Abstract
The effects of green tea extract (GTE) on N-methyl-N-nitrosourea (MNU)-induced photoreceptor cell apoptosis were examined, and the possible mechanisms of action of GTE were assessed. Alterations in the retinal morphological architecture were determined by hematoxylin-eosin staining, vimentin immunoreactivity, and photoreceptor cell apoptosis (TUNEL labeling). Expression of oxidant marker, heme oxygenase (HO)-1, mRNA levels in outer nuclear cells was assessed by laser capture microdissection (LCM). Sprague-Dawley rats were given 40 mg/kg MNU at 7 weeks of age in the absence and presence of 250 mg/kg GTE treatment (once daily from 3 days prior to MNU for a maximum 10 days). Although photoreceptor cell degeneration began 24 hr after MNU, the morphological effects of GTE at the time point were not definitive. However, GTE lowered TUNEL labeling and HO-1 mRNA expression. At 7 days after MNU, photoreceptor damage was attenuated by GTE treatment. Therefore, the ability of GTE to reduce MNU-induced photoreceptor cell apoptosis may be due to its antioxidant properties.
Keywords: apoptosis, green tea extract, heme oxygenase-1, laser capture microdissection, N-methyl-N-nitrosourea, photoreceptor cell
Retinitis pigmentosa (RP) is one of the most common forms of inherited blindness worldwide; the pathology begins with early night blindness followed by peripheral visual field alterations (tunnel vision) and eventually leads to blindness1, 2. Identification of an effective therapeutic agent against RP is desired because of the current absence of such treatments for RP. Therefore, animal models of RP are important for understanding human RP. Among the various RP animal models, the N-methyl-N-nitrosourea (MNU) model causes photoreceptor cell apoptosis similar to the mechanism of human RP3, 4. MNU-induced photoreceptor cell apoptosis is useful in the search for human RP treatments, as it is complete within 7 days following a single systemic administration of MNU to various animal species5, 6. Oxidative stress is thought to play a major role in patients with RP7. Oxidative stress contributes to the progression of MNU-induced photoreceptor cell apoptosis8, 9, and chemicals such as edaravone, a free radical scavenger8, and curcumin, a naturally occurring yellow pigment of turmeric and constituent of curry powder10 that possesses an antioxidant property, suppress photoreceptor cell loss in the MNU model. Edaravone and curcumin inhibit MNU-induced photoreceptor cell apoptosis by suppressing oxidative stress by decreasing the levels of 8-hydroxy-2’-deoxyguanosine8, 10. Green tea extract (GTE) has been reported to suppress MNU-induced photoreceptor apoptosis in Sprague-Dawley rats11. GTE possess pleiotropic effects such as antioxidative, anti inflammatory, and anticancerous properties12. GTE contains epigallocatechin gallate (EGCG) as a major ingredient, and EGCG acts as an antioxidant to reduce photoreceptor cell damage13,14,15. However, the mechanisms of action of GTE on MNU-induced photoreceptor apoptosis have not been elucidated.
Heme oxygenase (HO), the rate-limiting enzyme in the heme degradative pathway, is induced in many cell types in response to a variety of stimuli. Three HO isoenzymes are expressed, including HO-1, HO-2, and HO-3. Among these subtypes, HO-1 is an inducible isoform that is involved in the response to oxidative stress16 and may be a marker of cellular responses to oxidative damage. HO activation plays a role in photoreceptor cell loss and functions protectively by reducing oxidative injury17. However, it is not clear whether GTE-induced changes in HO-1 expression occur in photoreceptor cells after MNU damage. In the present study, the role of HO-1 in MNU-induced photoreceptor cell apoptosis was studied.
Six-week-old female Sprague-Dawley rats [Crl:CD(SD)] purchased from Charles River Laboratories Japan (Osaka, Japan) were housed in groups of 3 in plastic cages with paper bedding (Paper Clean, SLC, Hamamatsu, Japan) in a specific pathogen-free environment maintained at 22 ± 2°C and 60 ± 10% relative humidity, with a 12-hr light/dark cycle (lights on from 8:00 a.m. to 8:00 p.m.; illumination intensity less than 60 lux at the cage level). Animals were maintained on a commercial pellet diet (CMF 30 kGy, Oriental Yeast, Chiba, Japan) and had free access to water. The study protocol and animal procedures were approved by the animal care and use committee of Kansai Medical University (Permit Number: 15-021). Throughout the experiments, animals were cared for in accordance with the Guidelines for Animal Experimentation of Kansai Medical University.
