Abstract
Triclosan (TCS) is one of the most widespread emerging contaminants and has adverse impact on aquatic ecosystem, yet little is known about its complete biodegradation mechanism in bacteria. Sphingomonas sp, strain YL-JM2C, isolated from activated sludge of a wastewater treatment plant, was very effective on degrading TCS. Response surface methodology (RSM) was applied to optimize the conditions like temperature and pH. From RSM, the optimal TCS degradation conditions were found to be 30 °C and pH 7.0. Under optimal conditions, strain YL-JM2C completely mineralized TCS (5 mg L−1) within 72 h. Gas chromatography-mass spectrometry analysis revealed that 2,4-dichlorophenol, 2-chlorohydroquinone and hydroquinone are three main by-products of TCS. Furthermore, stable isotope experimental results revealed that the 13C12-TCS was completely mineralized into CO2 and part of heavier carbon (13C) of labeled TCS was utilized by strain YL-JM2C to synthesize fatty acids (PLFAs). Cell surface hydrophobicity (CSH) and degradation test results suggested that the strain could enhance degradation capacity of TCS through increasing CSH. In addition, the bacterium also completely degraded spiked TCS (5 mg L−1) in wastewater collected from the wastewater treatment plant. Hence, these results suggest that the strain has potential to remediate TCS in the environment.
Triclosan [5-chloro-2-(2,4-dichlorophenoxy)-phenol, TCS], also known as Ingrasan, is a biocide that inhibits the enoyl-acyl-carrier protein-reductase, which is an essential enzyme for the synthesis of fatty acids to develop bacterial cells1. Due to its strong inhibition activity against most of the bacteria, it has been widely used in many synthetic products like textiles, plastics, deodorants, soaps, toothpastes, etc2. Therefore, TCS can be released during usage and enter the sewage system and reach the wastewater treatment plant (WWTP). In WWTPs, TCS could only be partially removed and the removal efficiency varies significantly among different plants. Because of the effluent discharge and/or leaching from biosolids applied to agricultural fields, TCS is constantly released into the ecosystem3. As a result, TCS has been frequently detected with its by-products in the environmental matrices like ground water, wastewater, sediments, river, and sea1. For example, in the southeastern part of China, TCS was detected in influent as well as effluent of the WWTP located in Xiamen and was also detected in all of the 31 sampling sites in Jiulong river and its estuary in Fujian province4,5.
Studies suggest that TCS has weak androgenic activity against aquatic organisms and revealed both androgenic as well as estrogenic responses in human breast cancer cells6,7. Even under short term exposure to low concentration of TCS (environmentally relevant levels), fathead minnow gut microbiome was rapidly and significantly affected along with distorted8. In the environment, TCS might be transformed into chlorodioxins and dibenzofurans, when it was exposed to UV radiations or heat, and these transformed by-products are highly toxic than the parent compound9. Hence, researchers have raised great concerns over its potential adverse effects on environment. Since TCS is considered as an emerging contaminant, researchers have employed various chemical treatment methods for its degradation10,11,12. However, incomplete degradation of TCS in the chemical treatment could result in the production of toxic by- products like chlorophenoxy-phenols, chlorophenols, trihalomethanes, and dioxins, which are known to be carcinogenic13,14,15,16,17,18.
Biodegradation of triclosan in the environment and wastewater has recently attracted more attention. To date, some researches have used the stable isotope probing by 13C-labeled TCS to aid for tracking the movement of the heavier carbon (13C) from the labeled compound into the DNA (genetic biomarker) of the active TCS-utilizing microorganisms in the microbial consortia1,3. However, for the isolated TCS-degrading bacteria, there was not enough conclusive evidence to support that bacteria could utilize TCS for biosynthesis. Though two soil bacteria Pseudomonas putida and Alcaligenes xylosoxidans subsp. denitrificans were reported to grow on the TCS-containing agar plates19, most of the isolated TCS-degraders such as Sphingomonas sp. Rd1, Nitromonas europaea, Sphingomonas sp. PH-07 and Sphingopyxis strain KCY1 were not shown to use TCS as a sole source of carbon9,20,21,22. The bacterial cell surface hydrophobicity (CSH) is a major parameter that governs bacterial adhesion, uptake and degradation of organic pollutants23. TCS is a hydrophobic antibacterial drug but the relationships between CSH and TCS degradation is still unclear. In bacteria, TCS was transformed into various by-products such as monohydroxy-TCS, dihydroxy-TCS, 2,4-dichlorophenol, 4-chlorophenol, 4-chlorocatechol, 3,5-dichlorocatechol, phenol, 2-methoxy-3,5-dichlorophenol, catechol and 2-chlorohydroquinone21,24,25, some of which are also potential carcinogens. Therefore, understanding the TCS degradation mechanism and pathway in different wastewater bacteria are critical for enhancing effective biodegradation of TCS in wastewater.
In this study, we isolated and identified TCS-degrading bacterium strain YL-JM2C and optimized conditions for TCS degradation by Response surface methodology (RSM). We demonstrated that strain YL-JM2C could utilize TCS as the carbon source by analyzing the flow of heavier carbon (13C) from 13C12-TCS into bacterial cellular fatty acids (phospholipid fatty acids, PLFAs). The changes of the bacterial CSH during biodegradation of TCS were also studied to provide a theoretical basis for the bacterial interaction of emerging contaminants like TCS in the ecosystem. In the end, the aim of this study was to address the unanswered issues for TCS biodegradation.
Results
Identification of a TCS-degrading bacterium
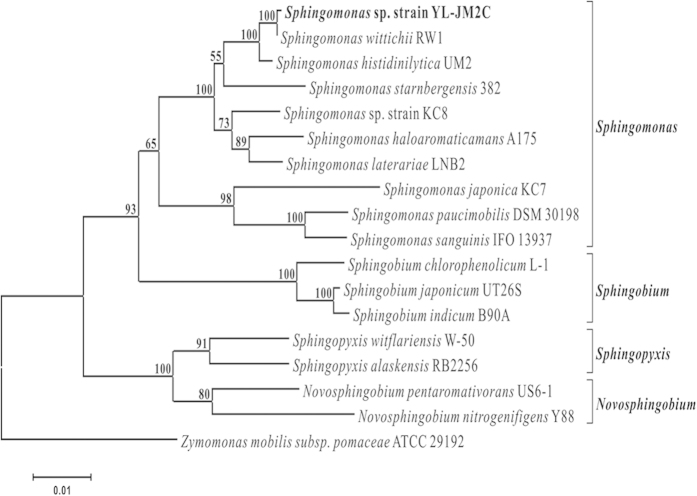
A TCS-degrading bacterial strain YL-JM2C was isolated from a WWTP, Xiamen, China. The bacterium was identified as Sphingomonas sp. strain YL-JM2C on the basis of 16 S rRNA gene sequence analysis and its phylogenetic relationship with other bacteria was shown in Fig. 1. The detailed description of the cell morphology and biochemical characterization of the bacterium was provided in Supplementary Information (SI). Structural morphology of strain YL-JM2C was observed by scanning electron microscope (Figure S1 in SI). The draft genome sequence of Sphingomonas sp. strain YL-JM2C was submitted to the GenBank under accession number ASTM01000000.
