Patients with anosognosia for hemiplegia after right-hemisphere stroke deny the existence of contralesional motor deficits. Besharati et al. show that such patients are impaired in social cognition tasks requiring 3 rd person perspective-taking. A reduced ability to disengage from the 1 st person perspective may explain the patients’ reduced bodily self-awareness.
Keywords: anosognosia, self, awareness, mentalizing, Theory of Mind
Patients with anosognosia for hemiplegia after right-hemisphere stroke deny the existence of contralesional motor deficits. Besharati et al. show that such patients are impaired in social cognition tasks requiring 3 rd person perspective-taking. A reduced ability to disengage from the 1 st person perspective may explain the patients’ reduced bodily self-awareness.
Abstract
Following right-hemisphere damage, a specific disorder of motor awareness can occur called anosognosia for hemiplegia, i.e. the denial of motor deficits contralateral to a brain lesion. The study of anosognosia can offer unique insights into the neurocognitive basis of awareness. Typically, however, awareness is assessed as a first person judgement and the ability of patients to think about their bodies in more ‘objective’ (third person) terms is not directly assessed. This may be important as right-hemisphere spatial abilities may underlie our ability to take third person perspectives. This possibility was assessed for the first time in the present study. We investigated third person perspective taking using both visuospatial and verbal tasks in right-hemisphere stroke patients with anosognosia ( n = 15) and without anosognosia ( n = 15), as well as neurologically healthy control subjects ( n = 15). The anosognosic group performed worse than both control groups when having to perform the tasks from a third versus a first person perspective. Individual analysis further revealed a classical dissociation between most anosognosic patients and control subjects in mental (but not visuospatial) third person perspective taking abilities. Finally, the severity of unawareness in anosognosia patients was correlated to greater impairments in such third person, mental perspective taking abilities (but not visuospatial perspective taking). In voxel-based lesion mapping we also identified the lesion sites linked with such deficits, including some brain areas previously associated with inhibition, perspective taking and mentalizing, such as the inferior and middle frontal gyri, as well as the supramarginal and superior temporal gyri. These results suggest that neurocognitive deficits in mental perspective taking may contribute to anosognosia and provide novel insights regarding the relation between self-awareness and social cognition.
Introduction
The ability to integrate multimodal signals into an egocentric reference frame and assign the first person perspective to one’s bodily experiences is the hallmark of self-awareness ( Vogeley et al. , 2001 , 2004 ; Blanke et al. , 2002 ). By contrast, the cognitive ability to disengage from the first person perspective and adopt another person’s, third person visuospatial and mental perspective is considered a prerequisite to understand and infer the thoughts and feelings of others; the so-called ‘theory of mind’ (ToM) or mentalizing ( Frith and Frith, 2007 ). In recent decades, these research traditions, first person embodied cognition and third person social cognition, have received ample empirical attention. Far fewer neuroscientific studies have focused on the importance of the third person perspective on our bodily self.
In fact, most of the existing studies in cognitive neurology and neuroscience that have investigated the ability to mentally disengage from the first person, embodied perspective, have focused on how we mentally project our psychological selves to other positions in space ( Blanke et al. , 2004 ) or to other bodies ( Corradi-Dell’Acqua et al. , 2008 ). Yet the question of how we perceive the self from such allocentric perspectives has not been investigated. More generally, while the interaction and the potential overlap of networks that support self-referent processing and social cognition in the brain has been long recognized ( Lieberman, 2007 ; Uddin et al. , 2007 ), the precise ways in which such systems interact to influence self-awareness, and particularly our bodily self-awareness, remains to be understood.
In this respect, neurological disorders of bodily awareness can offer an additional window into the complicated relation between self-awareness, spatial and social cognition. In particular, this study aimed to investigate the relation between bodily self-awareness, spatial and social cognition in anosognosia for hemiplegia (AHP). AHP is characterized by the apparent unawareness of motor deficits contralateral to the lesioned hemisphere. Patients with AHP typically remain anosognosic when they view their paralysed limbs from a first person perspective, such as when their paralysed arm is brought into the ipsilateral visual field and its paralysis is demonstrated by the examiner ( Bisiach et al. , 1986 ). They also remain anosognosic during conventional ‘mirror therapy’ (where a mirror is placed perpendicular to the body and the intact arm appears in the expected position of the paralysed arm; Ramachandran, 1995 ).
By contrast, it has been demonstrated that patients show dramatic improvements in body recognition and awareness when they are provided with visual feedback of their own body in the third person perspective, i.e. when visual feedback of their paralysis is provided via mirrors or video replays ( Fotopoulou et al. , 2009 ; Jenkinson et al. , 2013 ; Besharati et al. , 2014 a ). Similarly, patients show more awareness of their paralyses when asked to make verbal judgements from third person perspectives ( Marcel et al. , 2004 ). These findings suggest that third person visuospatial perspectives, as well as more abstract third person verbal representations of the self may be intact in these patients, in the sense that they can perceive the current state of the body accurately from such perspectives. However, these results leave open the question as to why patients do not habitually use such third person perspectives and knowledge to inform and update their first person perspective on their bodily state. One possibility is that they have lost the cognitive ability to do so without explicit, experimental instructions or manipulations, i.e. they are less able than healthy individuals to spontaneously disengage from the first person perspective and take third person visuospatial or mental perspectives more generally ( Fotopoulou, 2014 ).
This possibility, which we tested in the present study, is also consistent with some of the lesion sites selectively associated with AHP, including the inferior and middle frontal gyri, insula, superior temporal gyrus, and temporo-parietal junction, all within the right hemisphere. These areas have been selectively associated with AHP ( Berti et al. , 2005 ; Karnath et al. , 2005 ; Fotopoulou et al. , 2010 ; Vocat et al. , 2010 ; Moro et al. , 2011 ; Besharati et al. , 2014 b ; Kortte et al. , 2015 ). Areas such as the right superior temporal gyrus and the temporo-parietal junction have also been implicated in the so-called ‘mentalizing network’ ( Siegal and Varley, 2002 ; Gallagher and Frith, 2003 ; Aichhorn et al. , 2009 ; Koster-Hale and Saxe, 2013 ), while damage to areas around the right inferior and middle frontal gyri have been shown to relate to a difficulty inhibiting the self perspective ( Samson et al. , 2005 ). Nevertheless, to our knowledge no behavioural or neuroimaging study has examined the relationship between AHP and social cognition. This was the aim of the current study.
Specifically, we aimed to examine both visuospatial perspective taking and reflective (verbal) facets of mentalizing in a group of patients with right-hemisphere damage and severe AHP. This group was compared to a control group of patients with right-hemisphere damage without AHP and a second control group of neurologically healthy participants. To this end, we designed and tested a visuospatial perspective taking experiment as well as a set of ToM stories that required participants to infer the mental states of agents in each story presented from different perspectives. Based on our hypothesis that AHP patients will be unable to spontaneously take third person perspectives and use such information to update their self-awareness (see above), we expected that they would perform worse than both control groups in the third person conditions on both tasks, while performing comparably to controls on the first person conditions. A secondary prediction was that such deficits would be associated with their degree of motor unawareness, as well as with some inhibition and set-shifting impairment.
Finally, group level lesion overlay maps were used to identify commonly damaged brain areas, and voxel-based lesion–symptom mapping (VLSM; Bates et al. , 2003 ; Rorden et al. , 2007 ) was used to identify brain areas associated with the behavioural scores in our experimental tasks regardless of the clinical grouping. To our knowledge, only three AHP studies have compared experimental scores with lesion data ( Fotopoulou et al. , 2010 ; Moro et al. , 2011 ; Besharati et al. , 2014 b ) and no lesion study has investigated this association in relation to AHP and social cognition. We predicted that lesions to the right inferior and middle frontal gyri, the supramarginal gyrus (i.e. temporo-parietal junction) and the superior temporal gyrus would be associated with impaired performance on the experimental tasks, with the last two areas being implicated more in visuospatial versus verbal perspective taking, respectively.
Materials and methods
Participants
Thirty right-handed, adult neurological patients with right-hemisphere lesions and contralateral hemiplegia [16 females, mean age = 68.44 years, standard deviation (SD) = 12.73 years] participated in the study. Patients were recruited from consecutive admissions to three acute stroke wards using the following inclusion criteria: (i) imaging-confirmed right-hemisphere lesion; (ii) contralateral hemiplegia; and (iii) <4 months from symptom onset. Exclusion criteria were: (i) previous history of neurological or psychiatric illness; (ii) <7 years of education; (iii) medication with significant cognitive or mood side-effects; and (iv) language impairments that precluded completion of the study assessments.
Four eligible and screened patients (two patients with AHP and two with hemiplegia; see below) were excluded from the study as one patient had another stroke and passed away; two were transferred before they could be tested and one became too medically unwell to be tested on our neuropsychological and experimental tasks. There were no other exclusions. The remaining patients were divided into two groups based on their clinical diagnosis of AHP. This classification was based on the Berti structured interview ( Berti et al. , 1996 ), which includes questions regarding motor ability (e.g. ‘Can you move your left arm?’), and ‘confrontation’ questions (e.g. ‘Please touch my hand with your left hand. Have you done it?’). The interview is scored on a 3-point scale, with scores ≥ 1 indicating AHP.
The Feinberg et al. (2000) scale was used as a secondary measure of unawareness severity, providing a continuous, total score used in the experimental and neuroimaging analysis (see below). The scale consists of 10 different questions regarding patients motor deficits, including confrontation questions (e.g. ‘Please try and move your left arm for me. Did you move it?’). Responses were scored by the examiner for each item (0 = no awareness, 0.5 = partial unawareness, and 1 = complete unawareness), and summed to produce a total ‘Feinberg awareness score’ (0 = no awareness, 10 = complete unawareness).
Based on the Berti interview, 15 patients were classified as having AHP (nine females, mean age = 66.53 years, SD = 13.67 years, age range: 47–88 years) and 15 patients were classified as hemiplegic control subjects (hemiplegic group; seven females, mean age = 67.13 years, SD = 16.02 years, age range: 36–86 years). This classification was confirmed by the Feinberg scale in all patients. Fifteen age-matched healthy control subjects were recruited at the same hospital sites, among visitors (healthy control group; six females, mean = 71.67 years, SD = 6.98, age range: 60–90). The local National Health System Ethics Committee approved the study, which was carried out in accordance to the Declaration of Helsinki.
