Abstract
Background and aims
The published data about the efficacy of the intercellular adhesion molecule-1 (ICAM-1) antisense oligonucleotide termed alicaforsen in inflammatory bowel disease (IBD) is rather inconsistent. This case series analyzes its efficacy in chronic refractory pouchitis, after proctocolectomy.
Methods
We performed a retrospective analysis on all patients who had received at least one dose of alicaforsen for IBD at three referral centers in Switzerland. We assessed the drug’s efficacy in patients treated for chronic refractory pouchitis, by comparing the clinical and/or endoscopic disease activity at baseline with a 2–3-month follow-up visit.
Results
We identified 22 patients who had received at least one dose. Among them, 13 patients were being treated for chronic refractory pouchitis. These patients had a median age of 38.0 years (95% CI 21.0–69.0) and five were female (38.5%). The median time since pouch surgery was 102.5 months (95% CI 16.0–288.0), with a median pouchitis duration of 16.0 months (95% CI 4.0–216.0). At 2–3 months after therapy, clinical and endoscopic disease activity was significantly reduced (stool frequency 9.0 versus 6.0, the Pouchitis Disease Activity Index (PDAI) clinical subscore was 4.0 versus 1.0, and the endoscopic disease activity was 4.0 versus 2.0). Clinical improvement was achieved in 11 out of 13 pouchitis patients (84.6%); however, a relapse was observed in nine of these patients (81.8%). The median time from clinical improvement to relapse was 16 weeks (95% CI 9.0–23.0).
Conclusions
Alicaforsen seemed to be efficacious in inducing clinical and/or endoscopic improvement in chronic refractory pouchitis and may be a promising treatment alternative in those patients; however, given the high proportion of relapse, one 6-week course of alicaforsen may not be sufficient.
Keywords: Alicaforsen, antisense oligonucleotide, chronic pouchitis, ICAM-1, inflammatory bowel disease, relapse
Introduction
In recent years, the targeting of leukocyte trafficking to the bowel as gut-specific immunosuppression has become a major field of interest in inflammatory bowel disease (IBD) treatment research.1 Agents that specifically block the interaction between leukocytes and endothelial or colonic epithelial cells are evolving, promising at least similar efficacy, but with lower rates of adverse events, as compared to tumor necrosis factor alpha (TNFα) antagonists.2 Leukocyte trafficking is a multistep process, where highly specific interactions between proteins on the surface of leukocytes and their corresponding ligands are crucial. Attachment and adhesion of leukocytes to endothelial cells is facilitated by integrins,1 which are capable of binding to different molecules of the immunoglobulin superfamily, such as the intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule (VCAM) or mucosal vascular addressin cell adhesion molecule (MAdCAM).3 ICAM-1 is a transmembrane glycoprotein expressed on the surface of intestinal epithelial cells and vascular endothelial cells.4 Its expression is upregulated by pro-inflammatory mediators such as TFNα, interleukin-1 (IL-1), interferon gamma (IFN-γ) or lipopolysaccharide (LPS).5 Thus, ICAM-1, as other ICAMs, contributes to leukocyte adhesion, migration, local lymphocyte stimulation and plays an important role in intestinal T-lymphocyte trafficking.6
Alicaforsen, a human ICAM-1 antisense oligonucleotide, blocks ICAM-1 production by complementary hybridization to the messenger ribonucleic acid (mRNA) of the target gene, which leads to hydrolysis of the created deoxyribonucleic acid-ribonucleic acid (DNA-RNA) complex by a RNase enzyme, therefore blocking protein translation.7 So far, published data about its potential role in IBD is inconsistent: Randomized, controlled trials fail to show efficacy of intravenous drug administration in Crohn’s disease (CD)8–10; however, a post-hoc analysis of one study suggests a correlation between the area under the curve (AUC) of drug levels and the probability of remission.10 Consecutive studies with alicaforsen as an enema formulation in ulcerative colitis (UC) patients also fail to show significant effects regarding primary outcomes, such as disease activity and remission rates after six weeks. Nevertheless, significantly higher response rates and lower relapse rates, compared to either placebo or mesalazine several weeks after treatment termination, suggest a disease-modifying effect.11,12 Interestingly, one open-label study evaluating alicaforsen as an enema formulation in chronic refractory pouchitis could show efficacy regarding disease activity and remission rates;13 however, no randomized, controlled trial has been conducted so far.