Figure 1. A maximum-likelihood phylogenetic tree showing the position of Sphingomonas sp. strain YL-JM2C relative to other type strains within the genus Sphingomonas.
Building of the tree also involves a bootstrapping process repeated 100 times to generate a majority consensus tree. Zymomonas mobilis subsp. pomaceae served as the outgroup for the analysis. The scale bar indicates the number of substitutions/site.
Optimization of growth and degradation studies
RSM based on Box Behnken design was applied to investigate the main and interactive effects of significant parameters including temperature (X1), pH (X2) and biomass (X3) on TCS degradation by strain YL-JM2C. Design established conditions of variables collectively with the experimental responses are given in Table 1. SAS software containing Response surface regression procedure was used to assess the data (Table 1) with some of non-significant interaction coefficients (p < 0.05) eliminated. The following equation was obtained to clarify the TCS degradation in strain YL-JM2C.
Table 1. Box-Behnken experimental design and the response of dependent variable for TCS degradation in strain YL-JM2C.
| Run | X1 | X2 | X3 | Response |
|---|---|---|---|---|
| [TCS Degradation (mg L−1) YYL−JM2C] | ||||
| 1 | 0 | 0 | 0 | 5 |
| 2 | 0 | −1 | 1 | 0.85 |
| 3 | 1 | 1 | 0 | 4.79 |
| 4 | 0 | 0 | 0 | 5 |
| 5 | 1 | 0 | -1 | 4.84 |
| 6 | 0 | 1 | 1 | 4.72 |
| 7 | −1 | 1 | 0 | 0.47 |
| 8 | −1 | 0 | −1 | 0.73 |
| 9 | 0 | 0 | 0 | 5 |
| 10 | 1 | 0 | 1 | 4.89 |
| 11 | −1 | −1 | 0 | 0.45 |
| 12 | 1 | −1 | 0 | 0.11 |
| 13 | 0 | 1 | −1 | 4.57 |
| 14 | 0 | −1 | −1 | 0.89 |
| 15 | −1 | 0 | 1 | 0.86 |
Values are means ± standard deviations of three replicates.
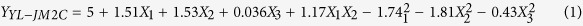 |
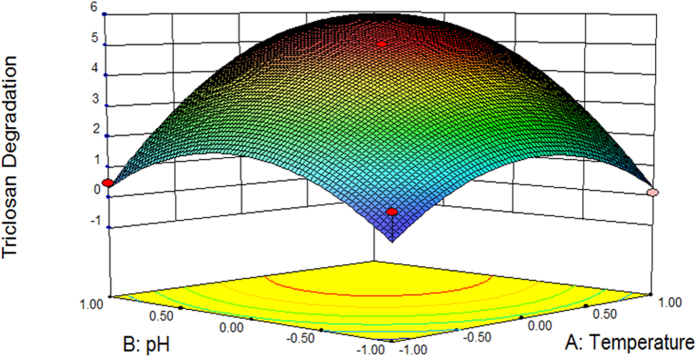
YYL−JM2C is the predicted TCS degradation by strain YL-JM2C. X1, X2, and X3 are the coded values of temperature, pH and biomass, respectively. An R2 of 0.9527 indicated that 95.27% of the randomness in response could be covered by the model, signifying that the predicted values of the model were in agreement with the experimental values. The results of regression analysis showed that the linear term coefficient of temperature (X1) as well as pH (X2), the square term coefficient of temperature (X1) as well as pH (X2) and the cross-product term coefficient of temperature (X1) and pH (X2) showed significant effects (p < 0.05) on the TCS degradation by strain YL-JM2C whereas the linear term coefficient of biomass (X3) and square term coefficient of biomass (X3) were not significant (p > 0.05). As the value of inoculum biomass (non-significant variable) fixed at 0.2 (g L−1), the three dimensional response surface was plotted to directly show the effects of temperature and pH on TCS degradation in strain YL-JM2C (Fig. 2). As shown in Fig. 2, the plot of TCS degradation by strain YL-JM2C had a theoretical maximum value 5 mg L−1 at the stationary point. At the stationary point, the optimum levels for the two parameters of X1 and X2 were found to be 0.000 and 0.000 in terms of the coded units, i.e., temperature 30 °C and pH 7.00, respectively. Hence, the optimal conditions for TCS degradation in strain YL-JM2C were found to be 30 °C and pH 7.00 with inoculum biomass at 0.2 (g L−1).
Figure 2. Three-dimensional plot of RSM showing the effects of temperature and pH on the degradation of TCS (5 mg L−1) by strain YL-JM2C with biomass (0.2 g L−1).
Values are means ± standard deviations of three replicates.
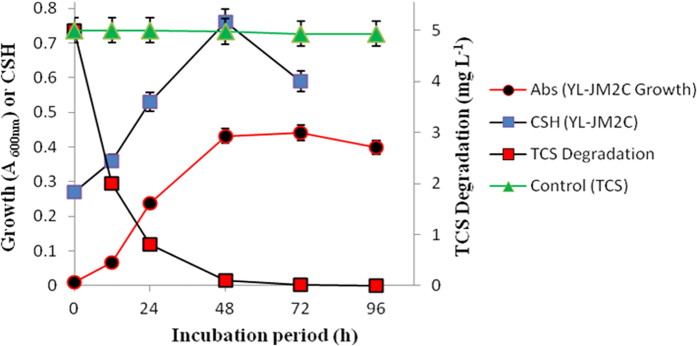
For growth studies, Sphingomonas sp. strain YL-JM2C was grown on yeast extract (0.04%)-containing ammonium mineral salts medium (AMS) with TCS (5 mg L−1). The growth of the strain was monitored on spectrophotometer with the increase in optical density at 600 nm and the maximum absorbance of growth was recorded as 0.44. The strain degraded TCS completely within 72 h (Fig. 3) with the corresponding release of chloride ions (Fig. S2). The bacterial strain degraded TCS when the range of the concentration was from 4.0 to 7.0 mg L−1 (Fig. 3 and Figure S3) and was able to degrade up to 99% of TCS at a given concentration of 7 mg L−1. However, when TCS concentration was 7 mg L−1, the growth of strain YL-JM2C was somewhat inhibited since the maximum absorbance of growth was around 0.32, which is less than 0.44 for 5 mg L−1 TCS.
