Abstract
Background
In May/June 2011, the new Shiga-like toxin-producing Escherichia coli (STEC) strain O104:H4 caused the severest outbreak ever recorded of hemorrhagic enterocolitis in 3842 patients in Germany.
Objectives
As bacterial enterocolitis is an established risk factor of subsequent irritable bowel syndrome (IBS), we aimed to estimate prevalence and incidence of post-infectious (PI)-IBS after six and 12 months in a cohort of STEC O104:H4 patients and to prospectively identify associated somatic and psychometric risk factors.
Methods
A total of 389 patients were studied prospectively at baseline and at six and 12 months after STEC infection using STEC disease-related questionnaires and validated instruments for IBS (Rome III) and psychological factors. Frequencies and logistic regression models using multiple imputations were applied to assess predictor variables.
Results
Prevalence of IBS increased from 9.8% prior to STEC infection to 23.6% at six and 25.3% at 12 months after STEC infection. In patients without IBS symptoms prior to STEC infection, incidence of new IBS was 16.9%. Logistic regression models indicated higher somatization and anxiety scores as risk factors for, and mesalazine treatment during, STEC infection as the only significant protective factor against IBS. No other factor analyzed, including disease severity, showed an association.
Conclusions
PI-IBS rates following this unusually severe STEC outbreak were similar to what has been observed after other infectious gastroenteritis outbreaks. Our findings suggest that mesalazine may have reduced the risk of subsequent PI-IBS. As altered mucosal immune activity is a pivotal pathogenic factor in PI-IBS, our observation of a potential protective effect of mesalazine might be explained by its known modulatory action on mucosal immunity, and may warrant further investigation.
Keywords: Mesalazine, EHEC
Introduction
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal (GI) diseases. It is characterized by chronic abdominal pain, cramps and bloating in association with altered bowel habits such as diarrhea, constipation or a mix of both. In the absence of structural disease biomarkers in routine diagnostic tests, IBS is diagnosed based on defined symptom criteria with the Rome-III criteria being the current diagnostic standard.1 Recent research was able to identify a variety of abnormalities in IBS patients such as altered intestinal immune and barrier functions, altered gut microbiome, and increased activation of the enteric nervous system. All of these alterations may be involved in disturbed gut functions such as increased or decreased motility, secretion and sensation, all of which are suggested to contribute to symptom generation in IBS.2 The role of enteric immune functions and altered microbiome in the pathophysiology of IBS is also supported by the observation that bacterial enterocolitis is associated with an increased risk of developing a so-called “post-infectious” IBS (PI-IBS).3
Studies in travelers’ diarrhea as well as in larger enterocolitis outbreaks after food or water contamination, e.g. with Salmonella, Campylobacter or Escherichia coli, revealed increased IBS incidence and prevalence rates in patients following enterocolitis compared to non-infected people. Rates ranging from 4% to 36.2% and odds ratios (ORs) ranging from 2.61 to 17.15 have been reported.4–6 Although data on risk factors, which were often assessed retrospectively, are heterogeneous, duration and severity (e.g. bloody diarrhea, fever) of the preceding gastroenteritis, female gender, younger age and pre-existing psychological comorbidity such as anxiety and somatization are suggested as potential risk factors of PI-IBS.7–9
In May/June 2011, an outbreak of the Shiga-like toxin-producing E. coli (STEC) strain O104:H4, a new strain belonging to the enterohemorrhagic E. coli (EHEC) family, affected a total of 3842 patients with hemorrhagic enterocolitis mostly in Northern Germany.10,11 A total of 855 (22.3%) of the patients developed hemolytic uremic syndrome (HUS) and overall, 53 patients died.10,11 Thus, this was the most severe outbreak of E. coli-related hemorrhagic entercolitis ever recorded worldwide. Hence, this new STEC strain O104:H4 appears to have been far more aggressive than the commonly known EHEC strain O157:H7 that is associated with substantially lower complication rates.12 This increased virulence might be attributed to the combination of virulence genes of two different diarrhea-causing E. coli pathotypes, i.e. genes typical of enteroaggregative E. coli, located on a virulence plasmid, and the gene for a Shiga-toxin 2 variant (stx2a).11
While EHEC O157:H7 affects predominantly children, patients of this STEC O104:H4 outbreak were predominantly adult females, well educated, most likely reflecting a certain social and eating behavior, as infected organic sprouts were identified as the cause of the outbreak.10–12
The aim of the current study was to prospectively follow up a cohort of STEC O104:H4-infected patients in order to estimate the prevalence and incidence of IBS after six and 12 months and to prospectively identify associated somatic and psychometric risk factors.
Methods
Patient sample
This prospective cohort study was initiated in summer 2011 during the large outbreak of hemorrhagic enterocolitis and/or HUS disease in Northern Germany caused by the new STEC O104:H4. The ethics committee of the local medical association approved the research protocol for this study, and all participants gave written informed consent. We included adult patients (≥18 years) with the clinical diagnosis of STEC/HUS disease of stable medical status and who gave informed consent and had sufficient knowledge of the German language. Baseline assessment was performed within the first three months (T0) of acute STEC infection, hence including patients with still-active disease or during recovery. Only patients still being treated in intensive care units and suffering from prolonged severe neurological and/or renal impairment were not contacted because of their poor health status at the time. Follow-up assessments were conducted six months (T1) and 12 months (T2) after STEC infection. Thirteen hospitals in Northern Germany participated in the study. For the baseline assessment, patients who visited the specialized STEC clinics specifically set in place during the outbreak were recruited in the hospital waiting-room (26% of total sample). All other patients received a letter explaining the study, contact information for further questions, the informed consent form, and the study questionnaire by mail from their treating hospital. Patients who did not respond within three weeks were contacted by telephone and requested to participate in the study. For follow-up assessments, patients were contacted six months (T1) and 12 months (T2) after STEC infection. Patients could choose to fill out the follow-up questionnaires either online in a study-specific website or on paper. Patients who did not participate in the follow-up assessment within three weeks of contact received one reminder.