Neurological and neuropsychological assessment
The Medical Research Council scale (MRC; Guarantors of Brain, 1986) was used to assess limb motor strength. Proprioception was assessed with eyes closed by applying small, vertical, controlled movements to three joints (middle finger, wrist and elbow), at three time intervals (correct = 1; incorrect = 0; Vocat et al. , 2010 ). The customary ‘confrontation’ technique was administered to test visual fields and tactile extinction ( Bisiach et al. , 1986 ). Orientation in time, space and person, was assessed using the Mini-Mental State Examination (MMSE; Folstein, 1975 ). Working memory was assessed using the digit span task from the Wechsler Adult Intelligence Scale III ( Wechsler, 1997 ). The Hospital Depression and Anxiety Scale (HADS; Zigmind and Snaith, 1983 ) was used to assess mood. Four subtests ( Table 1 ) of the Behavioural Inattention Test (BIT; Wilson et al. , 1987 ) were used to assess visuospatial neglect. Personal neglect was assessed using the ‘one item test’ ( Bisiach et al. , 1986 ) and the ‘comb/razor’ test ( Mcintosh et al. , 2000 ).
Table 1.
Groups’ demographic and neuropsychological profile
| AHP | HP | HC | Mann-whitney | Kruskal Wallis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Z | P | χ 2 | P | |
| n | 15 | − | 15 | − | 15 | − | − | − | − | − |
| Age (years) | 73.00 | 22.00 | 68.00 | 27.00 | 71.00 | 7.00 | −0.15 | 0.89 | 0.75 | 0.69 |
| Education (years) | 12.00 | 3.00 | 12.00 | 3.00 | 13.00 | 6.00 | −0.57 | 0.58 | 3.16 | 0.21 |
| Days from onset | 8.00 | 12.00 | 9.00 | 7.00 | − | − | −0.08 | 0.94 | − | − |
| MRC Left upper limb (max 5) | 0.00 | 0.00 | 0.00 | 0.00 | − | − | −0.54 | 1.00 | − | − |
| MRC left lower limb (max 5) | 0.00 | 1.25 | 1.00 | 2.00 | − | − | −0.53 | 0.68 | − | − |
| Premorbid IQ-WTAR (max 50) | 40.00 | 17.50 | 32.00 | 12.00 | 48.00 | 4.75 | −0.04 | 0.98 | 8.01 | 0.02 |
| Berti awareness interview (max 3) | 2.00 | 1.00 | 0.00 | 0.00 | − | − | −4.99 | <0.001 * | − | − |
| Feinberg awareness scale (max 10) | 6.00 | 4.00 | 0.00 | 0.00 | − | − | −4.83 | <0.001 * | − | − |
| Orientation (max 3) | 3.00 | 0.00 | 3.00 | 0.00 | − | − | −1.39 | 0.52 | − | − |
| Digit span forwards (max number repeated) | 6.00 | 2.00 | 6.00 | 2.00 | − | − | −0.30 | 0.78 | − | − |
| Digit span backwards (max number repeated) | 3.00 | 2.00 | 2.00 | 2.50 | − | − | −0.62 | 0.53 | − | − |
| MOCA memory (max 5) | 3.50 | 2.00 | 5.00 | 1.00 | − | − | −1.13 | 0.31 | 0.99 | 0.32 |
| MOCA (max 30) | 24.00 | 9.25 | 25.00 | 4.50 | − | − | −0.99 | 0.36 | 2.58 | 0.95 |
| Visual fields (max 6) | 3.50 | 2.25 | 2.00 | 4.00 | − | − | −0.68 | 0.52 | − | − |
| Somatosensory (max 6) | 3.00 | 2.00 | 2.00 | 1.50 | − | − | −1.84 | 0.08 | − | − |
| Proprioception (max 9) | 4.00 | 3.00 | 7.00 | 1.00 | − | − | −3.17 | <0.001 * | − | − |
| Comb/razor test bias (% bias) | −0.44 | −0.32 | −0.23 | −0.38 | − | − | −1.96 | 0.08 | − | − |
| Comb/razor test left (number of strokes) | 3.00 | 3.50 | 7.50 | 4.75 | − | − | −3.13 | 0.007 * | − | − |
| Comb/razor test right (number of strokes) | 11.00 | 4.25 | 10.50 | 5.55 | − | − | −0.36 | 0.73 | − | − |
| Comb/razor test ambiguous (number of strokes) | 4.50 | 3.25 | 4.00 | 4.25 | − | − | −0.58 | 0.58 | − | − |
| Bisiach one item test (max 3) | 1.00 | 1.25 | 1.00 | 1.00 | − | − | −1.03 | 0.38 | − | − |
| Star cancelation (max 54) | 12.50 | 7.50 | 40.00 | 35.50 | − | − | −2.46 | 0.013 | − | − |
| Line bisection (max 9) | 0.00 | 2.25 | 2.00 | 2.00 | − | − | −2.08 | 0.04 | − | − |
| Copy (max 3) | 0.00 | 0.25 | 1.00 | 1.00 | − | − | −1.94 | 0.06 | − | − |
| Representational drawing (max 1) | 0.00 | 0.25 | 1.00 | 1.00 | − | − | −2.40 | 0.03 | − | − |
| Cognitive estimates (max 30) | 9.00 | 7.75 | 8.00 | 7.00 | 6.50 | 5.75 | −0.04 | 0.98 | 3.71 | 0.16 |
| FAB total score (max 18) | 10.00 | 4.00 | 15.00 | 3.50 | 16.00 | 3.00 | −3.05 | <0.001 * | 21.18 | <0.001 * |
| Similarities (max 3) | 2.00 | 1.00 | 2.00 | 0.00 | 2.00 | 1.00 | −2.02 | 0.05 | 8.78 | 0.01 |
| Lexial fluency (max 3) | 2.00 | 1.00 | 3.00 | 1.00 | 3.00 | 1.00 | −2.19 | 0.02 | 8.27 | 0.02 |
| Motor series (max 3) | 2.00 | 1.00 | 3.00 | 1.50 | 3.00 | 1.00 | −1.35 | 0.20 | 4.07 | 0.13 |
| Conflict Ins (max 3) | 2.00 | 1.00 | 3.00 | 0.25 | 3.00 | 1.00 | −3.25 | 0.001 * | 16.47 | <0.001 * |
| Go/No-go (max 3) | 0.00 | 1.00 | 3.00 | 1.00 | 2.00 | 1.00 | −4.04 | <0.001 * | 22.69 | <0.001 * |
| Pres Behav (max 3) | 2.00 | 1.00 | 3.00 | 0.00 | 3.00 | 0.00 | −3.17 | 0.002 * | 17.77 | <0.001 * |
| HADS depression (max 21) | 6.00 | 6.25 | 7.00 | 6.50 | − | − | −1.37 | 0.18 | − | − |
| HADS anxiety (max 21) | 7.00 | 6.25 | 7.00 | 9.00 | − | − | −0.37 | 0.73 | − | − |
HP = hemiplegic group; HC = healthy control group; IQR = interquartile range; Medical Research Council (Guarantors of Brain, 1986); MOCA = The Montreal Cognitive Assessment ( Nasreddine, 2005 ); Comb/razor test = tests of personal neglect ( McIntoch et al. , 2000 ; % bias = left – right strokes/ left + ambiguous + right strokes); Bisiach one item test = test of personal neglect; Visual fields and somatosensory = customary ‘confrontation’ technique = ( Bisiach et al. , 1986 ); line crossing, star cancellation, copy and representational drawing = conventional sub-tests of Behavioural Inattention Test ( Wilson et al. , 1987 ); FAB = Frontal Assessment Battery ( Dubois et al. , 2000 ); HADS = Hospital Anxiety and Depression scale ( Zigmond and Snaith, 1983 ).
a Scores below tests’ cut-off points or more than 1 standard deviation below average mean.
*Significant difference between groups ( P < 0.01).
Patients and healthy controls were also assessed on the following neuropsychological measures. General cognitive functioning together with long-term verbal recall was assessed using the Montreal Cognitive Assessment (MoCA; Nasreddine, 2005 ). Premorbid intelligence was assessed using the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001 ). Executive and reasoning abilities were assessed using the Cognitive Estimates Test ( Shallice and Evans, 1978 ) and the six subtests ( Table 1 ) of the Frontal Assessment Battery (FAB; Dubois et al. , 2000 ).
Experiment 1: Visuospatial perspective taking
Design
To assess visuospatial perspective taking we designed a visuospatial task that required participants to count the number of items observed from different visuospatial perspectives (see below). We used a 3 × 3 design with one between-subject factor (Group: AHP versus Hemiplegia versus Healthy control) and one within-subject factor (Perspective: first person perspective taking versus third person perspective taking animate versus third person perspective taking inanimate). The main dependent variable was the total number of correct responses in each trial (1 = correct and 0 = incorrect). Total scores were converted into percentages for statistical analyses.
Materials and procedure
To construct a suitable visuospatial perspective taking task for our patient populations we adapted and piloted ( Supplementary material ) an existing task ( Langdon and Coltheart, 2001 ; Samson et al. , 2005 ). The task involved three visuospatial positions and corresponding perspectives: (i) the participant seated in his/her wheelchair in front of a table (first person perspective); (ii) the experimenter seated directly opposite the participant (at a 180° angle; third person perspective animate); and (iii) a photo-camera (placed on a table at the right-hand side of the patient to account for left visuospatial neglect) at a 90° angle (third person perspective inanimate; Fig. 1 ). Six transparent plastic cups were placed on a tray, which was placed at the centre of the table. The experiment only proceeded if the participant could see the tray and count all cups during practice items and at regular intervals between conditions. Following questions controlling for visuospatial neglect (for patients only), all participants were asked four types of questions about the cups presented in a pseudo-randomized order:
Figure 1.
