Abstract
Background and aim
The efficacy of using proton pump inhibitors (PPIs) prior to gastric endoscopic submucosal dissection (ESD) to reduce gastric bleeding remains controversial. This study aimed to systematically review the literature to evaluate the efficacy of preoperative PPI use to reduce post-ESD bleeding.
Methods
PubMed, the Cochrane library, and the Igaku-Chuo-Zasshi database were searched to identify randomized trials eligible for inclusion in the systematic review. Data from four studies (406 patients) were combined to calculate a pooled risk difference (RD) for developing post-ESD bleeding.
Results
Compared with patients who received no premedication, the pooled RD for post-ESD bleeding in patients who received preoperavive PPI was –0.027 (95% confidence interval: –0.070–0.017, p = 0.228), without significant heterogeneity. Preoperavive PPI use significantly increased gastric pH (weighted mean difference: 1.289, 95% CI: 0.227–2.352, p = 0.0174).
Conclusions
This systematic review and meta-analysis showed that premedication with PPI had no advantage for the prevention of post-ESD bleeding, despite increasing gastric pH.
Keywords: Proton pump inhibitor, premedication, systematic review, gastric endoscopic submucosal dissection, gastric bleeding
Introduction
Endoscopic submucosal dissection (ESD) was developed in Japan as an advanced endoscopic resection technique for early gastric cancer.1,2 Although ESD is considered safe, bleeding is still a common complication.3,4 A meta-analysis in 2011 showed that proton pump inhibitors (PPIs) prevented bleeding due to ESD-induced gastric ulcers more effectively than histamine H2-receptor antagonists.5 However, delayed bleeding occurs in approximately 5% of patients who undergo gastric ESD.6
PPIs produce a high gastric pH by irreversibly binding to the proton pumps on gastric parietal cells.7 The optimal time for the full effect of PPIs is reported to be several days.8 Several randomized controlled trials (RCTs) evaluated the efficacy of PPI administration before ESD. Watanabe et al. reported that preoperative PPI administration was useful for preventing post-ESD bleeding.9 However, other studies showed that premedication did not influence post-ESD bleeding rates.10–12 We proposed that systematically pooling data from all published reports might provide a better understanding of the efficacy of preoperative PPI use for preventing post-ESD bleeding. Our objective was to perform a systematic review and meta-analysis of RCTs comparing the impact of no premedication versus preoperative PPI use on post-ESD bleeding.
Methods
Before performing the meta-analysis, we developed a protocol to detail search strategies, study selection criteria, methods for relevant data extraction, quality assessment, and statistical analysis.
Search strategy
PubMed, the Cochrane library, and the Igaku-Chuo-Zasshi database in Japan (from 1950 to December 2014) were used to perform a systematic literature search. A combination of the following words was used for the search: (endoscopic submucosal dissection OR endoscopic mucosal resection) AND (proton pump inhibitor). Articles published in any language were included.
Inclusion and exclusion criteria
Studies meeting the following inclusion criteria were considered eligible: (1) study type: RCT; (2) population: patients who underwent gastric ESD; (3) intervention: PPI use from the day before ESD or earlier; (4) comparator: no PPI before the day of ESD; (5) outcome: post-ESD bleeding. Duplicate publications, reviews, and conference abstracts were excluded.
Outcome measures
The primary outcome of this study was clinical evidence of bleeding after ESD. Post-ESD bleeding included hematemesis, melena, reduced hemoglobin count by more than 2 g/dl, or endoscopically evident bleeding. The secondary outcome was gastric pH.
Data extraction
Standardized data abstraction sheets were prepared. Extracted data included study design, study quality, intervention, and outcomes. All articles were independently examined for eligibility by two reviewers (TN and TA). Disagreements were resolved by consulting a third reviewer (HS). We contacted the corresponding authors in order to clarify detail of studies.
