Abstract
Background
The MRI scanner environment induces marked psychological effects, but specific effects on pain perception and processing are unknown and relevant to all brain imaging studies.
Objectives and methods
We performed visceral and somatic quantitative sensory and pain testing and studied endogenous pain modulation by heterotopic stimulation outside and inside the functional MRI scanner in 11 healthy controls and 13 patients with irritable bowel syndrome.
Results
Rectal pain intensity (VAS 0–100) during identical distension pressures increased from 39 (95% confidence interval: 35–42) outside the scanner to 53 (43–63) inside the scanner in irritable bowel syndrome, and from 42 (31–52) to 49 (39–58), respectively, in controls (ANOVA for scanner effect: p = 0.006, group effect: p = 0.92). The difference in rectal pain outside versus inside correlated significantly with stress (r = −0.76, p = 0.006), anxiety (r = −0.68, p = 0.02) and depression scores (r = −0.67, p = 0.02) in controls, but not in irritable bowel syndrome patients, who a priori had significantly higher stress and anxiety scores. ANOVA analysis showed trends for effect of the scanner environment and subject group on endogenous pain modulation (p = 0.09 and p = 0.1, respectively), but not on somatic pain (p > 0.3).
Conclusion
The scanner environment significantly increased visceral, but not somatic, pain perception in irritable bowel syndrome patients and healthy controls in a protocol specifically aimed at investigating visceral pain. Psychological factors, including anxiety and stress, are the likely underlying causes, whereas classic endogenous pain modulation pathways activated by heterotopic stimulation play a lesser role. These results are highly relevant to a wide range of imaging applications and need to be taken into account in future pain research. Further controlled studies are indicated to clarify these findings.
Keywords: fMRI environment, visceral pain, endogenous pain modulation, irritable bowel syndrome, brain imaging, anxiety, conditioned pain modulation
Introduction
The perception and processing of pain is highly individualized and powerfully influenced by cognitive and emotional mechanisms, with genetics and physiologic factors also playing important roles.1,2 Attention, expectation, distraction, hypervigilance, reappraisal, anxiety and mood can affect pain intensity or cause painful experiences without any nociceptive input.3 These mechanisms play an important part in chronic functional pain syndromes, where an organic cause is not obvious. Irritable bowel syndrome (IBS) is one of the most prevalent functional pain disorders associated with changes in bowel habits and affecting approximately 10–15% of many populations.4 These patients often have co-morbid psychological disorders such as depression and anxiety,5 influencing central processing of pain and resulting in sensory hypersensitivity, sensitization and abnormal endogenous pain modulation (EPM).6–9 The symptom presentation in IBS can be affected by experimental manipulation of the psychological state, such as emotion induction and pharmacologic intervention.10–13 The hypersensitivity in IBS is also highly responsive to the expectation and anticipation effects underlying placebo and nocebo manipulations.14,15 Emotions have been shown to modulate experimentally-induced pain perception in healthy subjects, with negative emotions increasing and positive emotions decreasing pain perception.16 Moreover, various cognitive processes can bias pain perception and processing in the healthy brain.17 Therefore, the consideration of psychological factors in the interpretation of pain processing data is of paramount importance.
Brain functional magnetic resonance imaging (fMRI) is used to study pain processing by evaluating the haemodynamic response of the brain in relation to neural activity and has shown aberrant sensory processing in various pain disorders, including IBS.9,18,19 Areas consistently affected include those integrating and governing psychological responses and EPM, such as prefrontal regions, cingulate cortices, insula, amygdala, cerebellum and the dorsal brainstem.3,20 Good concordance was reported between the psychological abnormalities in chronic pain disorders and abnormal brain activation patterns in these regions. This has been shown especially for the important modulatory factors of anxiety, hypervigilance and expectation.14,21–24 To avoid artefacts in these sensitive imaging measures, a neutral and standardized testing environment is important. However, the highly artificial brain fMRI scanning environment itself, on which the definition of processing abnormalities is based, has received surprisingly little scrutiny despite undisputed induction of many psychological modulations known to affect pain processing. MRI examination is known to induce anxiety- and stress-related reactions. These reactions may involve the fear of enclosed places, and distress induced by the hammering noise and the temperature inside the tunnel, the expectation of being hurt, the worry of the unknown, and the stress of coping with the unusual environment.25–28 Patients who suffer from mood disorders are particularly vulnerable to the stressful properties of the MRI scanner environment.29 The effect of the fMRI environment on pain perception has to the best of our knowledge never been reported. Therefore, the current study investigated whether the fMRI scanning environment induces alterations in visceral and somatic pain testing, as well as in the endogenous modulation of pain, in IBS patients and healthy controls, and whether the IBS patients are affected differently from controls.
Materials and methods
This was a retrospective investigation of an imaging study performed in IBS patients and healthy controls in a single centre at the National University Hospital of Singapore (NUH), from February 2009 to December 2010. All patients included in the original imaging study were also included in this data re-analysis.