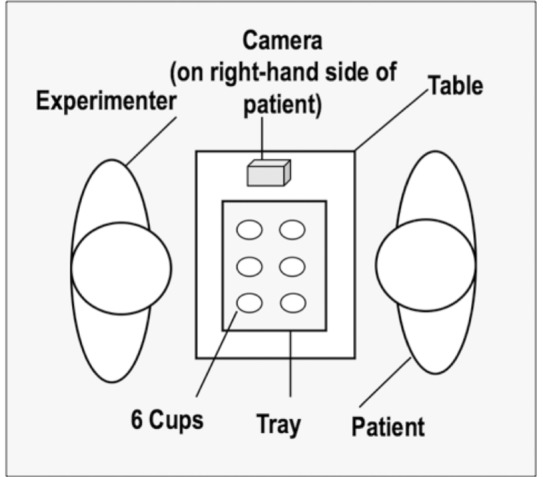
Schematic representation of the visuospatial perspective taking task. The experimenter sits directly in front of the participant (180° shift in perspective) and the camera on the right-hand side (90° shift in perspective); the position of the cups on the tray is changed from trial to trial, with the participant being asked how himself/herself (first person perspective), the experimenter (third person animate perspective) or the camera (third person inanimate perspective) would see the display.
Physical property judgement (quantity), control questions: e.g. ‘How many cups are there on the tray?’
First person perspective taking: ‘How many cups do YOU see in the front row?’
Third person perspective taking animate: ‘How many cups do I see in the front row?’
Third person perspective taking inanimate: ‘If the CAMERA took a picture , in the PICTURE , how many cups would be seen in the front row?’
The position of the cups on the tray was changed after each trial, with the number of cups in the ‘front row’ always differing for each visuospatial perspective (the participant, the experimenter and the camera). Five different arrangements were used ( Supplementary material ): two were used for the physical property control trials and three different arrangements were used for the visuospatial perspective taking trials. In total, the task consisted of six control trials and six visuospatial perspective taking trials (two per perspective condition).
Experiment 2: Theory of Mind stories
Design
To assess verbal ToM abilities we adapted previous story-based tests ( Hynes et al. , 2006 ), which required participants to understand the mental states (e.g. beliefs, intentions or emotions) of different people in the stories. The experimental design included one between-subject factor (Group: AHP versus Hemiplegia versus healthy controls) and two within-subject factors (Perspective: first person perspective taking versus third person perspective taking; and ToM order: First order versus Second order). Perspective was manipulated by changing the ‘person’ in which the protagonist of the stories was presented. First person perspective stories were expressed in the second person (e.g. ‘You are sitting by the TV …’), while third person perspective stories were expressed in the third person (e.g. ‘Eddie is sitting by the TV …’; see also Fotopoulou et al. , 2008 ). Order was manipulated by altering the questions participants were required to answer so that the participants had to understand a character’s mental state (first order) or a character’s belief about the mental state of another character in the story (second order). This design allowed for a 3 × 2 × 2 comparison on the main dependent variable of ToM accuracy, a composite score of spontaneous and multiple-choice answers (minimum score = 0, maximum score = 3; see details below). However, supplementary statistical analysis was also run using multiple choice answers only, showing the same pattern of results.
Materials and procedures
We created 20 stories in total: 16 target ToM stories and four control stories of carefully matched characteristics. All stories consisted of at least two characters and were followed first by an open ToM question and then by three multiple-choice responses ( Hynes et al. , 2006 ). Ten of the stories (eight ToM and two control) were expressed in the first person, while the other 10 were expressed in the third person ( Fig. 2 and Supplementary material ). Half of the ToM stories were followed by a first order question, while the other half extended the original story and were followed by a second order question (see above). The control stories were similar to the ToM stories and involved social situations, but the questions required inferential reasoning and semantic knowledge rather than perspective taking. ToM and control stories in both conditions did not differ in word length [ t (18) = 0.46, P = 0.87; mean = 42.5 words in length].
Figure 2.
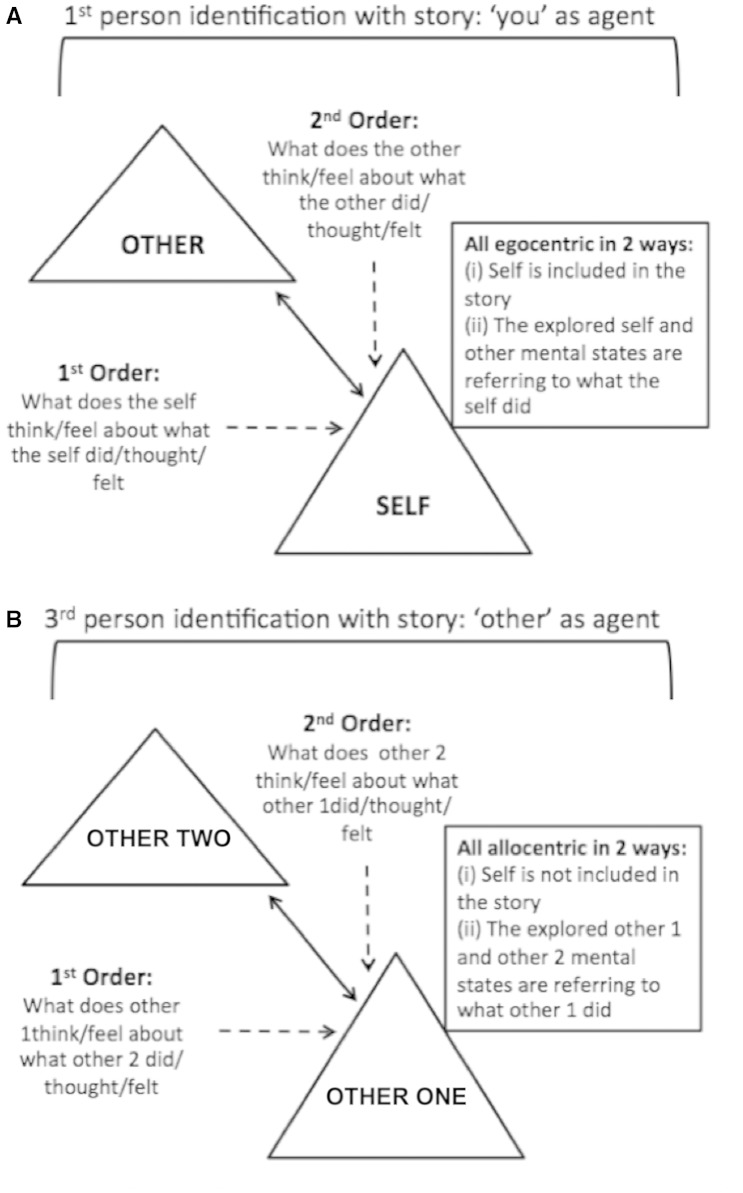
Figure representing first person and third person perspective taking sets of ToM stories. ( A ) first person perspective taking stories depicting the two actors (self and other) with ‘you’ as the agent. Questions are expressed in the second person and are egocentric (the self related to the other); the dotted arrows represent the first order and second order levels. ( B ) Third person perspective taking questions depicting the two actors (other one and other two) with the ‘other’ as the agent. Questions expressed in the third person and are allocentric (the other unrelated to the self); the dotted arrows represent the first order and second order levels.
Procedures
All scenarios and questions were read aloud to the participants in a slow pace and neutral tone. The participants were first required to make a spontaneous response, followed by multiple choice options. For each question a composite score was calculated using both the multiple choice answers and the spontaneous answer. Multiple choice answers were scored as 1 = correct and 0 = incorrect. Spontaneous answers were scored as 1 = correct, 0.5 = partially correct/inadequate, and 0 = incorrect. In the patient groups, testing was conducted in two successive sessions to avoid fatigue. The order of the presentation of the two sets (first person perspective taking and third person perspective taking) was counterbalanced. Each set began and ended with a control story and comprehension rating using a five-point Likert-type scale (max score = 5, full comprehension; Supplementary material ).
Control experiments
Two classic false belief tasks were used as a baseline measure of the participants’ ability to understand that others may have representations of the world that are false and/or different from their own ( Baron-Cohen et al. , 1985 ). Task 1 was an age-adapted version of the ‘Smarties’ task ( Gopnik and Astington, 1988 ), and Baron-Cohen et al. ’s (1985) ‘Sally-Anne’ false belief experiment was used for Task 2 ( Supplementary material ) . A mental rotation task ( Vandenberg and Kuse, 1978 ; Neuburger et al. , 2011 ) was added as an additional control task to assess whether deficits in visuospatial perspective could be attributed to impairments in mental rotation ability. This was tested on a random subset of patients (six AHP and hemiplegia patients, respectively; Supplementary material ).
Statistical analysis
All behavioural analyses were conducted in SPSS21 (IBM Corp. Released 2013). Non-parametric tests were used where the data were not normally distributed. For analysis of neurological and neuropsychological tests alpha significance level was set to 0.01 to account for multiple comparisons. For the experimental tasks, Bonferroni corrections were used where appropriate.
Furthermore, to investigate the specificity of the relationship between AHP and impairments in our visuospatial and verbal (ToM) tasks, modified t -tests (Revised Standardised Difference Test; Crawford et al. , 2010 ) were used to analyse on a case-by-case basis: (i) the incidence of perspective taking deficits and differential deficits (classical dissociations) in AHP and hemiplegia patients, according to the fully operational definitions proposed by Crawford et al. (2003) . Incidence was determined by examining the performance of an individual AHP or hemiplegia control patient on our target, third person perspective condition per se , as well as relative to the performance of the same patient on the control first person condition, in both cases in comparison to the performance of the healthy control group on the same task; and (ii) the severity of perspective taking deficits, as well as differential deficits (classical dissociations) in the AHP patients compared to hemiplegic control patients. Severity was determined by examining the performance of an individual AHP patient on our target, third person perspective condition per se , as well as relative to the performance of the same patient on the control first person condition, in both cases in comparison to the performance of the hemiplegia control group on the same task.