Assessment of methodological quality
The methodological quality of each study was assessed using the risk-of-bias tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0). Two reviewers (TN and HS) reviewed all studies and assessed six key RCT quality influencers: sequence generation, allocation concealment, blinding of participants and outcome assessors, management of incomplete outcome data, completeness of outcome reporting, and other potential threats to validity.
Statistical analysis
Data were entered into the StatsDirect statistical package (StatsDirect Ltd., Cheshire, UK). Separate analyses were performed for each outcome using a risk difference (RD) or weighted mean difference (WMD). We used a random-effect model to calculate summary RDs and 95% confidence intervals (CIs). We always used a random-effect model, regardless of the significance of the heterogeneity. Heterogeneity between studies was assessed by Cochran’s Q and I2 tests. Because of the low power of the Q test, a cut-off value (<0.10) was used to reject homogeneity, which thereby indicated heterogeneity.13 An I2 score of ≥50% indicates more than moderate heterogeneity. Some trials reported medians as the measure of treatment effect, with an accompanying interquartile range. For the purpose of analysis, medians were assumed to be equivalent to means, and standard deviation (SD) was estimated from interquartile ranges as follows: SD = interquartile range × 0.74.14,15 In all trials, PPIs were administered on the day of ESD either before or immediately after ESD. Therefore, all eligible trials were grouped into pre- and post-ESD groups and subgroup analysis was also performed. An analysis of sensitivity was performed in order to evaluate the stability of the results. Finally, we used funnel plot asymmetry to detect any publication bias in the meta-analysis and Egger’s regression test to measure funnel plot asymmetry.
Results
Search results
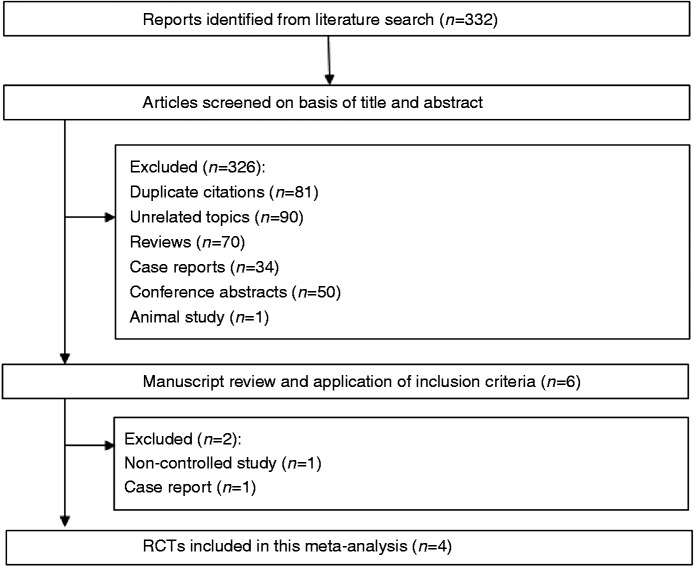
Our database search yielded a total of 332 citations (Figure 1). After adjusting for duplicates, 251 studies remained. Of these, 245 studies were removed from consideration after abstract review based on the exclusion criteria (90 unrelated topics, 70 reviews, 34 case reports, 50 conference abstracts, and one animal study). The remaining six studies were examined in detail. Another two studies were then excluded (one lack of control16 and one case report17). Finally, four studies were included in the systematic review and meta-analysis (Clinical trials registration number: Baeg et al.; NCT00844675, Hikichi et al.; UMIN000011487). The characteristics of these studies are summarized in Table 1. The indication for ESD was early gastric cancer or adenoma in all four RCTs.
Figure 1.
Flow of RCTs included in the systematic review.
Table 1.
