Abstract
Background. Sepsis is a major cause of morbidity and mortality among older adults. The main goals of this study were to assess the association of sepsis at intensive care unit (ICU) admission with mortality and to identify predictors associated with increased mortality in older adults.
Methods. We conducted a prospective cohort study of 309 participants ≥60 years admitted to an ICU. Sepsis was defined as 2 of 4 systemic inflammatory response syndrome criteria plus a documented infection within 2 calendar days before or after admission. The main outcome measure was time to death within 1 year of ICU admission. Sepsis was evaluated as a predictor for mortality in a Cox proportional hazards model.
Results. Of 309 participants, 196 (63%) met the definition of sepsis. Among those admitted with and without sepsis, 75 (38%) vs 20 (18%) died within 1 month of ICU admission (P < .001) and 117 (60%) vs 48 (42%) died within 1 year (P < .001). When adjusting for baseline characteristics, sepsis had a significant impact on mortality (hazard ratio [HR] = 1.80; 95% confidence interval [CI], 1.28–2.52; P < .001); however, after adjusting for baseline characteristics and process covariates (antimicrobials and vasopressor use within 48 hours of admission), the impact of sepsis on mortality became nonsignificant (HR = 1.26; 95% CI, .87–1.84; P = .22).
Conclusions. The diagnosis of sepsis in older adults upon ICU admission was associated with an increase in mortality compared with those admitted without sepsis. After controlling for early use of antimicrobials and vasopressors for treatment, the association of sepsis with mortality was reduced.
Keywords: functional status, ICU, older adults, outcomes, sepsis
Sepsis is a systemic inflammatory host response to infection that can lead to acute organ dysfunction [1]. It is estimated that more than 750 000 adults in the United States develop severe sepsis each year, and >50% of these cases require intensive care unit (ICU) level care [2].
Older adults have higher rates of sepsis compared with younger adults and are more likely to die from sepsis [2]. Although age has been found in few studies to be an independent predictor of mortality in patients with sepsis [3], other research suggests that factors such as comorbidities and functional status impact long-term outcomes more than age [4].
Over the past several decades, evidence-based guidelines have been developed to address the high mortality associated with sepsis by standardizing care [5]. Although these guidelines have led to improvements in morbidity and mortality for adults admitted with sepsis [6], it is unclear whether they apply specifically to older adults [7]. As our population ages, the incidence of older adults admitted with sepsis is expected to increase, making our understanding of how sepsis impacts mortality and functional status in this population increasingly important. Furthermore, additional assessments on how interventions recommended by current guidelines impact outcomes in older adults are needed.
The primary aims of this study were to (1) describe the clinical characteristics of older adults admitted to an ICU with and without sepsis; (2) assess the association of sepsis at ICU admission with mortality; and (3) identify predictors associated with increased mortality in adults aged ≥60 years admitted with sepsis. We also describe changes in functional status in older survivors of sepsis at 1-month post-ICU-discharge.
METHODS
The study participants were ≥60 years and admitted to the medical ICU (28 beds) at Yale-New Haven Hospital [8]. Informed consent was obtained from the proxy and patient according to procedures by the Yale University Human Investigation Committee, who approved this study.
Data Collection
This was a prospective study conducted from 2002 to 2004. Details of the initial data collection have been previously described [8]. Proxies were the primary source of baseline information due to critical illness in participants at the time of ICU admission. If a proxy was not available, the patient was excluded from the study [9]. Baseline demographic characteristics, admitting diagnosis, chronic medical conditions, Charlson Comorbidity Index [10], Acute Physiology and Chronic Health Evaluation II (APACHE) score [11], and medication usage were obtained by chart review at time of ICU admission. Functional status before hospitalization was assessed with the Katz [12] activities of daily living (ADL) scale and with Lawton's [13] instrumental activities of daily living (IADL) scale. Both participants and proxies provided ADL information posthospitalization through interviews with experienced research nurses [8, 14]. After the study was completed, a retrospective chart review was subsequently performed to abstract data (ie, clinical signs and symptoms of infection, laboratory testing, microbiologic cultures, and imaging) to diagnose sepsis.