MNU in powder form was obtained from Sigma-Aldrich (St. Louis, MO, USA) and stored at 4°C in the dark. A 5 mg/mL solution was prepared by dissolving MNU in physiologic saline containing 0.1% acetic acid immediately before use. The GTE used was THEA-FLAN 90S (ITO EN, Ltd., Tokyo, Japan), a decaffeinated product. Approximately 70% of the polyphenols in GTE are tea catechins with a galloyl moiety; these include EGCG (54%), epicatechin (12.4%), gallocatechin gallate (2.8%), catechin gallate (0.4%), epigallocatechin (0.3%), gallocatechin (0.1%), caffeine (0.0%), and others (0.3%)11. GTE was dissolved at 40 mg/mL in sterile distilled water just prior to use.
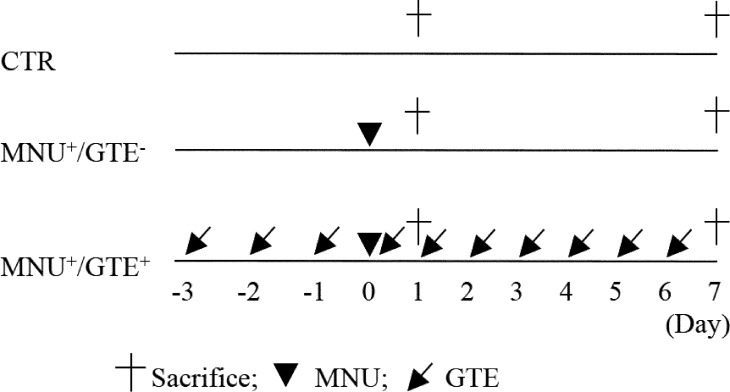
Female rats received a single intraperitoneal injection of 40 mg/kg MNU at 7 weeks of age. A daily dose of 250 mg/kg GTE was orally administered by gastric intubation starting 3 days prior to MNU administration, and administration was continued once daily for a maximum 10 days (Fig. 1). MNU and GTE doses were determined according to our previous report11. Control rats received an equivalent volume of saline instead of MNU and sterile distilled water instead of GTE at the same time points. Rats were divided into the following groups: control (CTR), MNU-exposed and GTE-untreated (MNU+/GTE−), and MNU-exposed and GTE-treated (MNU+/GTE+) groups. On the day of MNU exposure, GTE was administered 2 hr prior to MNU injection. All rats were observed daily for clinical signs of toxicity and were weighed on the day of MNU administration and on the day of sacrifice. Rats were sacrificed 24 hr and 7 days after MNU administration; GTE was not administered on the day of sacrifice. At the time of sacrifice, animals were euthanized under isoflurane (Wako, Osaka, Japan) anesthesia, and both eyes were quickly removed from each animal.
Fig. 1.
Schematic representation of the experimental protocol. The experiment included control (CTR), MNU-exposed and GTE-untreated (MNU+/GTE−), and MNU-exposed and GTE-treated (MNU+/GTE+) groups. CTR rats received equivalent volumes of saline and sterile distilled water instead of MNU and GTE, respectively, at the same time points.
One eye was fixed overnight in 10% neutral buffered formalin, and the other eye was fixed overnight in methacarn (60% methanol, 30% chloroform, and 10% acetic acid). Formalin- and methacarn-fixed samples were embedded in paraffin, sectioned at 4 μm, stained with hematoxylin and eosin (HE), and used for vimentin immunohistochemistry or TUNEL staining.
Methacarn-fixed sections were utilized to detect dynamic activities of Müller cells using anti-vimentin monoclonal antibody (×500; Santa Cruz, Dallas, TX, USA). The antigen antibody complexes were identified using a streptavidin biotin (LSAB) staining kit (Dako, Carpinteria, CA, USA), and the reaction products were visualized with 3,3′-diaminobenzidine tetrahydrochloride (DAB).
