Abstract
Background and aims
This systematic review and meta-analysis compares the safety and effectiveness of endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) in the treatment of flat and sessile colorectal lesions >20 mm preoperatively assessed as noninvasive.
Methods
We reviewed the literature published between January 2000 and March 2014. Pooled estimates of the proportion of patients with en bloc, R0 resection, complications, recurrence, and need for further treatment were compared in a meta-analysis using fixed and random effects.
Results
A total of 11 studies and 4678 patients were included. The en bloc resection rate was 89.9% for ESD vs 34.9% for EMR patients (RR 1.93 p < 0.001). The R0 resection rate was 79.6% for ESD vs 36.2% for EMR patients (RR 2.01 p < 0.001). The rate of perforation was 4.9% for the ESD group and 0.9% for EMR (RR 3.19, p < 0.001), while the rate of bleeding was 1.9% for ESD and 2.9% for EMR (RR 0.68, p = 0.070). Therefore, the overall need for further surgery, including surgery for oncologic reasons and surgery for complications, was 7.8% for ESD and 3.0% for EMR (RR 2.40, p < 0.001).
Conclusions
ESD achieves a higher rate of en bloc and R0 resection compared to EMR, at the cost of a higher risk of complications. This, added to an increased need for surgery for oncologic reasons for a plausible tendency to extend indication for endoscopic excision, increases the risk of further surgery after ESD.
Keywords: Colorectal adenoma, endoscopic mucosal resection, endoscopic submucosal dissection, systematic review, meta-analysis
Introduction
Almost 15 years after the first report,1 endoscopic submucosal dissection (ESD) is widely used in the treatment of colorectal lesions in Eastern countries, while its application is still debated in Europe and the United States (US), in favor of endoscopic mucosal resection (EMR). The complexity of the ESD procedure has prompted discussion about the need for a specific training and certification before undertaking clinical experience.
Supporters of the ESD technique affirm the need of an en bloc excision to allow a correct oncologic assessment of the lesion and the eventual submucosal invasion, as well as of the clearance of lateral and deep resection margins. Fragmentation may be responsible for an inadequate pathology examination according to the Hermanek2 assessed criteria to determine lesions at “low risk” for recurrence. With the advent of ESD,3 flexible endoscopy permitted a surgical-like technique for en bloc resection of superficial lesions of the digestive tract, representing an alternative to EMR for even the colon and rectum,4 aiming at an en bloc R0 excision even for lesions.
In truth, a definitive conclusion as to whether ESD is superior to EMR should consider the real need for further surgery for management of complications and with respect to oncologic indications. With the lack of a similar evaluation, and while long-term follow-up studies are awaited to focus on the oncologic adequacy of ESD and EMR treatment of colorectal lesions, a short-term analysis of safety and treatment implications may already be performed on existing data.
The aim of this study was to evaluate in a systematic review and meta-analysis whether there are clinically relevant short-term advantages in terms of safety and effectiveness of ESD compared to EMR in the treatment of large non-pedunculated colorectal lesions preoperatively assessed as noninvasive.
Methods
The methods for the analysis and generation of inclusion criteria were based on the Cochrane Collaboration guidelines5 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations.6 The study methods were documented in a protocol registered and accessible at http://www.crd.york.ac.uk/prospero/ (registration number: CRD42014010049).7
Criteria for identifying studies and eligibility
According to population, interventions, comparators, outcome measures, and setting (PICOS) criteria, we included patients from randomized or quasi-randomized studies, prospective and retrospective series that directly compared EMR and ESD for treatment of non-pedunculated colorectal lesions >20 mm preoperatively assessed as non-invasive according to Kudo classification8 and by the ability to lift when the submucosal layer was injected below the lesion. Exclusion criteria were the carcinoid nature of the lesion and the impossibility to hive-off data from mixed series.
EMR had to be performed en bloc when possible, or piecemeal when necessary. ESD could be performed by any of the techniques described in the literature, including using the different knives available.
Outcomes
Primary outcome was the effectiveness of resection, i.e. en bloc resection rate, defined as the rate of lesions excised in a single specimen, and R0 resection rate, defined as the rate of lesions excised with margins free of disease, as assessed by the pathologist. Secondary end-points were: size of lesions excised, time for completing the procedure, safety, i.e. post-procedural complications (bleeding and perforation), and the need for abdominal surgery to manage complications, recurrence rate as assessed by follow-up, the need for abdominal surgery for oncologic reasons, and finally the overall need for abdominal surgery. Abdominal surgery was defined as any kind of surgery performed through an abdominal access.
Search strategy
Searches were conducted on literature published in English between January 2000 and September 2014, identified by electronic searches of PubMed and EMBASE on October 1, 2014, using the string “endoscopic mucosal resection”/exp and “endoscopic submucosal dissection”/exp and (“colon”/exp or “rectum”/exp or “colorectal”) and (2000–2014)/py.
Study selection
Titles were screened by two authors (AA and NM). When the same data of a single research group were reported in multiple publications, only the study reporting on the largest cohort was included. A third investigator (RP) arbitrated in the event of lack of agreement.