Characteristics of studies included in the systematic review
| Author Year | Country | Premedication | PPI administration of ESD day | Follow-up endoscopy | Patients number | Age ±SD | Gender M/F | Post-ESD bleeding (%) | Gastric pH measurement | Mean pH ±SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Watanabe | Japan | LPZ 30 mg o.d. 7 days before ESD | Oral LPZ 30 mg | day 7, 56 | 51 | 72.5 ± 9.1 | 37/14 | 0 (0%) | Collecting gastric juice | 7.5 ± 0.7 |
| 2006 | No treatment | Oral LPZ 30 mg after ESD | 47 | 70.1 ± 8.0 | 37/10 | 3 (6.4%) | during ESD | 5.1 ± 1.4 | ||
| Ono | Japan | OPZ 20 mg o.d. 1 day before ESD | iv OPZ 20 mg before and after ESD | day 1, 7, 28 | 81 | 70.5 ± 8.3 | 51/30 | 6 (7.4%) | Collecting gastric juice | 7.3 ± 0.6 |
| 2009 | No treatment | iv OPZ 20 mg after ESD | 74 | 70.2 ± 9.1 | 60/14 | 6 (8.1%) | during ESD | 5.6 ± 3.7 | ||
| Baeg | Korea | RPZ 20 mg b.i.d. 5 days before ESD | iv PPZ 40 mg 2h before ESD | day 1, 30 | 45 | 59 ± 8.9 | 24/21 | 3 (6.7%) | 48-h pH monitoring | 6.7 ± 1.7 |
| 2014 | Placebo | iv PPZ 40 mg 2h before ESD | 53 | 58 ± 10 | 42/11 | 3 (5.7%) | after ESD | 6.5 ± 1.3 | ||
| Hikichi | Japan | RPZ 20 mg o.d. 3 days before ESD | oral RPZ 20 mg | day 7, 56 | 24 | 73.3 ± 7.8 | 18/6 | 0 (0%) | Collecting gastric juice | 7.2 ± 0.6 |
| 2014 | No treatment | oral RPZ 20 mg 7-8 h before ESD | 31 | 70.4 ± 9.0 | 21/10 | 1 (3.2%) | during ESD | 6.5 ± 1.1 |
ESD: endoscopic submucosal dissection; PPI: proton pump inhibitor, LPZ: lansoprazole, OPZ: omeprazole, PPZ: pantprazole, RPZ: rabeprazole; iv: intravenous.
Follow-up endoscopy: the days after the ESD.
Quality assessment
The risks of bias in the included RCTs are shown in Table 2. In general, the included RCTs were at low risk of bias for most of the aspects evaluated. All four RCTs described the specific methods used for random sequence generation, and one RCT did not perform allocation concealment. In three RCTs, blinding of participants and outcomes assessment were not performed. One RCT did not adequately assess incomplete outcomes. Avoidance of selective outcome reporting was found in all four RCTs. All four RCTs were free of other biases.
Table 2.
Evaluation of bias of RCTs included in the systematic review
| First | Random sequence | Allocation | Blinding of participants | Blinding of outcome | Adequate assessment | Selective reporting | No other |
|---|---|---|---|---|---|---|---|
| author | generation | concealment | and personnel | assessment | of incomplete outcome | avoided | bias |
| Watanabe | Yes | Yes | No | No | Yes | Yes | Yes |
| Ono | Yes | Yes | No | No | Yes | Yes | Yes |
| Baeg | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hikichi | Yes | No | No | No | No | Yes | Yes |
Yes: Low risk of bias.
No: High risk of bias.
Unclear: Unclear risk of bias.
Meta-analysis results
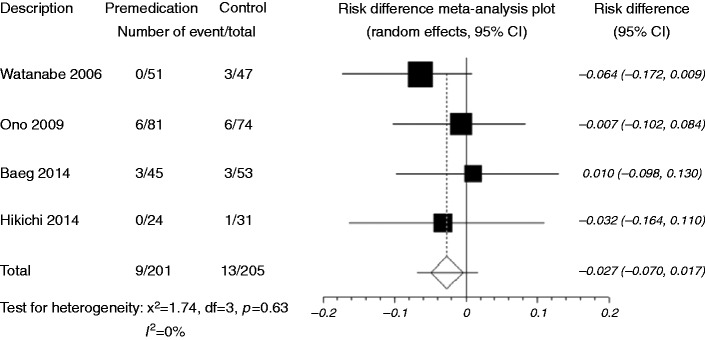
When data were pooled, post-ESD bleeding was reported in nine of 201 patients (4.5%) who received preoperative PPIs and in 13 of 205 patients (6.3%) who did not receive premedication (RD –0.027, 95% CI: –0.070–0.017, p = 0.228) (Figure 2, Table 1). There was no significant heterogeneity among the trial results (I2 = 0%, p = 0.63). The sensitivity analysis performed using sequential excluding of one trial at a time did not alter the results. The Egger test suggested no significant funnel plot asymmetry (p = 0.70), indicating no evidence of substantial publication bias.