Measures
Demographic characteristics, pre-existing conditions (including prior psychological illness), STEC-illness symptoms (with various questions assessing detailed aspects of disease severity) and treatment characteristics were assessed using structured, mostly binary self-report items. Given the lack of an established standard therapy for EHEC/STEC disease (besides supportive measures), most STEC therapies during the outbreak were just based on clinical judgment and initiated nearly “desperately” in light of the dramatic disease severity. In our analysis, we included all those therapies that were given to a larger number of patients and that could potentially have an effect on subsequent IBS development. Although there was “no standard approach” throughout all centers and not all therapies were used in all hospitals, the decision to start any given therapy was often driven by clinical disease severity, i.e. more severely ill patients were more likely to receive a therapy. These therapies included macrogol, which was given with the aim of increasing elimination (“clearance”) of the pathogens; the probiotic E. coli Nissle, which had been shown in vitro to inhibit growth of STEC strains; mesalazine,13 which was initiated with the aim of reducing intestinal inflammation; and the anticomplement C5 antibody eculizumab, which was given to patients with refractory HUS disease.
Subjective experiences were measured using Likert type self-report items. IBS diagnosis was assessed using the functional bowel disease module of the Rome III questionnaire, the current standard of symptom-based IBS diagnosis.1 At T0, the Rome III questionnaire was used retrospectively to assess the IBS status prior to STEC infection.
A large variety of psychological parameters were assessed using validated questionnaires:
The Patient Health Questionnaire-9 (PHQ-9)14 for depression, the Generalized Anxiety Disorder Scale (GAD-7)15,16 for anxiety severity, the Patient Health Questionnaire-15 (PHQ-15)17 for somatization, i.e. the overall-severity of the 15 most prevalent symptoms in primary care, the 12-Item Short Form Health Survey (SF-12)18 for health-related quality of life, the Self-Efficacy, Optimism, and Pessimism Scale (SWOP-K9)19 for self-efficacy, the Neuroticism-scale from the BIG-Five-Inventory (BFI-K)20 for neuroticism, and the F-SozU-questionnaire (K-14)21 for social support. Social support, neuroticism, self-efficacy, optimism, and pessimism were assessed only at baseline. All other self-report scales were applied both at baseline and at both follow-up assessments.
Statistical analysis
Statistical analyses included data from all patients who responded to the baseline questionnaires.
Frequencies of “IBS Rome III” and “IBS symptom complex” were calculated as percentages of patients fulfilling the respective criteria at each time point. “Full IBS Rome III” was based on the strictly defined Rome III criteria and “IBS symptom complex” was defined as fulfillment of all IBS symptom criteria with symptoms lasting >3 months, but with symptom onset less than six months ago.
Frequencies of “new IBS Rome III” and “new IBS symptom complex” one year after STEC infection were calculated as percentages of patients fulfilling the respective criteria at T2 and fulfilling neither of these criteria prior to STEC infection.
To account for possible confounding effects (e.g. gender and psychological variables), predictor analyses were performed with multivariate logistic regression analyses, for which missing values were replaced using multiple imputation methods22 with the creation of 200 datasets based on the candidate predictors. In addition, sensitivity analyses were performed using the original non-imputed data set. Regression analyses were performed based on the “forced entry” method, where all relevant variables are entered simultaneously based on their theoretical impact.23 P values <0.05, two tailed, were considered significant. Predictor analyses were performed for the following outcomes: “IBS Rome III” at T2, “new IBS Rome III” at T2, and the combined group of “new IBS Rome III or new IBS symptom complex” at T2. We tested for multicollinearity by running linear regression analyses using the same outcome and predictors. These analyses showed variance inflation factors (VIF) below 3.8 and tolerance values above 0.27, thus indicating no collinearity problem. All statistical analyses were performed using SPSS (version 20.0).
Results
Patient characteristics
The flowchart of patient recruitment is presented in Figure 1. At baseline, a total of 608 patients from 13 hospitals in the greater Hamburg metropolitan area were invited to participate in this study. Of those, 389 patients completed the questionnaires (response rate T0, 64%). There were no significant differences regarding age (40.4 ± 14.8 vs 42.7 ± 17.5 years; p = 0.42) and gender (70% vs 70% female; p = 1.0) between responders (n = 153) and non-responders (n = 113) from the hospital providing the largest patient sample. Of the 389 participating patients, 308 also responded at the six-month follow-up (response rate T1, 79%), and 300 also responded at the 12-month follow-up (response rate T2, 77.1%). Responders were significantly older than non-responders (47.03 ± 17.02 years vs 40.09 ± 16.55 years), but did not differ significantly with respect to any other variable.
Figure 1.