Inclusion and exclusion criteria
Thirteen male and female IBS patients diagnosed according to the Rome III criteria30 and aged 21–70 years were recruited from the NUH gastroenterology and colorectal clinics or by public advertising. Study inclusion criteria for IBS were an IBS symptom rating of at least 175 using the IBS severity scoring system (IBS-SSS; moderate intensity)31 in the last two weeks before study inclusion, with discomfort or pain due to IBS as the most prominent symptom. Main exclusion criteria included any organic gastrointestinal or other significant systemic disease, including cardiovascular, psychiatric, neurological and endocrine diseases, chronic or acute pain conditions, abdominal operations except appendectomy or hysterectomy, a history of brain disease or brain surgery, pregnancy or lactation, any medication known to affect nociception, drug abuse, claustrophobia or metal implants in the body. Eleven healthy volunteers matched for age and without any gastrointestinal pathology or history of significant abdominal pain, bowel disorders, bloating or discomfort during the last three months, as well as the above exclusion criteria and the absence of any ongoing medication were recruited by word of mouth. None of the patients or controls had previously participated in clinical trials or undergone MRI scanning.
Ethics approval and consideration
The study protocol was approved by the Institutional Review Board and Domain Specific Review Board of the National Healthcare Group, Singapore. All participants gave written informed consent and were remunerated for their participation. The trial was registered at Clinical Trials.gov with the number NCT00693732.
Study procedure
Subjects were seen on two occasions. At the first visit the IBS-SSS score, average abdominal pain and discomfort intensities in the past week on a computerized, anchored, horizontal visual analogue scale (COVAS; 0 = none, 100 = maximum bearable), average pain and discomfort duration, the Perceived Stress Scale 10-item questionnaire (PSS-10),32 the Hospital Anxiety and Depression Scale (HADS)33 and general demographic data were completed and subjects were familiarized with the study procedures. Patients then completed the two-week observation run-in period for prospective assessment of symptoms and pain. The actual experimental procedures were performed two weeks after the first visit. The sensory tests were always performed in the same time window in the morning to minimize diurnal rhythm influences and within 7–14 days after the end of the menstrual period in women to standardize the influence of the menstrual period. Identical instructions were given before each test and ambient test conditions were standardized and similar for the sessions inside and outside the scanner. Participants first underwent a series of stimulations lying in a supine position on an examination bed outside the scanner, in the preparation room connected to the MRI scanner room. The ambient conditions, including temperature and humidity, were identical to those within the scanner room. Rectal distension, foot heat, and simultaneous rectal distension with foot heat heterotopic stimulation were performed in randomized order (see below for details). Heterotopic stimulation, also described as conditioned pain modulation, is a standardized method for induction of EPM.34–36 Participants were then transferred to the scanner room, and moved into the MRI scanner magnet in supine position with the head coil and restraint in situ. They were instructed that they would experience rectal, foot, and rectal with foot stimulations in random sequence, exactly as had been performed outside the scanner, after a relaxation period of 30 min. Brain scanning was performed in subsequent sessions, but these data are not reported as part of this paper.
Rectal stimulation protocol
An enema of 300 ml of warm water was instilled for evacuation of the rectum. After testing for leakage by inflating with air under water, a lubricated 400 ml polyethylene bag (Mui Scientific, Toronto, Canada) attached to the top of a flexible catheter was inserted 5 cm into the subject’s rectum in left lateral position. The bag was slowly inflated with air to a pressure of 5 mmHg to ensure good contact between the bag and the rectal mucosa. The subject was positioned on a bed in a comfortable supine position with legs slightly apart, covered with a light sheet with the knees flexed and supported on pillows. After a 5-min acclimatization period, the preliminary quantitative sensory tests described below were commenced.
First sensation, first pain and pain tolerance rectal distension thresholds were determined using an ascending methods of limits (AML) protocol with incremental steps of 5 mmHg, 30-s distension and intermittent rest periods applied by a barostat (G&J Electronics Inc., Toronto, Canada), which was programmed to an inflation speed of 40 ml/s and a cut-off pressure of 60 mmHg. Additionally, within the AML protocol the pain intensity ratings were recorded by COVAS at the end of every stimulus step. Rectal distension thresholds were measured twice with a 30-s break and the results from the second run were used for analysis to avoid novelty effects. Five minutes later, the stimulation pressure which induced a moderate pain intensity (VAS 30–55) at the end of the 30-s distension was determined from the second stimulation run and used as the tonic rectal distension pressure, which was applied separately for 30 s. The pain intensities were recorded before and after the stimulation procedure on the COVAS scale.
Foot heat stimulation protocol
After a break of 10 min, a contact thermode (Medoc Pathway model, ATS/CHEPS system, Ramat Yishai, Israel) was placed halfway along the dorsum of the left foot and fastened by an elastic rubber strap. The first heat sensation, first pain and pain tolerance thresholds were determined using an AML protocol with incremental steps of 1℃ (beginning at 36℃ and with a maximum temperature of 48℃) and 30-s stimulation and rest periods. Pain intensity ratings were recorded by COVAS at the end of every stimulus step. Five minutes later, the stimulation temperature inducing moderate pain intensity (VAS 30–55) at the end of the 30-s stimulation was determined and used as the tonic foot heat pain stimulation, which was separately applied for 30 s. Pain intensity was recorded by COVAS before and after the stimulation.
Heterotopic (rectal plus foot) stimulation protocol – activating EPM
After a break of 10 min, foot heat pain and rectal distension pain at the titrated moderate intensities determined above were performed simultaneously (heterotopic stimulation for EPM) for 30 s. Subjects were instructed to focus on only their rectal pain during heterotopic stimulation. Rectal pain intensity was rated after 30 s on the COVAS scale.
Statistical analysis
Data were analysed using SAS 9.3 (SAS Institute, Cary, NC, USA). Continuous variables were expressed as the arithmetic mean with 95% confidence intervals (CIs), and categorical variables were expressed as frequencies and percentages. Significance thresholds of p < 0.05 were applied. EPM was defined as the rectal pain intensity rated after 30 s during heterotopic foot stimulation minus the rectal pain intensity rated after 30 s without heterotopic foot stimulation.
