Abstract
Background
The endoscopic use of argon plasma coagulation (APC) to achieve hemostasis for upper gastrointestinal tumor bleeding (UGITB) has not been adequately evaluated in controlled trials. This study aimed to evaluate the efficacy of APC for the treatment of upper gastrointestinal bleeding from malignant lesions.
Methods
Between January and September 2011, all patients with UGITB underwent high-potency APC therapy (up to 70 Watts). This group was compared with a historical cohort of patients admitted between January and December 2010, when the endoscopic treatment of bleeding malignancies was not routinely performed. Patients were stratified into two categories, grouping the Eastern Cooperative Oncology Group (ECOG) performance status scale: Category I (ECOG 0–2) patients with a good clinical status and Category II (ECOG 3–4) patients with a poor clinical status.
Results
Our study had 25 patients with UGITB whom underwent APC treatment and 28 patients whom received no endoscopic therapy. The clinical characteristics of the groups were similar, except for endoscopic active bleeding, which was more frequently detected in APC group. We had 15 patients in the APC group whom had active bleeding, and initial hemostasis was obtained in 11 of them (73.3%). In the control group, four patients had active bleeding. There were no differences in 30-day re-bleeding (33.3% in the APC group versus 14.3% in the control group; p = 0.104) and 30-day mortality rates (20.8% in the APC group, versus 42.9% in the control group; p = 0.091). When patients were categorized according to their ECOG status, we found that APC therapy had no impact in re-bleeding and mortality rates (Group I: APC versus no endoscopic treatment: re-bleeding p = 0.412, mortality p = 0.669; Group II: APC versus no endoscopic treatment: re-bleeding p = 0.505, mortality p = 0.580). Hematemesis and site of bleeding located at the esophagus or duodenum were associated with a higher 30-day mortality.
Conclusions
Endoscopic hemostasis of UGITB with APC has no significant impact on 30-day re-bleeding and mortality rates, irrespective of patient performance status.
Keywords: Argon plasma coagulation, bleeding, endoscopy, gastrointestinal cancer, gastrointestinal hemorrhage, hemostasis, mortality, tumor
Introduction
Gastrointestinal tumor bleeding (GITB) is a challenging clinical situation, not only because of the poor overall clinical status of the patients, but also because endoscopic treatment of this condition is difficult and eventually not feasible.
Argon plasma coagulation (APC) is a well-established therapy for the treatment of non-variceal gastrointestinal bleeding; and is widely available as a simple, safe, fast and low-cost method.1–5 While the efficacy of this method for patients with advanced neoplasms has been described, most studies focus on debulking to preserve luminal patency, rather than on the control of tumor bleeding.6,7
Kawamura et al.8 described successful hemostasis of a bleeding gastrointestinal (GI) stromal tumor with APC, as a bridge to surgery. Akhtar et al.7 reported using APC for the management of patients with esophageal and stomach neoplasms: Of five patients with gastric tumor bleeding, three achieved successful control of their bleeding. In addition to these anecdotal reports, no controlled trial has demonstrated APC efficacy thus far.
The purpose of this study was to evaluate the efficacy of APC for the treatment of bleeding from neoplasms of the upper GI tract, by comparing outcomes (successful hemostasis, re-bleeding rates and mortality) from APC-treated patients with a historical cohort whom received no treatment.
Methods
From January to September 2011, all patients with upper GI bleeding originating from malignancies of the GI tract (primary or metastatic) were selected for our study, according to a protocol approved by the review board of our institution.
Patients with upper GITB underwent high-potency APC hemostasis (Tübingen, Germany). The procedures were performed at a referral center for cancer treatment (Cancer Institute, University of São Paulo, Brazil). The endoscopic procedures were performed with standard Olympus endoscopes (GIF H180 and Q180; Olympus Co., Tokyo, Japan); under conscious sedation with midazolam, fentanyl and a small bolus of propofol, at the discretion of the attending endoscopist.