Additionally, we examined the relation between perspective taking (third person condition scores in both the visuospatial perspective taking and the ToM tasks) and anosognosia (using the Feinberg awareness scores) in the AHP group. We also examined the pattern of correlations between perspective taking in both groups and all neuropsychological tests in which the two patient groups showed statistically significant differences (corrected alpha = 0.01), i.e. proprioception and three subtests of the FAB battery. The two groups differed marginally ( P = 0.01) on the ‘star cancelation’ subtest so we also conducted a correlation between perspective taking and performance on this task too. Non-parametric Spearman’s rho tests, corrected for multiple comparisons, were used for all correlational analyses.
Lesion mapping methods
Routinely acquired clinical scans (CT and/or MRI) obtained on admission were collected for 29 patients (clinical dataset of one patient with hemiplegia was unavailable). Lesions were reconstructed onto axial slices of a standard template in MRIcron ( Rorden et al. , 2007 ). A binary lesion mask was created for all patients. A trained researcher (S.F.), blinded to the clinical information, groupings and study hypotheses, reviewed the reconstructions for accuracy and anatomical validity.
Lesion volume was extracted using FSL5 (FMRIB Software Library, http://fsl.fmrib.ox.ac.uk/fsl/ ). An independent t -test was used to assess mean differences between the clinical groups (AHP versus Hemiplegia). Group-level percentage lesion overlay maps for both groups and a subtraction map between them were computed in MRIcron. In addition, the binarized lesion masks were entered into a VLSM pipeline ( Bates et al. , 2003 ) using the NPM program implemented in MRIcron (non-parametric mapping; http://www.cabiatl.com/mricro/npm/ ; Rorden and Karnath, 2004 ). Separate VLSM analyses (including all patients, irrespective of diagnostic classification) were run for the following dependent variables (all scores were continuous): (i) inverted Feinberg awareness scores; (ii) third person perspective taking scores in the visuospatial tasks; and (iii) third person perspective taking in ToM stories. For these behavioural measures, a lower score corresponded to lower awareness and lower perspective-taking ability in both the visuospatial perspective taking and ToM tasks. A VLSM Brunner-Menzel analysis with voxel-based permutation (1000) was conducted ( Rorden et al. , 2007 ; Baldo et al. , 2012 ). Only voxels where at least 10% of patients had damage were included in the analysis to avoid lowering statistical power by including infrequently damaged voxels whilst increasing the number of computed comparisons. Results were then projected onto a high-resolution template ( Holmes et al. , 1998 ) in standard space. Anatomical locations were cross-referenced using the Juelich histological atlas ( Eickhoff et al. , 2007 ) implemented within FSL.
Results
Demographic and neuropsychological results
A summary of the neuropsychological and neurological profile of the participants is provided in Table 1 . No significant difference was observed for age, years of education, pre-morbid IQ, and general cognitive functioning between all three groups (all P ’s > 0.15). As expected, there was a significant difference in awareness scores (Berti interview: Z = −4.99, P < 0.001; Feinberg scale: Z = −4.83, P < 0.001) between the patient groups (AHP versus Hemiplegia). The patient groups did not differ in their time of symptom onset and assessment interval, orientation, long-term memory recall or working memory ( P ’s > 0.53). The scores of both patient groups were also within the normal range on the HADS (range: 0–7 normal, 8–10 borderline, 11+). There was a significant difference between the two groups on the test of proprioception (Z = −3.17, P < 0.001). Both patient groups presented with similar visual and sensory deficits as well as visuospatial and personal neglect ( Table 1 ). Neglect appeared to be marginally more impaired in the AHP group, with such differences not reaching statistically significant levels (alpha = 0.01; star cancelation showing the most marginal effect: Z = −2.46, P = 0.01; see correlational analysis below). Both patient groups performed outside the normal range on the Cognitive Estimates Test suggesting possible deficits in abstract reasoning, however, there was no statistical difference between groups (AHP versus Hemiplegia; Z = −0.04, P = 0.98). There was a significant difference between patient groups on FAB scores, with AHP patients preforming significantly worse overall (Z = −3.05, P < 0.001) and on three specific subtests: conflicting instructions (Z = −3.25, P = 0.001), inhibitory control (Go/No-go test; Z = −4.04, P < 0.001) and precision behaviour (Z = −3.17, P = 0.002). The healthy controls scored within the normal range.
Experiment 1: Visuospatial perspective taking task
Main effects
All participants answered the control questions correctly without any exceptions. An independent sample Kruskall-Wallis test confirmed a significant main effect of Group [ H (2) = 31.92, P < 0.001, r = 0.73]. Subsequent pairwise comparisons with Bonferroni corrections (α = 0.017) showed significant poorer performance in the AHP group (median = 33.3) compared to both control groups: Hemiplegia patients (median = 83.3; Z = −3.95, P < 0.001, r = 0.72) and healthy control subjects (median = 100; Z = −4.87, P < 0.001, r = 0.89). In addition performance was lower in the hemiplegia group relative to healthy controls ( Z = −2.90, P = 0.004, r = 0.53).
A Friedman test revealed a significant main effect of perspective [χ 2 (2) = 42.99, P < 0.001, r = 0.97]. Pairwise analysis with Bonferroni corrections (α = 0.017) showed a significant difference between first and third animate conditions ( Z = 3.40, P = 0.001, r = 0.5) as well as the first and third inanimate conditions ( Z = 4.33, P < 0.001, r = 0.65). However, there was no significant difference between third animate and third inanimate person perspective taking ( Z = 0.928, P = 0.35, r = 0.14) as well as no significant difference within groups for third animate and third inanimate person perspective taking ( P ’s > 0.32). Therefore the third animate and third inanimate conditions were combined to create a composite score for third person perspective taking and used in subsequent analyses below ( Supplementary materials ).
Two-way effects
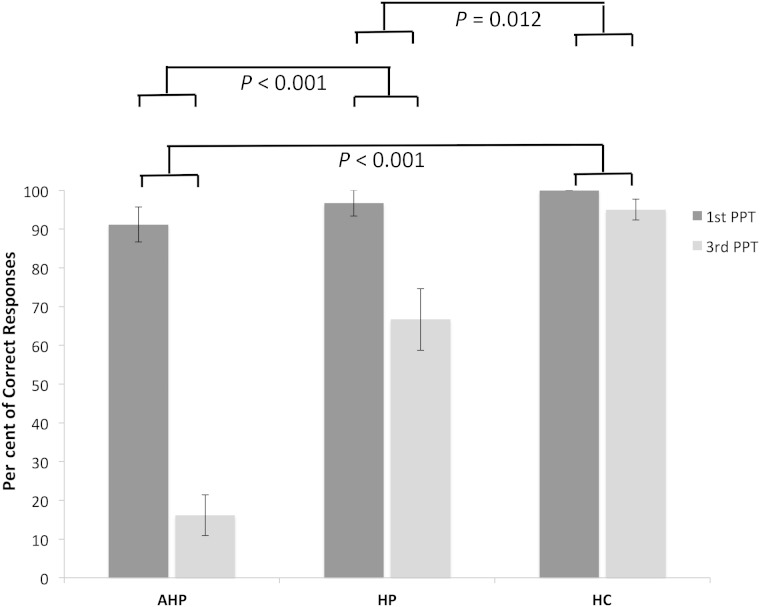
The interaction between Group and Perspective was analysed by calculating the difference between first person and third person perspective taking scores and comparing these between groups using a Kruskal-Wallis test, which showed a significant interaction [ H (2) = 27.88, P < 0.001, r = 0.63]. Pairwise group comparisons on these differential scores, using Bonferroni corrections (α = 0.017), showed a significant difference between AHP and hemiplegia patients ( Z = −3.45, P < 0.001, r = 0.89), AHP and healthy control groups ( Z = −4.804; P < 0.001, r = 1.24), and between the hemiplegia and healthy control groups ( Z = −2.55, P = 0.012, r = 0.657). AHP patients therefore presented with a ‘deficit’ in third person visuospatial perspective taking, in the sense that they performed significantly worse on this condition than they did in the otherwise-balanced, first person perspective condition of the experiment, when compared at the group level with the performance of hemiplegia patients and healthy controls on the same two conditions ( Fig. 3 ).
Figure 3.
Percentage of correct responses for visuospatial perspective taking across groups. Means and standard errors (SE) for first and third person perspective taking (third animate and third inanimate) conditions. AHP patients present with impairment in third person perspective taking, with no significant difference for first person perspective taking between the AHP and controls groups but with significant differences in third person perspective taking between the AHP and controls. Means and SE’s are used here for convention and illustration purposes.
Individual patient analyses
However, there were also statistically significant differences between the two control groups (i.e. the hemiplegia group may also show a deficit in third person perspective taking based on the same definition) and it was not possible to determine at the above group level whether patients with AHP show a ‘differential deficit’, or otherwise known, ‘classical dissociation’ (see Crawford et al. , 2003 for definitions) between the first person and third person perspective taking conditions, in relation to the hemiplegia group. Thus, we also conducted analysis at the individual level.
Specifically, using the Revised Standardized Difference Test (RSDT) ( Garthwaite and Crawford, 2004 ; Crawford and Garthwaite, 2005 ), we examined whether each patient with AHP, or with hemiplegia showed a deficit in third but not in first person conditions as compared with the performance of the healthy controls on these respective tasks, and whether this patient’s scores in the two tasks differed between them at statistically significant levels (the three criteria of a classical dissociation, Crawford et al. , 2003 ). These comparisons would allow us to establish how many patients in AHP and separately in the hemiplegia group showed a deficit and a differential deficit, or classical dissociation in third person perspective taking in relation to healthy controls. However, to further establish the specificity of this deficit in relation to AHP, we also needed to examine the severity (not just the incidence; see ‘Materials and methods’ section for definitions) of these deficits and differential deficits in the AHP group in relation to the hemiplegia group. To this end, we applied the same method on the comparison between each AHP patient with the performance of the hemiplegia controls on the two perspective conditions ( Supplementary Tables 1 and Supplementary Data ).