Formalin-fixed retinal tissues were evaluated by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP digoxigenin nick end-labeling (TUNEL) staining with an in situ apoptosis detection kit (TACS2 TdT; Trevigen, Gaithersburg, MD, USA), and the reaction products were visualized with DAB. Quantitative measurements were made from the central retina (approximately 400 μm from the optic nerve), and the number of TUNEL-positive cells per square millimeter was counted.
Three samples from 3 groups were used for laser capture microdissection (LCM). Formalin-fixed samples embedded in paraffin were sectioned at 10 μm × 3 slices. The sections were collected on Arcturus PEN Membrane Glass Slides (Applied Biosystems, Carlsbad, CA, USA), air dried overnight, and deparaffinized (2 minutes × 2 in xylol). Retinal layers were isolated using an ArcturusXT LCM System and Arcturus CapSure Macro LCM Caps (Applied Biosystems). LCM was performed by separately lifting the outer nuclear layer. RNA was isolated using a RecoverAll Total Nucleic Acid Isolation Kit (Ambion, Carlsbad, CA, USA), according to the manufacturer’s directions, and included a DNase treatment. Synthesis of cDNA was performed using a SuperScript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA) and analyzed by real-time PCR.
Levels of mRNA were assessed by real-time quantitative PCR using a Rotor-Gene SYBR Green PCR Kit (QIAGEN, Hilden, Germany) and Rotor-Gene Q (QIAGEN) with two standard curve methods, according to the manufacturer’s instructions. The following conditions were used: 95°C for 5 minutes (PCR initial activation step) followed by 40 cycles of 95°C for 5 sec (denaturation) and 60°C for 10 sec (combined annealing/extension). The mRNA level of the HO-1 gene was normalized to GAPDH. The GAPDH primers used were from the QuantiTect Primer Assay (QIAGEN). Master Mixes (22.5 µL) were added to 2.5 µL of cDNA. The HO-1 PCR primers produced a 110 bp product and were as follows: 5′-TCTATCGTGCTCGCATGAAC -3′ and 5′-CAGCTCCTCAAACAGCTCAA -3′18, 19. The intensity was quantified using image analysis software (Rotor-Gene Software ver 2.3.1.49, QIAGEN), and the results were standardized against the intensity of GAPDH.
Values were expressed as the mean ± SE and were analyzed using a one-tailed independent t-test for paired or unpaired samples after assuring the homogeneity of variances with the Excel Statistics Program File ystat 2008.xls. P values of <0.05 were accepted as statistically significant.
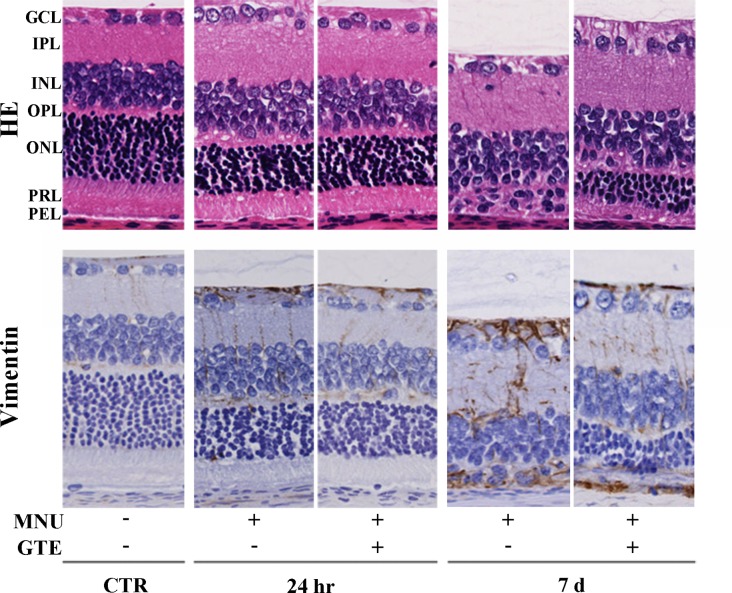
Twenty-four hours after MNU exposure, increased pyknosis and karyorrhexis of the photoreceptor nuclei and disarrangement of the photoreceptor segments were observed in HE sections (Fig. 2). However, although photoreceptor cell degeneration had begun by this time point, similar numbers of photoreceptor cells were found, and Müller cell proliferation was similar in both the GTE-treated and GTE-untreated rat retina according to HE staining and vimentin immunoreactivity, respectively. Therefore, the effect of GTE on disease suppression at this time point, from a structural perspective, was not conclusive. However, 7 days after MNU exposure, photoreceptor cells were virtually absent in the MNU+/GTE− retina, while GTE treatment partially ameliorated MNU injury with partial retention of photoreceptor cells and suppression of Müller cell proliferation, as seen by vimentin staining, in the MNU+/GTE+ retina.
