day of life had at least one sign of in utero involvement (e.g. hypokinesia, limb contractures or talipes) by 32 weeks gestation, and in 4/7 cases at least one abnormality was evident by 20 weeks.
External examination and autopsy findings
External examination was undertaken in all seven infants who died prematurely or at term. Six also underwent autopsy. Marked muscle hypoplasia was present in all seven. Hydrops was confirmed in six cases. External and autopsy examination revealed features associated with hydrops (e.g. pericardial and pleural effusions, ascites and/or hepatomegaly), in addition to the features associated with severe foetal hypokinesia (a small thorax, pulmonary hypoplasia, limb contractures and fractures). In addition to talipes (present in 6/7 cases), four had lower limb contractures; typically bilateral hip flexion contractures (3/4) with or without additional external rotation, and knee extension contractures (4/4). Five had upper limb contractures (finger flexion in 4/7, wrist 1/7, elbow flexion 2/7, shoulder flexion 1/7). In general, lower limb contractures were more marked than upper limb contractures. Limb fractures were present in 2/7 cases (left humerus and right mid-femur). Thin gracile ribs and/or thin long bones were noted in at least 2/7 cases. Several infants had additional facial features that were not shared by other family members. This included plagiocephaly (1/7), frontal bossing (2/7), deep set eyes (2/7 cases), downward-sloping lateral orbital ridges (1/7), a flattened nose (3/7), ear abnormalities (3/7), retrognathia and/or micrognathia (3/7), and/or a small triangular or tented mouth (2/7). A subset of these (e.g. multiple joint contractures, hydrops/oedema, pulmonary hypoplasia, and retromicrognathia) are well-known features of foetal akinesia deformation sequence ( Ravenscroft et al. , 2011 ).
Clinical features in surviving cohort members with ‘classical’ congenital myopathy
The four surviving cohort members are currently aged 2.5, 8, 14 and 35 years, and all have features consistent with ‘classical’ congenital myopathy.
In utero and birth history
Pregnancy records were available for three of the four surviving cohort members, with limited data available for the fourth. In utero features were generally less marked than in severe foetal hypokinesia cases; however, at least one abnormality (hypokinesia, polyhydramnios or talipes) was present in three of the four cases. Foetal hypokinesia was noted during the pregnancies of two of the four surviving cohort members (onset at 20 and 31 weeks, respectively), but appears to have been less marked than in more severely affected cohort members. Polyhydramnios complicated the pregnancies of 2/4 surviving cohort members and was first noted slightly later (31 to 40 weeks). None of the surviving cohort members had limb contractures or talipes detected on prenatal ultrasound, or a history of hydrops. All four were born at or close to term. Breech presentation, or transverse lie, complicated delivery of three of these four affected individuals. One required resuscitation following delivery.
Congenital features
All four surviving cohort members had moderate-to-severe hypotonia and facial, neck, axial and limb weakness, which was either present at birth (3/4) or developed within the first few days of life (as was the case for the proband from Family 3). A weak cry, a generalized reduction in muscle bulk, and reduced or absent limb reflexes were also each noted in two or more surviving infants. Talipes was present in only one of the four, and this was not detected until delivery. Another child had mild bilateral hip contractures first noted at birth, which resolved with stretching exercises.
Bulbar involvement
Swallowing difficulties, severe enough to warrant supplemental tube and/or parenteral gastrostomy (PEG) feeding, were present from birth in 3/4 surviving cohort members. Reliance on supplemental feeding typically declined over time, and ceased during infancy or early childhood. The two youngest cohort members (now aged 2.5 and 8 years) have evidence of persisting oromotor weakness and expressive language delay suggestive of ongoing bulbar involvement, however both have experienced improvement in their feeding and language status, over time.
Pattern of muscle involvement and contractures
All surviving cohort members had mild-to-moderate upper and lower limb weakness, which was typically more marked proximally (3/4 cases). Hip extension and abduction were the weakest manoeuvres in 2/4 cases. Neck weakness was universal (4/4), and typically more pronounced than limb weakness. Neck flexion was usually weaker than extension. Shoulder, neck and truncal muscle atrophy was often more marked than limb muscle atrophy. Muscle hypertrophy, muscle pain and fasciculations were not present in any cohort member.
Postnatal-onset lower limb contractures were noted in only 1/4 surviving cohort member. Abnormal foot positioning was noted in 2/4 (pes planus in 1/4, hindfoot valgus/forefoot pronation deformities in 1/4).
Progression in axial weakness was documented in at least one surviving cohort member. This resulted in the development of respiratory insufficiency severe enough to warrant institution of non-invasive nocturnal ventilation at age 6 years. Two of the four surviving cohort members needed non-invasive respiratory support at or soon after birth, and a third required invasive ventilation. The duration of support varied; however, two were weaned from respiratory intervention by the age of 2 years. The third remains reliant on nocturnal BiPAP (bilevel positive airway pressure) at age 2.5.
Postnatal-onset scoliosis, kyphosis and spinal rigidity each were also reported in at least one additional case.
Facial and eye involvement
Mild-to-moderate facial weakness and myopathic facial features including an elongated face and/or a high arched palate were present in all four cases ( Fig. 3 ). Additional dysmorphisms noted in at least 1/4 included dolichocephaly, synophrys, proptosis, large down-slanting palpebral fissures, and a broad nasal root and bridge. A subset of the dysmorphic facial features noted in cohort members with severe foetal hypokinesia, including deep set eyes, frontal bossing and micrognathia were present in at least one surviving individual. Mild weakness of up-gaze developed in one child at age 3 years. Generalized ophthalmoplegia was noted in a second affected individual. Ptosis was not present in any cohort member.