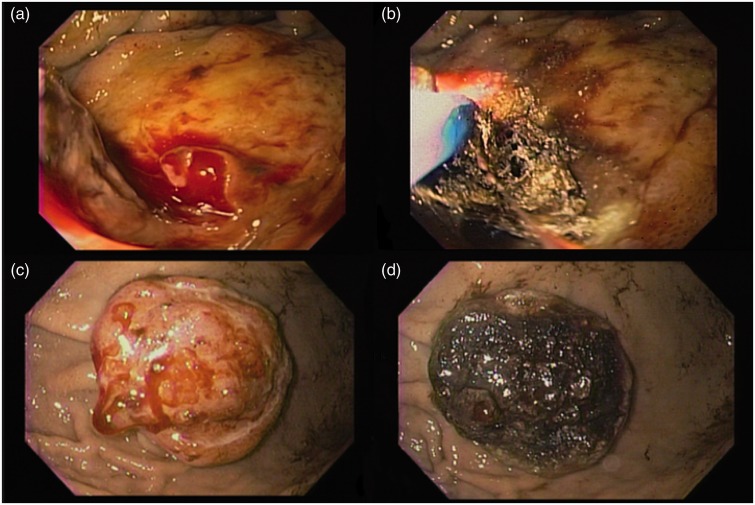
The diagnosis of tumor bleeding was made when active tumor bleeding was observed at endoscopy (pulsatile or oozing), or when coffee-ground stasis was observed in the stomach with bleeding stigmata visible in the tumor (clots or visible vessel) and no other source of bleeding detected. In the APC group, high-potency forced-mode APC (60–70 W, 1.5–2.0 L/min for esophageal and gastric lesions; and 40–50 W, 1.5 L/min for duodenal lesions; Erbe VIO 300D, Germany) was applied after careful evaluation and cleaning of the lesion, starting with the most obvious source of bleeding and then spreading the argon coagulation to the entire tumor surface (Figure 1). Immediate hemostasis was defined when a complete stop to bleeding from the lesion was achieved. No follow-up endoscopy was scheduled. The patients were discharged according to clinical status, as determined by the attending physician.
Figure 1.
(a, b) Argon plasma coagulation of bleeding from a pancreatic cancer invading the duodenal bulb. (c, d) Bleeding metastatic tumor in the stomach treated with APC.
The primary outcomes were re-bleeding and 30-day mortality rates. The results of the APC group were compared with a historical cohort of 28 patients (the control group) whom had received no endoscopic treatment, as treatment of GITB was not an established procedure in our department at that time (January to December 2010). The Eastern Cooperative Oncology Group (ECOG) scale was adopted to stratify patients’ performance status.9 Patients were classified into two categories:
Patients with good clinical status were classified as category I (ECOG 0–2); and
Patients with poor clinical status were classified as category II (ECOG 3–4).
Statistical analysis
Continuous variables were evaluated using Student’s t test; and categorical variables were examined using the Pearson Chi-Square and Fisher exact tests, or a likelihood ratio. All of the statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Results
In our study, 25 patients with GITB received endoscopic APC therapy between January and September 2011, including 14 men and 11 women with a mean age of 57 years (range, 19–79 years). One patient was lost to follow-up and was excluded from the analysis. As a control, from January 2010 to December 2010 we also had 28 patients (22 men and 6 women, mean age 62 years, range 31–81 years) with GITB whom received no endoscopic therapy. The duration of follow-up for both groups was up to 27 months (mean 5.13; median 1.5). The demographic characteristics of both APC and control groups were similar (Table 1).
Table 1.