We found that the incidence of first person deficits in the AHP and hemiplegia groups was low and comparable (2 and 1 of 15 patients, respectively, showed deficits in comparison to healthy controls), while 15 AHP and 10 hemiplegia patients showed deficits in third person perspective in comparison to healthy controls. All 15 patients with AHP showed ‘differential deficits’ in third person versus first person perspective when compared with healthy controls, while 10 of the hemiplegia patients showed such differential deficits.
When comparing between the two patient groups to examine severity of deficits, only eight patients with AHP showed a third person perspective deficit in comparison to hemiplegia controls, while only two patients with AHP showed a first person deficit and there were no differential deficits, although several patients’ scores showed trends towards significance. Taken together these results suggest that there is a higher incidence of third person visuospatial perspective taking ‘deficits’ and ‘differential deficits’ in patients with than without AHP, but that the severity of such deficits seem comparable between the two groups and there is no evidence of classical dissociation between groups.
Experiment 2: Theory of Mind stories
Control condition and comprehension ratings
All participants performed close to the ceiling level for physical control stories (AHP: 97.5%; Hemiplegia: 98.33% and Healthy controls: 99.65%). There was no significant difference between groups [ H (2) = 4.96, P = 0.1, r = 0.1]. All participants reported comprehension ratings between 4 and 5 (maximum score = 5, full comprehension).
Main effects
An independent sample Kruskall-Wallis test confirmed a significant main effect of Group [ H (2) = 20.65, P < 0.001, r = 0.47]. Pairwise comparisons with Bonferroni corrections (α = 0.017) showed a significant difference between the AHP and hemiplegia groups ( Z = −3.3, P = 0.001, r = 0.6), AHP and healthy control group ( Z = −3.94, P = 0.72, r = 0.72) and hemiplegia and healthy control groups ( Z = −2.34, P = 0.02, r = 0.42). Therefore overall, the patients with AHP (median = 56.25) performed worse on the social stories when compared to patients with hemiplegia (median = 75) and healthy controls (median = 89.06). A Wilcoxon signed rank test showed a significant main effect of Perspective ( Z = 3.92, P < 0.001, r = 0.58) with participants preforming significantly worse on third person perspective taking (median = 68.75) than first person perspective taking questions (median = 81.25). The main effect of Order was also significant ( Z = −5.23, P < 0.001, r = 0.82) with participants performing significantly worse on second order questions (median = 59.38) compared to first order (median = 84.38).
Two- and three-way interactions
The interaction between Group and Perspective was analysed by calculating the difference between first person perspective taking and third person perspective taking scores and comparing these between groups. A Kruskal-Wallis test revealed a significant interaction [ H (2) = 22.73, P < 0.001, r = 0.52]. Pairwise comparisons on these differential scores, with Bonferroni corrections (α = 0.017), showed a significant difference between the AHP (median = 43.75) and hemiplegia patients (median = 12.5; Z = −4.09, P < 0.001; r = 0.82), and AHP and healthy control groups (median = 3.125; Z = −4.14; P < 0.001; r = 1.07). However, there was no significant difference between hemiplegia and healthy control groups (Z = − 0.83; P = 0.39; r = 0.21). Finally, the interaction between Group and Order, Perspective and Order, as well as Group, Perspective and Order were likewise analysed, showing no significant interaction (all P ’s > 0.30).
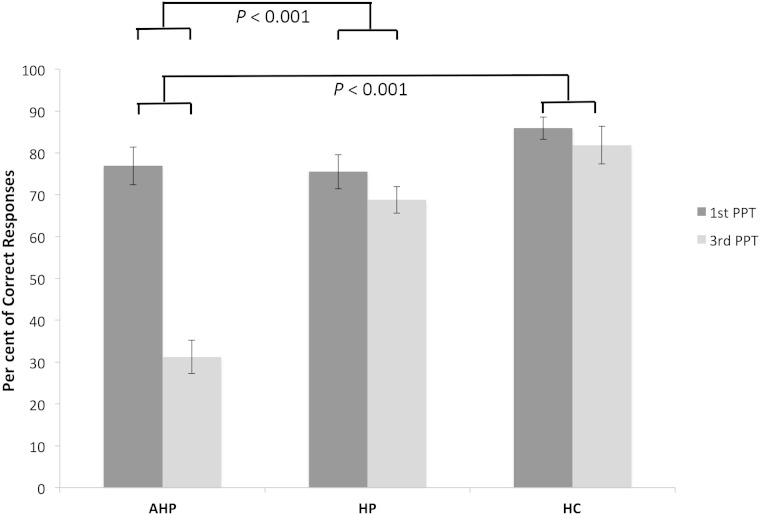
AHP patients (median = 31.25) therefore presented with a ‘deficit’ in third person perspective taking in ToM stories in the sense that they performed significantly worse on this condition than they did in the otherwise-balanced, first person perspective condition of the experiment, when compared at the group level with the performance of hemiplegia patients (median = 68.75) and healthy controls (median = 87.5) on the same two conditions (see Crawford et al. , 2003 for fully operational definitions of ‘deficits’ in neuropsychology; see Fig. 4 for illustration).
Figure 4.
Percentage of correct responses for ToM stories across groups. Means and SE’s for first person perspective taking and third person perspective taking conditions. There is no significant difference in first person perspective taking between the AHP patients and controls. However, there is a significant difference in third person perspective taking between the AHP patient and hemiplegia patients, as well as healthy controls. Means and SE’s are used here for convention and illustration purposes.
Individual patient analyses
As the above two-way results revealed that there was not a statistically significant difference between the differential scores of the two control groups, one could infer that most patients with AHP showed a ‘differential deficit’, or otherwise known, ‘classical dissociation’ (see Crawford et al. , 2003 for definitions) between the first person and third person perspective taking conditions, in relation to the hemiplegia group. However, to establish the reliability of this claim at the individual versus the group level, specialized analyses at the individual level were required as in the previous section ( Supplementary Tables 3 and Supplementary Data ).
Specifically, using the RSDT ( Garthwaite and Crawford, 2004 ; Crawford and Garthwaite, 2005 ), we found that the incidence of first person deficits in the AHP and hemiplegia groups was low and comparable (4 of 15 patients in both groups showed deficits in comparison to the healthy controls), while 15 patients with AHP versus one (score of 43.75%) patient with hemiplegia showed deficits in third person perspective in comparison to healthy controls. Twelve patients with AHP (two of those showed a trend effect) showed ‘differential deficits’ in third person versus first person perspective when compared with healthy controls, while none of the hemiplegia patients showed such differential deficits (in fact three patients showed the opposite dissociation).
When comparing between the two patient groups to examine severity of deficits, 12 patients with AHP showed a third person perspective deficit in comparison to hemiplegia controls, while none of the patients with AHP showed a first person deficit. Finally, 12 of 15 patients with AHP showed a ‘differential deficit’ (one of those showed a trend effect). Taken together these results suggest that there is a higher incidence of third person ToM ‘deficits’ and ‘differential deficits’ in patients with than without AHP and the severity of such deficits seems greater in the majority of patients with AHP, with evidence of classical dissociation in at least 11 of 15 patients with AHP.
Correlations between neuropsychological results and experimental results
Clinical unawareness and perspective taking
In the visuospatial perspective taking task, there was no significant relationship between the third person perspective taking and Feinberg awareness scores [ rs (13) = 0.17, P = 0.53] in the AHP group. In the ToM task there was a significant negative correlation between third person perspective taking and Feinberg awareness scores [ rs (13) = −0.66, P = 0.015] in the AHP group. This indicates that the more unaware the AHP patients were (i.e. the higher the Feinberg scores) the greater their impairment in the ToM task.
Neuropsychological differences and perspective taking
There were no statistically significant correlations (corrected alpha = 0.01) between proprioception scores, or neglect scores (star cancellation) and third person perspective taking in either the visuospatial perspective taking, or the ToM task in either group (all r s ’s < 0.3, P ’s > 0.4). There was a strong correlation in the Go/No-go subtest of the FAB and third person perspective taking in both the visuospatial perspective taking [ rs (13) = 0.75, P < 0.001] and ToM tasks [ rs (13) = 0.67, P = 0.001] in the AHP, but not the hemiplegia groups. This did not apply for the other two FAB subtests that the two patients groups have been found to differ between them ( Table 1 ). In sum, it appears that the worse the AHP patients’ performance in the Go/No-go subtest (i.e. inhibition/set-shifting) of executive functioning the worse their third person perspective taking ability in both experiments.
Lesion mapping results
Lesion overlay
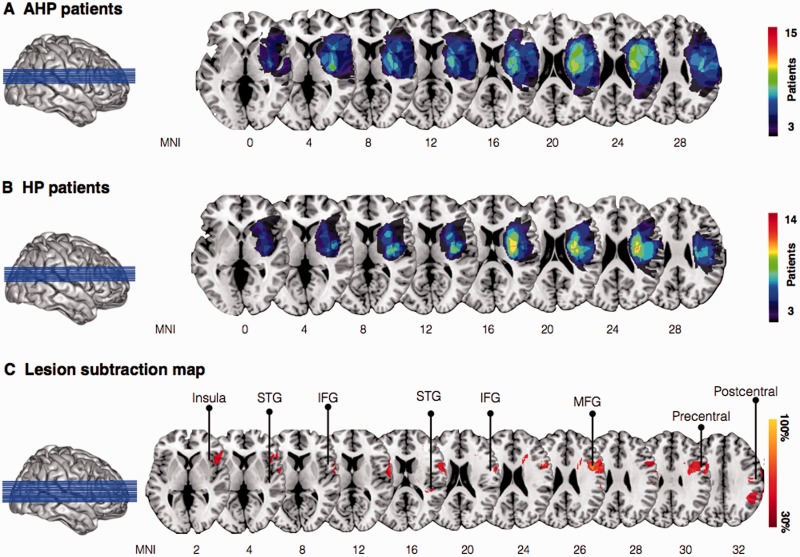
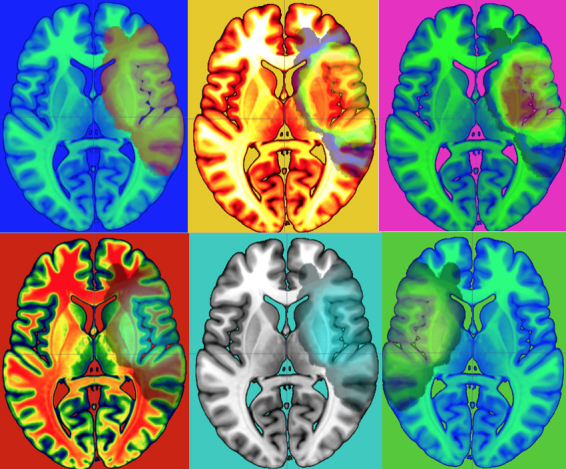
Group level percentage lesion overlay maps for the AHP group ( n = 15) identified involvement of the fronto-parietal-temporal cortices. Commonly damaged areas included the inferior and middle frontal gyri, the posterior insula ribbon, the inferior parietal lobe, and dorsal frontal white matter ( Fig. 5 A). The hemiplegia group ( n = 14) in comparison presented with a more focal damage largely involving subcortical regions with extension into surrounding fronto-parietal white matter ( Fig. 5 B). Overall lesion volume was comparable between patient groups [AHP: mean = 8.49 cm 3 , SD = 8.00; Hemiplegia: mean = 5.28 cm 3 , SD = 7.29; t (27) = −1.13, P = 0.27]. Subtraction maps identified clusters within the anterior insula ribbon, inferior frontal gyrus, middle frontal gyrus, superior temporal gyrus, and the pre- and postcentral gyri to differ between the patients groups ( Fig. 5 C).