Functional abilities
Delay in early motor milestones was universal. Age when independent ambulation was first achieved ranged from 18 months to 2 years 9 months. At least two cohort members were eventually able to run, albeit slowly and with difficulty. Gait was typically broad-based, slow, and waddling. Two of four used assistive devices (splints and/or frame), and 2/4 used a wheelchair. At least two cohort members had additional fine motor delay. All four experienced clearly documented improvements in strength and motor skills over time. All three that achieved independent ambulation remain able to walk. The youngest (2.5 years old) member is making good progress towards walking independently.
Additional features
Other features noted in two or more affected individuals included chest wall deformities (2/4), generalized joint hypermobility (at least 2/4), unilateral or bilateral scapular winging (one case each) ( Fig. 3 ). One surviving cohort member had tricuspid insufficiency at birth, which resolved with time. No cardiac defects were present in the other three individuals, and none have developed additional cardiac complications over time.
Intermittent exacerbations in muscle weakness and increased fatigability
Marked fatigability with walking and/or writing was a feature in at least 3/4 of the surviving cohort members.
The proband from Family 1 (carrying heterozygous p.R1135C), who is now 14 years of age, began to experience exercise- and illness-associated exacerbations in her level of muscle weakness from the age of 12, and an activity-limiting increase in her fatigability from age 13. These episodes can last a few minutes and while they could be considered reminiscent of periodic paralysis, her repetitive stimulation and single fibre EMG results were normal, with no evidence of alteration of the amplitude or the total area of the compound muscle action potential during exercise test performed after cooling or after a long exercise test.
The oldest cohort member (now aged 35) has experienced a deterioration in her strength and motor abilities, and increase in fatigability over the past 5 years. She also has an abnormal repetitive stimulation test result suggestive of an additional neuromuscular junction abnormality ( Table 2 ), which may be contributing to her fatigability. One of her mutations was reported previously in a family with autosomal dominant non-dystrophic myotonia ( Lee et al. , 2009 ); however, myotonia was notably absent in this and all other cohort members.
Table 2.
Investigations performed in cohort members with ‘classical’ congenital myopathy
| Cohort | Family F1 | Family F2 | Family F3 | Family F4 |
|---|---|---|---|---|
| Country of origin | UK | Denmark | Norway | Canada |
| Creatine kinase (CK) level | ||||
| Normal/Abnormal (level) | Normal (21 U/l) | Normal (118 U/l) | Normal (62 U/l) | Normal (30 U/l) |
| Neurophysiology results | ||||
| EMG |
|
|
|
|
| Single fibre EMG | At age 5 years 2 months: Normal single fibre EMG result | Insufficient signal | _ | _ |
| Repetitive nerve stimulation | At age of 4 years: No decrement at 3Hz | At age 35 years: No decrement at 3 Hz, 60% decrement at 10 Hz | At 6 weeks of age: Normal | _ |
| Nerve conduction velocity | At 14 years and 8 months: Normal | At age 35 years: Reduced motor amplitudes probably due to abnormal membrane function |
|
|
| Muscle MRI | ||||
| Pelvis/upper leg: muscles significantly involved | At age 6 years 5 months: Severe involvement of glutei, marked bilateral involvement of sartorius and adductor magnus | At age 35 years: Severe involvement of glutei, marked bilateral involvement of sartorius and adductor magnus | _ | _ |
| Lower leg: muscles significantly involved | Soleus | Soleus | _ | _ |
| Other relevant findings | No oedema | No oedema | _ | _ |
| Other relevant investigations/results | ||||
| Cranial MRI | _ | _ | At 15 months of age: Normal | Undertaken in Patient II.1: At 14 days: Normal |
| Spinal MRI | At age 9 years 8 months: Scoliosis present. Normal intraspinal appearances. Erector spinae muscles atrophic with fatty replacement. | _ | _ | _ |
| Mitochondrial studies | _ | _ | _ | Respiratory chain enzyme defects noted (significant reduction in activity of all complexes including the marker enzyme, citrate synthase): all thought to be secondary changes |
- = not done; MUP = motor unit potential.
Status of confirmed carriers
Segregation studies confirmed that all 12 parents, and one unaffected sibling (from Family 4) were carriers of one SCN4A mutation. Eight parents (from Families 1, 4, 5 and 6) and the sibling carrier were examined by an experienced neurologist and/or clinical geneticist and showed no evidence of myopathy, myotonia or other abnormal neurological features. This included the paternal carrier of p.R1135C mutation (previously reported in an unusual recessive form of hypokalaemic periodic paralysis). The remaining four parents were unavailable for formal examination but by report, had no neurological symptoms or signs. This included the maternal carrier of the p.R225W mutation (previously reported in dominant non-dystrophic myotonia).
Investigations
Investigations performed in the cohort members are summarized in Table 2 . The creatine kinase level was normal (21–118 U/l) in all four surviving cohort members.
Neurophysiology
Neurophysiology testing was undertaken in all four cohort members who survived infancy. EMG findings were normal in 2/4. Mild abnormalities were present in 1/4. In Patient II.1 from Family 4, there were widespread myopathic changes in all four limbs at age 14 months, however on repeat testing at age 6 years all abnormalities had normalized, in parallel with a gradual improvement in strength and motor abilities. Single fibre EMG was normal in one cohort member. Repetitive stimulation testing was undertaken in two cohort members. Neither had a positive decrement at 3 Hz. In the oldest cohort member there was, however, a 60% decrement at 10 Hz.
Nerve conduction velocities were normal in 2/4 members. Testing undertaken in the oldest cohort member, at age 35 years, showed reduced motor amplitudes. The fourth surviving cohort member had sensory conduction abnormalities at age 45 days and 14 months, but these had normalized by 6 years of age.
Muscle, spinal and cerebral magnetic resonance imaging
Lower limb muscle MRI was undertaken in Patients II.1 Family 1 (at age 6 years) and II.1 Family 2 (at age 35 years) ( Fig. 3 ). Both patients had severe involvement of the glutei, marked bilateral, symmetric involvement of sartorius, adductor magnus and soleus. Spinal MRI was undertaken in one cohort member and showed a scoliosis and erector spinae muscle atrophy but a normal spinal cord.