Comparison between APC and control groups
| APC (n = 24) | Control (n = 28) | Total (n = 52) | P value | |
|---|---|---|---|---|
| Gender (%) | .061 | |||
| Male | 13 (54.2) | 22 (78.6) | 35 (67.3) | |
| Female | 11 (45.8) | 6 (21.4) | 17 (32.7) | |
| Age (years ± SD) | 57 ± 13 | 62 ± 11 | 60 ± 12 | .159 |
| Hemoglobin (g/dL ± SD) | 7.3 ± 2.7 | 8.2 ± 2.3 | 7.8 ± 2.5 | .177 |
| Clinical presentation (%) | .516 | |||
| Hematemesis | 9 (37.5) | 13 (46.4) | 22 (42.3) | |
| Others | 15 (62.5) | 15 (53.6) | 30 (57.7) | |
| ECOG | .376 | |||
| I (good PS) | 18 (75) | 17 (60.7) | 35 (67.3) | |
| II (poor PS) | 6 (25) | 11 (39.3) | 17 (32.7) | |
| Bleeding site (%) | .108 | |||
| Duodenum | 7 (29.2) | 3 (10.7) | 10 (19.2) | |
| Esophagus | 3 (12.5) | 9 (32.1) | 12 (23.1) | |
| Stomach | 14 (58.3) | 16 (57.1) | 30 (57.7) | |
| Active bleeding (%) | 15 (62.5) | 4 (14.3) | 19 (36.5) | < .001 |
| Additional treatment (%) | 9 (37.5) | 8 (28.6) | 17 (32.7) | .494 |
| Re-bleeding (%) | 8 (33.3) | 4 (14.3) | 12 (23.1) | .104 |
| Mortality (%) | 5 (20.8) | 12 (42.9) | 17 (32.7) | .091 |
APC: argon plasma coagulation; ECOG: Eastern Cooperative Oncology Group performance status (Group I: good performance status (ECOG 0–2); Group II: poor performance status (ECOG 3–4)); PS: performance status; SD: standard deviation.
The stomach was the most common site of bleeding for both the APC (58.3%) and control groups (57.1%). Active bleeding was more frequent in the APC group (62.5% versus 14.3%, p < 0.001), and immediate hemostasis was successfully obtained in 73.3% of those patients (11 out of 15). Additional treatment to achieve hemostasis was performed in nine patients of the APC group: Eight patients underwent hemostatic radiotherapy and one underwent surgical resection. Two of these patients died within 30 days post-operation. In the control group, additional treatment was given to eight patients: Six underwent hemostatic radiotherapy and two underwent surgical resection; and two of these patients died within 30 days post-intervention.
The 30-day re-bleeding rates were similar in the APC and control groups (33.3% versus 14.3%; p = 0.104). The 30-day mortality was higher in the control group; however, this difference was not statistically significant (20.8% APC versus 42.9% control; p = 0.091). The APC treatment did not offer any advantage among patients with good performance; i.e. the Category I group (re-bleeding 33% APC group versus 18% in the control group, p = 0.412; and also mortality 17% APC versus 35% in the control group, p = 0.669) and similar findings in Category II patients with poor performance (re-bleeding 33% APC versus 9% in the control group, p = 0.505; mortality 33% APC versus 55% in the control group, p = 0.580). These results are summarized in Table 2.
Table 2.
Rebleeding and mortality of APC and control patients, grouped according to ECOG performance status
| Re-bleeding | p | mortality | p | |
|---|---|---|---|---|
| Group I | 0.412 | 0.669 | ||
| APC | 6/18 (33%) | 3/18 (17%) | ||
| Control | 3/17 (18%) | 6/17 (35%) | ||
| Group II | 0.505 | 0.580 | ||
| APC | 2/6 (33%) | 2/6 (33%) | ||
| Control | 1/11 (9%) | 6/11 (55%) |
APC: argon plasma coagulation; ECOG: Eastern Cooperative Oncology Group score; Group I: good performance status (ECOG 0–2); Group II: poor performance status (ECOG 3–4).
Risk factors for 30-day mortality are summarized in Table 3. Hematemesis at presentation was significantly associated with higher 30-day mortality (p < 0.001). The organ site of bleeding seemed to have an influence in mortality (p = 0.051). Patients with bleeding gastric neoplasms presented lower mortality, when compared to patients where the bleeding sites were located at the esophagus or duodenum.
Table 3.