The sensory and pain thresholds measured outside of the scanner were compared between IBS patients and healthy controls by unpaired Student’s t-test. To compare mean pain ratings in response to tonic pain stimulation outside and inside the scanner, as well as between IBS patients and controls, a two-way mixed ANOVA with ‘group’ (IBS versus controls) as the between-subject factor and ‘scanner’ (inside versus outside scanner) as the within-subject factor, was used. To test for a differential effect of the scanner environment between IBS patients and controls, a ‘group’-by-‘scanner’ interaction effect was included.
Analysis of covariance with ‘group’ and ‘pain ratings inside the scanner’ as categorical and continuous independent variables, respectively, and ‘pain ratings outside the scanner’ as the dependent variable was estimated; the ‘group’-by-‘pain ratings inside the scanner’ interaction effect was tested to check whether the relationship between pain ratings inside and outside the scanner differed significantly between IBS patients and controls.
Results
Participants
Thirteen IBS patients, mean age 37.2 years (95% CI 33.0–44.5) and seven females, and eleven healthy controls, mean age 37.1 years (95% CI 30.1–44.1) and seven females, were recruited in this study. The demographic data and psychological characteristics of the participants are listed in Table 1. IBS patients were closely matched with controls for age, gender and race, but demonstrated significantly higher PSS-10 and HADS anxiety scores. HADS depression scores were within the normal range and similar in both groups. The numbers of patients with the different IBS subtypes were balanced. Among 13 patients, four (30.8%) had IBS-constipation, four (30.8%) had IBS-diarrhoea and five (38.5%) had IBS-alternating. The mean IBS-SSS score was 220.9 (189.2–252.6).
Table 1.
Subject characteristics
| IBS patients | Healthy volunteers | p value | |
|---|---|---|---|
| Number of subjects: N | 13 | 11 | – |
| Age (95% CI): years | 37.2 (33.0–44.5) | 37.1 (30.1–44.1) | 0.978 |
| Female subjects: n (%) | 7/13 (53.8%) | 7/11 (63.6%) | 0.697 |
| Chinese subjects: n (%) | 10/13 (76.9%) | 7/11 (63.6%) | 0.659 |
| PSS-10 scoresa: mean (95% CI) | 21.6 (19.3 –23.9) | 15.0 (10.0–20.8) | 0.019 |
| PSS-10 scoresa >18: n (%) | 10/13 (76.9%) | 4/11 (36.4%) | 0.095 |
| Anxiety HADS scoresb: mean (95% CI) | 9.3 (7.1–11.5) | 4.4 (2.3–6.4) | 0.0008 |
| Anxiety HADS scoresb > 7: n (%) | 9/13 (69.2%) | 1/11 (9.1%) | 0.005 |
| Depression HADS scoresb: mean (95% CI) | 4.7 (2.6–6.8) | 2.8 (0.9 – 4.9) | 0.19 |
| Depression HADS scoresb >7: n (%) | 3/13 (23.1%) | 3/11 (27.3%) | 1.000 |
Scores of stress levels range from 0 to 40.
Scores of anxiety and depression range from 0 to 21, with < 7 defined as the normal range.
IBS: irritable bowel syndrome; CI: confidence interval; PSS-10: Perceived Stress Scale 10-item questionnaire; HADS: Hospital Anxiety Depression Scale.
Rectal thresholds and pain intensity during stimulation outside and inside the scanner
Rectal distension threshold pressures outside the scanner were similar between IBS patients and controls for first perception, first pain, moderate pain and pain tolerance (Table 2). As tonic rectal distension was titrated outside the scanner to induce moderate pain in all subjects, IBS and control subjects reported similar pain scores (38.9 (34.9–42.9) and 41.5 (37.5–45.5), respectively, p = 0.50).
Table 2.
Titration of visceral and somatic thresholds and moderate pain outside scanner
| Titration of stimulation thresholds outside scanner | IBS (N = 13) | Controls (N = 11) | p value | |
|---|---|---|---|---|
| Rectal distension (mmHg) | First perception | 18.0 (16.0–20.0) | 19.5 (16.8–22.1) | 0.22 |
| First pain | 31.5 (25.9–37.2) | 36.3 (28.6–44.0) | 0.27 | |
| Moderate paina | 40.1 (35.0–45.2) | 46.3 (39.4–53.3) | 0.12 | |
| Pain tolerance | 51.2 (46.3–55.0) | 55.0 (49.6–60.5) | 0.26 | |
| Foot heat (℃) | First perception | 38.4 (37.5–39.3) | 38.3 (37.4–39.2) | 0.77 |
| First pain | 42.3 (41.4–43.2) | 41.5 (40.3–42.6) | 0.20 | |
| Moderate paina | 44.5 (43.7–45.3) | 44.5 (43.6–45.5) | 0.94 | |
| Pain tolerance | 45.8 (45.0–46.5) | 45.8 (45.0–46.6) | 0.92 | |
Results expressed as mean (95% confidence interval).
Pain computerized visual analogue scale (COVAS) range from 30 to 55.