From each report, reviewers independently collected the following data when available: (a) year of publication, (b) prospective or retrospective study design, (c) enrollment period, (d) number of patients included, (e) mean age, (f) gender distribution, (g) indication for treatment, (h) Kudo pit-pattern classification, (i) means of submucosal injection, (j) type of device used, (k) mean operating time, (l) mean tumor size, (m) complications rate, (n) rate of surgery due to complications, (o) histology (adenoma, carcinoma in situ, invasive cancer, carcinoid), (p) rate of histologically verified en bloc resection, (q) rate of histologically verified complete resection (R0), (r) rate of surgery for oncologic reasons, (s) follow-up, (t) histologically demonstrated recurrence, and (u) need for further treatment for disease recurrence.
Quality assessment
Methodological quality and risk of bias of each study was determined according to the Cochrane Collaboration guidelines4 for randomized controlled trials (RCTs) and to the Newcastle-Ottawa Scale for non-RCTs9 by three reviewers (AA, NM and RP).
Statistical analyses
All analyses were performed according to original treatment allocation (intention-to-treat analysis). For binary outcome data, the relative risks (RR) and 95% confidence intervals (CIs) were estimated using the Mantel-Haenszel method. For continuous outcome data, the mean differences (MD) and 95% CIs were estimated using the inverse variance weighting; when means and/or standard deviations (SDs) were not reported, they were estimated from reported medians, ranges and sample size as described by Hozo et al.10
A fixed-effects model was used in all meta-analyses, always recalculating the same analyses by a random-effects model. Heterogeneity was assessed by the I2 measure of inconsistency, statistically significant if I2 >50%.
Potential sources of heterogeneity were explored by different sensitivity analyses: comparing fixed- vs random-effects models (thus incorporating heterogeneity by using the second method), performing sub-groups analyses (comparing full-text articles vs abstracts), checking the results of cumulative (sequentially including studies by date of publication) and influence analyses (calculating pooled estimates omitting one study at a time). Publication bias was assessed, generating a funnel plot and performing a linear regression test for funnel plot asymmetry. All analyses were conducted by R 3.1.0 using R package meta.11
Results
The search retrieved 381 studies (Figure 1) of which 11 studies12–22 met the inclusion criteria, including a total of 4678 patients: 1517 had an ESD (32.4%) and 3161 an EMR (67.6%) (Table 1). Five are full-text articles and six are abstracts to congresses. None of the studies included randomization. All were retrospective observational cohort studies, in one case with patient sequential inclusion. Indications for EMR and ESD were lesions ≥20 mm in all but three studies13,15,21 in which ESD was indicated for non-granular laterally spreading tumors (LST-NG) ≥20 mm and granular laterally spreading tumors (LST-G) ≥30 mm or ≥40 mm. Only three of the full-text articles and none of the abstracts gave a clear definition of “complete resection.”
Figure 1.
Flowchart diagram illustrating the systematic search and study selection strategy.
Table 1.
Principal characteristics of the included studies
| Study | Year | Center, city, country | Type of publication | Time interval considered | Number of lesions | Indication for EMR or ESD | Definition of complete resection |
|---|---|---|---|---|---|---|---|
| Iizuka | 2009 | Maebashi Red Cross Hospital, Maebashi, Japan | Full-text | January 2000–December 2004 | 121 | LST ≥20 mm | One-piece resection with histopathologically tumor-free margins, i.e. at least 0.5 mm between the tumor edge and the margin |
| Kobayashi | 2009 | Tochigi Cancer Center Hospital, Tochigi, Japan | Abstract | January 2000–June 2007 | 147 | ESD: LST-NG lesions ≥20 mm and LST-G lesions ≥30 mm EMR/EPMR: LST-G lesions ≥20 mm and ≤30 mm | NA |
| Nakae | 2010 | Juntendo University School of Medicine, Tokyo, Japan | Abstract | January 1995–December 2008 | 251 | LST ≥20 mm | NA |
| Saito | 2010 | National Cancer Center Hospital, Tokyo, Japan | Full-text | January 2003–December 2006 | 373 | ESD: LST-NG lesions ≥20 mm and LST-G lesions ≥40 mm EMR/EPMR: LST-G lesions ≥20 mm and ≤40 mm | NA |
| Tajika | 2011 | Aichi Cancer Center Hospital, Nagoya, Japan | Full-text | January 1995–December 2009 | 212 | LST ≥20 mm | Lateral and vertical margins both negative for tumor cells |
| Terasaki | 2011 | Hiroshima University Hospital, Hiroshima, Japan | Full-text | April 2006–December 2009 | 239 | ESD: lesions ≥20 mm unsuitable for en bloc resection by snare EMR; LST-NG; type VI pit pattern; cancer with submucosal infiltration; large depressed-type tumor fibrosis caused by biopsy or peristalsis of the lesions; local residual early cancer after endoscopic resection; sporadic localized tumors in chronic inflammation, such as ulcerative colitis. | NA |
| Sudo | 2011 | Northern Yokohama Hospital, Yokohama, Japan | Abstract | NA | 676 | LST ≥20 mm | NA |
| Lee | 2012 | Daehang Hospital, Seoul, Korea | Full-text | January 2004–November 2009 | 523 | LST ≥20 mm | Mucosal and submucosal margins of a specimen both free of adenoma and adenocarcinoma |
| Coumaros | 2012 | Clinique Sainte Barbe, Strasbourg, France | Abstract | 2005–2010 | 112 | LST ≥20 mm | NA |
| Kang | 2012 | Bongseng Memorial Hospital, Busan, Korea | Abstract | February 2009–March 2011 | 57 | LST ≥20 mm | NA |
| Kudo | 2013 | Showa University Northern Yokohama, Japan | Abstract | April 2011–December 2012 | 1922 | LST ≥20 mm | NA |
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; LST-NG: non-granular laterally spreading tumors; LST-G: granular laterally spreading tumors.