Risk factors for mortality
| No (n = 35) | Yes (n = 17) | Total (n = 52) | P value | |
|---|---|---|---|---|
| Gender (%) | .725 | |||
| Male | 23 (65.7) | 12 (34.2) | 35 (100) | |
| Female | 12 (70.6) | 5 (29.4) | 17 (100) | |
| Hemoglobin (g/dL ± SD) | 7.7 ± 2.6 | 8 ± 2.3 | 7.8 ± 2.5 | .763 |
| Clinical presentation (%) | < .001 | |||
| Hematemesis | 11 (50) | 11 (50) | 22 (100) | |
| Others | 24 (80) | 6 (20) | 30 (100) | |
| ECOG | .127 | |||
| I | 26 (74.3) | 9 (25.7) | 35 (100) | |
| II | 9 (52.9) | 8 (47.1) | 17 (100) | |
| Bleeding site (%) | .051 | |||
| Duodenum | 6 (60) | 4 (40) | 10 (100) | |
| Esophagus | 5 (41.6) | 7 (58.3) | 12 (100) | |
| Stomach | 24 (80) | 6 (20) | 30 (100) | |
| Active bleeding (%) | 15 (78.9) | 4 (21.1) | 19 (100) | .175 |
| Additional treatment (%) | 13 (76.5) | 4 (23.5) | 17 (100) | .326 |
| Re-bleeding (%) | 8 (66.7) | 4 (33.3) | 12 (100) | .957 |
| Groups (%) | .091 | |||
| APC | 19 (79.2) | 5 (20.8) | 24 (100) | |
| Control | 16 (57.1) | 12 (42.9) | 28 (100) |
APC: argon plasma coagulation; ECOG: Eastern Cooperative Oncology Group performance status.
Discussion
GI tumor bleeding is a challenging clinical condition with a high mortality rate. In a retrospective study,10 our group identified 41 patients with bleeding from malignancies in the upper GI tract, seven of whom received endoscopic therapy with successful initial hemostasis in six (85.7%) of them. Mortality rates were high and did not differ between patients who received endoscopic treatment and those whom did not (43.9% versus 44.1%, p = 0.677).
Bleeding from a neoplastic lesion is usually chronic and occurs diffusely on the surface of the tumor, rather than at a focal point. Given this fact, APC therapy is an attractive option because it can be applied over large surfaces. Furthermore, APC is widely available, easy to use and low cost; however, there is scarce data on the use of APC to control tumor bleeding. Akhtar et al.7 report a series of 48 patients with esophagogastric cancer treated with APC. They used high-potency APC (70 W, 2.0 L/min) in five patients whom had bleeding, achieving successful initial hemostasis in three of the patients (60%). Wahab et al.5 attempted hemostasis with APC and monopolar snare electrocoagulation in seven patients with rectosigmoid cancers presenting with tumor bleeding, with success in four patients (57%), without major complications.
The three most important factors influencing APC thermal impact are: the duration of application, the power setting and the probe-to-tissue distance. In order to take full advantage of APC therapy, we applied continuous high-power forced APC across the entire tumor surface, intending to reach as deep as we could into the tumor tissue. The initial success rate was 68.7%, which was comparable to the findings of previous studies.6,7 We used a second-generation APC, Erbe VIO 300D, with effectiveness reported to be 30–50% superior to the previous generation equipment.3 Pulsed mode has been widely used because it produces more homogeneous, ‘spray-like’ ablation; however, the effect in deeper tissue layers is limited. In a non-randomized study11 comparing pulsed versus forced APC for diminishing of obstruction of esophageal, gastric and rectal tumors; the overall response rate was similar in both groups. The authors suggest that an optimal approach would be the combination of both modes: pulsed APC as a first-line therapy for the homogeneous and superficial ablation of large tumor areas, and then forced APC could be used to reach deeper tissue layers.11
Despite the initial good results, the 30-day re-bleeding rate for patients who underwent APC was 33.3%, compared with 14.3% in the control group (p = 0.104). This may have been partially attributable to the fact that the APC group presented with more active bleeding on initial endoscopy (62.5% versus 14.3%, p < 0.001); but one can also argue that APC could induce tissue injury, with subsequent re-bleeding. Additional therapy, i.e. hemostatic radiotherapy, was employed equally in both groups; thus, it probably did not influence the re-bleeding rates. These findings are in accordance with another study about the natural history of GI bleeding, due to tumors.12 The authors retrospectively reviewed 106 patients with UGITB. Active bleeding was seen in 32 patients (30%). They applied various endoscopic therapies in 14 patients (ethanol injection, epinephrine injection, bipolar electrocoagulation, APC and combined therapies), achieving hemostasis in 12 of them (86%); however, all 18 patients whom did not receive endoscopic therapy also had hemostasis. Re-bleeding rates were equal between the patients who received endoscopic therapy and those whom did not (p = 0.88). Upon multivariate analysis, only an age ≤60 years and hemodynamic instability were associated with re-bleeding.