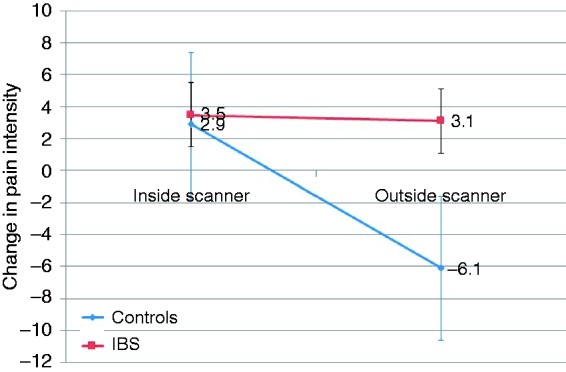
Two-way mixed ANOVA analysis showed a significant main effect of ‘scanner’ (F1,22 = 8.78, p = 0.006), due to significantly higher rectal pain ratings inside versus outside the scanner, but no significant main effect of ‘group’ (F1,22 = 0.01, p = 0.92), indicating similar pain ratings between groups over both measurements (Figure 1). Furthermore, there was no significant scanner-by-group interaction effect (F1,22 = 0.66, p = 0.43), indicating that the effect of the scanner environment did not differ significantly between IBS patients and healthy controls.
Figure 1.

Scanner environment effect on visceral pain in irritable bowel syndrome (IBS) subjects and controls.
A trend for the ‘group’-by-‘pain rating inside the scanner’ interaction effect in the analysis of covariance (β = 1.30 ± 0.68, p = 0.07) indicated a divergent relationship between pain outside and inside the scanner in IBS patients versus controls.
The difference in rectal pain outside versus inside the scanner (Δ rectal pain, calculated as VASrectal_pain_outside – VASrectal_pain_inside) correlated highly significantly with the stress (r = −0.76, p = 0.006), anxiety (r = −0.68, p = 0.02) and depression scores (r = −0.67, p = 0.02) in controls, but not in IBS patients (r = 0.35, p = 0.25; r = 0.43, p = 0.14; r = 0.48, p = 0.10, respectively). These divergent group effects were confirmed by analyses of covariance with Δ rectal pain as the dependent variable, which showed significant ‘group’-by-‘anxiety’, ‘group’-by-‘depression’ and ‘group’-by-‘stress’ interaction effects (β = 5.77 ± 1.82, p = 0.005; β = 5.85 ± 1.79, p = 0.004 and β = 3.20 ± 1.20, p = 0.015, respectively).
Endogenous visceral pain modulation outside and inside the scanner
EPM in IBS patients and controls is shown in Figure 2. The difference between IBS patients and controls in the change in rectal pain intensity during induction of EPM outside the scanner showed a trend (p = 0.09). The lack of significance may be due to the small sample size, and the effect size was large (Cohen’s d = 0.81). The mean change in pain intensity during EPM outside the scanner was an increase of 8% (3–20%) in IBS patients and a decrease of 9.6% (–75.4–56.1%) in controls. Two-way mixed ANOVA analysis showed neither a significant main effect of ‘scanner’ (F1,22 = 3.15, p = 0.09), due to insignificant effects on EPM outside versus inside the scanner, nor of ‘group’ (F1,22 = 3.01, p = 0.10), indicating largely similar EPM between groups over both measurements. The ‘scanner’-by-‘group’ interaction effect with EPM was also insignificant (F1,22 = 2.64, p = 0.12), demonstrating that the effect of the scanner environment did not differ significantly between IBS patients and healthy controls (see Figure 3). It should, however, be noted, that the ‘scanner’, ‘group’ and their interaction effects clearly yielded trends, which may be insignificant due to the small sample size. Figure 2 illustrates the divergent individual and group effects, with an overall switch from inhibition to facilitation of EPM in controls from outside to inside the scanner (Cohen’s d = 0.78, i.e. medium–large effect size), whereas IBS patients as a group maintained facilitation both outside and inside the scanner (Cohen’s d = 0.06, small effect size).
Figure 2.
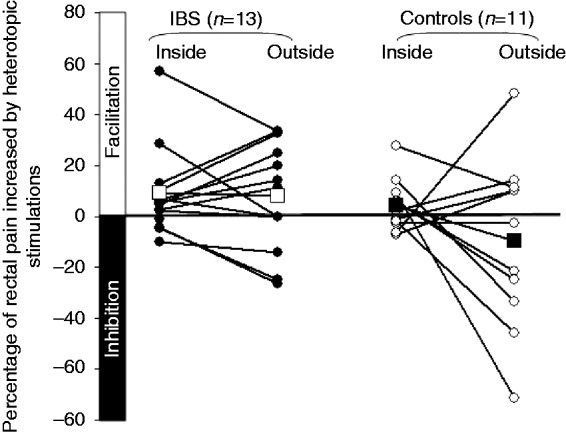
Individual endogenous pain modulation inside versus outside MRI scanner. (Individual data are indicated by round symbols, whereas mean values are signified by squares. The difference in mean values inside and outside the scanner is not significant in controls or in irritable bowel syndrome (IBS) patients.).
Figure 3.
Scanner environment effect on endogenous pain modulation in irritable bowel syndrome (IBS) subjects and controls.
The change in EPM outside versus inside the scanner (Δ EPM, calculated as EPMoutside – EPMinside) correlated strongly and significantly in IBS patients (r = 0.65, p = 0.02), but only weakly, inversely and non-significantly in controls (r = –0.33, p = 0.32). The significant effect in the ‘group’-by-‘EPM scanner’ interaction in the analysis of covariance (β = 0.64 ± 0.22, p = 0.009) confirmed the divergent relationship between EPM outside and inside the scanner in IBS patients versus controls suggested by the correlation results above.