Figure 5.
Group-level lesion overlay maps for patients with AHP and patients without anosognosia (HP). ( A ) Overlay of lesions in patients with AHP (3–15 AHP patients shown for illustration purposes). ( B ) Overlay of lesions in patients with hemiplegia (3–14 hemiplegia patients shown for illustration purposes). ( C ) Subtraction plot comparing the two populations of patients (AHP versus HP; 30% threshold is used for illustration purposes; the red percentages identify regions that are more common for AHP than HP). IFG = inferior frontal gryus; MFG = middle frontal gyrus; STG = superior temporal gyrus.
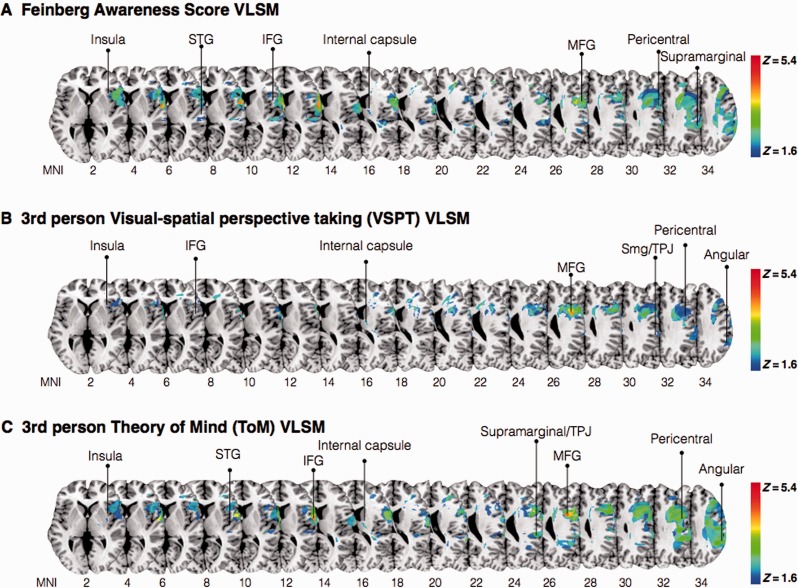
Voxel-based lesion–symptom mapping
VLSM analysis using the continuous Feinberg awareness scores showed that lesions involving voxels within the superior temporal gyrus, inferior and middle frontal gyri were significantly associated with motor unawareness, together with clusters in the anterior insula and pericentral gyri ( Figu. 6 A). VLSM analysis looking at deficits in third person perspective taking conditions (combining animate and inanimate conditions) in the visuospatial perspective taking experiment identified most significant cluster within the inferior frontal gyrus, middle frontal gyrus together with clusters within the pericentral gyri, inferior parietal lobe and dorsal frontal white matter ( Fig. 6 B). The cluster with the maximum Z ( Z = 5.6) corresponds to the inferior frontal gyrus (data not shown). The VLSM analysis for the continuous measure of ToM third person perspective taking ability returned significant voxels ( P < 0.05) in the fronto-parietal cortices including the precentral and postcentral gyrus, supramarginal gyrus and angular gyrus. The most predictive voxels were however centred within the middle frontal gyrus, inferior frontal gyrus ( Z = 5) and superior temporal gyrus ( Fig. 6 C).
Figure 6.
VLSM analysis. ( A ) Damaged MNI voxels predicting the severity of unawareness of symptom deficits (Feinberg scale, inverted, continuous measure). ( B ) Damaged MNI voxels predicting deficits in third person perspective-taking (animate and inanimate) condition(s) for visuospatial perspective taking task. ( C ) Damaged MNI voxels predicting deficits in third person perspective-taking ToM task. All measures were significant at the 5% level after 1000 permutations. IFG = inferior frontal gyrus; MFG = middle frontal gyrus; STG = superior temporal gyrus; SMG = supramarginal gyrus; TPJ = temporal-parietal junction.
Discussion
The present study used a visuospatial and a verbal (mental) perspective taking task to study perspective taking and mentalization in patients with AHP, compared to patients with right-hemisphere lesions but no AHP and healthy controls. The results partly confirmed our first prediction, in that AHP patients presented with differential deficits in third person mental (but not visuospatial) perspective taking compared to first person conditions, relative to both hemiplegic patients without AHP and healthy controls. Visuospatial perspective taking was also impaired in the AHP group, but individual analyses did not yield a clear pattern of dissociation from the hemiplegia control group. Secondly, it appears that the more severe the unawareness, the greater the deficit in mental (but not visuospatial) perspective taking. Finally, worse performance in both visuospatial and mental perspective taking was associated with executive, ‘set-shifting’ abilities. The main finding of the lesion analysis was that third person perspective taking in both visuospatial and mental perspective taking were associated with lesions to the inferior frontal gyrus, middle frontal gyrus, supramarginal gyrus, pericentral gyri as well as frontal white matter, with some of these regions being previously linked to motor awareness, inhibition and social cognition, as we discuss below.
To our knowledge this is the first experimental study to investigate the relationship between bodily self-awareness, spatial and social cognition. More importantly, our study is the first to demonstrate that patients with AHP have lost the cognitive capacity (but not necessarily its basic visuospatial aspects) to disengage from the first person perspective and take on the mental perspective of another. Furthermore, their difficulties in taking such third person perspectives and inferring other people’s mental states seemed associated with the degree of unawareness into their own deficits. These findings suggest that an accurate, mental appreciation of one’s body may require the ability to disengage from the first person perspective and reflect upon one’s body as though it was another’s. Moreover, it appears that the more fundamental visuospatial abilities that are needed to understand what another person is seeing from their own spatial perspective may not be critically related to self-awareness, in the sense that such a deficit was also frequently present in hemiplegic patients without anosognosia. Indeed, previous developmental and adult studies have shown that not all visuospatial perspective taking tasks require the ability to understand how the world is represented from another person’s perspective but may instead be accomplished by simply understanding what the other person is seeing ( Flavell et al. , 1986 ). These studies have further showed that only the first, more complex visuospatial perspective taking type is correlated with ToM deficits (for review see Pearson et al. , 2013 ).
Interestingly, patients with AHP, who typically remain anosognosic when they view their failed attempts to move their body, show dramatic improvements in body recognition and awareness when they are provided with visual feedback of their own body from third person perspectives (e.g. in mirrors; see ‘Introduction’ section). The current results suggest that without explicit, experimental suggestions or manipulations that promote third person reflection, instead of mere spatial perspective taking (see Fotopoulou et al. , 2009 for a clinical demonstration), these patients have lost their ability to mentally use such third person perspectives to inform and update their first person perspective on their bodily state in a more permanent manner.
We hypothesized that a deficit in third person mental perspective taking is causally related to anosognosia in that an ‘objective’ appreciation of one’s self, including one’s body state and abilities, entails an integration of feelings, sensations and more complex perceptual experiences registered in first and in third person perspectives. The idea that first-person perception of left-sided sensorimotor deficits is impaired in patients with AHP is intrinsic to the condition (for review see Fotopoulou, 2014 ). Indeed, as other authors and ourselves have shown in the past, the on-line, illusory experience that one is moving is experimentally associated with patients clinically diagnosed with AHP and it can be explained by neurocognitive processes involved in sensorimotor control ( Berti et al. , 2005 ; Fotopoulou et al. , 2008 ; Garbarini et al. , 2012 ). It appears, however, that such deficits may not be sufficient to explain the full clinical presentation of anosognosia (for extensive discussions see Fotopoulou et al. , 2010 ; Fotopoulou, 2014 ). Indeed, patients with AHP do not express mere uncertainty regarding the perception of sensations or movement from the left limbs, nor do they just complain of movement illusions. They instead ignore the wealth of evidence that they are paralysed (e.g. their disabilities, occasional accidents, others’ feedback) and adhere to the ‘delusional’ belief that they have functional limbs. The explanation of the latter belief (instead of the explanation of their illusory or non-veridical awareness, see above) requires the postulation of another dysfunction that prevents sensorimotor and other failures from being re-represented at a higher level of cognitive self-representation and integrated with more abstract, ‘objectified’ knowledge about the self and the world. We propose that such a deficit may be the selective deficit in third person mentalization and its associated inability to disengage from the first person perspective. This interpretation is consistent with a plethora of developmental, clinical and social psychology studies that have long shown that how we perceive or imagine the bodily self to be from the perspective of other people (e.g. as in physical mirrors, or during social mirroring) is critical for the development and maintenance of a coherent and ‘healthy’ self-awareness ( Fonagy and Target, 1997 ; Rochat, 2009 ).