Fig. 2.
Retinal morphology and vimentin immunoreactivity in normal animals and those treated with N-methyl-N-nitrosourea (MNU) (24 hr and 7 days), with or without green tea extract (GTE). Retinal regions depicted are as follows: GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PRL, photoreceptor layer; PEL, pigment epithelial layer.
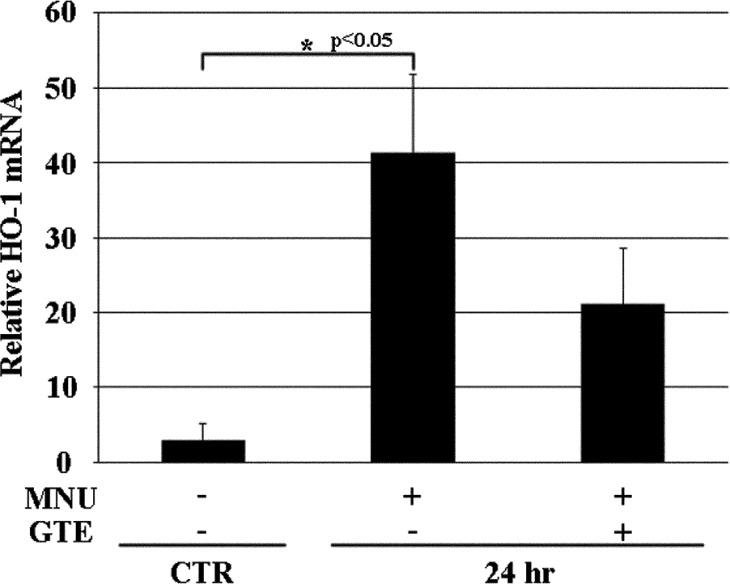
While the effect of GTE on disease suppression could be observed 7 days after MNU at the structural level, morphology alone could not be used to discern the effects of GTE 24 hr after MNU. Therefore, TUNEL staining and HO-1 expression were quantitatively evaluated 24 hr after MNU exposure. TUNEL-positive cells were not detected in the CTR retina, and MNU exposure produced more TUNEL-positive cells in the MNU+/GTE- retina compared with the MNU+/GTE+ retina (Fig. 3). HO-1 mRNA expression was barely detectable in CTR photoreceptor cells, while significantly higher expression occurred in the MNU+/GTE− retina; HO-1 expression tended to decrease in the MNU+/GTE+ retina compared with the MNU+/GTE− retina (Fig. 4).
Fig. 3.