The difference in EPM outside versus inside the scanner did not correlate significantly with the stress (r = 0.15, p = 0.66), anxiety (r = 0.03, p = 0.93) or depression scores (r = 0.29, p = 0.39) in either controls or IBS patients (r = 0.18, p = 0.55; r = −0.08, p = 0.79; r = 0.11, p = 0.72, respectively).
Somatic thresholds and pain intensity during stimulation outside and inside the scanner
Foot heat sensation, first pain, moderate pain and pain tolerance thresholds were similar in both subject groups (Table 2). No difference was observed in the titrated temperature for induction of moderate foot heat pain between the two groups and the induced mean pain intensity outside the scanner was similar in IBS (44.8 (39.0–50.5)) and control subjects (40.9 (29.3–52.5))(p = 0.52). Two-way mixed ANOVA analysis confirmed the absence of significant main effects for ‘scanner’ (F1,22 = 0.87, p = 0.36) and for ‘group’ (F1,22 = 1.10, p = 0.31) on somatic pain. Furthermore, there was no significant ‘scanner’-by-‘group’ interaction effect (F1,22 = 0.10, p = 0.76), indicating that the effect of the scanner environment on somatic pain was similar between IBS patients and controls (Figure 4).
Figure 4.
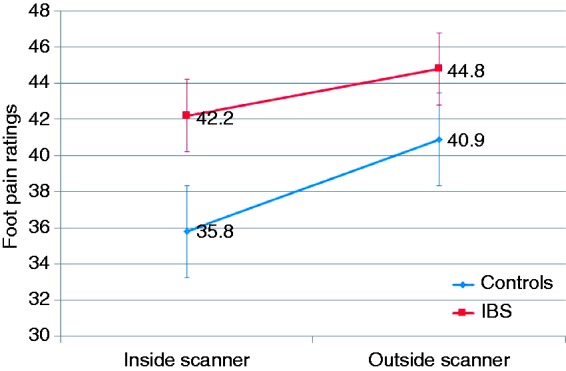
Scanner environment effect on somatic pain in irritable bowel syndrome (IBS) subjects and controls.
A significant ‘group’-by-‘pain’ rating inside the scanner interaction in the analysis of covariance (β = 1.32 ± 0.56, p = 0.03) suggested divergent correlations between IBS patients versus controls.
The difference in foot pain outside versus inside the scanner did not correlate significantly with the stress (r = −0.49, p = 0.12), anxiety (r = −0.58, p = 0.06) or depression scores (r = −0.28, p = 0.41) in controls or in IBS patients (r = 0.28, p = 0.36; r = −0.04, p = 0.91; r = −0.09, p = 0.78, respectively).
Discussion
The fMRI scanner environment significantly increased experimentally induced visceral pain intensity in patients with IBS and in healthy controls, whilst somatic pain was not affected. The increase in visceral pain in the scanner correlated closely with the trait anxiety, stress and depression scores in healthy controls, but not in IBS patients with ab initio increased anxiety and stress. There were trends towards differences in EPM between IBS patients and controls both outside and inside the scanner (‘group’ effect), as well as between outside and inside locations (‘scanner’ effect), which fell short of significance. These data suggest psychological processes underlie the selective visceral hyperalgesia induced by the scanner environment.
The scanner setting has previously been shown to enhance anxiety as well as other psychological factors in healthy subjects and in patients with pre-existing panic disorder and agoraphobia, to precipitate unintended mood alterations and to increase levels of the stress hormone, cortisol, in healthy volunteers.25,37 Anxiety levels are increased in anticipation of scanning and in the scanner, and it is recognized that augmented anxiety, as well as anxiety sensitivity – the fear of anxiety-related sensations and its perceived harmful consequences – are associated with enhanced pain and worsened pain outcomes.38 The expectation of pain is a proven principle factor in determining the intensity of subsequently experienced pain and is influenced by positive or negative mood and anxiety.14–16,39,40 The majority of evidence has indicated that negative mood or the expectation of increased pain significantly exacerbate pain, whereas positive mood, good coping skills or the expectation of reduced pain prominently attenuate pain.41–47 These factors may explain the increased pain reports inside the scanner. Several studies also examined the effect of MR scanner-associated noise on brain activation patterns. There was not only activation in the auditory processing regions, but also interference with the stimulation presentation and altered attentional demands.48–50
The ANOVA analysis indicated a divergent effect of the scanner environment between IBS patients and controls, which did not reach significance. However, the covariance analysis revealed a strong trend for the interaction of ‘group’ with ‘pain rating inside the scanner’, with a medium-large effect size, indicating significance would be reached with a larger sample size. Stress and anxiety responses vary considerably inter-individually and are related to underlying neuroendocrine and psychological factors, including the cortisol response, the level of arousal and the impact of individual trait differences.51 In our healthy controls a close correlation existed between trait anxiety, stress and depression levels and increased visceral pain inside the scanner, in accordance with the above-mentioned susceptibility factors. Such a correlation was not evident in IBS patients, who already had elevated anxiety and stress scores outside the scanner. The a priori elevated psychological arousal levels may explain the lack of linear relationship between background anxiety and stress and further pain changes in the scanner.35,52–55 The recording of state anxiety and anxiety sensitivity measures inside and outside the scanner and personality traits for characterization of further pain endophenotypes would have yielded additional useful information, but they were not available in this study.56
The increase seen in visceral pain inside the scanner was not reproduced for somatic pain and there also was no correlation between anxiety, stress and depression and somatic pain changes in either subject group. The psychological modulation of visceral and somatic pain consequently appears to be differentiated. Psychological variables in IBS have been extensively explored in visceral hyperalgesia, but not in somatic hyperalgesia. In one recent study in IBS, patients’ skin thermal hypersensitivity was considerably less associated with psychological factors than visceral hypersensitivity.15 A possible explanation for a selective visceral modulation could be a selective hypervigilance towards visceral stimuli based on the subjects’ awareness of the gastrointestinal focus of the study. In a recent psychophysiological study participants showed greater responses to an expected pain modality than to an unexpected one.57 An underlying mechanism for the selective effects may be the more extensive involvement of autonomic and affective brain modulation in visceral stimulation and the bias towards spatial encoding in somatic stimulation.58,59 An illustration of this is the greater activation of the nucleus cuneiformis, a nucleus of the brainstem reticular formation, during visceral pain than during somatic pain in a recent human fMRI study.59 This nucleus receives emotive, autonomic and executive input and has a potential facilitatory role in the development of central visceral sensitization.59,60 Activation in the periaqueductal grey, a brainstem area strongly implicated in pain modulation, was also shown to be correlated with anxiety during visceral stimulation but not somatic stimulation.59 We, therefore, speculate that psychological and affective factors involved in the expectation of visceral pain are more likely to influence visceral pain processing than somatic pain.