Figure 3. Bacterial growth and CSH of strain YL-JM2C during degradation of TCS (5 mg L−1).
Values are means ± standard deviations of three replicates.
Hydrophobicity of strain YL-JM2C during degradation of TCS
The bacterial degradation of TCS carried out under optimal condition obtained by RSM and the CSH against TCS degradation with respect to incubation period were plotted (Fig. 3). Initially during degradation of TCS, the bacterial CSH increased quickly and subsequently reached its maximum hydrophobicity of 0.76 within 48 h (Fig. 3), and in the meantime bacterial growth and TCS degradation reached maximum. After the complete degradation of TCS in the medium, the CSH of strain YL-JM2C declined rapidly.
Identification of metabolites
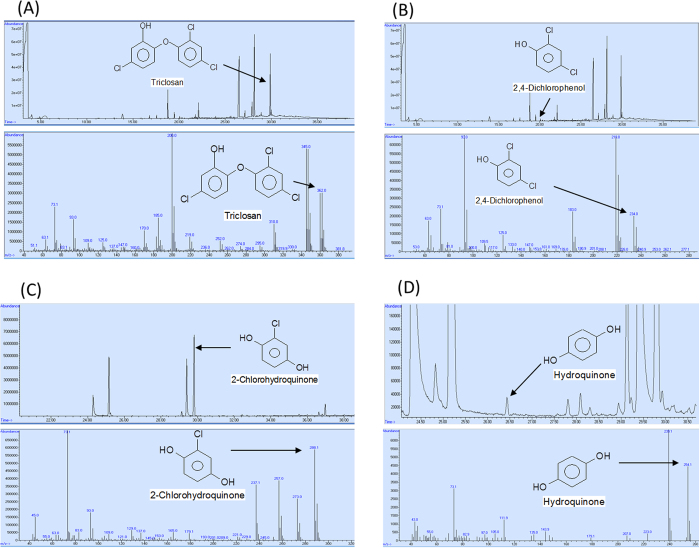
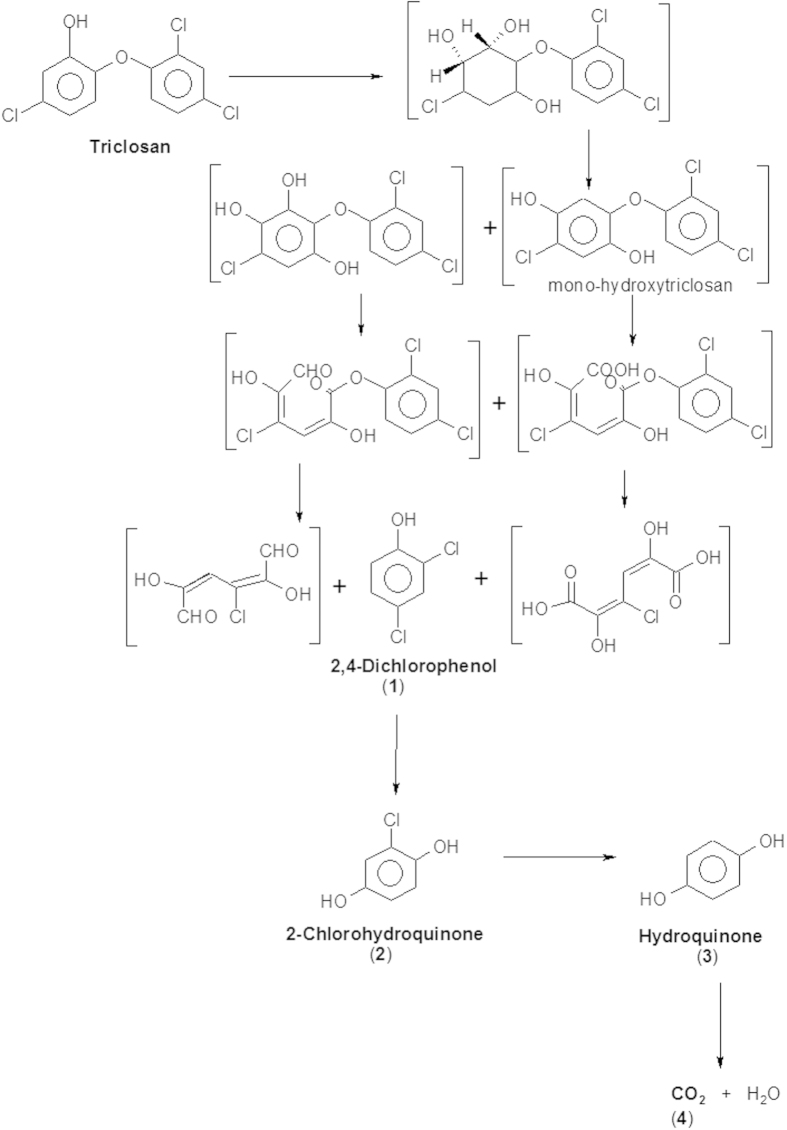
To elucidate the pathway of TCS degradation by Sphingomonas sp. strain YL-JM2C, TCS intermediates were extracted and confirmed by gas chromatography-mass spectrometry (GC-MS). The degradation by-products identified on the basis of retention time with respect to their mass spectrum were matched with authentic standard compounds and also reported data. The peak at retention time of 28.160 min corresponded to trimethylsilyl (TMS)-TCS and decreased over time and completely disappeared within 84 h. TCS disappeared with the release of three major intermediates (Fig. 4). In 0 h sample, a peak of TMS-TCS was detected with the retention time 28.106 min. In the sample of 8 h, the reduction of TMS-TCS was observed along with a peak of first metabolite at 19.487 min (retention time) (Fig. 4A,B). In the 54 h sample, the peak of the first metabolite disappeared with the occurrence of two new peaks (the second and third metabolites) at 29.822 and 26.440 min (retention time) (Fig. 4C,D), respectively and TCS was detected in decreasing quantities. On the basis of these results, the presence of three by-products of TCS biodegradation were identified as 2,4-dichlorophenol (first metabolite), 2-chlorohydroquinone (second metabolite) and hydroquinone (third metabolite) with their authentic compounds (Figure S4) as well as the previously reported data9,25.