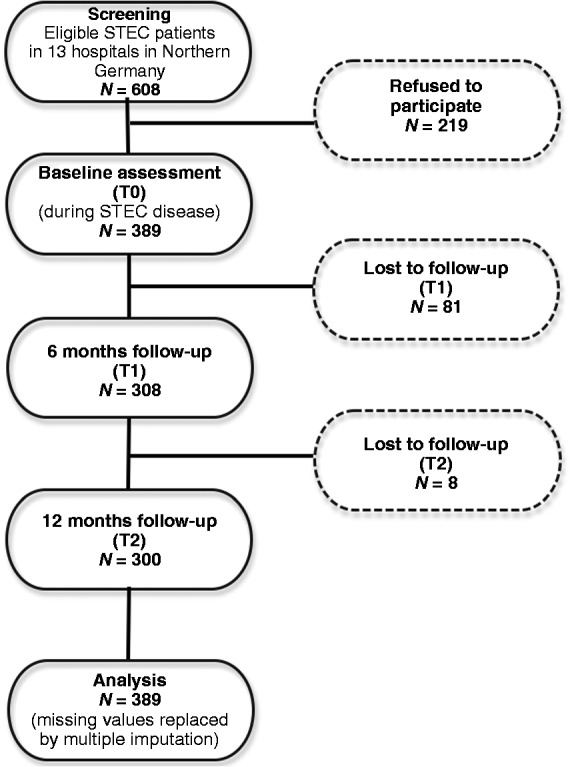
Flowchart of patient recruitment.
STEC: Shiga-like toxin-producing Escherichia coli.
The patient characteristics are displayed in Table 1, and the baseline values for psychological parameters in Table 2.
Table 1.
Characteristics of study participants (N = 389) at baseline assessment
| Sociodemographic characteristics | ||
|---|---|---|
| Female, no. (%) | 270 (69) | |
| Age, year | 46 ± 17 | |
| Educational level ( ≥ 10 years of schooling), no. (%) | 317 (82) | |
| Living alone/as single parent, no. (%) | 95 (24) | |
| BMI, mean score | 24 ± 4 | |
| STEC disease-related characteristics | ||
| Stayed in intensive care unit, no. (%) | 111 (30) | |
| Duration of hospitalization, no. (%) | ||
| 0 weeks: 77 (20), one week: 74 (20); two weeks: 86 (23) three weeks: 58 (15); ≥4 weeks: 82 (22) | ||
| Diagnosed with HUS, no. (%) | 119 (31) | |
| Dysfunction of nervous system, no. (%) | 148 (40) | |
| Fever, no. (%) | 61 (16) | |
| Duration of bloody diarrhea, no. (%) | ||
| 0 to 4 days | 198 (53) | |
| 5 or more days | 173 (47) | |
| ≥ 4 instances of diarrhea on ≥ 3 days, no. (%) | 257 (69) | |
| Abdominal pain, no. (%) | ||
| None | 20 (13) | |
| Mild or moderate | 105 (28) | |
| Severe | 225 (59) | |
| Eculizumab therapy, no. (%) | 76 (26) | |
| Mesalazine therapy, no. (%) | 52 (18) | |
| Makrogol therapy, no. (%) | 78 (27) | |
| E. coli Nissle therapy, no. (%) | 100 (35) | |
Plus-minus values are means ± SD. No. (%) refers to number of participants and percentage of the cohort. BMI: body mass index; STEC: Shiga-like toxin-producing Escherichia coli; HUS: hemolytic uremic syndrome.
Table 2.
Psychological parameters of study participants (N = 389) at baseline assessment
| Psychological parameters | Mean (±SD) | Value of the general population |
|---|---|---|
| Social support | 4.39 (± 0.6) | 3.9721 |
| Neuroticism | 2.75 (± 0.89) | Not available |
| Self efficacy | 2.98 (± 0.56) | Not available |
| Optimism | 3.20 (± 0.79) | Not available |
| Pessimism | 2.03 (± 0.73) | Not available |
| Depression | 6.10 (± 4.82) | 2.9122 |
| Anxiety | 4.44 (± 4.12) | 2.9515,16 |
| Somatization | 7.52 (± 5.15) | 3.823 |
| Number (%) | ||
| Fear of death | 183 (49) | Not available |
The following validated questionnaires were used: Patient Health Questionnaire-9 (PHQ-9)14 for depression, the Generalized Anxiety Disorder Scale (GAD-7)15,16 for anxiety severity, the Patient Health Questionnaire-15 (PHQ-15)17 for somatization, the Self-Efficacy, Optimism, and Pessimism Scale (SWOP-K9)19 and the Neuroticism-scale from the BIG-Five-Inventory (BFI-K)20 for neuroticism, the F-SozU-questionnaire (K-14)21 for social support.
Prevalence of “IBS Rome III” and “IBS symptom complex” at all time points (Figure 2)
Figure 2.

Prevalence of ‘IBS Rome III’ and ‘IBS symptom complex’ before and 6 and 12 months after STEC O104:H4 enterocolitis.
At T0, baseline, retrospective reporting of bowel symptoms prior to STEC infection revealed 9.8% of patients fulfilling all Rome III criteria for IBS. An additional 5.8% fulfilled all criteria for the IBS symptom complex. Hence, prior to STEC infection, a total of 15.6% of the patients suffered from any IBS symptom complex with symptoms > 3 months.
At T1, six months after STEC infection, 23.6% of patients fulfilled all criteria for “IBS Rome III.” An additional 12.3% fulfilled all criteria for the “IBS symptom complex.” Hence, at T1, a total of 35.9% of the patients suffered from any IBS symptom complex with symptoms > 3 months.