Moreover, this kind of ‘third person’ reflection on the body has typically been described as ‘self-objectification’ in other fields, and mismatches between the first person bodily experiences and third person objectifications of the body seem to partially underlie symptom formation in several psychopathologies, such as eating disorders and schizophrenia ( Langdon and Coltheart, 2001 ; Russell et al. , 2009 ). There is also now increased understanding of the relation between self-awareness difficulties and mentalizing impairments in these disorders ( Tchanturia et al. , 2004 ; Langdon et al. , 2006 ; Russell et al. , 2009 ). Our study is the first to examine the relationship between anosognosia, a neurological disorder of first person body awareness, and impaired perspective taking and mentalizing. Moreover, our VLSM analysis revealed that third person perspective taking difficulties in these patients were selectively associated with anatomical areas that have been previously identified as part of a ‘mentalizing network’ ( Koster-Hale and Saxe, 2013 ), specifically the right supramarginal gyrus (i.e. temporo-parietal junction) and superior temporal gyrus.
Moreover, our results suggest a relation between frontal inhibition and mental perspective taking. Although this debate lies beyond the scope of this paper, there is evidence in the literature supporting a strong association between executive function and ToM ( Ozonoff and McEvoy, 1994 ; Sabbagh et al. , 2006 ), yet the exact nature of this relationship is still unclear. Previous lesion-based studies have proposed that the right inferior frontal gyrus plays a significant role in set-shifting and inhibition, which is required for the suppression of the self-perspective ( Samson et al. , 2005 ; Griffin et al. , 2006 ). In comparison, our lesion results highlight the inferior and middle frontal gyri as having the highest association with deficits in third person perspective taking in both experimental tasks. Additionally, the right inferior frontal gyrus ( Uddin et al. , 2005 , 2007 ) has been implicated in facilitating the distinction between self and other mental states ( Ruby and Decety, 2001 ) through attentional systems. Consequently, it has been proposed that both the ventral and dorsal attentional systems act together through the middle frontal gyrus, linking attentional and mentalizing functions to process first and third person mental states ( Abu-Akel and Shamay-Tsoory, 2011 ). Damage to the inferior and middle frontal gyri may therefore compromise this capacity to spontaneously shift between perspectives ( Furlanetto et al. , 2013 ; Bradford et al. , 2015 ).
Limitations and future direction
The current study has shown that patients with AHP present with differential deficits in mental (but not visuospatial) perspective taking; however, future studies could explore visuospatial difficulties in greater samples and using more difficult tasks that may reveal further differences patients and controls. Moreover, future studies with larger samples could investigate such factors in groups fully balanced for neuropsychological performance, or with statistical tests allowing for co-variation of neuropsychological performance. It would also be of interest to investigate such deficits in direct relation to disability-related material to specify the current findings. Moreover, our results suggest that perspective taking (mental but not visuospatial), rather than mentalization was the critical deficit in this population (in the sense that they could perform the ToM task in the first person perspective and they passed easier false belief tasks). However, the precise relation between these abilities (mental perspective taking and mentalizing or ToM) goes beyond the scope of this paper and could be addressed in future studies. Furthermore, future studies should include a left-hemisphere damage patient group allowing for greater interpretation regarding laterality. Additionally, future studies should use more comprehensive executive tests to measure neuropsychological deficits in executive function with greater specificity. However, in working with acute brain damaged patients all experimental measures, as well as neuropsychological test, must be adapted and selected to accommodate patients’ needs and apply with bedside testing. Lastly, it is important to recognize that interpretation of the neuroanatomical correlates are limited by our small sample size and inherit limitations to our lesion mapping approach ( Rorden et al. , 2007 ; Geva et al. , 2012 ; Volle et al. , 2012 ). Nevertheless, all previous lesion mapping studies in AHP are subject to comparable limitations, with our study being one of the few that has directly compared experimental scores with lesion data. Future studies will have to use better structural lesion data and functional MRI paradigms to be able to more accurately identify brain areas related to AHP and its association with experimental measures.
Supplementary Material
Acknowledgements
We thank the patients and their relatives for their kindness and willingness to take part in the study, and to the staff and PACS office at participating hospital sites for their kind assistance with this study. We are also very grateful to Lindsey Bell for initial contributions with experimental design as well as Cristina Papadaki and Laura Crucianelli for help with patient recruitment and testing. No conflicts of interest were reported.
Funding
This work was funded by an European Research Council (ERC) Starting Investigator Award for the project ‘The Bodily Self’ N°313755 to A.F., a Neuropsychoanalysis Foundation Fellowship to P.M.J., and a Commonwealth Scholarship, an Oppenheimer Memorial Trust Fellowship, and a Neuropsychology International Fellowship Award from the British Psychological Society in conjunction with the British Neuropsychological Society to S.B.
Glossary
Abbreviations
- AHP
anosognosia for hemiplegia
- ToM
Theory of Mind
- VLSM
voxel-based lesion–symptom mapping
Supplementary material
Supplementary material is available at Brain online.
References
- Abu-Akel A, Shamay-Tsoory S . Neuroanatomical and neurochemical bases of theory of mind . Neuropsychologia 2011. ; 49 : 2971 – 84 . [DOI] [PubMed] [Google Scholar]
- Aichhorn M, Perner J, Weiss B, Kronbichler M, Staffen W, Ladurner G . Temporo-parietal junction activity in theory-of-mind tasks: falseness, beliefs, or attention . J Cogn Neurosci 2009. ; 21 : 1179 – 92 . [DOI] [PubMed] [Google Scholar]
- Baldo JV, Wilson SM, Dronkers NF . Uncovering the neural substrates of language: a voxel-based lesion symptom mapping approach. Advances in the Neural Substrates of Language: Toward a Synthesis of Basic Science and Clinical Research. Oxford: Wiley-Blackwell; 2012 . [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U . Does the autistic child have a “theory of mind”? Cognition 1985. ; 21 : 37 – 46 . [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. . Voxel-based lesion-symptom mapping . Nat Neurosci 2003. ; 6 : 448 – 50 . [DOI] [PubMed] [Google Scholar]
- Berti A, Bottini G, Gandola M, Pia L, Smania N, Stracciari A, et al. . Shared cortical anatomy for motor awareness and motor control . Science 2005. ; 309 : 488 – 91 . [DOI] [PubMed] [Google Scholar]
- Berti A, Làdavas E, Corte MD . Anosognosia for hemiplegia, neglect dyslexia, and drawing neglect: clinical findings and theoretical considerations . J Int Neuropsychol Soc 1996. ; 2 : 426 – 40 . [DOI] [PubMed] [Google Scholar]
- Besharati S, Kopelman M, Avesani R, Moro V, Fotopoulou AK . Another perspective on anosognosia: self-observation in video replay improves motor awareness . Neuropsychol Rehabil 2014a. ; 25 : 319 – 52 . [DOI] [PubMed] [Google Scholar]
- Besharati S, Forkel SJ, Kopelman M, Solms M, Jenkinson PM, Fotopoulou A . The affective modulation of motor awareness in anosognosia for hemiplegia: behavioural and lesion evidence . Cortex 2014b. ; 61 : 127 – 40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisiach E, Vallar G, Perani D, Papagno C, Berti A . Unawareness of disease following lesions of the right hemisphere: anosognosia for hemiplegia and anosognosia for hemianopia . Neuropsychologia 1986. ; 24 : 471 – 82 . [DOI] [PubMed] [Google Scholar]
- Blanke O, Landis T, Spinelli L, Seeck M . Out-of-body experience and autoscopy of neurological origin . Brain 2004. ; 127 : 243 – 58 . [DOI] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M . Neuropsychology: stimulating illusory own-body perceptions . Nature 2002. ; 419 : 269 – 70 . [DOI] [PubMed] [Google Scholar]
- Bradford EE, Jentzsch I, Gomez JC . From self to social cognition: theory of mind mechanisms and their relation to executive functioning . Cognition 2015. ; 138 : 21 – 34 . [DOI] [PubMed] [Google Scholar]
- Corradi-Dell’Acqua C, Ueno K, Ogawa A, Cheng K, Rumiati RI, Iriki A . Effects of shifting perspective of the self: an fMRI study . Neuroimage 2008. ; 40 : 1902 – 11 . [DOI] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH . Testing for suspected impairments and dissociations in single-case studies in neuropsychology: evaluation of alternatives using Monte Carlo simulations and revised tests for dissociations . Neuropsychology 2005. ; 19 : 318 – 31 . [DOI] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH, Porter S . Point and interval estimates of effect sizes for the case-controls design in neuropsychology: rationale, methods, implementations, and proposed reporting standards . Cogn Neuropsychol 2010. ; 27 : 245 – 60 . [DOI] [PubMed] [Google Scholar]
- Crawford JR, Gray CD, Garthwaite PH . Wanted: fully operational definitions of dissociations in single-case studies . Cortex 2003. ; 39 : 357 – 70 . [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B . The FAB-A frontal assessment battery at beside . Neurology 2000. ; 55 : 1621 – 6 . [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, et al. . Assignment of functional activations to probabilistic cytoarchitectonic areas revisited . Neuroimage 2007. ; 36 : 511 – 21 . [DOI] [PubMed] [Google Scholar]
- Feinberg TE, Roane DM, Ali J . Illusory limb movements in anosognosia for hemplegia . J Neurol Neurosurg Psychiatry 2000. ; 69 : 511 – 13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell JH, Green FL, Flavell ER, Watson MW, Campione JC . Development of knowledge about the appearance-reality distinction . Monogr Soc Res Child Dev 1986. ; 51 : i–v, 1–87 . [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR . “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician . J Psychiatr Res 1975. ; 12 : 89 – 198 . [DOI] [PubMed] [Google Scholar]
- Fonagy P, Target M . Attachment and reflective function: their role in self-organization . Dev Psychopathol 1997. ; 9 : 679 – 700 . [DOI] [PubMed] [Google Scholar]
- Fotopoulou A . The virtual bodily self: mentalisation of the body as revealed in anosognosia for hemiplegia . Conscious Cogn 2014. ; 33 : 1 – 11 . [DOI] [PubMed] [Google Scholar]
- Fotopoulou A, Pernigo S, Maeda R, Rudd A, Kopelman MA . Implicit awareness in anosognosia for hemiplegia: unconscious interference without conscious re-representation . Brain 2010. ; 133 : 3564 – 77 . [DOI] [PubMed] [Google Scholar]
- Fotopoulou A, Rudd A, Holmes P, Kopelman M . Self-observation reinstates motor awareness in anosognosia for hemiplegia . Neuropsychologia 2009. ; 47 : 1256 – 60 . [DOI] [PubMed] [Google Scholar]
- Fotopoulou A, Conway MA, Solms M, Tyrer S, Kopelman M . Self-serving confabulation in prose recall . Neuropsychologia 2008. ; 46 : 1429 – 41 . [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U . Social cognition in humans . Curr Biol 2007. ; 17 : 724 – 32 . [DOI] [PubMed] [Google Scholar]
- Furlanetto T, Cavallo A, Manera V, Tversky B, Becchio C . Through your eyes: incongruence of gaze and action increases spontaneous perspective taking . Front Hum Neurosci 2013. ; 7 : 455 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD . Functional imaging of “theory of mind .” Trends Cogn Sci 2003. ; 7 : 77 – 83 . [DOI] [PubMed] [Google Scholar]
- Garbarini F, Rabuffetti M, Piedimonte A, Pia L, Ferrarin M, Frassinetti F, Berti A . ‘Moving’ a paralysed hand: bimanual coupling effect in patients with anosognosia for hemiplegia . Brain 2012. ; 135 : 1486 – 97 . [DOI] [PubMed] [Google Scholar]
- Garthwaite PH, Crawford JR . The distribution of the difference between two t -variates . Biometrika 2004. ; 91 : 987 – 94 . [Google Scholar]
- Geva S, Baron JC, Jones PS, Price CJ, Warburton EA . A comparison of VLSM and VBM in a cohort of patients with post-stroke aphasia . NeuroImage 2012. ; 1 : 37 – 47 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopnik A, Astington JW . Children's understanding of representational change and its relation to the understanding of false belief and the appearance-reality distinction . Child Dev 1988. ; 59 : 26 – 37 . [DOI] [PubMed] [Google Scholar]
- Griffin R, Friedman O, Ween J, Winner E, Happé F, Brownell H . Theory of mind and the right cerebral hemisphere: refining the scope of impairment . Laterality 2006. : 11 ; 195 – 225 . [DOI] [PubMed] [Google Scholar]
- Guarantors of Brain. Aids to the examination of the peripheral nervous system. London: W.B. Saunders; 1986.