The primary predictor variable was sepsis at baseline. We defined sepsis as patients meeting 2 of 4 criteria for systemic inflammatory response syndrome (SIRS): (1) temperature >38.3 or <36°C; (2) heart rate >90 beats/minute; (3) respiratory rate >20 breaths/minute or PaCO2 <32 mmHg; (4) white blood cell count >12 000 cells/mm3, plus at least 1 documented infection [1, 5, 15] within 2 calendar days before and/or 2 calendar days after admission to the medical ICU to capture all patients with sepsis upon admission to the ICU [16, 17]. Documented infections in all participants were defined using the Centers for Disease Control and Prevention/National Health Surveillance (CDC/NHSN) definitions for healthcare-associated infections (HAI) [18]. Two physicians performed chart review and adjudicated all documented infections. If there was a disagreement in diagnosis of infection, the chart was rereviewed until both physicians agreed.
Main Outcome Measure
The main outcome measure was time to death within 1 year after ICU admission in participants admitted with an adjudicated diagnosis of sepsis. Study participants were censored at 365 days after ICU admission if they were alive at that time. Our comparison group was participants admitted to the ICU with a diagnosis of any condition other than sepsis. We also measured change in functional status, as determined by any change in count of ADLs and IADLs at 1 month after ICU discharge compared with baseline functional status before ICU admission.
Definition of Variables
Baseline characteristics included age, gender, race, and Charlson Comorbidity Index. Admission to ICU from medical floor was included, because previous studies have suggested worse outcomes in patients admitted from a medical floor compared with admission directly from an emergency room [19]. Control variables were identified based on clinical experience and prior literature review. Process variables included use of antimicrobials [20] and use of vasopressors, because these variables have been shown to impact outcomes in sepsis [5]. We defined antimicrobial and vasopressor use as any dose of antimicrobial or vasopressor given within 48 hours of admission to the ICU. Activities of daily living and IADLs were assessed at baseline and 1 month post-ICU discharge using an ordinal scale ranging from (1) help, (2) unable to do, and (3) no help. When describing change in functional status, we combined the first and second categories and compared them with the third category.
Statistical Analysis
Baseline demographic and admission characteristics were summarized overall and by (1) sepsis status, (2) means and standard deviations, (3) medians and interquartile ranges, and (4) counts and percentages. Significance tests for differences in these variables across the levels of the sepsis primary predictor were performed using t tests or Wilcoxon rank-sum test for continuous and count variables and Pearson χ2 test or Fisher's exact test for categorical variables. Percentages are also reported in graphical context for categories of documented infections and for organisms identified in sputum by culture or direct fluorescent-antibody by nasopharyngeal (DFA/NP) testing, urine cultures, and in blood cultures.
Three Cox proportional hazards models were fit in which sepsis at ICU admission was the primary predictor and death during 1 year of follow-up after ICU admission was the outcome. An unadjusted Cox model was fit with only sepsis as an explanatory variable; a second model was fit with the addition of several baseline admission covariates; and a third model was fit with the addition of 3 process covariates (admitted from floor, antimicrobials, and vasopressors within 48 hours of admission). All covariates were selected on clinical grounds. Study participants who were lost to follow-up or were alive at the end of the 1-year follow-up period were censored. A significance level of .05 was used in the interpretation of 2-sided significance tests. In testing the proportional hazards assumption, however, a significance level of .01 was used because of multiple tests of the same null hypothesis. Model goodness of fit was assessed with residual analyses and influence diagnostics. SAS software (version 9.4) was used in all analyses.
RESULTS
Baseline characteristics of the 309 participants are listed in Table 1. Of the 196 participants with sepsis (63% of the study cohort), 30 (15%) met 2 SIRS criteria, 72 (36%) met 3 SIRS criteria, and 94 (48%) met all 4 SIRS criteria. One hundred seventy-six (90%) participants diagnosed with sepsis received antimicrobials within 48 hours of ICU admission, and 84 (43%) received vasopressor support within 48 hours. Table 2 represents the unadjusted and adjusted survival models for the sepsis predictor. When adjusting for baseline admission covariates including age, race, sex, and Charlson comorbidity score, sepsis had a significant association with mortality (HR = 1.80; 95% CI, 1.28–2.52; P < .001). After adjusting for baseline characteristics and process covariates (ie, use of antimicrobials and/or vasopressors within 48 hours of admission, and admission from the medical floor), the association of sepsis with survival became nonsignificant (HR = 1.26; 95% CI, .87–1.84; P = .22). Use of antimicrobials and vasopressors within 48 hours together were responsible for almost all of the reduction in association for the sepsis variable (HR = 1.28; 95% CI, .88–1.85; P = .20). Inclusion of admission from the floor in the multivariable model did not appear to significantly reduce the association of sepsis with mortality.