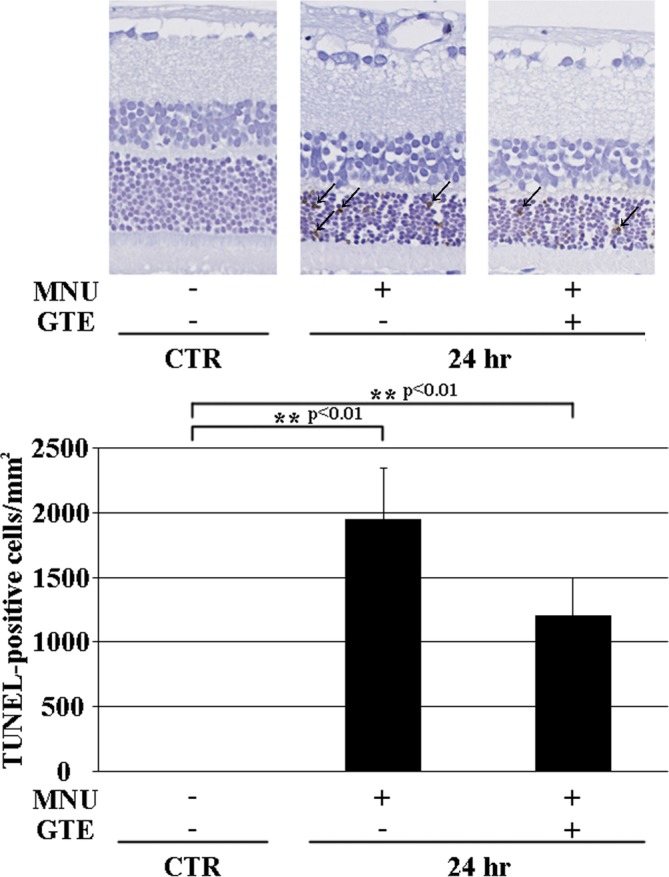
TUNEL labeling in normal animals and those treated with N-methyl-N-nitrosourea (MNU) (after 24 hr) with or without green tea extract (GTE). Arrows indicate TUNEL-positive photoreceptor cells.
Fig. 4.
mRNA expression of HO-1 in normal animals and those treated with N-methyl-N-nitrosourea (MNU) (after 24 hr) with or without green tea extract (GTE).
EGCG, a main component of GTE, has been shown to suppress photoreceptor cell damage caused by various agents13,14,15. In the present study, daily oral administration of 250 mg/kg GTE effectively attenuated MNU-induced photoreceptor cell apoptosis, and the compensatory proliferation of Müller cells was suppressed 7 days after MNU. At 24 hr after MNU, GTE suppressed photoreceptor cell apoptosis and downregulated HO-1 expression when retinal morphology was indistinguishable. LCM of the outer nuclear layer (photoreceptor cells) was performed, rather than isolation of total retinal RNA20, to obtain a more accurate comparison of mRNA HO-1 levels.
HO-1 mRNA and protein are barely detectable in the normal retina, whereas they are overexpressed in response to photooxidative (light) damage. The antioxidant dimethylthiourea suppresses light-induced photoreceptor damage by blocking the induction of HO-1; HO-1 induction is oxidatively driven21 and produces an antioxidative effect. The iron chelators deferiprone22 and α-lipoic acid23, as well as curcumin24, which has antioxidant properties, suppress light-induced photoreceptor damage accompanied by downregulation of HO-1 expression. The protease inhibitor bortezomib suppresses ischemia/reperfusion-induced inner retinal injury caused by oxidative stress; while expression of HO-1 mRNA is low in the normal retina, increased expression caused by ischemia/reperfusion is significantly lowered by bortezomib9. Antioxidants decrease oxidative stress and reduce induction of HO-1 in inner retinal cells and photoreceptor cells.
In general, antioxidant therapy has been reported to downregulate HO-1. This effect has been described extensively in oxidative stress-induced ocular disease17. However, upregulation of HO-1 is seen when curcumin treatment reduces H2O2-induced damage in cultured photoreceptor cells24 and when resveratrol treatment reduces inner retinal injury caused by retinal ischemic/reperfusion injury25. Adeno-associated virus moderates the levels of HO-1 expression in photoreceptors, resulting in cytoprotection from light damage by attenuating apoptosis26. However, while moderate levels of HO-1 expression protect against oxidative stress, higher levels of HO-1 expression result in susceptibility to oxidative stress, indicating the existence of paradoxical HO-1 actions27. As HO-1 expression increases after exposure to oxidative stress compared with the level in cells not exposed to stress, HO-1 acts as a defense mechanism. However, the present study and many other studies indicate that excessive stress can produce extremely high HO-1 expression, which may cause deleterious effects. Conversely, during moderate levels of stress, antioxidant treatment can produce increased HO-1 expression. Differences in the dose and degree of oxidative stress or in measurement timing may produce different results27,28,29. Using the present MNU dose regimen, GTE effectively attenuated photoreceptor cell apoptosis by lowering HO-1 mRNA expression.