At T2, 12 months after STEC infection, 25.3% of patients fulfilled all criteria for “IBS Rome III.” An additional 9.3% fulfilled all criteria for the “IBS symptom complex.” Hence, at T2, a total of 34.6% of the patients suffered from any IBS symptom complex with symptoms > 3 months.
Incidence of “new IBS Rome III” and “new IBS symptom complex” 12 months after STEC (T2) (Figure 3)
Figure 3.
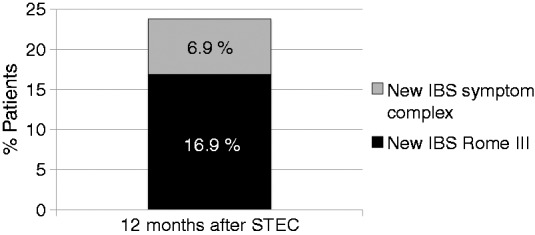
Incidence of ‘new IBS Rome III’ and ‘new IBS symptom complex’ 12 months after STEC O104:H4 enterocolitis.
The incidence of “new IBS Rome III” at T2 was 16.9%; the incidence of “new IBS symptom complex” at T2 was 6.9%. Hence, at T2, a total of 23.8% had acquired any new IBS symptom complex with symptoms > 3 months.
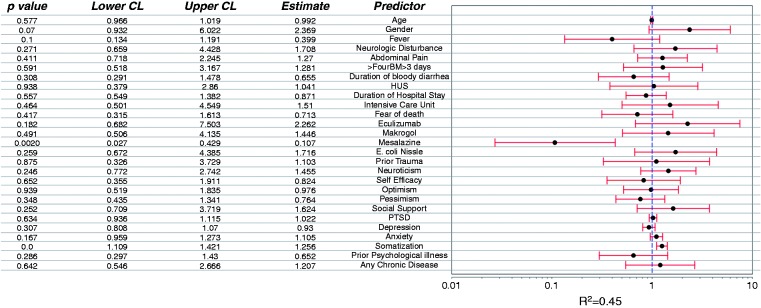
Predictors of “IBS Rome III” overall at T2, 12 months after STEC infection (Figure 4)
Figure 4.
Predictors for ‘IBS Rome III’ overall 12 months after STEC O104:H4 enterocolitis.
Treatment with mesalazine during STEC infection was a significant protective predictor, and high somatization score a significant negative predictor, i.e. risk factor, of IBS at T2. That was confirmed by sensitivity analysis in the original, non-imputed dataset. There was a trend for fever during STEC infection as a protective predictor and for female gender as a negative predictor. All other suspected factors tested in the regression model were not significant.
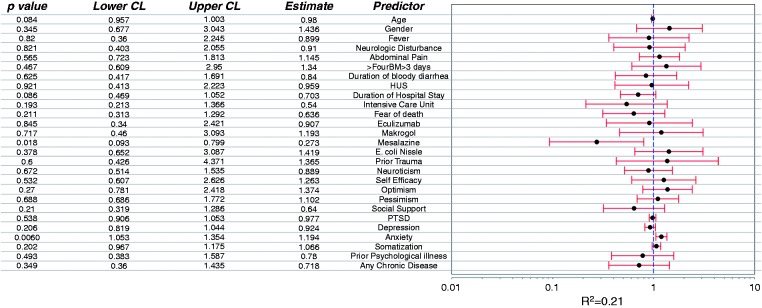
Predictors of “new IBS Rome III” at T2 in patients free of IBS or IBS symptom complex prior to STEC infection (Figure 5)
Figure 5.
Predictors for new ‘IBS Rome III' 12 months after STEC O104:H4 enterocolitis.
Treatment with mesalazine during STEC infection was the only significant protective predictor of “new IBS Rome III” at T2. That was confirmed by sensitivity analysis in the original, non-imputed dataset. There was a trend for high somatization score and high “generalized anxiety and depression score” as a negative predictor of new IBS at T2. All other suspected factors tested in the regression model were not significant.
Predictors of “new IBS Rome III or IBS symptom complex” at T2 in patients free of IBS or IBS symptom complex prior to STEC (Figure 6)
Figure 6.
Predictors for new ‘IBS Rome III or IBS symptom complex’ 12 months after STEC O104:H4 enterocolitis.
Treatment with mesalazine during STEC disease was a significant protective predictor, while increased anxiety during STEC disease was a significant negative predictor, i.e. risk factor, of “new IBS Rome III or IBS symptom complex” at T2. That was confirmed by sensitivity analysis in the original, non-imputed dataset. There was a trend for increased age and longer duration of hospital stay as protective predictors of “new IBS Rome III or IBS symptom complex” at T2. All other suspected factors tested in the regression model were not significant.
Discussion
This large cohort study with prospective 12-month follow-up analyzed occurrence and potential somatic and psychosocial risk factors of post-infectious IBS in a cohort of 389 patients affected by severe hemorrhagic enterocolitis with or without HUS during the outbreak caused by the new STEC type O104:H4 in early summer 2011 in northern Germany.
Our main findings can be summarized as follows: Prevalence of IBS increased from 9.8% prior to STEC infection up to 25.3% 12 months after STEC infection. In patients without IBS or IBS symptoms prior to STEC infection, incidence of new IBS Rome III was 16.9%. Logistic regression models indicated mesalazine treatment during STEC infection as a significant protective factor and higher somatization and anxiety scores as negative risk factors for IBS. Hence, the rates of PI-IBS were high, but only within the range reported after milder infections. Severity of enterocolitis, the main suspected risk factor of PI-IBS, could not be confirmed as a risk factor in this cohort.