Hypersensitivity in IBS is associated with dysfunctional EPM, as was also evident outside the scanner in the current study,15,54,55,61 where trends, albeit falling short of significance, for ‘scanner’ or ‘group’ effects on EPM, as well as their interaction, were demonstrated. The effect size analysis indicated that these insignificant trends are likely due to the small sample size. The pain facilitation seen in the majority of IBS patients was similar outside and inside the scanner, whilst in controls there was a net change from pain inhibition to facilitation from outside to inside the scanner, with considerable intra-individual variability. This variability is unlikely to be due to poor test reproducibility, as heterotopic stimulation has been validated as a reproducible method for induction of EPM in healthy volunteers.35,62 Our data show that trait stress, anxiety and depression do not play a major role in EPM, although state psychological factors may contribute to the variability in EPM.61 However, recent evidence in healthy controls indicates the predominant effect of anxiety or anxiety sensitivity on pain is relayed via cortical pathways and not via those assessed by heterotopic stimulation, which include major spinal and brainstem components.9,55,61,63 Within this context it appears probable that EPM is involved in the scanner-induced effects, but that the anxiety–expectation–fear network, constituted mainly by the amygdala, prefrontal and cingulate cortices, is the main driver of the response.64–67
This study has several limitations. The numbers of subjects included in the study are relatively small, as it was primarily powered and designed for imaging comparisons. For this reason, no subgroup analysis, including that between the IBS subtypes or genders, was performed. The lack of a randomized test sequence is a potential issue, which arose from this being an extended analysis of existing study data. However, we feel that this is unlikely to significantly compromise the results for the following reasons. In the case of an order effect, habituation or a training effect with resultant decreased pain perception would be assumed with repetition of pain testing.68 However, despite the MRI session consistently being the second session, a significant increase in visceral pain was observed. Sensitization is highly unlikely with a resting period of 30 minutes between sessions and would have affected both somatic and visceral pain perception, which was not the case in the current study. Interaction and order effects have been previously described between imaging and non-imaging environments and consequently a standardized test session sequence was chosen for the current study to reduce variability.68 The significant changes in sensory findings despite the limited sample size attest to the robustness of the effects of the scanner environment. Future studies must integrate more specific psychological assessment tools used at repeated time-points.
In conclusion, the results of the present study suggest that an fMRI environment significantly influences the outcome of pain processing compared with a non-scanner setting. The scanner environment affected visceral pain in both IBS patients and healthy controls in a protocol specifically aimed at investigating visceral pain. Anxiety and stress are the likely underlying modulating causes, whereas classic EPM pathways activated by heterotopic stimulation play a lesser role. These results are highly relevant to a wide range of imaging applications and need to be taken into account in future pain research. Although further controlled studies are warranted, these results are likely to have salience in the design of future neuroimaging studies of visceral pain.
Acknowledgements
We wish to thank Ms Tung Siew Lai, the research assistant for this study, for her expert help in study coordination and subject recruitment. We also wish to thank Mr Christopher Au and Ms Regine Teo, for their expert help with the fMRI scanning.
Funding
This work was supported by the Singapore National Medical Research Council, Individual Research Grant (grant number: NMRC/1086/2006).
Conflict of interest
The authors have no competing interests or relevant financial conflicts to declare.