Table 1.
Participant Characteristicsa
| Baseline Demographics | Overall Cohort N = 309 |
Sepsis N = 196 |
No Sepsis N = 113 |
P Value |
|---|---|---|---|---|
| Age in years, mean (SD) | 74.7 (8.5) | 74.6 (8.3) | 74.9 (8.8) | .76 |
| Male gender, n (%) | 145 (47) | 96 (49) | 49 (43) | .34 |
| Nonwhite race, n (%) | 51 (17) | 28 (14) | 23 (20) | .17 |
| Admission | ||||
| Admitted from ER, n (%) | 205 (66) | 123 (63) | 82 (73) | .08 |
| Admitted from floor, n (%) | 97 (31) | 70 (36) | 27 (24) | .03 |
| Admitted from other location, n (%) | 7 (2) | 3 (2) | 4 (4) | .26 |
| Location Before Admission | ||||
| Admitted from nursing home, n (%) | 55 (18) | 38 (19) | 17 (15) | .34 |
| Admitted from home, n (%) | 241 (78) | 148 (76) | 93 (82) | .17 |
| Admitted from other, n (%) | 13 (4) | 10 (5) | 3 (3) | .39 |
| Baseline Medical Status | ||||
| Dementia by IQCODE score, n (%) | 95 (31) | 65 (33) | 30 (27) | .25 |
| History of depression | 85 (28) | 59 (30) | 26 (23) | .18 |
| Charlson Comorbidity Index score, median (IQR) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | .79 |
| APACHE II score, mean (SD) | 23.5 (6.4) | 24.8 (6.3) | 21.2 (6.0) | <.001 |
| Any impairment in activities of daily living, 7-item, n (%) | 115 (37) | 75 (38) | 40 (35) | .62 |
| Any impairment in instrumental activities of daily living, 7-item, n (%) | 264 (85) | 169 (86) | 95 (84) | .61 |
| Full code status on admission, n (%) | 265 (86) | 172 (88) | 93 (82) | .19 |
| Total no. ICU delirium days, median (IQR) | 3 (1, 7) | 4 (2, 9) | 1 (0, 3) | <.001 |
| Admission Diagnosis (ICD-9 Code) | ||||
| Respiratory, n (%) | 156 (50) | 115 (59) | 41 (36) | <.001 |
| Gastrointestinal hemorrhage, n (%) | 52 (17) | 19 (10) | 33 (29) | <.001 |
| Sepsisb, n (%) | 51 (17) | 40 (20) | 11 (10) | .01 |
| Neurologic, n (%) | 5 (2) | 1 (1) | 4 (4) | .06 |
| Other causes, n (%) | 45 (15) | 21 (11) | 24 (21) | .01 |
| Admitting Physiologic Variables | ||||
| Temp >38°C or <36°C | 215 (70) | 144 (73) | 71 (63) | .05 |
| Heart rate >90 beats/min | 253 (82) | 172 (88) | 81 (72) | <.001 |
| Respiratory rate >20 breaths/min or PaCO2 <32 mmHG | 282 (91) | 187 (95) | 95 (84) | <.001 |
| WBC >12 000 cells/mm3 or <4000 cells/mm3 | 196 (63) | 149 (76) | 47 (42) | <.001 |
| Mean arterial pressure mmHg, mean (SD) | 59.8 (15.1) | 57.5 (13.4) | 63.9 (17.0) | .001 |
| Medications | ||||
| Received antimicrobialsc within 48 h | 241 (78) | 176 (90) | 65 (58) | <.001 |
| Received vasopressor support within 48 h | 101 (33) | 84 (43) | 17 (15) | <.001 |
| Documented Infection | ||||
| No. of patients with at least 1 documented infection | 200 (65) | 196 (100) | 4 (4) | <.001 |
| Outcome | ||||
| Death within 1 month | 95 (31) | 75 (38) | 20 (18) | <.001 |
| Death within 1 year | 165 (53) | 117 (60) | 48 (42) | .004 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICD-9, International Classification of Diseases, Ninth Revision; ICU, intensive care unit; ER, emergency room; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; IQR, interquartile range; SD, standard deviation; Temp, temperature; WBC, white blood cells.