In this cohort study, IBS rates were already increased after six months and slightly increased further up to 12 months, suggesting long-term persistence of bowel symptoms rapidly following symptomatic hemorrhagic enterocolitis in a subset of STEC patients at risk of PI-IBS. Due to the prospective follow-up design of our study with assessment of IBS status prior to STEC-infection immediately at baseline, we were also able to estimate IBS incidence in patients without chronic GI symptoms prior to STEC infection (i.e. no IBS and no IBS symptom complex). Incidence of new chronic IBS symptoms within 12 months after STEC infection was altogether 25.8%, with 16.9% of these fulfilling criteria for new full IBS Rome III, and 6.9% only criteria of the IBS symptom complex.
The observation that the rates of “full IBS” still increase between six and 12 months, whereas the rates of “IBS symptom complex” decrease, suggests that some patients have developed their IBS with some delay and fulfill only the “duration of symptoms” criterion 12 months after the initial GI infection. Similar observations were reported by Mearin and colleagues.26 In view of current pathogenic concepts of PI-IBS, it is well conceivable that not all involved mechanisms are “switched on” immediately during the infection, but rather develop gradually, which would be associated with a clinical lag time with no or little symptoms after the acute infection until the unequivocal manifestation of “full” IBS some time later. However, future research is needed to elucidate these important aspects of PI-IBS development.
Overall, the substantial increase of IBS prevalence and incidence following STEC O104:H4 enterocolitis is in accordance with prior reports of PI-IBS after enterocolitis caused by various bacterial pathogens.4–6 The wide range of observed incidence rates between 4% and 36% may be due to differences in study design (e.g. retrospective or prospective cohort study or case-control study), IBS definitions (Rome I, Rome II, Rome III) and preceding intestinal infections (e.g. benign travelers’ diarrhea vs severe hemorrhagic enterocolitis caused by STEC/EHEC). The latter might be of specific importance, not because of the causative pathogen itself (with different PI-IBS rates in different studies of the same pathogen, e.g. for campylobacter) but rather in relation to the severity of the initial enterocolitis disease, which has been suggested to be an important risk factor for PI-IBS.7–9
In our study cohort both severity of the underlying enterocolitis as well as the causative pathogen were similar to those recorded in the Walkerton Health Study. This cohort study was initiated in 2002 two years after a large outbreak of acute gastroenteritis in 2300 residents due to contamination of the municipal water with Campylobacter species and the commonly known EHEC strain O157:H7 that is associated with substantially lower complication rates compared to the new STEC O104:H4. In the Walkerton cohort, the incidence of PI-IBS two to three years after EHEC/Campylobacter gastroenteritis was 36.2% in patients with clinically suspected gastroenteritis.27 This was among the highest reported PI-IBS-incidence rates and suggested to be due to the severity of the initial gastroenteritis. However, this interpretation is not supported by our study, which found far lower IBS rates even though the clinical severity of the preceding hemorrhagic enterocolitis was clearly higher than in Walkerton. Several factors might conceivably contribute to this ostensible discrepancy: First, different time points during the follow-up after EHEC were analyzed, i.e. six and 12 months, compared with two to three years, and it might be hypothesized that in a subgroup of afflicted patients, late-onset PI-IBS develops after a brief asymptomatic lag period, before IBS prevalence gradually decreases in the long term.28 Second, the use of different IBS definitions (Rome III in our study, Rome I criteria in the Walkerton studies) might also explain this difference. On the other hand, studies comparing the different Rome criteria have shown that by applying Rome III criteria, more rather than fewer suspected IBS patients were included than by using Rome I.29 Hence, using Rome III should not underestimate IBS rates compared to Rome I. Third, a methodological bias toward an over-reporting of IBS symptoms might have played a role in the Walkerton cohort in light of ongoing lawsuits regarding charges of negligence and claims by victims for large sums of money for compensation,30 whereas no comparable legal and financial issues were pursued in the German outbreak.
Finally, the underlying infection was not identical in the two cohorts: The Walkerton outbreak was caused by water contamination with mixed pathogens, mainly Campylobacter species and EHEC O157:H7,27 whereas the 2011 outbreak in Northern Germany was exclusively related to STEC O104:H4-contaminated sprouts.10 And although STEC O104:H4 is closely related to the commonly known EHEC O157:H7 of Walkerton, both strains with Shiga-like toxin production, STEC O104:H4 also carries large similarities to an enteroaggregative E. coli (EAEC) strain, resulting in a more severe course of disease with a dramatically severe enterocolitis, a strikingly high incidence of HUS and neurological symptoms, and substantial mortality.10–12 Conceivably, differences in immunologic responses as well as different impacts on microbiota adaptation following various types of intestinal pathogens might account for, or contribute to, different risks of PI-IBS, and might be more important triggers than initial clinical disease severity alone. This is supported by recent research underlining the important role of microbiota changes in PI-IBS.3,31–33
Identification of risk factors for PI-IBS is key for an improved understanding of the disease and for the potential development of preventive measures. Previous studies of PI-IBS have already tried to identify potential risk or protective factors, though some of the studies assessed the factors retrospectively. Our study, on the contrary, assessed both somatic and psychometric factors immediately during the STEC infection period, and patients were followed prospectively for the development of IBS. Moreover, we assessed a broad spectrum of suspected predictive factors including medical treatments for STEC disease, which could be possible confounders for the effects of disease severity.