Figure 4. GC-MS analysis of the metabolites produced from TCS degradation by Sphingomonas sp. strain YL-JM2C.
The retention times of the compounds were 28.106 (m/z, 362), 19.487 (m/z, 234), 29.822 (m/z, 288) and 26.440 (m/z, 254) min, respectively, which were identified as TCS-TMS, 2,4-dichlorophenol-TMS, 2-chlorohydroquinone-TMS and hydroquinone-TMS. TCS-TMS and 2,4-dichlorophenol-TMS were identified and compared by reported data9,25 whereas 2-chlorohydroquinone-TMS and hydroquinone-TMS were identified by their authentic compounds (Provided in supplementary information Figure S4).
Enzyme assay for chlorohydroquinone dehydrogenase
In the crude extract of the TCS induced cells of strain YL-JM2C, we have detected enzyme activity for chlorohydroquinone dehalogenase. During the enzyme assay we observed stoichiometric release of chloride ions (data not shown), which suggested the conversion of 2-chlorohydroquinone to hydroquinone. GC-MS analysis of the sample confirmed the formation of hydroquinone.
Tracking experiment studied by 13C12-TCS
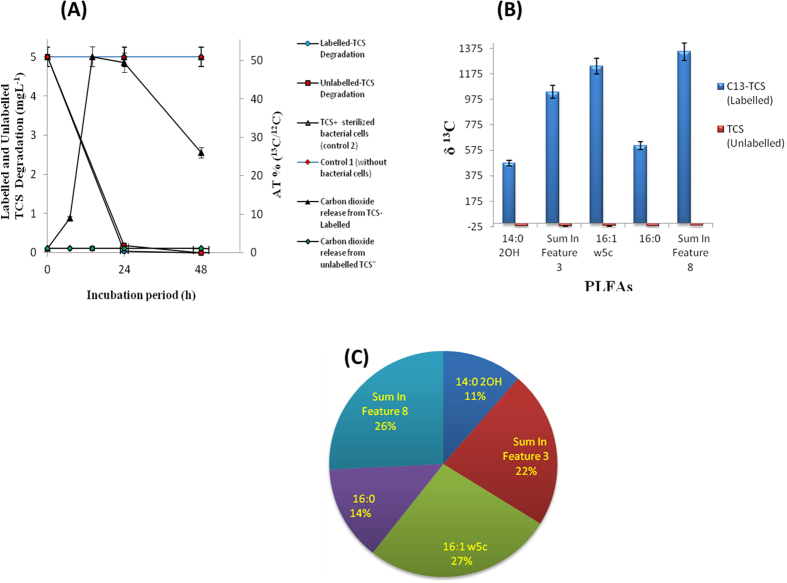
In the tracking experiment using 13C12-TCS, the percentage of 13C/12C of the headspace carbon dioxide (CO2) rapidly increased to about 51% within 24 h. Yet, in unlabeled TCS, the percentage of 13C/12C of the headspace CO2 remained low around 1% (Fig. 5A). Hence, the results suggest that the strain could completely mineralize the given concentration of TCS into CO2. In addition, we also studied whether the bacterium would also utilize TCS as a carbon source for anabolism. To clarify the ambiguity, the PLFAs of strain YL-JM2C were also extracted and analyzed by stable isotope ratio mass spectrometer (SIRMS) after incubation with 13C12-TCS in the microcosm. Results of δ13C of PLFAs showed that the PLFAs of 14:0 2OH, 16:0, sum in feature 3, sum in feature 8 and 16:1 w5c have contained significant quantity of heavier carbon (13C) from labeled TCS (Fig. 5B). From these results, strain YL-JM2C not only mineralized TCS, the microorganism also synthesized its fatty acids by utilizing TCS as a sole source of carbon. Further, we had estimated the percentage of 13C assimilated into bacterial PLFAs. Analytical result indicated that strain YL-JM2C incorporated 61.58% of added 13C into PLFAs and the incorporation proportions of 13C were about 6.99%, 8.37%, 13.84%, 15.9% and 16.48% for the PLFAs of 14:0 2OH, 16:0, sum in feature 3, sum in feature 8 and 16:1 w5c, respectively. Moreover, the distribution of assimilated 13C was approximately 11%, 14%, 22%, 26% and 27% for the PLFAs of 14:0 2OH, 16:0, sum in feature 3, sum in feature 8 and 16:1 w5c (Fig. 5C). Incorporation percentages were related to the content of individual PLFA in the bacteria.
Figure 5. Results of 13C12-TCS labeling study in Sphingomonas sp. strain Yl-JM2C.
(A) Release of carbon dioxide during degradation of labeled and unlabeled TCS; (B) the δ values of PLFAs; (C) distribution of assimilated 13C in individual PLFAs. Values are means ± standard deviations of three replicates.
Microcosm studies
In order to determine the ability of Sphingomonas sp. strain YL-JM2C to degrade TCS in wastewater, we performed microcosm studies by sterile influent wastewater, non sterile influent wastewater and non sterile effluent wastewater. Sphingomonas sp. strain YL-JM2C completely degraded TCS in the test microcosm with sterile and non sterile influent wastewater within 24 h (Figure S5A,B). In another test microcosm with non sterile effluent wastewater, the complete TCS depletion occurred also within 24 h (Figure S5C). In controls with sterile as well as non sterile influent wastewater and non sterile effluent wastewater, no degradation was observed even after 72 h (Figure S5).