a Missing data: Charlson Comorbidity Index Score (n = 1), education (n = 9), dementia by IQCODE score (n = 3), medications (n = 1). For categorical variables, χ2 or Fisher's test exact was used as appropriate. For continuous variables, t test or Wilcoxon test was used as appropriate.
b Diagnosis of sepsis based on ICD-9 Code.
c Antimicrobials included the following medications: acyclovir, amikacin, amoxicillin, amoxicillin-clavulanate, amphotericin B, ampicillin, ampicillin-sulbactam, aztreonam, azithromycin, cefazolin, ceftazidime, cefuroxime, ceftazidime, ceftriaxone, cephalexin, ciprofloxacin, clarithromycin, clindamycin, dapsone, diflucan, doxycycline, erythromycin, ethambutol, flagyl, ganciclovir, gatifloxacin, gentamicin, imipenem, isoniazid, meropenem, moxifloxacin, nafcillin, penicillin, piperacillin-tazobactam, pyrazinamide, rifampin, tobramycin, trimethoprim-sulfamethoxazole, ticarcillin-clavulanate, valganciclovir, vancomycin, voriconazole. The most common antimicrobial administered overall in this cohort was piperacillin-tazobactam followed by vancomycin.
Table 2.
Sepsis as a Predictor of Mortality Upon Admission to a Medical Intensive Care Unita
| Outcome | Regression Model |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Cox Proportional Hazards Model (N = 309) |
Cox Proportional Hazards Model Adjusted for Baseline Admission Covariates (N = 308)b |
Cox Proportional Hazards Model Adjusted for Baseline Admission and Process Covariates (N = 307) |
|||||||
| Hazard Ratio | 95% Confidence Interval | P Value | Hazard Ratio | 95% Confidence Interval | P Value | Hazard Ratio | 95% Confidence Interval | P Value | |
| Sepsis (yes vs no) | 1.76 | 1.26–2.46 | .001 | 1.80 | 1.28–2.52 | <.001 | 1.26 | .87–1.84 | .22 |
| Age (continuous) | 1.04 | 1.02–1.06 | <.001 | 1.04 | 1.02–1.06 | <.001 | |||
| Race (non-white vs white) | 1.05 | .68–1.62 | .83 | 1.11 | .71–1.71 | .65 | |||
| Sex (male vs female) | 1.16 | .85–1.58 | .34 | 1.27 | .93–1.74 | .13 | |||
| Charlson Comorbidity Index (continuous) | 1.14 | 1.06–1.22 | <.001 | 1.14 | 1.06–1.22 | <.001 | |||
| Admitted from floor (yes vs no) | 1.57 | 1.13–2.16 | .006 | ||||||
| Antimicrobials by 48 h (yes vs no) | 1.93 | 1.17–3.17 | .01 | ||||||
| Vasopressor by 48 h (yes vs no) | 1.36 | .97–1.91 | .08 | ||||||
Additional sepsis results upon adding each individual process variable to the adjusted for baseline characteristics cox proportional hazards model.
Abbreviations: HR, hazard ratio.
a Sepsis results upon adding process variable to the adjusted cox proportional hazards model.
b Adjusted for baseline admission covariates (sepsis HR = 1.80, P < .001).
Two hundred participants on whom complete data were available were assessed for a change in ADL at 1-month postdischarge. Of the 113 participants with sepsis who were assessed at 1-month, 72 (64%) had a change in at least 1 ADL and 47 of 60 (78%) participants had a change in at least 1 IADL. In patients without sepsis, 45 of 87 (52%) had a change in at least 1 ADL at 1 month and 50 of 66 (76%) had a change in at least 1 IADL. Change in ADL or IADL was not significantly different between those with sepsis and those without sepsis.