Cerebral MRI was performed in two cohort members (one at 14 days, the other at 15 months of age) and was normal in both.
Muscle pathology
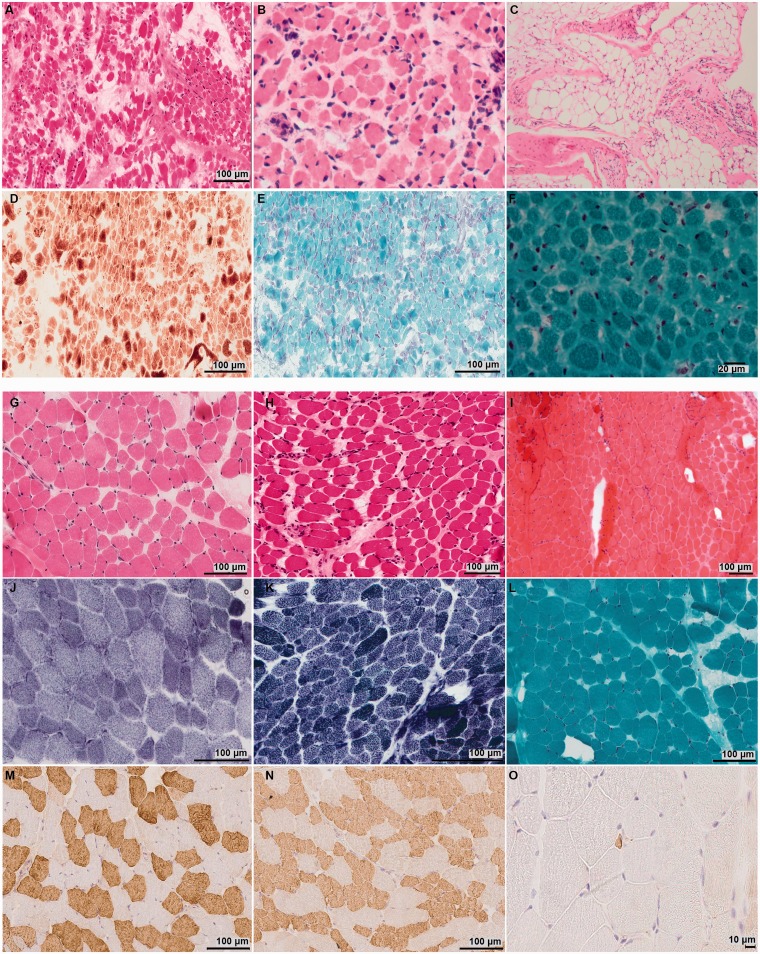
Post-mortem muscle samples were taken from all foetal hypokinesia affected cohort members: Patient II.2 Family 4 (gestational age 40/40); Family 5 Patient II.1 (33/40), Patient II.2 (31/40), and Patient II.3 (36/40); and Family 6 Patient II.1 (29/40), Patient II.2 (32/40), and Patient II.3 (28/40). Muscle histopathology findings included abnormal fibre size variation with a mixture of small and large fibres across fascicles without overt grouping. Presence of fibrofatty tissue, which was particularly severe in Patient II.1 Family 6, was noted in all cases ( Fig. 4 A–C). Necrosis and regeneration were not seen. Rods, other inclusions, cores or other structural abnormalities were absent ( Fig. 4 ).
Figure 4.
Muscle pathology in the foetal hypokinesia and ‘classical’ congenital myopathy affected individuals. Haematoxylin and eosin staining performed in the muscle samples taken from the foetal hypokinesia cases showed abnormal fibre size variation ( A–C ). Presence of fibrofatty tissue was noted in the muscle samples taken from affected foetuses II.2 from Family 4 and II.2 from Family 6 ( A and B ). Marked end-stage presence of fibrofatty tissue was seen in the post-mortem sections from Patient II.1 Family 6 ( C ). No mitochondrial abnormalities ( D ), rods or other inclusions were present in the muscle sample from affected foetus II.2 from Family 4 ( D and E ). Rods or other inclusions were also absent in Patient II.2 from Family 5 ( F ). Haematoxylin and eosin staining in the biopsies from ‘classical’ congenital myopathy cases showed myopathic features with abnormal fibre size variation without necrosis and regeneration ( G – I ). Mild fibrofatty replacement was present in the quadriceps biopsy taken from Patient II.1 Family 1 (age 2 years) ( G ), Patient II.1 Family 4 (age 1 month) ( H ) and Patient II.1 Family 3 (age 1.5 months) ( I ). NADH oxidative enzyme staining showed a population of small type 1 fibres with slow myosin in the biopsy of Patient II.1 Family 1 cohort member ( J ). This was confirmed with myosin heavy chain immunolabelling [fast myosin ( M ); slow myosin ( N )]. Foetal myosin ( O ) showed scattered, abnormal, very small fibres measuring <5 µm. NADH histochemistry showed preserved fibre typing without cores or minicores in Patient II.1 Family 4 ( K ). Rods or other inclusions were absent in the Gomori Trichrome stain (Patient II.1 Family 3) ( L ).
Quadriceps muscle biopsies taken from the congenital myopathy cohort members showed milder myopathic features ( Fig. 4 ). A combination of slow fibre hypotrophy with normal to larger sized fast fibres and mild slow predominance was seen in Patient II.1 Family 1. No overt structural abnormalities or nemaline rods were present ( Fig. 4 ).
Where fibre typing data were available, muscle samples in the foetal hypokinesia cohort showed fast predominance, probably related to immaturity, while mild to marked slow predominance was observed in the biopsies from the congenital myopathy cohort.