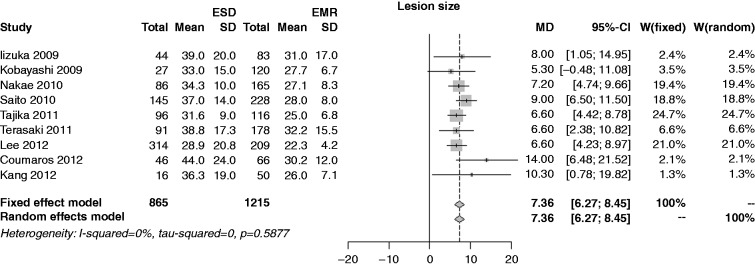
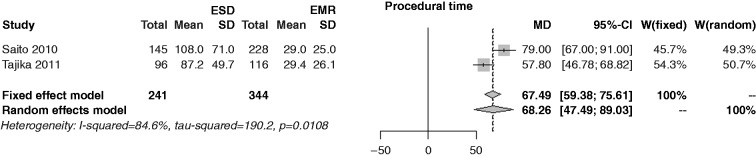
Characteristics of patients are shown in Table 2. The mean polyp size in the ESD series was 33.7 mm vs 27.4 mm in the EMR series (p < 0.001) (Figure 2). The operating time in the ESD series was 66.5 minutes vs 29.1 minutes in the EMR series (p < 0.001) (Figure 3).
Table 2.
Characteristics of patients treated by endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR)
| Study | Treatment | Number of lesions | Mean age in years ± SD (range) | Male/Female | EUS reported (yes/no) | Instruments | Injection solution | Mean follow-up months ± SD (range) |
|---|---|---|---|---|---|---|---|---|
| Iizuka | ESD | 44 | 69 ± 12 (34–86) | 24/20 | Yes | Flex knife | Sodium hyaluronate | NA |
| EMR | 83 | 66 ± 12 (32–91) | 242/140 | Yes | NA | |||
| Kobayashi | ESD | 27 | NA | NA | No | NA | NA | NA |
| EMR | 120 | NA | NA | No | NA | |||
| Nakae | ESD | 86 | NA | NA | No | NA | NA | NA |
| EMR | 165 | NA | NA | No | NA | NA | ||
| Saito | ESD | 145 | 64 ± 11 | NA | No | B-knife, IT-knife | Sodium hyaluronate, glycerol | 20 ± 13 (6–61) |
| EMR | 228 | 64 ± 4 | NA | No | Sodium hyaluronate | 26 ± 17 (6–68) | ||
| Tajika | ESD | 96 | 64.3 ± 9.2 | 49/36 | No | B-knife | Sodium hyaluronate or saline | 14.3 ± 13.4 (3–53) |
| EMR | 116 | 59.9 ± 10.6 | 61/39 | No | Saline | 53.8 ± 44.6 (3–191) | ||
| Terasaki | ESD | 91 | 66.7 ± 10.7 (42–86) | 38/23 | No | Dual knife, Flex knife, Hook knife, SB knife Jr | Sodium hyaluronate | NA |
| EMR | 178 | 67.9 ± 11.3 (39–89) | 99/79 | No | Glycerol | NA | ||
| Sudo | ESD | 167 | NA | NA | No | NA | NA | NA |
| EMR | 509 | NA | NA | No | NA | NA | ||
| Lee | ESD | 314 | 61 (25–85) | NA | No | Flex knife, Hook knife | Sodium hyaluronate, saline | 17 |
| EMR | 209 | 63 (23–90) | NA | No | Glycerol or saline | 26 | ||
| Coumaros | ESD | 46 | NA | NA | No | NA | NA | NA |
| EMR | 66 | NA | NA | No | NA | NA | ||
| Kang | ESD | 16 | NA | NA | No | NA | NA | 4.9 ± 3.7 (3–16) |
| EMR | 50 | NA | NA | No | NA | 6.1 ± 5.0 (3–24) | ||
| Kudo | ESD | 485 | NA | NA | No | NA | NA | NA |
| EMR | 1437 | NA | NA | No | NA | NA |
EUS: endoscopic ultrasound.