Discussion
A TCS-degrading bacterium was identified as a Sphingomonas sp. strain YL-JM2C and characterized its ability to degrade TCS under different parameters. Previously, many studies have shown that the pH and temperature are the two important variables concerned and strongly influence the degradation of toxic pollutants by microorganisms23,26,27,28,29. RSM is an efficient method and has been successfully applied to get optimized conditions for different microorganisms involved in the degradation of xenobiotics23,27,30,31. Hence, it is used in this study to identify optimal conditions for TCS degradation in strain YL-JM2C. The results of our study indicated that strain YL-JM2C degraded TCS at different temperature (20–40 °C) and pH (5–9). In the present study we have developed a standard mathematical model, which could be efficiently used to predict and optimize conditions for the degradation of TCS in strain YL-JM2C within a given parameters. The bacterium could effectively degrade 5 mg L−1 of TCS (Fig. 3) under its optimized conditions. The bacterium was also able to degrade up to 99% of TCS at a given concentration of 7 mg L−1 (Figure S3). 2,4-Dichlorophenol, 2-chlorohydroquinone and hydroquinone are identified as major metabolites of the TCS degradation pathway in strain YL-JM2C (Fig. 6). This is the first report of the formation of hydroquinone in the degradation pathway of TCS. The initial mechanism of degradation of TCS by strain YL-JM2C was similar to other microorganisms9,21,25. In strain YL-JM2C, TCS was oxidatively transformed into 2,4-dichlorophenol by breaking ether bond present between two aromatic rings of TCS similar to other bacteria like strain PH-07, strain KCY1 and strain RHA1. The bacterium draft genome of strain YL-JM2C has 45 monooxygenase and 101 dioxygenase enzymes32 which are probably involved in these reactions9,25. TCS was also transformed to 2,4-dichlorophenol in fungus like Trametes versicolor and Aspergillus versicolor33,34. 2,4-Dichlorophenol was further transformed into 2-chlorohydroquinone by dechlorination. Similar result was observed in strain RHA1 whereas in strain PH-07, 2,4-Dichlorophenol was transformed into 3,5-dichlorocatechol and 4-chlorocatechol via two different routes. In this study, 2-chlorohydroquinone was dechlorinated and formed hydroquinone. Similar pathway was observed in strain RKJ 80035 during the degradation of 2-chloro-4-nitrophenol. Finally, this non-chlorinated compound hydroquinone was transformed into CO2 (Fig. 6).
Figure 6. A proposed pathway for the degradation of TCS by Sphingomonas sp. strain YL-JM2C.
The compounds shown in the brackets are hypothetical metabolites.
The results of CSH indicated that the hydrophobicity of strain YL-JM2C might be associated with the concentration and toxic effect of TCS. Several research groups reported that the higher CSH was potentially useful in pesticide as well as petroleum bioremediation and survival for bacteria in the environment23,36. CSH is one of the most important factors that govern bacterial adhesion, uptake and degradation of hydrophobic organic compounds. Previous study has shown that high CSH of strains could enhance the degradation of hydrophobic pesticides23. However, there is no report on the relationship between high CSH and degradation ability of TCS which is also a hydrophobic compound. In our study we found that the presence of TCS could increase the hydrophobicity of strain YL-JM2C. As shown in the Fig. 3, with the increasing CSH of the strain YL-JM2C, bacterial growth as well as the TCS degradation increased. When the CSH maintained its maximum level at 48 h, the bacterial growth and TCS degradation efficiency reached their highest level. After the complete degradation of TCS, the CSH of strain YL-JM2C decreased rapidly. These results suggested that the CSH which governed adherence to the surface of the hydrophobic compounds could help the capability of the strain YL-JM2C to absorb and degrade TCS. Therefore, the strain with high hydrophobicity could be potentially useful for the remediation of wastewater containing hydrophobic emerging contaminants such as TCS.
In strain YL-JM2C, to confirm complete mineralization of TCS and also the use of TCS as a sole source of carbon for anabolism, stable isotope labeled TCS (13C12-TCS) was used and no yeast extract was added throughout the experiment. By the determination of heavier carbon (13C) released from labeled TCS into CO2, we can confirm TCS is completely mineralized by strain YL-JM2C into CO2. In addition, since fatty acids are necessary for building cell membrane of living microorganisms, we have also confirmed that the 13C from labeled TCS was used by strain YL-JM2C to synthesize its cellular fatty acids. These results suggest that the bacterium is not only able to mineralize TCS into CO2 but also utilize TCS as a sole source of carbon for its biosynthesis. Due to the low solubility and antimicrobial activity of TCS, we only used 4–7 mg L−1 TCS in the medium, and this low concentration of carbon source was unable to cause significant bacterial growth observed using optical density at 600 nm (data not shown). Yeast extract was added to help strain YL-JM2C to show more significant growth in the presence of TCS. The stable isotope result demonstrated that strain YL-JM2C could mineralize and utilize TCS for biosynthesis in the absence of yeast extract. In addition, TCS was used to block an enzyme called NADH dependent enoyl-acyl carrier protein reductase (encoded by fabI gene), which blocks the synthesis of fatty acids in the bacteria. In this study, stable isotope experiment of PLFAs demonstrated that strain YL-JM2C could assimilate the carbons located in TCS into fatty acids like 14:0 2OH, 16:0, sum in feature 3, sum in feature 8 and 16:1 w5c. This result suggested that strain YL-JM2C has novel mechanism to overcome the antimicrobial activity of TCS. Future studies are needed to quantify the amount of 13C-TCS transformed into each of its by-products, calculate the concentration of 13C-TCS entering into bacterial cell biomolecules, and also identification of responsible genes for the resistance and degradation of TCS in Sphingomonas sp. strain YL-JM2C.
Conclusion
In conclusion, Sphingomonas sp. strain YL-JM2C isolated in the present study appeared to be efficient in degrading TCS. RSM was successfully applied to optimize the conditions for TCS degradation by strain YL-JM2C. Three different metabolites, 2,4-Dichlorophenol, 2-chlorohydroquinone and hydroquinone, were detected during degradation of TCS. Our results demonstrated that 13C12-TCS was completely mineralized into CO2 and the heavier carbon (13C) of labeled TCS was utilized by strain YL-JM2C for fatty acids synthesis. The CSH of strain YL-JM2C increased during the degradation of TCS and the increasing CSH helped the ability of strain YL-JM2C to degrade TCS. Overall, this study suggested that strain YL-JM2C could be a good candidate for the remediation of TCS in the contaminated wastewater.
Materials and Methods
Chemicals and culture media
Irgasan (Triclosan, TCS; 97% HPLC Grade) was purchased from Sigma-Aldrich Co., USA. Triclosan (13C12, 99%) was purchased from Cambridge Isotope Laboratories, Inc, USA. Acetone, acetonitrile and methanol were purchased from Merck, Germany. All other chemicals were of pure analytical-grade or highest grade available commercially. Stock solution (5 g L−1) of TCS was prepared with acetone and stored in brown bottles at −20 °C before use. AMS composition: K2SO4, 0.98 mM; KH2PO4, 3.9 mM; Na2HPO4.12 H2O, 6.1 mM; (NH4)2SO4, 5.88 mM; MgSO4.7 H2O, 0.15 mM; CaSO4.2 H2O, 0.07 mM; CoMoO4, 0.004 mM; KI, 0.001 mM; ZnSO4.7 H2O, 0.002 mM; MnSO4.H2O, 0.002 mM; H3BO3, 0.002 mM; FeSO4.H2O, 0.08 mM; H2SO4, 0.1 mM. The AMS medium was set to pH 7.00 (using 2 M NaOH or 2 M H2SO4) and sterilized in autoclave at 121 °C.