SCN4A nonsense, essential splice site and frameshift mutations alter essential functional domains of the Na v 1.4 channel
The truncating mutations p.Q470X and p.Y1593X would delete 1366 and 240 amino acids, respectively from the C-terminal end of the Na v 1.4 protein. They would result in nonsense-mediated decay of the SCN4A transcript or if translated in non-functional channels.
To investigate the effect of the c.3145-2A>C mutation on SCN4A mRNA splicing, we performed reverse transcription PCR in peripheral blood cells from the proband, mother and father from Family 4. Using primers flanking exon 15–17, two distinct fragments were amplified. A smaller fragment was obtained in all three samples while a larger fragment was only present in the index case and the father. Sanger sequencing demonstrated the smaller to be a 424 bp fragment corresponding to the wild-type SCN4A transcript of exons 15–17. The larger 971 bp fragment contained exons 15–17 and an additional 547 bp of intron 16 ( Supplementary Fig. 1 ). The retained intron 16 sequence introduces a stop codon in the SCN4A mRNA coding sequence at amino acid position 1099 (p.A1049VfsX50) that would generate a truncated Na v 1.4 protein. The deletion of 738 C-terminal amino acids is predicted to induce complete loss of Na v 1.4 channel function.
The frameshift p.H1782Qfs65 located at the channel C-terminus caused by c.5345 duplication generates a protein that is nine amino acids longer than the wild-type. The mutated channels would have C-terminal sequence that is completely altered compared to wild-type channels. The effect of C-terminus frameshift mutations is largely unknown and therefore p.H1782Qfs65 was included in the functional analyses.
In vitro electrophysiological characterization of the SCN4A mutations
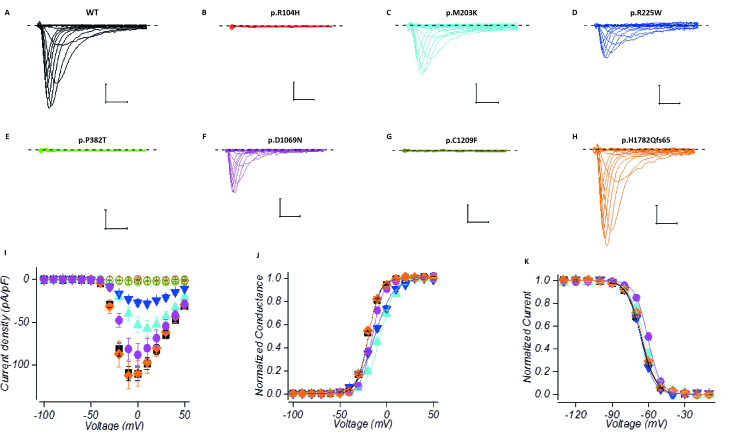
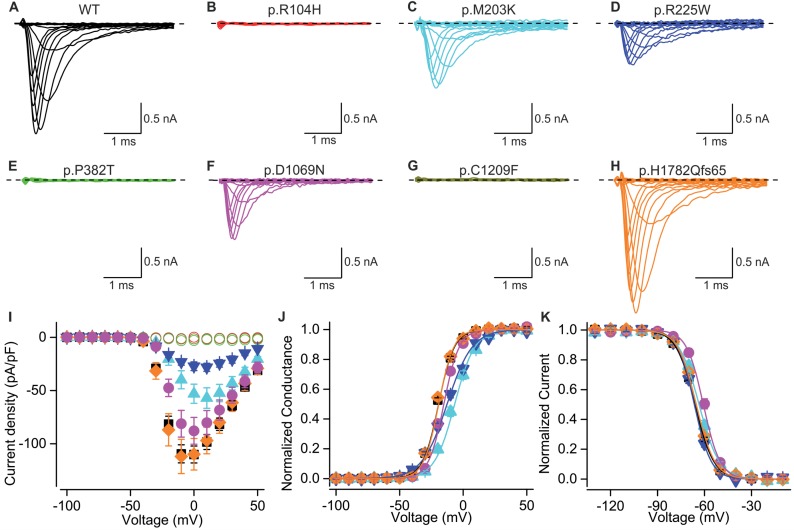
Three of the variants (p.R104H, p.P382T and p.C1209F) did not display any detectable sodium currents when expressed in HEK293 cells ( n > 10) ( Fig. 5 ), suggesting that they render the channel completely non-functional.
Figure 5.
Electrophysiological characterization of congenital myopathy channel variants . Current traces in response to depolarizing test voltages for wild-type (WT; A ), p.R104H ( B ), p.M203K ( C ), p.R225W ( D ), p.P382T ( E ), p.D1069N ( F ) p.C1209F ( G ) and p.H1782Qfs65 ( H ) channels expressed in HEK293 cells. Scale-bars are 1 ms ( x -axis), 0.5 nA ( y -axis). The dashed line represents the baseline current level at holding voltage. Mean current density ( I ) and normalized conductance ( J ) response to test voltages for channels expressed in HEK293 cells. Solid lines represent fit of Boltzmann equation to the mean data. Variants are colour coded as in A–H . Symbols: wild-type (square), p.M203K (triangle), p.R225W (inverted triangle), p.D1069N (circle), p.H1782Qfs65 (diamond). p.R104H, p.P382T and p.C1209F are all represented by open circles. Mean normalized currents in response to test voltage of −10 mV (HEK293) following a prepulse steps of 150 ms to voltages indicated in x -axis ( K ). Lines represent fit of Boltzmann equation to the mean data. Colour coding and symbols are as in I .
Variants p.M203K, p.R225W and p.D1069N displayed currents with reduced amplitude ( P < 0.001 for M203K and R225W, P = 0.07 for D1069N). The voltage dependence of channel activation for these three variants was shifted to depolarizing direction ( P < 0.001) ( Fig. 5 and Table 3 ). The data clearly demonstrate that the function of these channels is impaired. The loss-of-function effects of the p. D1069N mutation were confirmed in an alternate heterologous expression system, Chinese hamster ovary (CHO) cells (data not shown).