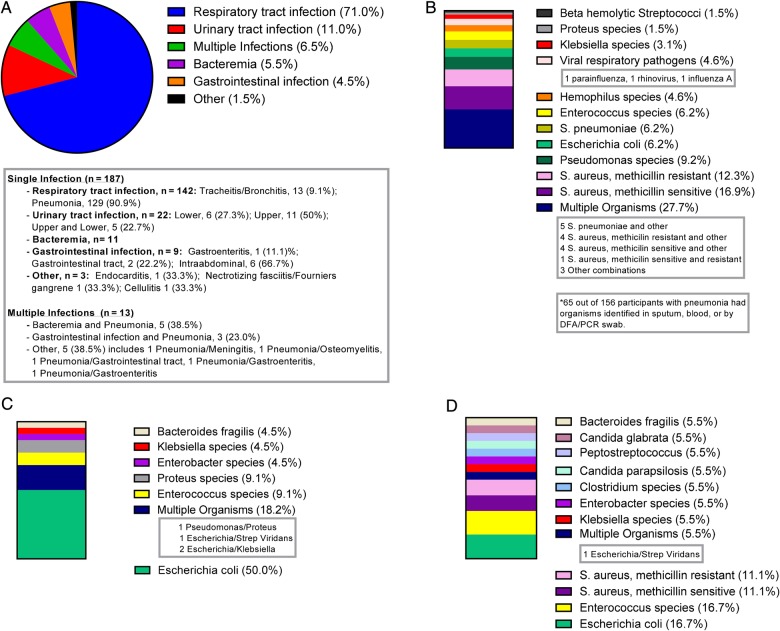
In this cohort, 200 participants had a documented infection based on the CDC/NHSN definitions for HAI; 187 participants were diagnosed with 1 infection and 13 had multiple infections. The most common site of infection was respiratory tract (71%), followed by urinary tract (11%), bloodstream (5.5%), and gastrointestinal infection (4.5%) (Figure 1A). Organisms cultured from participants with an infection are listed in Figure 1. Of patients diagnosed with pneumonia, 27.7% had multiple organisms identified by blood, sputum, or DFA/NP swab. The most common organism cultured for pneumonia was methicillin-sensitive Staphylococcus aureus (16.9%), followed by methicillin-resistant S aureus (12.3%) (Figure 1B). The most common organism identified by urine culture in participants with an infection of the urinary tract was Escherichia coli (50%) (Figure 1C). Enterococcus species and E coli were the most common organisms identified in blood cultures of patients identified as having bacteremia (Figure 1D).
Figure 1.
The graphic illustrates infections and identified pathogens. (A) Categories of documented infections (n = 200) are shown. (B) Pneumonia (n = 65*): percentage of organisms identified in sputum, blood, or by direct fluorescent-antibody by nasopharyngeal (DFA/NP) swab results. (C) Urinary tract infection (n = 22): percentage of organisms identified in urine cultures. (D) Bacteremia (n = 18): percentage of organisms identified in blood cultures.
DISCUSSION
We identified that the diagnosis of sepsis in adults ≥60 years upon admission to an ICU is associated with an increase in mortality compared with those admitted without sepsis. This study suggests that early use of antimicrobials and vasopressors for treatment of sepsis in older adults may be associated with a decrease in mortality associated with sepsis, although overall mortality still remains high. In addition, the majority of patients who survived hospitalization had a decline from baseline admission in at least 1 ADL or IADL 1-month post-ICU admission.
Similar to previous studies [3, 21], our results show that older adults with sepsis have a high mortality rate. The Surviving Sepsis Campaign recommends early use of broad-spectrum antimicrobials and adequate resuscitation to achieve and maintain a mean arterial pressure ≥65 mmHg [5]. These recommendations are mostly based on a randomized control trial of participants with a mean age of 65.8 years with a standard deviation of 17.3 years [22]. The application of these guidelines specifically focusing on older adults with sepsis has not been extensively evaluated [7, 23]. A prior study suggests that more aggressive therapies (eg, vasopressor use, mechanical ventilation, and renal replacement therapy) increased survival in older adults admitted to an ICU; however, this study included patients with diagnoses other than sepsis [24]. The mean age of participants in our study is almost a decade older than prior studies for which sepsis guidelines are based [22]. Our results show that even in an older cohort, the association of sepsis with mortality was decreased when accounting for the timely use of antimicrobials and vasopressors, suggesting that these interventions may improve outcomes.