Isolation and genome sequencing of Sphingomonas sp. strain YL-JM2C
Sphingomonas sp. strain YL-JM2C was isolated from activated sludge in a WWTP, Xiamen (China), through enrichment on TCS. For enrichment, 5 mL of activated sludge was added to 250 mL Erlenmeyer flask containing 95 mL of AMS with TCS (5 mg L−1) as a sole source of carbon (acetone free). The enrichment flask was incubated at 30 °C under dark condition on shaker at 150 rpm. Upon complete degradation, 5 mL of cultures were transferred to a new flask containing 95 mL of AMS with TCS (5 mg L−1). After 6 months of enrichment, one milliliter of the enrichment culture was serially diluted in R2A plates having TCS (5 mg L−1). Microorganisms grown on R2A agar plates containing TCS were further purified, and only one colony showed rapid degradation of TCS. The microorganism was designated as strain YL-JM2C. The microorganism was identified by using 16 S rRNA gene sequence analysis37. The whole genome sequence of YL-JM2C has been deposited in GenBank under accession number ASTM0100000032.
Optimal condition, bacterial growth and TCS degradation
The experiments were conducted to elaborate important parameters like temperature, pH and biomass to get optimal condition for the degradation of TCS in strain YL-JM2C. In single-factor experiments, we studied the optimal ranges of three main variables such as temperature (20–40 °C), pH (5–9) and biomass (0.1–0.3 g L−1). RSM based on Box-Behnken design was used to optimize the above mentioned parameters and their interaction which significantly influenced TCS degradation in strain YL-JM2C23,38. Filter-sterilized yeast extract (0.04%) was added into AMS containing TCS (5 mg L−1). The microorganism was inoculated into the medium and the samples were collected after 72 h to quantify the residual TCS. A three-variable Box-Behnken design consisting of 15 experimental runs with 3 replicates at the midpoint was applied in this experiment. The experimental design was provided in Table 1 and the equation 2 shows the quadratic polynomial equation.
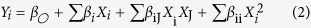 |
where Yi is the predicted response, Xi and Xj are variables, βo is the constant, βi is the linear coefficient, βii is the quadratic coefficient and βij is the interaction coefficient.
To monitor the effects of TCS on the growth of strain YL-JM2C, strain YL-JM2C was grown in 500 mL Erlenmeyer flask containing 100 mL of yeast extract (0.04%)-containing AMS with appropriate concentration of TCS (4–7 mg L−1). The bacterial growth was measured at 600 nm by UV-spectrophotometer (UV-5200 Spectrophotometer). For degradation studies, the TCS degradation was monitored by HPLC. Uninoculated culture flasks with the same concentrations of TCS were served as a control.
Identification of metabolites
The metabolic products of TCS in strain YL-JM2C cultures containing TCS (5 mg L−1) were extracted and identified by GC-MS. The samples were taken at regular intervals (0, 8, 24, 36, 54, 60, 72 and 84 h). The same bacterial culture supernatant lacking TCS was used as a negative control, and non inoculated control containing TCS (5 mg L−1) was included as well. The collected samples were centrifuged (7000 × g) for 20 min, adjusted to pH 2.0 with 2 M H2SO4 and were extracted with ethyl acetate. For extraction, supernatant was mixed with equal volume of ethyl acetate in a brown bottle and shaken for 6 h at 150 rpm on shaker. Further, the sample was transferred to separating funnel and allowed for 15 min to separate. The ethyl acetate layer was collected and the aqueous layer was used for one more time extraction with ethyl acetate. Both ethyl acetate layers were combined together and evaporated at room temperature on anhydrous sodium sulfate. The extracted samples were reconstituted in acetone (470 μL) and derivatized with BSTFA (30 μL). The derivatized samples were analyzed by the GC-MS. The metabolites identified by mass spectrometry analysis were matched with authentic standard compounds as well as reported data.
Measurement of the relative CSH
The relative CSH of the strain YL-JM2C during the growth and biodegradation of TCS was assessed by the microbial adherence to hydrocarbon method39. The p-xylene (hydrocarbon) was used for CSH determination of Sphingomonas sp. strain YL-JM2C. The CSH value was calculated using equation 3.
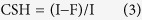 |
I and F represent the initial and the final optical densities at 600 nm of the aqueous phase.
Enzyme assays
The cells of strain YL-JM2C grown on yeast extract (0.04%)-containing AMS medium supplemented with TCS (5 mg L−1) were harvested by centrifugation (8,000 × g for 10 min at 4 °C), washed and resuspended in 50 mM phosphate buffer (pH 7.0). The cell-free extract were prepared through ultrasonication (Ultrasonic homogenizer JY92-IIN, Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) followed by centrifugation (12,500 × g for 40 min at 4 °C). The supernatant obtained was used as the crude enzyme for enzyme assays40. Chlorohydroquinone dehalogenase activity was assayed according to the previous literature35. The enzyme activity was determined as the total chloride released at 30 °C. The reaction mixture contained 100 mM Tris-acetate buffer (pH 7.5), 0.2 mM NADPH, 5-7 mg of cell extract and 2-chlorohydroquinone (30 μM). The final volume of the reaction mixture was 5 mL and samples were collected at regular intervals for chloride ion analysis as described below. Protein content was determined by BCA protein assay kit (Thermo Scientific, USA).
Stable Isotope Experiment
13C12-TCS was used to confirm TCS carbon utilization by the isolated bacterium strain YL-JM2C. Fifty milliliters of sterile ASM (without yeast extract) containing 13C12-TCS (5 mg L−1) was filled into a 120 mL serum bottle. Artificial air (nitrogen/oxygen, 21/79, v/v) was used to purge ASM to remove CO2. The serum bottle was then inoculated with strain YL-JM2C (optical densities at 600 nm = 0.25) and sealed by butyl rubber immediately. Meanwhile, unlabeled-TCS with the same concentration was also used in another set of bottles for comparison. Heat-killed culture (YL-JM2C) and uninoculated serum bottles, which were set up under identical conditions to those of the experimental cultures and served as controls. All treatments were incubated at 30 °C under dark conditions and kept on shaker at 150 rpm. 13C/12C ratio of the headspace CO2 was determined by SIRMS at 0, 7, 14, 25, and 48 h. Thereafter the bacterial cultures were harvested by centrifugation and washed twice with phosphate buffer. Bacterial fatty acids were extracted according to the manual of MIDI Sherlock Microbial Identification System and analyzed by SIRMS immediately. SIRMS conditions were described in the SI. The percentage of added 13C incorporated into a specific PLFA was calculated as: %13C incorporation = 100 × ((Flabeled − Funlabeled) × (PLFAi)labeled]/[13C added], with the concentrations of PLFA and 13C added in mg C kg−1 solution and F value as: F = 13C/(13C + 12C) = R/(R + 1). The carbon isotope ratio (R) was evaluated from δ13C values as: R = (δ13C/1000 + 1)RVPDB, with Pee Dee Belemnite standard RVPDB = 0.011241. The proportion of 13C incorporated into an individual PLFA of labeled PLFAs was expressed as: %13C incorporation = 100 × (13CPLFAi/Σ 13CPLFAi), where 13CPLFAi was the amount of 13C incorporated into the PLFA of “i”, which was calculated as: 13CPLFAi = (Flabeled − Funlabeled) × [PLFAi]labeled42.