Figure 2.
Forest plot displaying the risk difference and 95% CIs of each study for post-ESD bleeding.
Premedication, preoperavive PPI use; Control, no premedication
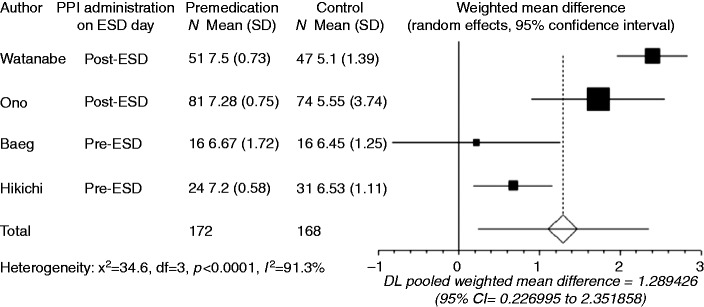
Gastric pH was recorded in all four studies. Baeg et al. reported the mean intragastric pH monitored for 48 h after ESD,11 while gastric juice was collected during ESD in the other studies. Since the number of reports was limited, data obtained using the different measurement methods were combined in the present meta-analysis. Compared with no premedication, preoperative PPI use significantly increased gastric pH (WMD: 1.289, 95% CI: 0.227–2.352, p = 0.0174, Figure 3). However, there was significant heterogeneity among the trial results (I2 = 91.3%, p < 0.0001). Watanabe et al.9 and Ono et al.10 administered PPI after ESD on the day of the ESD (post-ESD group), while Baeg et al.11 and Hikichi et al.12 administered PPI before ESD on the day of the ESD (pre-ESD group). Subgroup analysis indicated a significantly higher gastric pH with preoperative PPI use compared with no premedication for both the pre- and post-ESD groups, and the heterogeneity disappeared (p = 0.43 and p = 0.17, respectively). The WMD in the post-ESD group (2.171, 95% CI: 1.548–2.794, p < 0.0001) was higher than that in the pre-ESD group (0.598, 95% CI: 0.182–1.015, p = 0.0049). Sensitivity analysis omitting the study that used 48-hour intragastric pH monitoring did not alter the findings.
Figure 3.
Forest plot displaying the weighted mean difference and 95% CIs of each study for gastric pH.
Premedication, preoperavive PPI use; Control, no premedication; Post-ESD, PPI administration on ESD day was post-operative; Pre-ESD, PPI administration on ESD day was preoperative.
Discussion
This systematic review and meta-analysis indicates that preoperative PPI use significantly increases gastric pH but confers no advantage for preventing post-ESD bleeding.
Ozawa et al. reported intragastric pH monitoring in H. pylori-positive patients treated with H2-receptor antagonist or PPI. The mean pH was 6.9 ± 0.3 in patients treated with PPI, and it was 4.3 ± 0.7 in patients treated with H2-receptor antagonist.18 In order to control bleeding, PPIs should be administered instead of H2-receptor antagonist after ESD.
Platelet aggregation is reduced to less than 50% at a pH of 6.4 or lower. When the pH falls below 5.4, platelet aggregation and plasma coagulation are virtually abolished. Below pH 4.0, fibrin clots are dissolved.19,20 Therefore, gastric pH must be kept >5.4 to prevent bleeding.