Although use of antimicrobials and vasopressors may improve outcomes, the mortality rate in older adults with sepsis remains high. Furthermore, older adults that survive sepsis are likely to suffer from a significant decline in functional status. Martin et al [3] identified that among elderly patients discharged after a hospitalization for sepsis, over half did not return home, with most requiring admission to a long-term care facility. A larger study by Iwashyna et al [25] found that older adults who survived severe sepsis were more likely to have new cognitive impairment and functional decline compared with those with nonsepsis admissions. Our descriptive results support prior research, which show that older adults have a significant decline in functional status after hospitalization for sepsis.
Our findings, along with the existing literature, may have important implications in the treatment of older adults with sepsis. Despite benefit of therapies recommended by the Surviving Sepsis Campaign, older adults have a high mortality rate and functional decline after ICU admission for sepsis. Efforts to improve outcomes of older adults with sepsis by evaluating measures that impact mortality are needed. However, it may be equally important to address end-of-life planning before and during such acute care episodes [26]. Furthermore, during a hospitalization for sepsis, patients, families, and physicians should be aware of the potential need for posthospitalization long-term care including need for admission to a nursing home or skilled nursing facility [27].
Our study has some limitations. First, diagnosis of sepsis was made retrospectively via chart review and did not use the expanded definition of sepsis, which includes several other inflammatory, hemodynamic, and organ dysfunction variables. However, we rigorously limited the definition of sepsis to include a documented infection (as designated by CDC/NHSN Surveillance Definitions for HAI and reviewed by 2 physicians) plus at least 2 of 4 SIRS criteria within 48 hours of admission to the ICU. In addition, we were unable to differentiate community-acquired infections from HAI. Second, we were unable to assess whether effective antimicrobials active against the identified organism and/or vasopressors were administered within 1 hour of sepsis diagnosis as suggested by the Surviving Sepsis Campaign; however, we were able to confirm use within 48 hours of ICU admission for sepsis. Because we did not have exact timings of antimicrobial administration, we were unable to evaluate time to effective therapy as a predictor of mortality. We were also unable to assess the type of empiric antimicrobial and whether it was active against the identified organism or concordant with recommendations from the surviving sepsis campaign. However, it is standard practice to use broad-spectrum antimicrobials in the ICU setting and anticipate that in most instances, identified pathogens would have been treated by the empiric regimens. Third, we acknowledge that data from our study was initially collected in 2002–2004. However, few studies since then have focused specifically on older adults [7]. Thus, our findings may still have important clinical implications in this population. Fourth, we did not have hemodynamic parameters to assess whether use of vasopressors was according to the surviving sepsis protocol, and we could not assess whether other interventions such as fluid resuscitation and glucose monitoring were performed. However, it is not clear that protocols driving central hemodynamic monitoring improves outcomes compared with usual care [28]. Fifth, there were many missing values for the ADL and IADL outcomes; thus, we were only able to provide descriptive results. Finally, our study was a single-center study and, thus, the results may not be generalizable.
CONCLUSIONS
Our study suggests that treating older adults with therapies recommended by the Surviving Sepsis Campaign may improve outcomes in this age group, although mortality remains high for older adults with sepsis. In addition, given our reported descriptive results, we can hypothesize that admission to an ICU with sepsis may be associated with a decline in functional status. This may be important for both physicians and families to consider when discussing health goals and site of care after discharge. Although studies evaluating the impact of each intervention (eg, class of antimicrobial, dosage, and timing) in improving mortality and functional status post-ICU admission in older adults with sepsis are needed, further studies that address patient treatment goals earlier in the trajectory of ICU care are also warranted.