Previous studies observed psychological comorbidity as potential risk factors for PI-IBS.7–9 This was typically referring to known psychological disturbances before the GI infection. Although in our study prior psychological illness, as assessed by self-report of the patients, was not a significant predictive factor, increased baseline anxiety and somatization significantly increased the risk for PI-IBS. However, while self-reports on prior psychological illness were comparable to the general population, mean baseline anxiety and somatization scores as assessed at baseline during STEC disease were most naturally higher than in the general population, possibly at least partly due to the potentially life-threatening STEC disease at that stage. The influence of these psychological factors during STEC disease on the development of PI-IBS could maybe indicate differences in coping strategies of persistent somatic symptoms after experiencing life-threatening organic disease. Moreover, there may also be an association of IBS with a persistent psychological impairment after STEC disease: In fact, a follow-up of this same patient cohort regarding the long-term psychological outcome after STEC disease revealed poor psychological health, fatigue, and impaired quality of life in many patients.34 This would be in line with a large body of evidence in the literature that IBS is associated with psychological impairment.1
We found that clinical severity of STEC enterocolitis, assumed to be a major risk factor of IBS, was not associated with subsequent PI-IBS in our cohort. This surprising finding is not explained easily. It might be speculated that this could be related to unique pathophysiological features of the new STEC O104:H4 strain. On the other hand, this observation might be related to differences in management of the acute STEC disease, depending on its severity.
It cannot be excluded that therapeutic interventions may have confounded the natural course of emerging PI-IBS; conversely, interference with the spontaneous development of IBS conceivably may reflect potential protective mechanisms.
Our unexpected observation that mesalazine treatment during STEC disease appeared to be a potential protective factor for PI-IBS deserves attention. Yet, as only 18% of all patients received mesalazine, these results need to be interpreted with some caution despite their statistical strength. On the other hand, they do not appear to be caused by a methodological bias insofar as mesalazine-treated patients did not represent a subgroup with milder STEC disease; rather, patients with more severe clinical symptoms of hemorrhagic enterocolitis were more likely to receive mesalazine.
Because of the methodological limitations of this study, the intriguing hypothesis that mesalazine may indeed have exerted protective effects could not be tested in our patient cohort, but carries theoretical as well as clinical appeal:
Established mechanisms of action of mesalazine include modulation of inflammatory responses in the gut wall, such as reduction of inflammatory cytokine release and downregulating of mast cell function.35–37 Conversely, in IBS increased mast cell infiltration and activation, as well as release of proinflammatory cytokines, count among the main features of immune activation and possibly symptom generation via activated enteric nervous system and increased neurotransmitters38 in IBS. Hence, early modulation of these alterations during enterocolitis might inhibit the persistent increase of mucosal immune reaction as the hallmark of PI-IBS and hence act as protective factor. Recent studies have also investigated the efficacy of mesalazine treatment in patients with existing IBS showing reduction of rectal inflammatory markers and some beneficial effects on symptoms, although results of the different studies are ambiguous.39–41
Some general limitations of our study should be acknowledged. One of them is the lack of a control group. This can be explained by the effort to start the assessment of STEC patients directly during the acute outbreak, which had a natural limitation of a few weeks. The time pressure for this rapid assessment needed all of our resources at the time. Another limitation may be the retrospective assessment of IBS status before STEC. However, the IBS prevalence rate of 9.8% prior to STEC disease in this cohort is comparable to the epidemiologic IBS prevalence estimates for Germany ranging from 7.4%42 to 12.5%,43 thereby underlining a valid assessment of baseline IBS status prior to STEC as well as a good representativeness of our study cohort.
Furthermore, we cannot exclude some sampling and response bias, e.g. toward more symptomatic patients, as only 64% of eligible patients participated in the study and some patients were lost to follow-up. However, overall response rates were very high compared to other questionnaire studies, and comparisons between responders and non-responders did not reveal relevant differences. Another sampling bias might be attributed to the exclusion of very severe cases requiring long intensive care unit treatment and suffering from neurological impairment and renal failure at the time. Due to their poor health status, these patients were unable to participate in a questionnaire study at the time. However, the severity of disease in these patients could mainly be attributed to HUS complications and not to the STEC enterocolitis. As HUS did not influence IBS incidence in our cohort, it is unlikely that omission of these patients affected the results.