The biology of neoplastic tissue may explain the tendency for a temporary effect of endoscopic hemostasis in bleeding neoplasms. Neoplasms present aberrant vascular growth and express neo-angiogenesis factors that might renders tissue more prone to bleeding.13,14
Although not statistically significant, the 30-day mortality rate was higher in the control group (70.6% versus 29.4%, p = 0.091); however, this trend disappeared when patients were grouped according to their ECOG scale. As expected, the clinical finding of hematemesis was predictive of 30-day mortality. The higher mortality in patients with bleeding sites located in the esophagus and duodenum, compared to bleeding gastric neoplasms, may be explained by the fact that a bleeding esophageal neoplasm is usually related to the erosion of larger vessels, such as bronchial arteries, and duodenal bleeding is usually caused by invasive biliopancreatic cancer.
Endoscopic therapy of malignant lesions, either with APC, laser, heater probe or injection, is not an established treatment for tumor bleeding.15 Currently, only small case series have been published without control groups and with re-bleeding rates ranging from 17% to 80%.15–18 Hemospray™ was recently introduced to the market as a therapeutic option for UGITB.19 Chen et al.20 report their preliminary experience with five cases of cancer-related GI hemorrhage with immediate hemostasis in all of their patients, and re-bleeding was observed in one patient. In another study, Hemospray™ was used in five patients with bleeding from upper GI malignancies, with immediate hemostasis achieved in all of the patients and recurrent bleeding at a 7-day follow-up in two patients.21 Therefore, Hemospray™ appears to be an effective option for treating bleeding malignancies. Additional prospective studies are warranted, to validate this method.
This current study had some limitations. Although the APC group was a prospective cohort, the control group was a retrospective cohort whom had received no endoscopic therapy in the year before use of the APC protocol. These patients might have been biased by selection, because more efforts may have been done in patients with a better prognosis (including any kind of endoscopic therapy), leaving the patients with a poorer clinical condition in the control group. This might explain the trend toward decreased mortality in the APC group. Selection bias might also explain the trend toward more re-bleeding in the APC group, because there were fewer patients with active bleeding in the control group (patients with active bleeding in the historical group might have been elected to receive some endoscopic therapy). Also, this was a single-center study and the selection bias inherent to a tertiary care academic center may have influenced the results, which could limit the external validity of our findings. Finally, the number of patients included in this study was small, and it is possible that the study was underpowered to demonstrate a protective effect of APC treatment.
In conclusion, although initial hemostasis could be achieved with APC in patients with UGITB, this effect appeared to be temporary. APC therapy did not have an impact on 30-day re-bleeding and mortality rates. While endoscopic therapy has not been proven as an effective therapy, additional treatment such as hemostatic radiotherapy, angiography or even surgery should be considered in this clinical situation.