Table 3.
Biophysical parameters of Na V 1.4 variants
| Clone |
Activation
|
Fast inactivation
|
|||||
|---|---|---|---|---|---|---|---|
| V 1/2 (mV) | V slope (mV) | I peak (at 0mV) (pA/pF) | n | V 1/2 (mV) | V slope (mV) | n | |
| Wild-type (HEK293) | −20.0 ± 0.4 | 6.36 ± 0.12 | −110.1 ± 8.0 | 51 | −65.8 ± 0.4 | 5.55 ± 0.09 | 52 |
| R104H | NA | NA | – | 15 | NA | NA | – |
| M203K | −7.0 ± 1.2*** | 8.47 ± 0.25*** | −53.0 ± 9.0*** | 12 | −63.1 ± 0.9* | 5.98 ± 0.46 | 13 |
| R225W | −12.3 ± 1.1*** | 10.68 ± 0.34*** | −27.1 ± 4.1*** | 9 | −66.0 ± 0.6 | 5.23 ± 0.28 | 13 |
| P382T | NA | NA | – | 13 | NA | NA | – |
| D1069N | −15.8 ± 0.7*** | 6.24 ± 0.28 | −87.8 ± 12.6 | 13 | −60.2 ± 0.8*** | 5.15 ± 0.22 | 15 |
| C1209F | NA | NA | – | 11 | NA | NA | – |
| H1782Qfs65 | −20.2 ± 0.8 | 6.11 ± 0.22 | −109.9 ± 14.8 | 16 | −64.8 ± 0.4 | 5.62 ± 0.14 | 19 |
Mean ± SEM are shown. Statistical comparison of the variants was done against the wild-type channel using Student’s t -test. Statistically significance is denoted by * P < 0.05, ** P < 0.01, *** P < 0.001.
V 1/2 = mid-point voltage; V slope = slope factor; I peak = peak current density at 0 mV; NA = not available.
The frameshift variant p.H1782Qfs65 did not display any loss of Na v 1.4 channel function properties upon heterologous expression. Neither the peak current density, the voltage dependencies of the channel activation, fast- or slow inactivation, nor the time courses of onset or recovery from fast inactivation were significantly different from wild-type channels ( Fig. 5 , Table 3 , Supplementary Fig. 2 and Supplementary Table 4 ).
In addition to impaired channel activation, the voltage dependence of fast inactivation was shifted to depolarizing direction for p.D1069N ( P < 0.001) and p.M203K ( P < 0.05) variants ( Fig. 5 and Table 3 ). Some substitutions also displayed a statistically significant change in the voltage dependence of slow inactivation or in the kinetics of fast inactivation. Analysis of these data is shown in Supplementary Fig. 2 and Supplementary Table 4 .
The p.R1135C channel has been comprehensively characterized ( Groome et al. , 2014 ). It causes loss of channel function by enhancing fast inactivation of the channel. In addition it causes gating pore currents, which are typical for hypokalaemic periodic paralysis mutant channel. We studied the gating pore currents of the p.R1135C channels using X. laevis oocyte expression system. We found that the gating pore current of the rat Na v 1.4-R1128C orthologue is active at hyperpolarized resting voltages even when the holding voltage is hyperpolarized ( Supplementary Fig. 3 ). This differs from previous studies ( Groome et al. , 2014 ) that detected the hyperpolarization activated gating pore currents only after a depolarizing prepulse.
Discussion
Using whole exome sequencing, we ascertained a cohort of 11 individuals from six unrelated and ethnically distinct kindreds with homozygous or compound heterozygous mutations in SCN4A . Affected individuals presented with congenital myopathy of variable severity. All had in utero - or neonatal-onset muscle weakness resulting in reduced foetal movements. Patients at the severe end of the spectrum had clinical features associated with foetal hypokinesia (maternal polyhydramnios, in utero upper/lower limb contractures, talipes and hydrops) resulting in intrauterine or early postnatal death. The patients at the less severe end of the spectrum presented with generalized hypotonia and weakness at birth or within the first few days of life. This was typically associated with mild-to-moderate facial muscle weakness without ptosis, and significant early respiratory and feeding difficulties. Surviving individuals had delayed motor milestones and achieved independent ambulation before the age of 3 years. Consistent with many forms of ‘classical’ congenital myopathy, all cohort members who survived infancy experienced improvement in their strength and motor skills over time, and concomitant resolution of early respiratory and feeding difficulties.
The phenotype of the cohort members described in this study differs from that of other sodium channelopathies (e.g. autosomal dominant myotonia and autosomal dominant periodic paralysis), and from the two reported SCN4A -related congenital myasthenia cases. In stark contrast to other sodium channelopathies, the congenital myopathy cohort members had in utero - or neonatal-onset permanent muscle weakness, rather than later-onset episodic muscle weakness. Although individuals with periodic paralysis and, in rare instances, paramyotonia congenita, sometimes develop permanent proximal muscle weakness ( Suetterlin et al. , 2014) , this is typically a late complication of the condition and not a presenting feature. In addition, none of the patients had the typical features of weakness aggravated by specific factors (cold, special food and exercise) that trigger a worsening of stiffness and/or weakness in SCN4A -related myotonia or periodic paralysis ( Cannon, 2015 ). While in one teenaged patient excessive muscle activity was associated with marked increase in fatigue, detailed electrophysiology examination failed to demonstrate neuromuscular junction defects or aspects evocative of periodic paralysis.
Other characteristic features of our patient cohort were the association with abnormal muscle histopathological features, and a selective pattern of muscle involvement on muscle MRI.