In conclusion: Although this STEC outbreak was the severest outbreak of hemorrhagic enterocolitis ever reported, rates of post-infectious IBS within 12 months increased only within the range reported after milder infections. Furthermore, mesalazine, which was given to 18% of the patients during STEC disease, emerged as a significant independent protective factor of PI-IBS. Since persistent increased mucosal immune activity is an important pathogenic factor in PI-IBS pathophysiology, the known modulatory effects of mesalazine on mucosal immune responses might have played a role. These findings may warrant further studies investigating a potential therapeutic role of mesalazine in the prevention of PI-IBS.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
We thank the patients and physicians who contributed to this study. The following members of the STEC Investigation Team have substantially contributed to the study by generously assisting with the data collection in their hospitals:
Professor Dr Torsten Kucharzik (Klinikum Lüneburg)
Professor Dr Klaus Fellermann, PD Dr Jürgen Büning, PD Dr Matthias J. Bahr (Universitätsklinikum und Sana Lübeck)
Professor Dr Rolf Stahl (Universitätsklinikum Hamburg-Eppendorf (UKE)
Professor Dr Friedrich Hagenmüller (Asklepios Klinik Hamburg Altona)
PD Dr Siegbert Faiss (Asklepios Klinik Hamburg Barmbek)
Professor Dr Klaus Herrlinger (Asklepios Klinik Hamburg Nord)
Professor Dr Stefan Ulrich Christl (Asklepios Klinik Hamburg Harburg)
Dr Anneke Wiese (Klinikum Rotenburg, Wümme)
Professor Dr Andreas de Weerth (Agaplesion Hamburg)
Professor Dr Irmtraut Koop (Amalie Sieveking Krankenhaus Hamburg)
Professor Dr Hugo Heidemann (Schön Klinik Hamburg Eilbek)
Professor Dr Andreas van de Loo (Marienkrankenhaus Hamburg)
Contributorship
Viola Andresen: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript
Bernd Löwe: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Wiebke Broicher: study concept and design, acquisition of data, statistical analysis, analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Björn Riegel: statistical analysis, analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Katharina Fraedrich: acquisition of data, study concept and design
Moritz von Wulffen: acquisition of data
Kerrin Gappmayer: acquisition of data
Karl Wegscheider: statistical analysis, analysis and interpretation of data
András Treszl: statistical analysis, analysis and interpretation of data
Matthias Rose: study concept and design, critical revision of the manuscript for important intellectual content
Peter Layer: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Ansgar Lohse: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Conflicts of interest
Viola Andresen has received consulting and/or speaker fees from AbbVie, Almirall, Ardeypharm, AstraZeneca, Falk, Mundipharma, Norgine and Shire.
Peter Layer has received consulting and/or speaker fees from Abbvie, Almirall, Norgine, Falk, Norgine and Shire.
Ansgar W. Lohse has received speaker fees from Norgine.
All other authors have nothing to declare.
References
- 1.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012; 303: G775–G785. [DOI] [PubMed] [Google Scholar]
- 3.Spiller R, Lam C. An update on post-infectious irritable bowel syndrome: Role of genetics, immune activation, serotonin and altered microbiome. J Neurogastroenterol Motil 2012; 18: 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwille-Kiuntke J, Frick JS, Zanger P, et al. Post-infectious irritable bowel syndrome—a review of the literature. Z Gastroenterol 2011; 49: 997–1003. [DOI] [PubMed] [Google Scholar]
- 5.Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther 2007; 26: 535–544. [DOI] [PubMed] [Google Scholar]
- 6.Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome—a meta-analysis. Am J Gastroenterol 2006; 101: 1894–1899. quiz 1942. [DOI] [PubMed] [Google Scholar]
- 7.Spiller R, Garsed K. Infection, inflammation, and the irritable bowel syndrome. Dig Liver Dis 2009; 41: 844–849. [DOI] [PubMed] [Google Scholar]
- 8.Ruigόmez A, García Rodríguez LA, Panés J. Risk of irritable bowel syndrome after an episode of bacterial gastroenteritis in general practice: Influence of comorbidities. Clin Gastroenterol Hepatol 2007; 5: 465–469. [DOI] [PubMed] [Google Scholar]
- 9.Thabane M, Simunovic M, Akhtar-Danesh N, et al. Development and validation of a risk score for post-infectious irritable bowel syndrome. Am J Gastroenterol 2009; 104: 2267–2274. [DOI] [PubMed] [Google Scholar]
- 10.Robert Koch Institut. Abschließende Darstellung und Bewertung der epidemiologischen Erkenntnisse im EHEC O104:H4 Ausbruch [Final description and evaluation of the epidemiological results regarding the EHEC O104:H4 outbreak]. Berlin: Robert Koch Institut, 2011.
- 11.Frank C, Werber D, Cramer JP, et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med 2011; 365: 1771–1780. [DOI] [PubMed] [Google Scholar]
- 12.Beutin L, Martin A. Outbreak of Shiga toxin-producing Escherichia coli (STEC) O104:H4 infection in Germany causes a paradigm shift with regard to human pathogenicity of STEC strains. J Food Prot 2012; 75: 408–418. [DOI] [PubMed] [Google Scholar]
- 13.Reissbrodt R, Hammes WP, dal Bello F, et al. Inhibition of growth of Shiga toxin-producing Escherichia coli by nonpathogenic Escherichia coli. FEMS Microbiol Lett 2009; 290: 62–69. [DOI] [PubMed] [Google Scholar]
- 14.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001; 16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med 2006; 166: 1092–1097. [DOI] [PubMed] [Google Scholar]
- 16.Löwe B, Decker O, Müller S, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care 2008; 46: 266–274. [DOI] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002; 64: 258–266. [DOI] [PubMed] [Google Scholar]
- 18.Bullinger M and Kirchberger I. Fragebogen zum Gesundheitszustand, Kurzform SF-12. [12-Item Short Form Health Survey—German version]. Göttingen, Germany: Hogrefe, 1998.