Figure 2.
Forest plot for size of lesions.
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; MD: mean difference; CI: confidence interval.
Figure 3.
Forest plot for procedural time.
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; MD: mean difference; CI: confidence interval.
Risk of bias of included studies
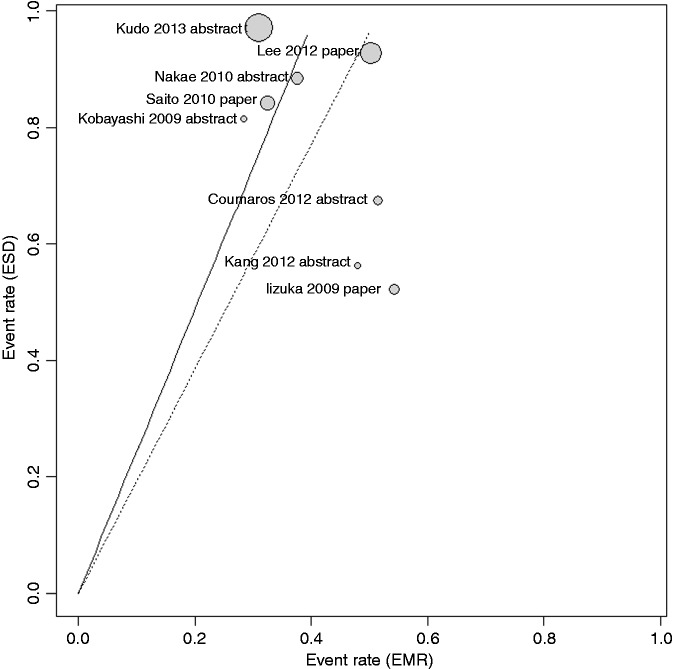
Assessment of quality according to the Newcastle-Ottawa Scale showed an average value of 5.6 (range 4–8) (Table 3). A L’Abbé plot for en bloc resection reporting the potential sources of heterogeneity within all studies showed a quite homogeneous distribution of studies (Figure 4).
Table 3.
Quality assessment of the included prospective controlled clinical trials based on the Newcastle-Ottawa scale
| Study | Selectiona |
Comparabilityb |
Outcome assessmentc |
Score | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4# | 5 | 6 | 7 | ||
| Iizuka | * | * | * | ** | * | 6 | ||
| Kobayashi | * | * | * | * | 4 | |||
| Nakae | * | * | * | * | * | 5 | ||
| Saito | * | * | * | * | * | * | * | 7 |
| Tajika | * | * | * | ** | ** | * | 8 | |
| Terasaki | * | * | * | * | * | 5 | ||
| Sudo | * | * | * | * | 4 | |||
| Lee | * | * | * | * | ** | * | * | 8 |
| Coumaros | * | * | * | * | * | 5 | ||
| Kang | * | * | * | * | * | 5 | ||
| Kudo | * | * | * | * | 4 | |||
Selection: (1) assignment for treatment (if yes, one point). (2) How representative was the ESD group in comparison to the general population undergoing treatments (if yes, one point; no points if the patients were selected or selection of group was not described). (3) How representative was the EMR group in comparison to the general population undergoing treatments (if yes, one point; no points if the patients were selected or selection of group was not described).
Comparability: (4) group comparable for 1–2 (if yes, two points; one point if one of these two characteristics was not reported even if there were no other differences between the two groups and other characteristics had been controlled for; no points were assigned if the two groups differed). (5) Group comparable for 3–5 (if yes, two points; one point if one of these three characteristics was not reported even if there were no other differences between the two groups and other characteristics had been controlled for; no points were assigned if the two groups differed). Comparability variables: 1 = age, 2 = gender, 3 = tumor location, 4 = stage, 5 = procedure.
Outcome assessment: (6) clearly defined outcome of interest (if yes, one point for information ascertained by medical records or interview; no points if this information was not reported). (7) Follow-up equal between the two groups (if yes, one point; no points if follow-up not reported).
It was not possible to evaluate the American Society of Anesthesiology Score.
Figure 4.
L’Abbé plot for rate of en bloc resection.
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection.
Primary outcomes
The meta-analysis investigated as primary outcomes en bloc and R0 resection rates (Table 4).
Table 4.