Acknowledgments
Author contributions. T. R. and M. J.-M. provided study concept and design, analysis, and interpretation of data, and preparation of manuscript. K. L. B. A. participated in acquisition of subjects and data, analysis of data, and preparation of manuscript. M. A. P. participated in acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript. P. H. V. N. participated in analysis and interpretation of data and preparation of manuscript. All authors have read and approved of the final version of the manuscript.
Financial support. T. R. received funding from the National Institutes of Health Training Grant (T32 AI007517-12). M. J.-M. received funding from a National Institutes of Health Mentored Career Development Award (K23 AG028691). M. J.-M. and P. H. V. N. received funding from the Claude D. Pepper Older Americans Independence Center Award (P30 AG021342), National Institute on Aging, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Bone RC, Balk RA, Cerra FB et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–55. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–10. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 2006; 34:15–21. [DOI] [PubMed] [Google Scholar]
- 4.Boumendil A, Maury E, Reinhard I et al. Prognosis of patients aged 80 years and over admitted in medical intensive care unit. Intensive Care Med 2004; 30:647–54. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Rhodes A et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637. [DOI] [PubMed] [Google Scholar]
- 6.Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA et al. Impact of the surviving sepsis campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med 2010; 38:1036–43. [DOI] [PubMed] [Google Scholar]
- 7.Rajapakse S, Rajapakse A. Age bias in clinical trials in sepsis: how relevant are guidelines to older people? J Crit Care 2009; 24:609–13. [DOI] [PubMed] [Google Scholar]
- 8.Pisani MA, Murphy TE, Van Ness PH et al. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med 2007; 167:1629–34. [DOI] [PubMed] [Google Scholar]
- 9.Pisani MA, Inouye SK, McNicoll L, Redlich CA. Screening for preexisting cognitive impairment in older intensive care unit patients: use of proxy assessment. J Am Geriatr Soc 2003; 51:689–93. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 11.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–29. [PubMed] [Google Scholar]
- 12.Katz S, Ford AB, Moskowitz RW et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963; 185:914–9. [DOI] [PubMed] [Google Scholar]
- 13.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9:179–86. [PubMed] [Google Scholar]
- 14.Ahasic AM, Van Ness PH, Murphy TE et al. Functional status after critical illness: agreement between patient and proxy assessments. Age Ageing 2015; 44:506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bone RC. A critical evaluation of new agents for the treatment of sepsis. JAMA 1991; 266:1686–91. [PubMed] [Google Scholar]
- 16.Knaus WA, Sun X, Nystrom O, Wagner DP. Evaluation of definitions for sepsis. Chest 1992; 101:1656–62. [DOI] [PubMed] [Google Scholar]
- 17.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–74. [PubMed] [Google Scholar]
- 18.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309–32. [DOI] [PubMed] [Google Scholar]
- 19.Lundberg JS, Perl TM, Wiblin T et al. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med 1998; 26:1020–4. [DOI] [PubMed] [Google Scholar]
- 20.Gaieski DF, Mikkelsen ME, Band RA et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010; 38:1045–53. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Yang KS, Hsann YM et al. The effect of comorbidity and age on hospital mortality and length of stay in patients with sepsis. J Crit Care 2010; 25:398–405. [DOI] [PubMed] [Google Scholar]
- 22.Rivers E, Nguyen B, Havstad S et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–77. [DOI] [PubMed] [Google Scholar]
- 23.Girard TD, Opal SM, Ely EW. Insights into severe sepsis in older patients: from epidemiology to evidence-based management. Clin Infect Dis 2005; 40:719–27. [DOI] [PubMed] [Google Scholar]
- 24.Lerolle N, Trinquart L, Bornstain C et al. Increased intensity of treatment and decreased mortality in elderly patients in an intensive care unit over a decade. Crit Care Med 2010; 38:59–64. [DOI] [PubMed] [Google Scholar]
- 25.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304:1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer S, Min SJ, Cervantes L, Kutner J. Where do you want to spend your last days of life? Low concordance between preferred and actual site of death among hospitalized adults. J Hosp Med 2013; 8:178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odden AJ, Rohde JM, Bonham C et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis 2013; 13:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pro CI, Yealy DM, Kellum JA et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]