Acknowledgments
This project was supported by a grant from the MEXT-Supported Programs for the Strategic Research Foundations at Private Universities. We are grateful to Ms. T. Akamatsu for her technical help and Ms. A. Shudo for manuscript preparation.
Footnotes
Disclosure of potential conflicts of interest: In connection with this paper, there are no conflicts of interest with any companies to be disclosed.
References
- 1.Hartong DT, Berson EL, and Dryja TP. Retinitis pigmentosa. Lancet. 368: 1795–1809. 2006. [DOI] [PubMed] [Google Scholar]
- 2.Davis RJ, Hsu CW, Tsai YT, Wert KJ, Sancho-Pelluz J, Lin CS, and Tsang SH. Therapeutic margins in a novel preclinical model of retinitis pigmentosa. J Neurosci. 33: 13475–13483. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakajima M, Yuge K, Senzaki H, Shikata N, Miki H, Uyama M, and Tsubura A. Photoreceptor apoptosis induced by a single systemic administration of N-methyl-N-nitrosourea in the rat retina. Am J Pathol. 148: 631–641. 1996. [PMC free article] [PubMed] [Google Scholar]
- 4.Tsubura A, Yoshizawa K, and Sasaki T. Methylnitrosourea. In: Encyclopedia of Toxicology, 3rd ed., Vol. 3. P Wexler (ed). Academic Press/Elsevier, Oxford. 321–323. 2014. [Google Scholar]
- 5.Tsubura A, Yoshizawa K, and Kuro M. N-Methyl-N-nitrosourea animal models for retinitis pigmentosa. In: Animal Models for the Study of Human Disease. PM Conn (ed). Academic Press/Elsevier, Oxford. 117–142. 2013. [Google Scholar]
- 6.Yoshizawa K, Emoto Y, and Tsubura A. N-Methyl-N-nitrosourea-induced retinal degeneration in rats: a reliable animal model of retinitis pigmentosa for the development of new therapeutic strategies. In: Recent Advances in Eye Research. A Hogarth (ed). Nova Science Publishers, Hauppauge. 53–97. 2015. [Google Scholar]
- 7.Campochiaro PA, Strauss RW, Lu L, Hafiz G, Wolfson Y, Shah SM, Sophie R, Mir TA, and Scholl HP. Is there excess oxidative stress and damage in eyes of patients with retinitis pigmentosa? Antioxid Redox Signal. 23: 643–648. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuruma K, Yamauchi M, Inokuchi Y, Sugitani S, Shimazawa M, and Hara H. Role of oxidative stress in retinal photoreceptor cell death in N-methyl-N-nitrosourea-treated mice. J Pharmacol Sci. 118: 351–362. 2012. [DOI] [PubMed] [Google Scholar]
- 9.Chen FT, Yang CM, and Yang CH. The protective effects of the proteasome inhibitor bortezomib (velcade) on ischemia-reperfusion injury in the rat retina. PLoS ONE. 8: e64262 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emoto Y, Yoshizawa K, Uehara N, Kinoshita Y, Yuri T, Shikata N, and Tsubura A. Curcumin suppresses N-methyl-N-nitrosourea-induced photoreceptor apoptosis in Sprague-Dawley rats. In Vivo. 27: 583–590. 2013. [PubMed] [Google Scholar]
- 11.Emoto Y, Yoshizawa K, Kinoshita Y, Yuri T, Yuki M, Sayama K, Shikata N, and Tsubura A. Green tea extract suppresses N-methyl-N-nitrosourea-induced photoreceptor apoptosis in Sprague-Dawley rats. Graefes Arch Clin Exp Ophthalmol. 252: 1377–1384. 2014. [DOI] [PubMed] [Google Scholar]
- 12.Feng WY. Metabolism of green tea catechins: an overview. Curr Drug Metab. 7: 755–809. 2006. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, and Osborne NN. Oxidative-induced retinal degeneration is attenuated by epigallocatechin gallate. Brain Res. 1124: 176–187. 2006. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B, Safa R, Rusciano D, and Osborne NN. Epigallocatechin gallate, an active ingredient from green tea, attenuates damaging influences to the retina caused by ischemia/reperfusion. Brain Res. 1159: 40–53. 2007. [DOI] [PubMed] [Google Scholar]