References
- 1.Norbury TA, MacGregor AJ, Urwin J, et al. Heritability of responses to painful stimuli in women: A classical twin study. Brain 2007; 130: 3041–3049. [DOI] [PubMed] [Google Scholar]
- 2.Hocking LJ, Morris AD, Dominiczak AF, et al. Heritability of chronic pain in 2195 extended families. Eur J Pain 2012; 16: 1053–1063. [DOI] [PubMed] [Google Scholar]
- 3.Tracey I. Imaging pain. Br J Anaesth 2008; 101: 32–39. [DOI] [PubMed] [Google Scholar]
- 4.Choung RS, Locke GR., 3rd Epidemiology of IBS. Gastroenterol Clin North Am 2011; 40: 1–10. [DOI] [PubMed] [Google Scholar]
- 5.Tosic-Golubovic S, Miljkovic S, Nagorni A, et al. Irritable bowel syndrome, anxiety, depression and personality characteristics. Psychiatr Danub 2010; 22: 418–424. [PubMed] [Google Scholar]
- 6.Elsenbruch S, Rosenberger C, Enck P, et al. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: An fMRI study. Gut 2010; 59: 489–495. [DOI] [PubMed] [Google Scholar]
- 7.Dunphy RC, Bridgewater L, Price DD, et al. Visceral and cutaneous hypersensitivity in Persian Gulf war veterans with chronic gastrointestinal symptoms. Pain 2003; 102: 79–85. [DOI] [PubMed] [Google Scholar]
- 8.Ringel Y, Drossman DA, Leserman JL, et al. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: An FMRI study. Gastroenterology 2008; 134: 396–404. [DOI] [PubMed] [Google Scholar]
- 9.Wilder-Smith CH. The balancing act: Endogenous modulation of pain in functional gastrointestinal disorders. Gut 2011; 60: 1589–9915. [DOI] [PubMed]
- 10.Houghton LA, Calvert EL, Jackson NA, et al. Visceral sensation and emotion: A study using hypnosis. Gut 2002; 51: 701–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posserud I, Agerforz P, Ekman R, et al. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut 2004; 53: 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut 1998; 42: 845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan V, Pickens D, Gautam S, et al. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 2005; 54: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price DD, Craggs JG, Zhou Q, et al. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: Evidence from human psychophysics, animal models, and neuroimaging. Neuroimage 2009; 47: 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piche M, Arsenault M, Poitras P, et al. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain 2010; 148: 49–58. [DOI] [PubMed] [Google Scholar]
- 16.Wiech K, Tracey I. The influence of negative emotions on pain: Behavioral effects and neural mechanisms. Neuroimage 2009; 47: 987–994. [DOI] [PubMed] [Google Scholar]
- 17.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci 2008; 12: 306–313. [DOI] [PubMed] [Google Scholar]
- 18.Tracey I. Can neuroimaging studies identify pain endophenotypes in humans? Nat Rev Neurol 2011; 7: 173–181. [DOI] [PubMed] [Google Scholar]
- 19.Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 2: How, where, and what to look for using functional imaging. Discov Med 2011; 11: 209–219. [PubMed] [Google Scholar]
- 20.Van Oudenhove L, Aziz Q. Recent insights on central processing and psychological processes in functional gastrointestinal disorders. Dig Liver Dis 2009; 41: 781–787. [DOI] [PubMed] [Google Scholar]
- 21.Drossman DA, Creed FH, Olden KW, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gut 1999; 45(Suppl. 2): II25–II30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Oudenhove L, Vandenberghe J, Demyttenaere K, et al. Psychosocial factors, psychiatric illness and functional gastrointestinal disorders: A historical perspective. Digestion 2010; 82: 201–210. [DOI] [PubMed] [Google Scholar]
- 23.Kearney DJ, McDermott K, Martinez M, et al. Association of participation in a mindfulness programme with bowel symptoms, gastrointestinal symptom-specific anxiety and quality of life. Aliment Pharmacol Ther 2011; 34: 363–373. [DOI] [PubMed] [Google Scholar]
- 24.Posserud I, Svedlund J, Wallin J, et al. Hypervigilance in irritable bowel syndrome compared with organic gastrointestinal disease. J Psychosom Res 2009; 66: 399–405. [DOI] [PubMed] [Google Scholar]
- 25.Muehlhan M, Lueken U, Wittchen HU, et al. The scanner as a stressor: Evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. Int J Psychophysiol 2011; 79: 118–126. [DOI] [PubMed] [Google Scholar]
- 26.Katz RC, Wilson L, Frazer N. Anxiety and its determinants in patients undergoing magnetic resonance imaging. J Behav Ther Exp Psychiatry 1994; 25: 131–134. [DOI] [PubMed] [Google Scholar]
- 27.Tornqvist E, Mansson A, Larsson EM, et al. It’s like being in another world – patients’ lived experience of magnetic resonance imaging. J Clin Nurs 2006; 15: 954–961. [DOI] [PubMed] [Google Scholar]
- 28.MacKenzie R, Sims C, Owens RG, et al. Patients’ perceptions of magnetic resonance imaging. Clin Radiol 1995; 50: 137–143. [DOI] [PubMed] [Google Scholar]
- 29.Lueken U, Muehlhan M, Wittchen HU, et al. (Don’t) panic in the scanner! How panic patients with agoraphobia experience a functional magnetic resonance imaging session. Eur Neuropsychopharmacol 2011; 21: 516–525. [DOI] [PubMed] [Google Scholar]
- 30.Coffin B. [Diagnosis of irritable bowel syndrome]. Gastroenterol Clin Biol 2009; 33(Suppl. 1): S9–S16. [DOI] [PubMed] [Google Scholar]
- 31.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997; 11: 395–402. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983; 24: 385–396. [PubMed] [Google Scholar]
- 33.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 34.Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 2009; 144: 16–19. [DOI] [PubMed] [Google Scholar]
- 35.Wilder-Smith CH, Song G, Yeoh KG, et al. Activating endogenous visceral pain modulation: A comparison of heterotopic stimulation methods in healthy controls. Eur J Pain 2009; 13: 836–842. [DOI] [PubMed] [Google Scholar]