Efficacy and safety of EMR and ESD for the treatment of colorectal lesions >20 mm
| Study | Treatment | Number of lesions | Mean size in mm ± SD (range) | Number of en bloc resections, (%) | Number of complete resections, (%) |
|---|---|---|---|---|---|
| Iizuka | ESD | 44 | 39 ± 20a | 23 (52%) | 22 (50%) |
| EMR | 83 | 31 ± 17 | 45 (54%) | 31 (37%) | |
| Kobayashi | ESD | 27 | 33.0 (20–80) | 22 (81%) | NA |
| EMR | 120 | 27.7 (20–60) | 34 (28%) | NA | |
| Nakae | ESD | 86 | 34.3 (20–81) | 76 (88%) | NA |
| EMR | 165 | 27.1 (20–70) | 62 (37%) | NA | |
| Saito | ESD | 145 | 37 ± 14 (20–140) | 122 (84%) | NA |
| EMR | 228 | 28 ± 8 (20–95) | 74 (33%) | NA | |
| Tajika | ESD | 96 | 31.6 ± 9.0 (20–54)b | NA | 73 (76%)b |
| EMR | 116 | 25 ± 6.8 (20–55)b | NA | 41 (35%)b | |
| Terasaki | ESD | 91 | 38.8 ± 17.3 | NA | NA |
| EMR | 178 | 32.2 ± 15.5 | NA | NA | |
| Sudo | ESD | 167 | NA | NA | NA |
| EMR | 509 | NA | NA | NA | |
| Lee | ESD | 314 | 28.9 (20–145) | 291 (93%) | 275 (88%) |
| EMR | 209 | 22.3 (20–45) | 105 (50%) | 87 (42%) | |
| Coumaros | ESD | 46 | 44 ± 24 | 31 (67%) | NA |
| EMR | 66 | 30 ± 12 | 34 (52%) | NA | |
| Kang | ESD | 16 | NA | 6 (86%) | 4 (25%) |
| EMR | 50 | NA | 24 (48%) | 7 (14%) | |
| Kudo | ESD | 485 | NA | 471 (97%) | NA |
| EMR | 1437 | NA | 444 (31%) | NA |
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection. aExcluding seven patients addressed for surgery for complications during ESD. bExcluding 23 patients addressed for surgery for oncologic reasons.
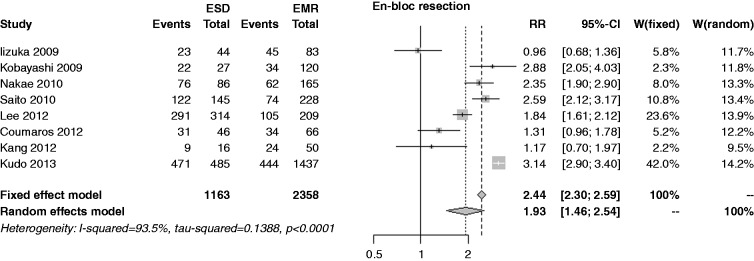
The en bloc resection rate was reported in eight studies; the rate was 89.9% in the ESD group and 34.9% in the EMR. Due to extreme heterogeneity (I2 = 93.5%) the random-effects model was used, showing an overall RR of 1.93 (95% CI 1.46–2.54, p < 0.001), with extreme publication bias (p < 0.001) (Figure 5). Performing a cumulative meta-analysis, RR ranged from 1.7 to 2.1 except for the Iizuka et al. study;22 performing an influence analysis, the RR varied in the same range. In the sensitivity analysis, the RR was 1.71 (1.12–2.62) among full papers, and 2.09 (1.47–2.97) among abstracts (p = 0.478).
Figure 5.
Forest plot for rate of en bloc resection.
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; MD: mean difference; CI: confidence interval.
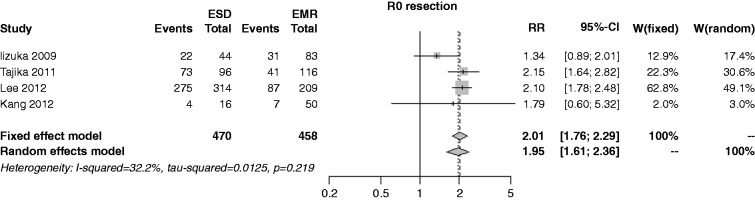
The R0 resection rate was reported in four studies; the rate was 79.6% in the ESD group and 36.2% in the EMR. Due to the low heterogeneity (I2 = 32.2%) the fixed-effects model was used, showing an overall RR of 2.01 (95% CI 1.76–2.29, p < 0.001), with marginal publication bias (p = 0.076) (Figure 6). Performing a cumulative meta-analysis, the RR increased from 1.3 to 2.0 in the time course. At influence analysis, the RR was affected by the Iizuka study,22 which represented the only source of heterogeneity: Omitting this trial, the RR estimate was 2.11 (1.83–2.43, p < 0.001, I2 = 0%). In the sensitivity analysis, the RR was 2.01 (1.76–2.30) among full papers, and 1.79 (0.60–5.32) among abstracts (p = 0.830).
Figure 6.
Forest plot for rate of R0 resection.
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; MD: mean difference; CI: confidence interval.