Microcosm study for the degradation of TCS by strain YL-JM2C in wastewater
Wastewater samples were collected from raw sewage (influent) and treated wastewater (effluent) of a WWTP, Xiamen (China). The total nitrogen (TN), total phosphorus (TP) and total chemical oxygen demand (TCOD) of collected influent wastewater were 33.8, 2.5 and 95.6 mg L−1, respectively, whereas the TN, TP and TCOD of collected effluent wastewater were 15.6, 1.4 and 43.0 mg L−1, respectively. The pH of the samples was adjusted to pH 7.00 (using 1 M NaOH or 1 M H2SO4). For microcosm studies, the following four experiments with TCS (5 mg L−1) spiked wastewater were conducted: (I) test microcosm with sterile influent in which cells of strain YL-JM2C were inoculated (2.66 × 107 CFU per 100 mL of wastewater) in the autoclaved influent; (II) test microcosm with non sterile influent in which cells of strain YL-JM2C were inoculated in natural influent; (III) test microcosm with non sterile effluent in which cells of strain YL-JM2C were inoculated in natural effluent; and (IV) heat-killed controls consisted of autoclaved cultures and non-inoculated controls without Sphingomonas sp. strain YL-JM2C, were set up under identical conditions to those of the experimental cultures at 30 °C.
Analytical Methods
Metabolites of TCS in strain YL-JM2C were analyzed by GC-MS. TCS and 13C12-TCS residues at different intervals were determined by HPLC (Dionex Ultimate 3000, USA). Chloride ion analysis was carried out using Ion Chromatography. Detailed description of the analytical methods is provided in SI.
Additional Information
How to cite this article: Mulla, S. I. et al. Characterization of triclosan metabolism in Sphingomonas sp. strain YL-JM2C. Sci. Rep. 6, 21965; doi: 10.1038/srep21965 (2016).
Supplementary Material
Acknowledgments
This research was supported by Hundred Talents Program of Fujian Province, China, International Young Scientists by National Natural Science Foundation of China (Grant No. 31450110426), Science and Technology Planning Project of Xiamen, China (3502Z20142021), Young Talents Project of Institute of Urban Environment, CAS (IUEMS201401), and the financial assistance (2013FFZB0003) from The World Academy of Sciences-Chinese Academy of Sciences (TWAS-CAS) under its Post-Doctoral fellowships for Young International Scientists. We also would like to thank Dr. Han Zhang for her help with stable isotope analysis.
Footnotes
The authors declare no competing financial interests.
Author Contributions S.M. and C.Y. conceived and designed the study. S.M., H.W., Q.S. and A.H. performed experimental works. S.M., H.W., Q.S., A.H. and C.Y. analyzed the data and S.M. and C.Y. wrote the paper. All authors agreed with the contents of the manuscript.
References
- Lolas I. B., Chen X., Bester K. & Nielsen J. L. Identification of triclosan-degrading bacteria using stable isotope probing, fluorescence in situ hybridization and microautoradiography. Microbiology 158, 2796–2804 (2012). [DOI] [PubMed] [Google Scholar]
- Rodricks J. V., Swenberg J. A., Borzelleca J. F., Maronpot R. R. & Shipp A. M. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol 40, 422–484 (2010). [DOI] [PubMed] [Google Scholar]
- Lee D. G., Cho K. C. & Chu K. H. Identification of triclosan-degrading bacteria in a triclosan enrichment culture using stable isotope probing. Biodegradation 25, 55–65 (2014). [DOI] [PubMed] [Google Scholar]
- Lv M. et al. Pharmaceuticals and personal care products in a mesoscale subtropical watershed and their application as sewage markers. J Hazard Mater 280, 696–705 (2014). [DOI] [PubMed] [Google Scholar]
- Sun Q., Lv M., Hu A., Yang X. & Yu C. P. Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China. J Hazard Mater 277, 69–75 (2014). [DOI] [PubMed] [Google Scholar]
- Foran C. M., Bennett E. R. & Benson W. H. Developmental evaluation of a potential non-steroidal estrogen: triclosan. Mar Environ Res 50, 153e156 (2000). [DOI] [PubMed] [Google Scholar]
- Gee R. H., Charles A., Taylor N. & Darbre P. D. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J Appl Toxicol 28, 78–91 (2008). [DOI] [PubMed] [Google Scholar]
- Narrowe A. B. et al. Perturbation and restoration of the fathead minnow gut microbiome after low-level triclosan exposure. Microbiome 3, 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. G., Zhao F., Rezenom Y. H., Russell D. H. & Chu K. H. Biodegradation of triclosan by a wastewater microorganism. Water Res 46, 4226–4234 (2012). [DOI] [PubMed] [Google Scholar]
- Gao Y., Ji Y., Li G. & An T. Mechanism, kinetics and toxicity assessment of OH-initiated transformation of triclosan in aquatic environments. Water Res 49, 360–370 (2014). [DOI] [PubMed] [Google Scholar]
- Jiang J., Pang S. Y. & Ma J. Oxidation of Triclosan by Permanganate (Mn(VII)): Importance of Ligands and In Situ Formed Manganese Oxides. Environ Sci Technol 43, 8326–8331 (2009). [DOI] [PubMed] [Google Scholar]
- Latch D. E. et al. Aqueous photochemistry of triclosan: formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products. Environ Toxicol Chem 24, 517–525 (2005). [DOI] [PubMed] [Google Scholar]
- Liyanapatirana C., Gwaltney S. R. & Xia K. Transformation of Triclosan by Fe(III)-saturated montmorillonite. Environ Sci Technol 44, 668–674 (2010). [DOI] [PubMed] [Google Scholar]
- Methatham T., Lu M.–C. & Ratanatamskul C. Effect of operating parameters on triclosan degradation by Fenton’s reagents combined with an electrochemical system. Desalin Water Treat 52, 920–928 (2013). [Google Scholar]