The majority of SCN4A mutations associated with periodic paralysis and myotonia are dominantly inherited and cause gain-of-function predominantly by enhancing channel activation, attenuating fast inactivation or by inducing gating pore currents. Mild enhancement of fast inactivation leading to a loss-of-function effect has been reported for some Na v 1.4 mutations in hypokalaemic periodic paralysis. Thus far, there are only two reports of loss of Na v 1.4 channel function and recessive inheritance of SCN4A mutations in congenital myasthenic syndrome ( Tsujino et al. , 2003 ; Arnold et al. , 2015 ). The loss-of-function effects were caused by enhanced fast inactivation in both cases.
Recessive loss-of-function Na v 1.4 mutations as a pathomechanism of congenital myopathy
In this study, we establish that recessive SCN4A loss-of-function mutations are the underlying pathomechanism in patients affected by a novel form of congenital myopathy.
All 12 parents and one unaffected sibling were confirmed to be carriers of one SCN4A mutation but were unaffected clinically, based on formal examination in nine cases, and on history and verbal report in the other four. The absence of clinical findings in confirmed carriers suggests that the loss of function in only one allele is insufficient to cause a clinical phenotype, and further suggest an autosomal recessive mechanism of inheritance.
The inheritance of full loss-of-function mutations on both alleles appears to cause a particularly severe phenotype resulting in early lethality, evidenced by the homozygous p.P382T mutation detected in the three affected individuals from Family 5. This suggests that muscles without functional Na v 1.4 channels are unable to support life.
The remaining cohort members carried compound heterozygous SCN4A mutations. All had one mutation that resulted in a completely non-functional channel, either as a consequence of Na v 1.4 protein truncation or as a result of a mutation that led to a complete abolition of the sodium flux through the channel (p.R104H, p.C1209F, p.Q470X, p.A1049VfsX50, p.Y1593X in Families 1–4 and 6, respectively). Affected individuals from four of the five families with compound heterozygous mutations had a second mutation, in the trans allele, which resulted in partial Na v 1.4 loss-of-function mainly by attenuating channel activation (p.R225W, p.D1069N and p.M203K, Families 2, 4 and 6, respectively) or by enhancing channel inactivation (p.R1135C, Family 1). In Family 3, there were no detectable loss-of-function properties in HEK293 cells for one of the Na v 1.4 variants (discussed below).
Some of the affected individuals with compound heterozygous mutations (Families 6 and 4) also died in utero , or at birth, implying that some combinations of complete loss-of-function/partial loss-of-function mutations also result in a severe clinical phenotype. Interfamilial variability was, however, also clearly evident. This is best demonstrated in Family 4, where one affected infant developed severe in utero -onset hypokinesia and died within a few hours of birth. The other affected infant survived infancy albeit with significant feeding, and respiratory support. She is now 8 years of age, has a phenotype consistent with ‘classical’ congenital myopathy and continues to improve clinically over time, a feature often associated with ‘classical’ congenital myopathies. Further studies in a large cohort of patients are required to delineate more precise correlation of functional and clinical expression of SCN4A mutations.
One of the mutations present in the 2.5-year-old male cohort member from Family 3 (p.H1782Qfs65) did not result in loss of channel function when studied in HEK293 cells. This child has a similar clinical phenotype to other cohort members. He was born following a pregnancy complicated by polyhydramnios (first noted at delivery), had marked neonatal-onset weakness and hypotonia associated with facial weakness, required significant feeding and respiratory support from birth, but continues to improve clinically. The frameshift mutation is predicted to alter the C-terminal of the protein and extends it by nine residues compared to the wild-type channel. Our analysis suggests that in HEK293 cells, Na v 1.4 channel C-terminus has no detectable effects on the biophysical properties of the channel. This is in agreement with previous data showing that a deletion of the C-terminal 100 residues has no effect on channel gating ( Herzog et al. , 2003 ). However, it is possible that the frameshift mutation disrupts channel function in a tissue-specific manner, which cannot be detected in a heterologous expression system, or it might cause loss-of-function effect not elicited by the functional analyses undertaken in this study. For example, the last five C-terminal amino acid residues play an important role in the interaction of Na v 1.4 with the PDZ domain of syntrophin ( Schultz et al. , 1998 ), a scaffold protein that is a part of the dystrophin-associated protein complex and has a vital role in the maintenance of muscle integrity ( Ehmsen et al. , 2002 ). Alterations of the sequence of the C-terminus may also disrupt signals responsible for channel trafficking, targeting, activity or stability. Additional studies are required to further investigate these possibilities.
Interestingly, we identified compound heterozygosity for p.R1135C and a full loss-of-function mutation in a 14-year-old girl with a ‘classical’ congenital myopathy phenotype. Homozygous p.R1135C has been previously described in a patient with an unusual recessive form of hypokalaemic periodic paralysis ( Groome et al. , 2014 ). Consistent for periodic paralysis, the mutant channels display gating pore currents. The mutation also causes loss-of-function effects on the main pore current by enhancing fast inactivation, which results in reduced channel availability. However, the heterozygous carrier of this mutation in our family had no periodic weakness. Furthermore the muscle weakness of the proband, carrying a heterozygous p.R1135C and a full loss-of-function SCN4A mutation in trans, has been permanent rather that periodic, suggesting that the reduced main pore sodium current is the likely pathomechanism of the myopathy. Although this patient, who is now 14 years old, has started to complain of recurrent brief episodes of increased muscle weakness, a detailed electrophysiological study failed to identify any abnormalities typical of periodic paralysis, myotonia or neuromuscular junction abnormalities, but only demonstrated myopathic features.