Secondary outcomes
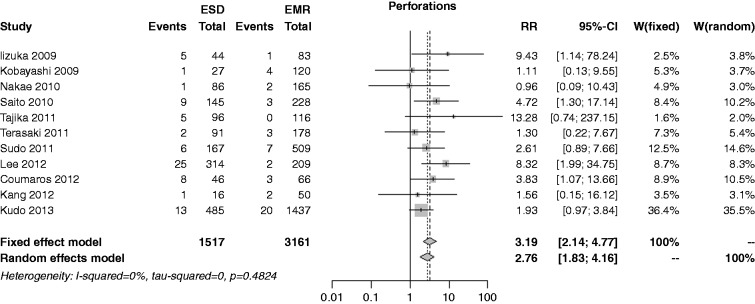
The risk of perforation was reported in 11 studies; the rate was 4.9% in the ESD group and 0.9% in the EMR, with an overall RR of 3.19 (I2 = 0%, 95% CI 2.14–4.77, p < 0.001), and no publication bias (p = 0.515) (Figure 7). In both the cumulative meta-analysis and influence analysis, the RR was quite constant.
Figure 7.
Forest plot for rate of perforation.
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; MD: mean difference; CI: confidence interval.
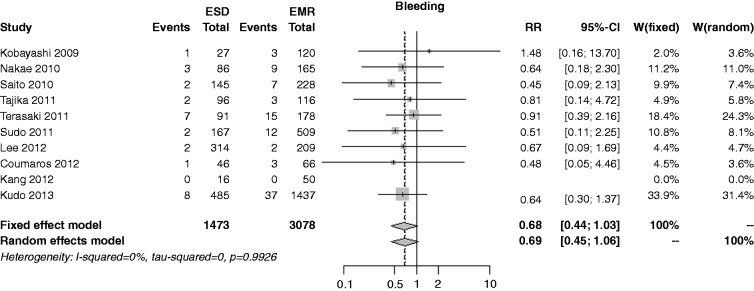
The bleeding risk was reported in 10 studies; the rate was 1.9% in the ESD group and 2.9% in the EMR one, with an overall RR of 0.68 (I2 = 0%, 95% CI 0.44–1.03, p = 0.070), and no publication bias (p = 0.788) (Figure 8). In both the cumulative meta-analysis and influence analysis, the RR was quite constant.
Figure 8.
Forest plot for rate of bleeding.
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; MD: mean difference; CI: confidence interval.
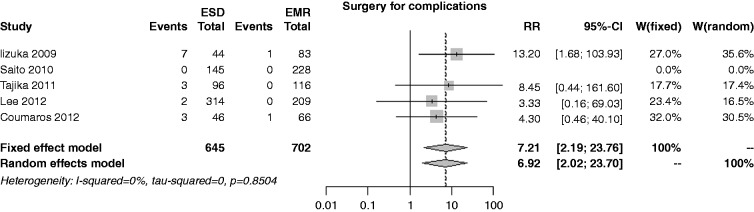
The risk of surgery for complications was reported in four studies; the rate was 3.0% in the ESD group and 0.4% in the EMR one, with an overall RR of 7.21 (I2 = 0%, 95% CI 2.19–23.76, p = 0.001), and no publication bias (p = 0.884) (Figure 9). In both the cumulative meta-analysis and influence analysis, the RR was quite constant.
Figure 9.
Forest plot for rate of surgery for complications.
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; MD: mean difference; CI: confidence interval.
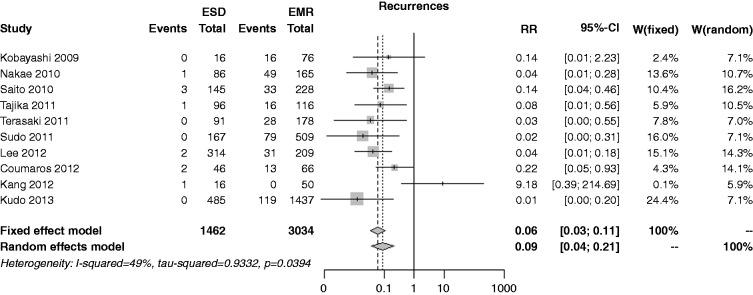
The recurrence risk was reported in 10 studies; the rate was 0.7% in the ESD group and 12.7% in the EMR one, with an overall RR of 0.06 (I2 = 49.0%, 95% CI 0.03–0.11, p < 0.001), and no publication bias (p = 0.840) (Figure 10). In both the cumulative meta-analysis and influence analysis, the RR was quite constant.
Figure 10.
Forest plot for recurrence rate.
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; MD: mean difference; CI: confidence interval.
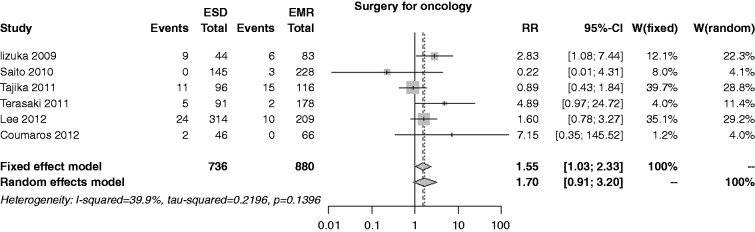
The risk of surgery for oncology was reported in six studies; the rate was 6.9% in the ESD group and 4.1% in the EMR, with an overall RR of 1.55 (I2 = 39.9%, 95% CI 1.03–2.33, p = 0.034), and no publication bias (p = 0.626) (Figure 11). In both the cumulative meta-analysis and influence analysis, the RR was quite constant.