The mean gastric pH in patients who did not receive premedication in all four RCTs was relatively high (5.9 ± 2.5; 5.1–6.5). This might be because most patients with gastric neoplasms have atrophic gastritis. As corpus atrophy results in a reduced number of acid-producing parietal cells, the stomach becomes hypochlorhydric. The reduced number of parietal cells would require a lower PPI dose in order to be fully inhibited, resulting in no clinically significant difference in post-ESD bleeding rates between preoperavive PPI use and no premedication.
This systematic review has several limitations that should be taken into account. Most study participants were Japanese and Korean, so the results may not be generalizable to other races. Although the outcome parameter is rather objective (hematemesis, melena, etc), the risk of bias imposed by lacking blinding in three RCTs must be considered. The different time of follow-up endoscopy in the four RCTs may be considered as a source of heterogeneity. Due to the limited number of eligible studies, this study may be statistically underpowered. Patients who took antiplatelet or antithrombotic drugs perioperatively were excluded from three RCTs, and antiplatelet medication was stopped from 7 days before ESD to 7 days after ESD in one RCT. Three RCTs excluded patients with a high risk of bleeding, such as those who had chronic renal failure or liver cirrhosis. In patients with a high risk of bleeding, intensive acid inhibition by preoperative PPI use might be effective. Further studies with large numbers of patients are warranted to clarify the efficacy of PPI administration before ESD.
In conclusion, premedication with PPI had no advantage for the prevention of post-ESD bleeding, despite increasing gastric pH.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
During the last 2 years, Author H.S. received scholarship funds for the research from Astellas Pharm Inc., Astra-Zeneca K.K., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Zeria Pharmaceutical Co., Ltd. and received service honoraria from Astellas Pharm Inc., Astra-Zeneca K.K., Eisai Co., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Zeria Pharmaceutical Co., Ltd. Author T.K. received scholarship funds for the research from Astellas Pharm Inc., Astra-Zeneca K.K., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eisai Pharmaceutical Co., Ltd., Zeria Pharmaceutical Co., Ltd., Tanabe Mitsubishi Pharmaceutical Co., Ltd. JIMRO Co., Ltd., Kyorin Pharmaceutical Co. Ltd., and received service honoraria from Astellas Pharm Inc., Eisai Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Tanabe Mitsubidhi Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Miyarisan Pharmaceutical Co. Ltd., and Zeria Pharmaceutical Co., Ltd. Author N.Y. received scholarship funds for the research from Astra-Zeneca K.K., Takeda Pharmaceutical Co., Ltd., Eisai Co., Top Corporation, Kaigen Pharm Co., Ltd., ASKA Pharmaceutical Co., Ltd., FUJIFILM Corporation, Boston Scientific Japan K.K., Century Medical Inc., and Covidien Japan Inc. The funding source had no role in the design, practice or analysis of this study. There are no other conflicts of Interests for this article.