Our findings are in agreement with Na v 1.4 being the main skeletal muscle voltage gated sodium channel isoform, including the foetal and neonatal muscle, critical to muscle function ( Zhou and Hoffman, 1994 ). Expression of a second isoform, Na v 1.5, which is tetrodotoxin resistant, has been shown in developing and denervated muscle ( Harris and Thesleff, 1971 ; Weiss and Horn, 1986 ). The tetrodotoxin resistant sodium current may be able to generate action potentials in muscle of newborn rats ( Harris and Marshall, 1973 ). However, these action potentials are defective and disappear soon after birth, confirming a key role for tetrodotoxin sensitive currents in generating skeletal muscle action potentials. Possible differences in Na v 1.5 expression in foetal muscle may, however, contribute to the variable clinical presentation in the myopathy cohort. No mutations in our cohort were detected in SCN5A that encodes Na v 1.5, the principal sodium channel responsible for the initiation of the cardiac action potential ( George et al. , 1995 ). This is consistent with the lack of cardiac involvement in the cohort members.
In conclusion, we have identified homozygous or compound heterozygous recessive mutations in SCN4A in six congenital myopathy families. At least one of the mutations in each patient renders the Na v 1.4 channel fully non-functional. The mutation on the second allele resulted in full or partial loss of Na v 1.4 channel function in five out of six families. This constitutes the first report describing full loss-of-function SCN4A mutations as associated with a clinical phenotype.
The Na v 1.4 channel is crucial for the initiation and propagation of the muscle action potential. The link between the depolarization of the membrane and the Ca 2+ release triggering the muscle contraction is mediated by the interaction between the voltage-sensing DHPR and the Ca 2+ release channel RYR1, main components of the excitation–contraction coupling. Mutations in RYR1 are an established cause of several types of congenital myopathy ( Zhang et al. , 1993 ; Jungbluth et al. , 2002 ; Monnier et al. , 2003 ). Here, we propose that the combined effects of two loss-of-function mutant Na v 1.4 channels, one of which is a full loss-of-function, attenuates the action potential amplitude in skeletal muscle to a level insufficient to sustain normal muscle force.
These findings have important implications for genetic diagnosis as they confirm a new form of recessive SCN4A -related congenital myopathy, a discovery which will undoubtedly result in improved genetic diagnosis rates in patients with this group of disorders. Furthermore, this study indicates that SCN4A is another example of a growing number of genes encoding for ion channels recently found to be involved in congenital myopathies. Our study also opens research avenues aimed at improving the therapeutic management of these patients.
Supplementary Material
Acknowledgements
We gratefully acknowledge the clinical and scientific support from our colleagues Dr Mariacristina Scoto and Darren Chambers from the Dubowitz Neuromuscular Centre, UCL Institute of Child Health, London and Dr W. Wasserman, Dr M. Tarailo-Graovac, Dr C.J. Ross, Dr H. Gill, Dr K. Selby, Dr B. Sayson, Dr P Eydoux, Ms M. Balicki, Ms Chieko Chijiwa from the University of British Columbia, Vancouver CA.
Glossary
Abbreviations
- ExAC
Exome Aggregation Consortium
- HEK293
human embryonic kidney 293
Funding
The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 2012-305121 ‘Integrated European –omics research project for diagnosis and therapy in rare neuromuscular and neurodegenerative diseases (NEUROMICS)’. This study was also supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London (F.M.). The support of the Medical Research Council Neuromuscular Centre Biobank and of the Muscular Dystrophy Campaign study coordinators is also gratefully acknowledged (F.M.; M.G.H.). M.G.T. was funded by a Medical Research Council Centre for Neuromuscular Diseases studentship. R.M. was funded by United Kingdom Medical Research Council project grant MR/M006948/1. We also acknowledge support of the UCLH Biomedical Research Centre. E.C.O. was gratefully funded by a Winston Churchill Memorial Trust of Australia fellowship and by a National Health and Medical Research (NHMRC) early career research fellowship (grant number: GNT1090428). This research was also supported by the National Health and Medical Research Council of Australia (Early Career Researcher Fellowship #1035955 to G.R., Research Fellowship APP1002147 to N.G.L. and Project Grant APP1022707; EU Collaborative grant APP1055295); the Association Francaise contre les Myopathies (#15734). We acknowledge funding support from the B.C. Children’s Hospital Foundation (Treatable Intellectual disability Endeavour in British Columbia: 1st Collaborative Area of Innovation www.tidebc.org ), Genome BC (SOF-195) and Canadian Institutes of Health Research (#301221). Dr. C. van Karnebeek is a recipient of the Michael Smith Foundation for Health Research Scholar Award. M.G.H. and R.M. work is supported by the UCLH Biomedical Research Centre.
Supplementary material
Supplementary material is available at Brain online.