Funding statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Herrera S, Bordas JM, Llach J, et al. The beneficial effects of argon plasma coagulation in the management of different types of gastric vascular ectasia lesions in patients admitted for GI hemorrhage. Gastrointest Endosc 2008; 68: 440–446. [DOI] [PubMed] [Google Scholar]
- 2.Pavey DA, Craig PI. Endoscopic therapy for upper-GI vascular ectasias. Gastrointest Endosc 2004; 59: 233–238. [DOI] [PubMed] [Google Scholar]
- 3.Manner H. Argon plasma coagulation therapy. Curr Opin Gastroenterol 2008; 24: 612–616. [DOI] [PubMed] [Google Scholar]
- 4.Kanai M, Hamada A, Endo Y, et al. Efficacy of argon plasma coagulation in nonvariceal upper gastrointestinal bleeding. Endoscopy 2004; 36: 1085–1088. [DOI] [PubMed] [Google Scholar]
- 5.Canard JM, Védrenne B. Clinical application of argon plasma coagulation in gastrointestinal endoscopy: Has the time come to replace the laser? Endoscopy 2001; 33: 353–357. [DOI] [PubMed] [Google Scholar]
- 6.Wahab PJ, Mulder CJ, Den Hartog G, et al. Argon plasma coagulation in flexible gastrointestinal endoscopy: Pilot experiences. Endoscopy 1997; 29: 176–181. [DOI] [PubMed] [Google Scholar]
- 7.Akhtar K, Byrne JP, Bancewicz J, et al. Argon beam plasma coagulation in the management of cancers of the esophagus and stomach. Surg Endosc 2000; 14: 1127–1130. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura H, Inamori M, Akiyama T, et al. Argon plasma coagulation for a bleeding gastrointestinal stromal tumor. Digestion 2007; 75: 164–164. [DOI] [PubMed] [Google Scholar]
- 9.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–655. [PubMed] [Google Scholar]
- 10.Maluf-Filho F, Martins BC, De Lima MS, et al. Etiology, endoscopic management and mortality of upper gastrointestinal bleeding in patients with cancer. United Eur Gastroenterol J 2013; 1: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eickhoff A, Jakobs R, Schilling D, et al. Prospective non-randomized comparison of two modes of argon beamer (APC) tumor desobstruction: Effectiveness of the new pulsed APC versus forced APC. Endoscopy 2007; 39: 637–642. [DOI] [PubMed] [Google Scholar]
- 12.Sheibani S, Kim JJ, Chen B, et al. Natural history of acute upper GI bleeding due to tumours: Short-term success and long-term recurrence with or without endoscopic therapy. Aliment Pharmacol Ther 2013; 38: 144–150. [DOI] [PubMed] [Google Scholar]
- 13.Reinmuth N, Parikh AA, Ahmad SA, et al. Biology of angiogenesis in tumors of the gastrointestinal tract. Microsc Res Tech 2003; 60: 199–207. [DOI] [PubMed] [Google Scholar]
- 14.Tiwari AK, Crawford SE, Radosevich A, et al. Neo-angiogenesis and the premalignant micro-circulatory augmentation of early colon carcinogenesis. Cancer Lett 2011; 306: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heller SJ, Tokar JL, Nguyen MT, et al. Management of bleeding GI tumors. Gastrointest Endosc 2010; 72: 817–824. [DOI] [PubMed] [Google Scholar]
- 16.Savides TJ, Jensen DM, Cohen J, et al. Severe upper gastrointestinal tumor bleeding: Endoscopic findings, treatment and outcome. Endoscopy 1996; 28: 244–248. [DOI] [PubMed] [Google Scholar]
- 17.Loftus EV, Alexander GL, Ahlquist DA, et al. Endoscopic treatment of major bleeding from advanced gastroduodenal malignant lesions. Mayo Clin Proc 1994; 69: 736–740. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Miho O, Watanabe Y, et al. Endoscopic laser therapy in the curative and palliative treatment of upper gastrointestinal cancer. World J Surg 1989; 13: 158–164. [DOI] [PubMed] [Google Scholar]
- 19.Sung JJY, Luo D, Wu JCY, et al. Early clinical experience of the safety and effectiveness of Hemospray in achieving hemostasis in patients with acute peptic ulcer bleeding. Endoscopy 2011; 43: 291–295. [DOI] [PubMed]
- 20.Chen Y-I, Barkun AN, Soulellis C, et al. Use of the endoscopically applied hemostatic powder TC-325 in cancer-related upper GI hemorrhage: Preliminary experience. Gastrointest Endosc 2012; 75: 1278–1281. [DOI] [PubMed] [Google Scholar]
- 21.Leblanc S, Vienne A, Dhooge M, et al. Early experience with a novel hemostatic powder used to treat upper GI bleeding related to malignancies or after therapeutic interventions. Gastrointest Endosc 2013; 78: 169–175. [DOI] [PubMed] [Google Scholar]