References
- 1.Kodashima S, Fujishiro M, Yahagi N, et al. Endoscopic submucosal dissection using flexknife. J Clin Gastroenterol 2006; 40: 378–384. [DOI] [PubMed] [Google Scholar]
- 2.Uraoka T, Parra-Blanco A, Yahagi N. Colorectal endoscopic submucosal dissection in Japan and Western countries. Dig Endosc 2012; 24(Suppl 1): 80–83. [DOI] [PubMed] [Google Scholar]
- 3.Fujishiro M, Chiu PW, Wang HP. Role of antisecretory agents for gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(Suppl 1): 86–93. [DOI] [PubMed] [Google Scholar]
- 4.Nishizawa T, Suzuki H, Kinoshita S, et al. Second-look endoscopy after endoscopic submucosal dissection for gastric neoplasms: A systematic review. Dig Endosc 2015; 27: 279–284. [DOI] [PubMed] [Google Scholar]
- 5.Yang Z, Wu Q, Liu Z, Wu K, et al. Proton pump inhibitors versus histamine-2-receptor antagonists for the management of iatrogenic gastric ulcer after endoscopic mucosal resection or endoscopic submucosal dissection: A meta-analysis of randomized trials. Digestion 2011; 84: 315–320. [DOI] [PubMed] [Google Scholar]
- 6.Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection – an analysis of risk factors. Endoscopy 2008; 40: 179–183. [DOI] [PubMed] [Google Scholar]
- 7.Sachs G, Shin JM, Briving C, et al. The pharmacology of the gastric acid pump: The H+,K+ ATPase. Annu Rev Pharmacol Toxicol 1995; 35: 277–305. [DOI] [PubMed] [Google Scholar]
- 8.Jansen JB, Lundborg P, Baak LC, et al. Effect of single and repeated intravenous doses of omeprazole on pentagastrin stimulated gastric acid secretion and pharmacokinetics in man. Gut 1988; 29: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe Y, Kato N, Maehata T, et al. Safer endoscopic gastric mucosal resection: Preoperative proton pump inhibitor administration. J Gastroenterol Hepatol 2006; 21: 1675–1680. [DOI] [PubMed] [Google Scholar]
- 10.Ono S, Kato M, Ono Y, et al. Effects of preoperative administration of omeprazole on bleeding after endoscopic submucosal dissection: A prospective randomized controlled trial. Endoscopy 2009; 41: 299–303. [DOI] [PubMed] [Google Scholar]
- 11.Baeg MK, Choi MG, Moon SJ, et al. Preprocedural rabeprazole treatment before endoscopic submucosal dissection for gastric neoplasms. Dig Dis Sci 2014; 59: 2243–2248. [DOI] [PubMed] [Google Scholar]
- 12.Hikichi T, Sato M, Watanabe K, et al. Oral rabeprazole administration on a procedure day suppresses bleeding after endoscopic submucosal dissection for gastric neoplasms. Fukushima J Med Sci 2014; 60: 68–74. [DOI] [PubMed] [Google Scholar]
- 13.Nishizawa T, Suzuki H, Kanai T, et al. Proton pump inhibitor alone vs proton pump inhibitor plus mucosal protective agents for endoscopic submucosal dissection-induced ulcer: A systematic review. J Clin Biochem Nutr 2015; 56: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser GL, Devlin JW, Worby CP, et al. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: A systematic review and meta-analysis of randomized trials. Crit Care Med 2013; 41(9 Suppl 1): S30–S38. [DOI] [PubMed] [Google Scholar]
- 15.Nishizawa T, Suzuki H, Sagara S, et al. Dexmedetomidine versus midazolam for gastrointestinal endoscopy: A meta-analysis. Dig Endosc 2015; 27: 8–15. [DOI] [PubMed] [Google Scholar]
- 16.Niimi K, Fujishiro M, Goto O, et al. Prospective single-arm trial of two-week rabeprazole treatment for ulcer healing after gastric endoscopic submucosal dissection. Dig Endosc 2012; 24: 110–116. [DOI] [PubMed] [Google Scholar]
- 17.Kurosawa M, Ishibashi K, Iizuka Y, et al. Prevention of delayed bleeding after gastric endoscopic mucosal resection; pretreatment with omeprazole. J Aizu General Hospital 1999; 15: 29–31. [Google Scholar]
- 18.Ozawa T, Yoshikawa N, Tomita T, et al. The influence of feeding on gastric acid suppression in Helicobacter pylori-positive patients treated with a proton pump inhibitor or an H2-receptor antagonist after bleeding from a gastric ulcer. J Gastroenterol 2003; 38: 844–848. [DOI] [PubMed] [Google Scholar]
- 19.Green FW, Jr., Kaplan MM, Curtis LE, et al. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology 1978; 74: 38–43. [PubMed] [Google Scholar]
- 20.Sugimoto M, Jang JS, Yoshizawa Y, et al. Proton pump inhibitor therapy before and after endoscopic submucosal dissection: A review. Diagn Ther Endosc 2012; 2012: 791873–791873. [DOI] [PMC free article] [PubMed] [Google Scholar]