Figure 11.
Forest plot for rate of surgery for oncologic adequacy.
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; MD: mean difference; CI: confidence interval.
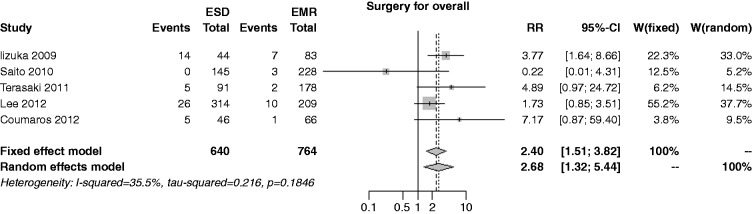
The overall risk of surgery was reported in five studies; the rate was 7.8% in the ESD group and 3.0% in the EMR, with an overall RR of 2.40 (I2 = 35.5%, 95% CI 1.51–3.82, p < 0.001), and no publication bias (p = 0.997) (Figure 12). In both the cumulative meta-analysis and the influence analysis, the RR was quite constant.
Figure 12.
Forest plot for overall rate of surgery.
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; MD: mean difference; CI: confidence interval.
Discussion
Although a meta-analysis of only RCTs would be ideal, case control and cohort studies data are the only evidence available to date. The major limitation of meta-analyzing these data is the potential confounding by a systematic difference in patient characteristics between the two groups. We therefore restricted the inclusion to those studies that used a unique criteria for both groups, i.e. sessile lesions >20 mm, according to the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines.23
Since the year 2000, 11 studies12–22 have been published comparing EMR and ESD for the treatment of colorectal resection, although six are in abstract form only. Nevertheless, statistical analysis showed an acceptable level of evidence, as confirmed by risk of bias analysis and heterogeneity test. The sensitivity analyses showed that no study played an influential role on RR. In truth, the mean size of lesions resected by ESD was slightly but significantly larger than EMR. Furthermore, in three studies the indication for ESD included larger LST-NG lesions, for their higher incidence of concomitant invasive carcinoma, which might have influenced the results, although no influence of these studies was statistically detected in any of the analyses.
As primary outcomes the rate of en bloc resection and R0 resection was used, as this seems the most relevant innovation introduced by ESD. In fact ESD demonstrated in our analysis a consistently higher rate of en bloc resections, close to 90% on average, as well as of R0 resection although these data are incomprehensibly reported in fewer studies. Still, the R0 resection rate is quite far from ideal demonstrating that current techniques and technologies need to improve consistently to allow a real surgical dissection. This is somehow confirmed by the three times higher rate of complications, mainly perforations, occurring during ESD compared to EMR, requiring surgical management; fortunately most of these complications are managed endoscopically. The most important risk factor for recurrence is an R1 resection,24 and in fact, ESD offers a significantly lower incidence of recurrence, although common experience is that the majority of these recurrences can be easily retreated endoscopically.25
A reasonable expectation would be that the much higher incidence of en bloc R0 resection in the ESD group should influence the need for further surgery for oncologic reasons, for the higher rate of clear margins. The uncertain pathology assessment that a piecemeal EMR entails necessitates, in cases of unexpected carcinoma invasion, radical surgery. Interestingly, on the contrary, the present analysis shows a significantly higher incidence of surgery for oncologic adequacy in the ESD group compared to EMR. This cannot be justified by the fact that in some studies indication for ESD was extended compared to EMR,12,14,20 as these were not the studies in which ESD scored a higher incidence of further surgery for oncology.14,22 The reasons might be searched for, instead, in the diffuse tendency to extend indication of ESD to difficult lesions, such as those not ideally lifted, or hidden between two folds, which were considered contraindications for EMR until recently. As a consequence, overall incidence of further surgery for any reason, including management of complications and oncologic adequacy, was more than twice in the ESD group.
These results should be interpreted cautiously as the present analysis is biased by some limitations. First, most of the studies are of relatively low quality according to acknowledged scientific criteria such as the Newcastle-Ottawa Scale. Secondly, most of the studies did not have en bloc and R0 resection as the primary outcome. Finally, scarce data regarding patients’ selection were reported in the majority of the studies, so that heterogeneity can be imagined among overall analyzed patients.