- 19.Scholler G, Fliege H, Klapp BF. Questionnaire of self-efficacy, optimism and pessimism: Reconstruction, selection of items and validation of an instrument by means of examinations of clinical samples [article in German]. Psychother Psychosom Med Psychol 1999; 49: 275–283. [PubMed] [Google Scholar]
- 20.Rammstedt B, John OP. Kurzversion des Big Five Inventory (BFI-K). Diagnostica 2005; 51: 195–206. [Google Scholar]
- 21.Fydrich T, Sommer G, Tydecks S, et al. Fragebogen zur Sozialen Unterstützung (F-SozU): Normierung der Kurzform (K-14). Z Med Psychol 2009; 18: 43–48. [Google Scholar]
- 22.Kocalevent RD, Hinz A, Brähler E. Standardization of the depression screener patient health questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry 2013; 35: 551–555. [DOI] [PubMed] [Google Scholar]
- 23.Kocalevent RD, Hinz A, Brähler E. Standardization of a screening instrument (PHQ-15) for somatization syndromes in the general population. BMC Psychiatry 2013; 13: 91–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Buuren S, Groothuis-Oudshoon K. MICE: Multivariate imputation by chained equations in R. J Stat Softw 2011; 45: 1–67. [Google Scholar]
- 25.Field A. Discovering statistics using SPSS, 3rd ed London: SAGE Publications Ltd., 2009. [Google Scholar]
- 26.Mearin F, Pérez-Oliveras M, Perellό A, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: One-year follow-up cohort study. Gastroenterology 2005; 129: 98–104. [DOI] [PubMed] [Google Scholar]
- 27.Marshall JK, Thabane M, Garg AX, et al. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology 2006; 131: 445–450. quiz 660. [DOI] [PubMed] [Google Scholar]
- 28.Marshall JK, Thabane M, Garg AX, et al. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut 2010; 59: 605–611. [DOI] [PubMed] [Google Scholar]
- 29.Ghoshal UC, Abraham P, Bhatia SJ, et al. Comparison of Manning, Rome I, II, and III, and Asian diagnostic criteria: Report of the Multicentric Indian Irritable Bowel Syndrome (MIIBS) study. Indian J Gastroenterol 2013; 32: 369–375. [DOI] [PubMed] [Google Scholar]
- 30.Grabow WOK, Müller EE, Ehlers MM, et al. Occurrence of E. coli O157:H7 and other pathogenic E. coli strains in water sources intended for direct and indirect consumption. In Final Report to the Water Research Commission, WRC Report No 1068/1/03. Department of Medical Virology, University of Pretoria, and Rand Water Scientific Services, Vereeniging. Pretoria: 2003.
- 31.Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013; 62: 159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundin J, Rangel I, Fuentes S, et al. Altered faecal and mucosal microbial composition in post-infectious irritable bowel syndrome patients correlates with mucosal lymphocyte phenotypes and psychological distress. Aliment Pharmacol Ther 2015; 41: 342–351. [DOI] [PubMed] [Google Scholar]
- 33.Jalanka-Tuovinen J, Salojärvi J, Salonen A, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014; 63: 1737–1745. [DOI] [PubMed] [Google Scholar]
- 34.Löwe B, Andresen V, Fraedrich K, et al. Psychological outcome, fatigue, and quality of life after infection with Shiga toxin-producing Escherichia coli O104. Clin Gastroenterol Hepatol 2014; 12: 1848–1855. [DOI] [PubMed] [Google Scholar]
- 35.Corinaldesi R, Stanghellini V, Cremon C, et al. Effect of mesalazine on mucosal immune biomarkers in irritable bowel syndrome: A randomized controlled proof-of-concept study. Aliment Pharmacol Ther 2009; 30: 245–252. [DOI] [PubMed] [Google Scholar]
- 36.Barbara G, Stanghellini V, Cremon C, et al. Aminosalicylates and other anti-inflammatory compounds for irritable bowel syndrome. Dig Dis 2009; 27 (Suppl 1): 115–121. [DOI] [PubMed] [Google Scholar]
- 37.Andrews CN, Griffiths TA, Kaufman J, et al. Mesalazine (5-aminosalicylic acid) alters faecal bacterial profiles, but not mucosal proteolytic activity in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2011; 34: 374–383. [DOI] [PubMed] [Google Scholar]
- 38.Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 2009; 137: 1425–1434. [DOI] [PubMed] [Google Scholar]
- 39.Barbara G, Cremon C, Annese V, et al. Randomised controlled trial of mesalazine in IBS. Gut. Epub ahead of print 22 December 2014. DOI: 10.1136/gutjnl-2014-308188. [DOI] [PMC free article] [PubMed]
- 40.Lam C, Tan W, Leighton M, et al. A multi-centre, parallel group, randomised placebo controlled trial of mesalazine for treatment of diarrhea-predominant irritable bowel syndrome (IBS-D). Gastroenterology 2014; 146: S123–S124. [Google Scholar]
- 41.Castro Tejera V, Jerlstad P, Venge P, et al. Su2086 A pilot study of mesalazine treatment in IBS, evaluating the tolerance and usefulness of the mucosal patch technique for measurement of rectal inflammatory markers. Gastroenterology 2014; 146(Suppl 1): S–542. [Google Scholar]
- 42.Hungin AP, Whorwell PJ, Tack J, et al. The prevalence, patterns and impact of irritable bowel syndrome: An international survey of 40,000 subjects. Aliment Pharmacol Ther 2003; 17: 643–650. [DOI] [PubMed] [Google Scholar]
- 43.Icks A, Haastert B, Enck P, et al. Prevalence of functional bowel disorders and related health care seeking: A population-based study. Z Gastroenterol 2002; 40: 177–183. [DOI] [PubMed] [Google Scholar]