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC . Enhancement of MR images using registration for signal averaging . J Comput Assist Tomogr 1998. ; 22 : 324 – 33 . [DOI] [PubMed] [Google Scholar]
- Hynes CA, Baird AA, Grafton ST . Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking . Neuropsychologia 2006. ; 44 : 374 – 83 . [DOI] [PubMed] [Google Scholar]
- Jenkinson PM, Haggard P, Ferreira NC, Fotopoulou A . (2013) . Body ownership and attention in the mirror: insights from somatoparaphrenia and the rubber hand illusion . Neuropsychologia 2013. ; 51 : 1453 – 62 . [DOI] [PubMed] [Google Scholar]
- Karnath HO, Baier B, Nägele T . Awareness of the functioning of one’s own limbs mediated by the insular cortex? J Neurosci 2005. ; 25 : 7134 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortte KB, McWhorter JW, Pawlak MA, Slentz J, Sur S, Hillis AE . Anosognosia for Hemiplegia: the contributory role of right inferior frontal Gyrus . Neuropyshcology 2015. ; 29 : 421 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster-Hale J, Saxe R . Theory of mind: a neural prediction problem . Neuron 2013. ; 79 : 836 – 48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon R, Coltheart M . Visual perspective-taking and schizotypy: evidence for a simulation-based account of mentalizing in normal adults . Cognition 2001. ; 82 : 1 – 26 . [DOI] [PubMed] [Google Scholar]
- Langdon R, Corner T, McLaren J, Ward PB, Coltheart M . Externalizing and personalizing biases in persecutory delusions: the relationship with poor insight and theory-of-mind . Behav Res Ther 2006. ; 44 : 699 – 713 . [DOI] [PubMed] [Google Scholar]
- Lieberman MD . Social cognitive neuroscience: a review of core processes . Ann Rev Psychol 2007. ; 58 : 259 – 89 . [DOI] [PubMed] [Google Scholar]
- Marcel AJ, Tegnér R, Nimmo-Smith I . Anosognosia for plegia: specificity, extension, partiality and disunity of bodily unawareness . Cortex 2004. ; 40 : 19 – 40 . [DOI] [PubMed] [Google Scholar]
- Mcintosh RD, Brodie EE, Beschin N, Robertson IH . Imporving the clinical diagnosis of personal neglect: a reformulated comb and razor test . Cortex 2000. ; 4 : 289 – 92 . [DOI] [PubMed] [Google Scholar]
- Moro V, Pernigo S, Zapparoli P, Cordioli Z, Aglioti SM . Phenomenology and neural correlates of implicit and emergent motor awareness in patients with anosognosia for hemiplegia . Behav Brain Res 2011. ; 225 : 259 – 69 . [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. . The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment . J Am Geriatr Soc 2005. ; 53 : 695 – 99 . [DOI] [PubMed] [Google Scholar]
- Neuburger S, Jansen P, Heil M, Quaiser-Pohl C . Gender differences in pre-adolescents’ mental-rotation performance: do they depend on grade and stimulus type? Pers Individ Dif 2011. ; 50 : 1238 – 42 . [Google Scholar]
- Ozonoff S, McEvoy RE . A longitudinal study of executive function and theory of mind development in autism . Dev Psychopathol 1994. ; 6 : 415 – 31 . [Google Scholar]
- Pearson A, Ropar D, Hamilton A . A review of visual perspective taking in autism spectrum disorder . Frontiers in human neuroscience 2013. ; 7 : 652 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS . Anosognosia in parietal lobe syndrome . Conscious Cogn 1995. ; 4 : 22 – 51 . [DOI] [PubMed] [Google Scholar]
- Rochat P . Others in mind: social origins of self-consciousness . New York: : Cambridge University Press; ; 2009. . [Google Scholar]
- Rorden C, Karnath HO, Bonilha L . Improving lesion-symptom mapping . J Cogn Neurosci 2007. ; 19 : 1081 – 8 . [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H . Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci 2004. ; 5 : 3 – 9 . [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J . Effect of subjective perspective taking during simulation of action: a PET investigation of agency . Nat Neurosci 2001. ; 4 : 546 – 50 . [DOI] [PubMed] [Google Scholar]
- Russell TA, Schmidt U, Doherty L, Young V, Tchanturia K . Aspects of social cognition in anorexia nervosa: affective and cognitive theory of mind . Psychiatry Res 2009. ; 168 : 181 – 5 . [DOI] [PubMed] [Google Scholar]
- Sabbagh MA, Xu F, Carlson SM, Moses LJ, Lee K . The development of executive functioning and theory of mind a comparison of Chinese and US preschoolers . Psychol Sci 2006. ; 17 : 74 – 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Kathirgamanathan U, Humphreys GW . Seeing it my way: a case of a selective deficit in inhibiting self-perspective . Brain 2005. ; 128 : 1102 – 11 . [DOI] [PubMed] [Google Scholar]
- Shallice T, Evans ME . The involvement of the frontal lobes in cognitive estimation . Cortex 1978. ; 14 : 294 – 303 . [DOI] [PubMed] [Google Scholar]
- Siegal M, Varley R . Neural systems involved in “theory of mind ”. Nat Rev Neurosci 2002. ; 3 : 463 – 71 . [DOI] [PubMed] [Google Scholar]
- Tchanturia K, Happé F . (2004) . “Theory of mind”in anorexia nervosa . Eur Eat 2004. ; 366 : 361 – 66 . [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP . The self and social cognition: the role of cortical midline structures and mirror neurons . Trends Cogn Sci 2007. ; 11 : 153 – 7 . [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kaplan JT, Molnar-Szakacs I, Zaidel E, Iacoboni M . Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: an event-related fMRI study . NeuroImage 2005. ; 25 : 926 – 35 . [DOI] [PubMed] [Google Scholar]
- Vandenberg SG, Kuse AR . Mental rotations, a group test of three-dimensional spatial visualization . Percept Mot Skills 1978. ; 47 : 599 – 604 . [DOI] [PubMed] [Google Scholar]
- Vocat R, Staub F, Stroppini T, Vuilleumier P . Anosognosia for hemiplegia: a clinical-anatomical prospective study . Brain 2010. ; 133 : 3578 – 97 . [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happé F, Falkai P, et al. . Mind reading: neural mechanisms of theory of mind and self-perspective . NeuroImage 2001. ; 14 : 170 – 81 . [DOI] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR . Neural correlates of first-person perspective as one constituent of human self-consciousness . J Cogn Neurosci 2004. ; 16 : 817 – 27 . [DOI] [PubMed] [Google Scholar]
- Volle E, de Lacy Costello A, Coates LM, McGuire C, Towgood K, Gilbert S, et al. . Dissociation between verbal response initiation and suppression after prefrontal lesions . Cereb Cortex 2012. ; 22 : 2428 – 40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D . WAIS-III: administration and scoring manual: Wechsler adult intelligence scale . San Antonio, TX: Psychological Corporation ; 1997. . [Google Scholar]
- Wechsler D . Wechsler test of adult reading: WTAR . San Antonio, TX: Psychological Corporation ; 2001. . [Google Scholar]
- Wilson BA, Cockburn J, Halligan PW . Behavioural Inattention Test (BIT). Bury St Edmunds: Thames Valley Test Company; 1987 . [Google Scholar]
- Zigmond AS, Snaith RP . The hospital anxiety and depression scale . Acta Psychiatr Scand 1983. ; 67 : 361 – 70 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.