- 36.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 1979; 6: 283–304. [DOI] [PubMed] [Google Scholar]
- 37.Tessner KD, Walker EF, Hochman K, et al. Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Hum Brain Mapp 2006; 27: 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strigo IA, Simmons AN, Matthews SC, et al. The relationship between amygdala activation and passive exposure time to an aversive cue during a continuous performance task. PLoS One 2010; 5: e15093–e15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci 2009; 1156: 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benedetti F. Placebo analgesia. Neurol Sci 2006; 27(Suppl. 2): S100–S102. [DOI] [PubMed] [Google Scholar]
- 41.Zelman DC, Howland EW, Nichols SN, et al. The effects of induced mood on laboratory pain. Pain 1991; 46: 105–111. [DOI] [PubMed] [Google Scholar]
- 42.Zillmann D, de Wied M, King-Jablonski C, et al. Drama-induced affect and pain sensitivity. Psychosom Med 1996; 58: 333–341. [DOI] [PubMed] [Google Scholar]
- 43.Weisenberg M, Raz T, Hener T. The influence of film-induced mood on pain perception. Pain 1998; 76: 365–375. [DOI] [PubMed] [Google Scholar]
- 44.Meagher MW, Arnau RC, Rhudy JL. Pain and emotion: Effects of affective picture modulation. Psychosom Med 2001; 63: 79–90. [DOI] [PubMed] [Google Scholar]
- 45.Rhudy JL, Meagher MW. Negative affect: Effects on an evaluative measure of human pain. Pain 2003; 104: 617–626. [DOI] [PubMed] [Google Scholar]
- 46.Wunsch A, Philippot P, Plaghki L. Affective associative learning modifies the sensory perception of nociceptive stimuli without participant’s awareness. Pain 2003; 102: 27–38. [DOI] [PubMed] [Google Scholar]
- 47.Kenntner-Mabiala R, Pauli P. Affective modulation of brain potentials to painful and nonpainful stimuli. Psychophysiology 2005; 42: 559–567. [DOI] [PubMed] [Google Scholar]
- 48.Amaro E, Jr, Williams SC, Shergill SS, et al. Acoustic noise and functional magnetic resonance imaging: Current strategies and future prospects. J Magn Reson Imaging 2002; 16: 497–510. [DOI] [PubMed] [Google Scholar]
- 49.Tomasi D, Caparelli EC, Chang L, et al. fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage 2005; 27: 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaab N, Gabrieli JD, Glover GH. Resting in peace or noise: Scanner background noise suppresses default-mode network. Hum Brain Mapp 2008; 29: 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ochsner KN, Ludlow DH, Knierim K, et al. Neural correlates of individual differences in pain-related fear and anxiety. Pain 2006; 120: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: A review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun 2011; 25: 386–394. [DOI] [PubMed] [Google Scholar]
- 53.North CS, Hong BA, Alpers DH. Relationship of functional gastrointestinal disorders and psychiatric disorders: Implications for treatment. World J Gastroenterol 2007; 13: 2020–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol 2007; 13: 3699–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song GH, Venkatraman V, Ho KY, et al. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain 2006; 126: 79–90. [DOI] [PubMed] [Google Scholar]
- 56.Farmer AD, Coen SJ, Kano M, et al. Psychophysiological responses to pain identify reproducible human clusters. Pain 2013; 154: 2266–2276. [DOI] [PubMed] [Google Scholar]
- 57.Spence C, Bentley DE, Phillips N, et al. Selective attention to pain: A psychophysical investigation. Exp Brain Res 2002; 145: 395–402. [DOI] [PubMed] [Google Scholar]
- 58.Dunckley P, Wise RG, Aziz Q, et al. Cortical processing of visceral and somatic stimulation: Differentiating pain intensity from unpleasantness. Neuroscience 2005; 133: 533–542. [DOI] [PubMed] [Google Scholar]
- 59.Dunckley P, Wise RG, Fairhurst M, et al. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci 2005; 25: 7333–7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zambreanu L, Wise RG, Brooks JC, et al. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain 2005; 114: 397–407. [DOI] [PubMed] [Google Scholar]
- 61.Heymen S, Maixner W, Whitehead WE, et al. Central processing of noxious somatic stimuli in patients with irritable bowel syndrome compared with healthy controls. Clin J Pain 2010; 26: 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cathcart S, Winefield AH, Rolan P, et al. Reliability of temporal summation and diffuse noxious inhibitory control. Pain Res Manag 2009; 14: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terry EL, Kerr KL, DelVentura JL, et al. Anxiety sensitivity does not enhance pain signaling at the spinal level. Clin J Pain 2012; 28: 505–510. [DOI] [PubMed] [Google Scholar]
- 64.Ji G, Fu Y, Ruppert KA, et al. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain 2007; 3: 13–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adwanikar H, Ji G, Li W, et al. Spinal CGRP1 receptors contribute to supraspinally organized pain behavior and pain-related sensitization of amygdala neurons. Pain 2007; 132: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elsenbruch S, Rosenberger C, Bingel U, et al. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology 2010; 139: 1310–1319. [DOI] [PubMed] [Google Scholar]
- 67.Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci 2009; 29: 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lueken U, Muehlhan M, Evens R, et al. Within and between session changes in subjective and neuroendocrine stress parameters during magnetic resonance imaging: A controlled scanner training study. Psychoneuroendocrinology 2012; 37: 1299–1308. [DOI] [PubMed] [Google Scholar]