References
- Arnold WD, Feldman D, Ramirez S, He L, Kassar D, Quick A, et al. . Defective fast inactivation recovery of NaV1.4 in congenital myasthenic syndrome . Ann Neurol 2015. ; 77 : 840 – 50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon SC . Channelopathies of skeletal muscle excitability [Review] . Compr Physiol 2015. ; 5 : 761 – 90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrochano S, Männikkö R, Joyce PI, McGoldrick P, Wettstein J, Lassi G, et al. . Novel mutations in human and mouse SCN4A implicate AMPK in myotonia and periodic paralysis . Brain 2014. ; 137 : 3171 – 85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, et al. . Diagnostic exome sequencing in persons with severe intellectual disability . N Engl J Med 2012. ; 367 : 1921 – 9 . [DOI] [PubMed] [Google Scholar]
- Ehmsen J, Poon E, Davies K . The dystrophin-associated protein complex [Review] . J Cell Sci 2002. ; 115 : 2801 – 3 . [DOI] [PubMed] [Google Scholar]
- George AL, Jr, Varkony TA, Drabkin HA, Han J, Knops JF, Finley WH, et al. . Assignment of the human heart tetrodotoxin-resistant voltage-gated Na+ channel alpha-subunit gene (SCN5A) to band 3p21 . Cytogenet Cell Genet 1995. ; 68 : 67 – 70 . [DOI] [PubMed] [Google Scholar]
- Groome JR, Lehmann-Horn F, Fan C, Wolf M, Winston V, Merlini L, et al. . NaV1.4 mutations cause hypokalaemic periodic paralysis by disrupting IIIS4 movement during recovery . Brain 2014. ; 137 : 998 – 1008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JB, Marshall MW . Tetrodotoxin-resistant action potentials in newborn rat muscles . Nat New Biol 1973. ; 243 : 191 – 2 . [DOI] [PubMed] [Google Scholar]
- Harris JB, Thesleff S . Studies on tetrodotoxin dependent action potentials in denervated skeletal muscle . Acta Physiol Scand 1971. ; 83 : 382 – 8 . [DOI] [PubMed] [Google Scholar]
- Herzog RI, Liu C, Waxman SG, Cummins TR . Calmodulin binds to the C terminus of sodium channels Nav1.4 and Nav1.6 and differentially modulates their functional properties . J Neurosci 2003. ; 23 : 8261 – 70 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbluth H, Müller CR, Halliger-Keller B, Brockington M, Brown SC, Feng L, et al. . Autosomal recessive inheritance of RYR1 mutations in a congenital myopathy with cores . Neurology 2002. ; 59 : 284 – 7 . [DOI] [PubMed] [Google Scholar]
- Lee SC, Kim HS, Park YE, Choi YC, Park KH, Kim DS . Clinical diversity of SCN4A-mutation-associated skeletal muscle sodium channelopathy . J Clin Neurol 2009. ; 5 : 186 – 91 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E, Guet A, Mayer M, Vicart S, Pemble S, Sternberg D, et al. . Neonatal hypotonia can be a sodium channelopathy: regognition of a new phenotype . Neurology 2008. ; 71 : 1740 – 2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier N, Ferreiro A, Marty I, Labarre-Vila A, Mezin P, Lunardi J . A homozygous splicing mutation causing a depletion of skeletal muscle RYR1 is associated with multi-minicore disease congenital myopathy with ophthalmoplegia . Hum Mol Genet 2003. ; 12 : 1171 – 8 . [DOI] [PubMed] [Google Scholar]
- Nance JR, Dowling JJ, Gibbs EM, Bönnemann CG . Congenital myopathies: an update [Review] . Curr Neurol Neurosci Rep 2012. ; 12 : 165 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenscroft G, Laing NG, Bönnemann CG . Pathophysiological concepts in the congenital myopathies: blurring the boundaries, sharpening the focus [Review] . Brain 2015. ; 138 : 246 – 68 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenscroft G, Sollis E, Charles AK, North KN, Baynam G, Laing NG . Fetal akinesia: review of the genetics of the neuromuscular causes [Review] . J Med Genet 2011. ; 48 : 793 – 801 . [DOI] [PubMed] [Google Scholar]
- Ryan AM, Matthews E, Hanna MG . Skeletal-muscle channelopathies: periodic paralysis and nondystrophic myotonias [Review] . Curr Opin Neurol 2007. ; 20 : 558 – 63 . [DOI] [PubMed] [Google Scholar]
- Schultz J, Hoffmüller U, Krause G, Ashurst J, Macias MJ, Schmieder P, et al. . Specific interactions between the syntrophin PDZ domain and voltage-gated sodium channels . Nat Struct Biol 1998. ; 5 : 19 – 24 . [DOI] [PubMed] [Google Scholar]
- Sewry CA, Jimenez-Mallebrera C, Muntoni F . Congenital myopathies [Review] . Curr Opin Neurol 2008. ; 21 : 569 – 75 . [DOI] [PubMed] [Google Scholar]
- Stühmer W, Conti F, Suzuki H, Wang XD, Noda M, Yahagi N, et al. . Structural parts involved in activation and inactivation of the sodium channel . Nature 1989. ; 339 : 597 – 603 . [DOI] [PubMed] [Google Scholar]
- Suetterlin K, Männikkö R, Hanna MG . Muscle channelopathies: recent advances in genetics, pathophysiology and therapy [Review] . Curr Opin Neurol 2014. ; 27 : 583 – 90 . [DOI] [PubMed] [Google Scholar]
- Trimmer JS, Cooperman SS, Tomiko SA, Zhou JY, Crean SM, Boyle MB, et al. . Primary structure and functional expression of a mammalian skeletal muscle sodium channel . Neuron 1989. ; 3 : 33 – 49 . [DOI] [PubMed] [Google Scholar]
- Tsujino A, Maertens C, Ohno K, Shen XM, Fukuda T, Harper CM, et al. . Myasthenic syndrome caused by mutation of the SCN4A sodium channel . Proc Natl Acad Sci USA 2003. ; 100 : 7377 – 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicart S, Sternberg D, Fontaine B, Meola G . Human skeletal muscle sodium channelopathies [Review] . Neurol Sci 2005. ; 26 : 194 – 202 . [DOI] [PubMed] [Google Scholar]
- Wallgren-Pettersson C, Laing N. The congenital myopathies . In: Karpati G, Hilton-Jones D, Bushby K, Griggs RC , editors. Disorders of voluntary muscle . 8th ednCambridge: : Cambridge University Press; ; 2010. . pp. 282 – 98 . [Google Scholar]
- Weiss RE, Horn R . Functional differences between two classes of sodium channels in developing rat skeletal muscle . Science 1986. ; 233 : 361 – 4 . [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen HS, Khanna VK, De Leon S, Phillips MS, Schappert K, et al. . A mutation in the human ryanodine receptor gene associated with central core disease . Nat Genet 1993. ; 5 : 46 – 50 . [DOI] [PubMed] [Google Scholar]
- Zhou J, Hoffman EP . Pathophysiology of sodium channelopathies. Studies of sodium channel expression by quantitative multiplex fluorescence polymerase chain reaction . J Biol Chem 1994. ; 269 : 18563 – 71 . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.