Notwithstanding the above-mentioned limitations, the pooled results of the present systematic review indicate that in case of non-pedunculated superficial lesions of the colon and rectum >20 mm ESD achieves a higher en bloc and R0 resection rate compared to EMR, but is technically demanding with current equipment, requiring a longer time to complete and entailing a higher rate of perioperative complications. ESD shows no benefit in terms of reduced need for further surgery rather than expected, as already speculated.26 How these results will ultimately translate into common daily clinical practice remains unclear. No randomized, head-to-head comparisons between EMR and ESD have yet been performed. Our review clearly highlights the need for a large randomized study to obtain unbiased results on the effectiveness and safety of these two strategies in patients with large superficial colorectal neoplasms.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Gotoda T, Kondo H, Ono H, et al. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: Report of two cases. Gastrointest Endosc 1999; 50: 560–563. [DOI] [PubMed] [Google Scholar]
- 2.Hermanek P, Gall FP. Early (microinvasive) colorectal carcinoma. Pathology, diagnosis, surgical treatment. Int J Colorectal Dis 1986; 1: 79–84. [DOI] [PubMed] [Google Scholar]
- 3.Ohkuwa M, Hosokawa K, Boku N, et al. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy 2001; 33: 221–226. [DOI] [PubMed] [Google Scholar]
- 4.Tamegai Y, Saito Y, Masaki N, et al. Endoscopic submucosal dissection: A safe technique for colorectal tumors. Endoscopy 2007; 39: 418–422. [DOI] [PubMed] [Google Scholar]
- 5.Higgins JPT and Green S (eds) Cochrane handbook for systematic reviews of interventions, version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
- 6.Moher D, Liberati A, Tetzlaff J, et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 7.University of York Centre for Reviews and Dissemination, http://www.crd.york.ac.uk/PROSPERO (2015, accessed 1 November 2014).
- 8.Kudo S, Rubio CA, Teixeira CR, et al. Pit pattern in colorectal neoplasia: Endoscopic magnifying view. Endoscopy 2001; 33: 367–373. [DOI] [PubMed] [Google Scholar]
- 9.Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003; 7: iii–x,. 1–173. [DOI] [PubMed] [Google Scholar]
- 10.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarzer G. meta: An R package for meta-analysis. R News 2007; 7: 40–45. [Google Scholar]
- 12.Tajika M, Niwa Y, Bhatia V, et al. Comparison of endoscopic submucosal dissection and endoscopic mucosal resection for large colorectal tumors. Eur J Gastroenterol Hepatol 2011; 23: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 13.Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24: 343–352. [DOI] [PubMed] [Google Scholar]
- 14.Lee EJ, Lee JB, Lee SH, et al. Endoscopic treatment of large colorectal tumors: Comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc 2012; 26: 2220–2230. [DOI] [PubMed] [Google Scholar]
- 15.Terasaki M, Tanaka S, Oka S, et al. Clinical outcomes of endoscopic submucosal dissection and endoscopic mucosal resection for laterally spreading tumors larger than 20 mm. J Gastroenterol Hepatol 2012; 27: 734–740. [DOI] [PubMed] [Google Scholar]
- 16.Kudo K, Kudo S, Hayashi T, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal laterally spreading tumors. J Gastroenterol Hepatol 2013; 28: 1–22. [Google Scholar]
- 17.Coumaros D, Vincent F. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) for superficial colorectal neoplastic lesions: A European experience. Gastrointest Endosc 2012; 75: AB427–AB427. [Google Scholar]
- 18.Kang DH, Kim HW, Choi CW, et al. Outcomes of endoscopic mucosal resection for large sessile polyps and LST of colorectum. Gastrointest Endosc 2012; 75: AB419–AB419. [Google Scholar]
- 19.Sudo K, Kudo SE, Hayashi T, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal laterally spreading tumors over 20 mm in diameter. Gastrointest Endosc 2011; 73: AB229–AB229. [Google Scholar]
- 20.Nakae K, Sakamoto N, Osada T, et al. Comparison of endoscopic submucosal dissection (ESD) versus endoscopic mucosal resection (EMR) for large superficial colorectal tumor. Gastrointest Endosc 2010; 71: AB340–AB340. [Google Scholar]
- 21.Kobayashi N, Hattori S, Kurihara H, et al. Retrospective comparison between endoscopic mucosal resection and endoscopic submucosal dissection for large colorectal tumors. Gastrointest Endosc 2009; 69: AB283–AB284. [Google Scholar]
- 22.Iizuka H, Okamura S, Onozato Y, et al. Endoscopic submucosal dissection for colorectal tumors. Gastroentérol Clin Biol 2009; 33: 1004–1011. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 2012; 17: 1–29. [DOI] [PubMed] [Google Scholar]
- 24.Morino M, Allaix ME, Caldart M, et al. Risk factors for recurrence after transanal endoscopic microsurgery for rectal malignant neoplasm. Surg Endosc 2011; 25: 3683–3690. [DOI] [PubMed] [Google Scholar]
- 25.Barendse RM, van den Broek FJ, Dekker E, et al. Systematic review of endoscopic mucosal resection versus transanal endoscopic microsurgery for large rectal adenomas. Endoscopy 2011; 43: 941–949. [DOI] [PubMed] [Google Scholar]
- 26.Bahin FF, Rasouli KN, McLeod D, et al. The role of a universal or selective endoscopic submucosal dissection strategy for large sessile and laterally spreading colorectal tumors in a Western tertiary referral center. J Gastroenterol Hepatol 2014; 29(S2): 28–28. [Google Scholar]