- Rafqah S., Wong-Wah-Chung P., Nelieu S., Einhorn J. & Sarakha M. Phototransformation of triclosan in the presence of TiO2 in aqueous suspension: Mechanistic approach. Appl Catal B-Environ 66, 119–125 (2006). [Google Scholar]
- Son H. S., Ko G. & Zoh K. D. Kinetics and mechanism of photolysis and TiO2 photocatalysis of triclosan. J Hazard Mater 166, 954–960 (2009). [DOI] [PubMed] [Google Scholar]
- Song Z. et al. Efficient oxidative degradation of triclosan by using an enhanced Fenton-like process. Chem Eng J 198-199, 379–387 (2012). [Google Scholar]
- Suarez S., Dodd M. C., Omil F. & von Gunten U. Kinetics of triclosan oxidation by aqueous ozone and consequent loss of antibacterial activity: relevance to municipal wastewater ozonation. Water Res 41, 2481–2490 (2007). [DOI] [PubMed] [Google Scholar]
- Meade M. J., Waddell R. L. & Callahan T. M. Soil bacteria Pseudomonas putida and Alcaligenes xylosoxidans subsp. denitrificans inactivate triclosan in liquid and solid substrates. FEMS Microbiol Lett 204, 45–48 (2001). [DOI] [PubMed] [Google Scholar]
- Hay A. G., Dees P. M. & Sayler G. S. Growth of a bacterial consortium on triclosan. FEMS Microbiol Ecol 36, 105–112 (2001). [DOI] [PubMed] [Google Scholar]
- Kim Y.-M. et al. Triclosan susceptibility and co-metabolism-a comparison for three aerobic pollutant-degrading bacteria. Bioresource Technol 102, 2206–2212 (2011). [DOI] [PubMed] [Google Scholar]
- Roh H. et al. Biodegradation potential of wastewater micropollutants by ammonia-oxidizing bacteria. Chemosphere 77, 1084–1089 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang C. et al. Biodegradation of beta-cypermethrin by two Serratia spp. with different cell surface hydrophobicity. Bioresource Technol 101, 3423–3429 (2010). [DOI] [PubMed] [Google Scholar]
- Gangadharan P. V. P., Nadaraja A. V., Bhasi A., Khan S. & Bhaskaran K. Degradation of triclosan under aerobic, anoxic, and anaerobic conditions. Appl Biochem Biotech 167, 1603–1612 (2012). [DOI] [PubMed] [Google Scholar]
- Lee, D., G. & Chu, K. H. Effects of growth substrate on triclosan biodegradation potential of oxygenase-expressing bacteria. Chemosphere 93, 1904–1911 (2013). [DOI] [PubMed] [Google Scholar]
- Anwar S., Liaquat F., Khan Q. M., Khalid Z. M. & Iqbal S. Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by Bacillus pumilus strain C2A1. J Hazard Mater 168, 400–405 (2009). [DOI] [PubMed] [Google Scholar]
- Chen S. et al. Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by a new fungal strain Cladosporium cladosporioides Hu-01. PloS one 7, e47205 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycon M., Wojcik M. & Piotrowska-Seget Z. Biodegradation of the organophosphorus insecticide diazinon by Serratia sp. and Pseudomonas sp. and their use in bioremediation of contaminated soil. Chemosphere 76, 494–501 (2009). [DOI] [PubMed] [Google Scholar]
- Yus Azila Y., Mashitah M. D. & Bhatia S. Process optimization studies of lead (Pb(II)) biosorption onto immobilized cells of Pycnoporus sanguineus using response surface methodolog. Bioresource Technol 99, 8549–8552 (2008). [DOI] [PubMed] [Google Scholar]
- Chen S. et al. Isolation and characterization of a fungus able to degrade pyrethroids and 3-phenoxybenzaldehyde. Bioresource Technol 102, 8110–8116 (2011). [DOI] [PubMed] [Google Scholar]
- Dritsa V., Rigas F., Doulia D., Avramides E. J. & Hatzianestis I. Optimization of Culture Conditions for the Biodegradation of Lindane by the Polypore Fungus Ganoderma australe. Water Air Soil Pollut 204, 19–27 (2009). [Google Scholar]
- Mulla S. I., Hu A., Xu H. & Yu C.-P. Draft genome sequence of triclosan degrading bacterium Sphingomonas sp. strain YL-JM2C isolated from a wastewater treatment plant in China. Genome Announc 3, e00603–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertit Tastan B. & Donmez G. Biodegradation of pesticide triclosan by A. versicolor in simulated wastewater and semi-synthetic media. Pestic biochem phys 118, 33–37 (2015). [DOI] [PubMed] [Google Scholar]
- Hundt K. et al. Transformation of triclosan by Trametes versicolor and Pycnoporus cinnabarinus. Appl Environ Microb 66, 4157–4160 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P. K. & Jain R. K. Metabolism of 2-chloro-4-nitrophenol in a gram negative bacterium, Burkholderia sp. RKJ 800. PloS one 7, e38676 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza G. A., Ulfig K. & Brigmon R. L. Surface active properties of bacterial strains isolated from petroleum hydrocarbon-bioremediated soil. Pol J Microbiol 54, 161–167 (2005). [PubMed] [Google Scholar]
- Feng C. et al. Characterization of exoelectrogenic bacteria enterobacter strains isolated from a microbial fuel cell exposed to copper shock load. PloS one 9, e113379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Luo J., Hu M., Geng P. & Zhang Y. Microbial detoxification of bifenthrin by a novel yeast and its potential for contaminated soils treatment. PloS one 7, e30862 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Gutnick D. & Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9, 29–33 (1980). [Google Scholar]
- Mulla S. I., Manjunatha T. P., Hoskeri R. S., Tallur P. N. & Ninnekar H. Z. Biodegradation of 3-Nitrobenzoate by Bacillus flexus strain XJU-4. World J Microbiol Biotechnol 27, 1587–1592 (2011). [Google Scholar]
- Boschker H. T. S. et al. Direct linking of microbial populations to specific biogeochemical processes by 13C-labelling of biomarkers. Nature 392, 801–805 (1998). [Google Scholar]
- Moore-Kucera J. & Dick R. P. Application of 13C-labeled litter and root materials for in situ decomposition studies using phospholipid fatty acids. Soil Biol Biochem 40, 2485–2493 (2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.