- 15.Costa BL, Fawcett R, Li GY, Safa R, and Osborne NN. Orally administered epigallocatechin gallate attenuates light-induced photoreceptor damage. Brain Res Bull. 76: 412–423. 2008. [DOI] [PubMed] [Google Scholar]
- 16.Stocker R. Induction of haem oxygenase as a defence against oxidative stress. Free Radic Res Commun. 9: 101–112. 1990. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J, Tan S, Liu F, Zhang Y, Su M, and Sun D. Heme oxygenase and ocular disease: a review of the literature. Curr Eye Res. 37: 955–960. 2012. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida T, Kurella M, Beato F, Min H, Ingelfinger JR, Stears RL, Swinford RD, Gullans SR, and Tang SS. Monitoring changes in gene expression in renal ischemia-reperfusion in the rat. Kidney Int. 61: 1646–1654. 2002. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T, and Nangaku M. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol. 14: 1825–1832. 2003. [DOI] [PubMed] [Google Scholar]
- 20.Lohr HR, Kuntchithapautham K, Sharma AK, and Rohrer B. Multiple, parallel cellular suicide mechanisms participate in photoreceptor cell death. Exp Eye Res. 83: 380–389. 2006. [DOI] [PubMed] [Google Scholar]
- 21.Kutty RK, Kutty G, Wiggert B, Chader GJ, Darrow RM, and Organisciak DT. Induction of heme oxygenase 1 in the retina by intense visible light: suppression by the antioxidant dimethylthiourea. Proc Natl Acad Sci USA. 92: 1177–1181. 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song D, Song Y, Hadziahmetovic M, Zhong Y, and Dunaief JL. Systemic administration of the iron chelator deferiprone protects against light-induced photoreceptor degeneration in the mouse retina. Free Radic Biol Med. 53: 64–71. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao L, Wang C, Song D, Li Y, Song Y, Su G, and Dunaief JL. Systemic administration of the antioxidant/iron chelator α-lipoic acid protects against light-induced photoreceptor degeneration in the mouse retina. Invest Ophthalmol Vis Sci. 55: 5979–5988. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandal MN, Patlolla JM, Zheng L, Agbaga MP, Tran JT, Wicker L, Kasus-Jacobi A, Elliott MH, Rao CV, and Anderson RE. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Med. 46: 672–679. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu XQ, Wu BJ, Pan WH, Zhang XM, Liu JH, Chen MM, Chao FP, and Chao HM. Resveratrol mitigates rat retinal ischemic injury: the roles of matrix metalloproteinase-9, inducible nitric oxide, and heme oxygenase-1. J Ocul Pharmacol Ther. 29: 33–40. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun MH, Pang JH, Chen SL, Kuo PC, Chen KJ, Kao LY, Wu JY, Lin KK, and Tsao YP. Photoreceptor protection against light damage by AAV-mediated overexpression of heme oxygenase-1. Invest Ophthalmol Vis Sci. 48: 5699–5707. 2007. [DOI] [PubMed] [Google Scholar]
- 27.Maruhashi K, Kasahara Y, Ohta K, Wada T, Ohta K, Nakamura N, Toma T, Koizumi S, and Yachie A. Paradoxical enhancement of oxidative cell injury by overexpression of heme oxygenase-1 in an anchorage-dependent cell ECV304. J Cell Biochem. 93: 552–562. 2004. [DOI] [PubMed] [Google Scholar]
- 28.Picard E, Ranchon-Cole I, Jonet L, Beaumont C, Behar-Cohen F, Courtois Y, and Jeanny JC. Light-induced retinal degeneration correlates with changes in iron metabolism gene expression, ferritin level, and aging. Invest Ophthalmol Vis Sci. 52: 1261–1274. 2011. [DOI] [PubMed] [Google Scholar]
- 29.He M, Pan H, Xiao C, and Pu M. Roles for redox signaling by NADPH oxidase in hyperglycemia-induced heme oxygenase-1 expression in the diabetic retina. Invest Ophthalmol Vis Sci. 54: 4092–4101. 2013. [DOI] [PubMed] [Google Scholar]