Pouchitis remains the major long-term complication after proctocolectomy in UC patients, with an incidence of 15–53%; and 4.5–5.5% experience a chronic refractory disease course, which represents a very difficult-to-treat entity.14 Up to one-third of them will eventually require surgery.15 Interestingly, Patel et al.16 report increased serum levels of soluble ICAM-1 in patients with pouchitis, suggesting that ICAM-1 is a key player in chronic inflammatory processes and is a potential target for future therapy options. To our knowledge, this is the only case series investigating the role and potential efficacy of the ICAM-1 antisense oligonucleotide alicaforsen in chronic refractory pouchitis in UC patients after proctocolectomy, besides one open-label study with 12 patients.13
Materials and methods
Subjects
We identified all IBD patients who had received the ICAM-1 antisense oligonucleotide alicaforsen at three IBD referral centers in Switzerland (University Hospital Zurich, Gastrozentrum Hirslanden Zurich and Tiefenauspital Bern) from the patient information systems of each individual hospital. All clinical case notes were reviewed. Patients were excluded if they were younger than 16 years old. Diagnosis of underlying condition and the indication for alicaforsen treatment (such as chronic refractory pouchitis in UC patients, fistulizing CD or UC proctitis) were made by the clinical course, and confirmed by endoscopy and histology. Given the off-label status of alicaforsen, approval by each individual’s health care insurance for reimbursement and by the Swiss Agency for Therapeutic Products (Swissmedic) had to be obtained for every patient, prior to treatment initiation. For a systematic overview of alicaforsen use in Switzerland, all patients treated for IBD were included. For a treatment outcome analysis, only those UC patients treated for chronic refractory pouchitis (with a duration >4 weeks) after proctocolectomy were included, while we excluded those treated for other indications such as fistulizing CD or proctitis from analysis. All the patients in this case series had had prior inclusion in the Swiss IBD cohort study, which an ethical approval is available for, and written informed consent had been obtained from all 22 patients.
Data collection
The following data was collected: Patient demographics, diagnosis, disease extent, prior medications and treatments (including surgery), current medication, symptom severity (stool frequency, abdominal cramps, fecal urgency, rectal bleeding and fever), laboratory parameters (full blood count, C-reactive protein (CRP) and fecal calprotectin), endoscopy (colonoscopy or sigmoidoscopy), histology and details about their alicaforsen use (indication, dosage, efficacy and tolerance). For all patients treated for chronic refractory pouchitis, the Pouchitis Disease Activity Index (PDAI) clinical subscore17 was calculated from the symptoms reported in each individual’s patient chart. If one symptom was not elucidative in the patient charts, PDAI was excluded from analysis. Due to the retrospective nature of this study, we did not have standardized endoscopic disease activity for calculation of the PDAI endoscopic subscore. Given the subjective interpretation by each individual endoscopist, we graduated the disease activity in a 6-scale score, ranging from no significant pouchitis (0) to severe pouchitis (5). Histology was included in the analysis if the Moskowitz score, which is a validated histologic grading system developed in 1986,18 was available.
We defined clinical improvement and treatment success of alicaforsen as the fulfillment of all the following criteria:
Reduction of stool frequency;
Reduction of PDAI clinical subscore; and
Interpretation as clinical improvement by the responsible clinician in synopsis of clinical symptoms, quality of life and, if applicable, endoscopy.
According to other studies investigating alicaforsen in IBD, we evaluated the clinical and/or endoscopic response and re-assessed disease activity at a 2–3-month follow-up visit. Relapse was defined as the time point when increasing clinical or endoscopic disease activity was reported in the patient charts, interpreted by the responsible clinician as relapsing pouchitis. We recorded the time from clinical improvement to relapse, for our Kaplan Meier analysis. Any symptom reported in the patient charts during alicaforsen treatment or within the next few weeks that was interpreted by the responsible clinician as related to therapy, was interpreted as a possible adverse drug event.
Statistical analysis
For the statistical analysis, we used the IBM Software SPSS Statistics Version 22.0.0 (2013 SPSS Science Inc., Chicago, IL, USA). We compared the clinical and/or endoscopic disease activity scores, at baseline and at a 2–3-month follow-up visit, using the Wilcoxon signed rank test. For calculation of the clinical improvement to relapse time, a Kaplan Meier analysis was used. A two-sided p value of <0.05 was regarded as statistically significant.
Results
Overview of patients treated with alicaforsen
A total of 22 patients treated with alicaforsen for IBD were identified. In 13 patients (59.1%), the treatment indication was chronic refractory pouchitis after proctocolectomy for UC. The remaining nine patients were treated for refractory ulcerative proctitis (seven patients, 31.8%), ischemic pouchitis (one patient, 4.5%) and fistulizing CD (one patient, 4.5%). Overall, 10 patients were female (45.5%) and the median age was 37.0 (95% CI 21.0–68.0). Patient demographics are shown in Table 1.
Table 1.
Demographic data of all patients
| Age (median in years) | 37.0 (95% CI 21.0–68.0) |
| Gender (male/female) | 12/10 (54.5%/45.5%) |
| Indication | |
| chronic pouchitis | 13 (59.1%) |
| ischemic pouchitis | 1 (4.5%) |
| UC proctitis | 7 (31.8%) |
| Fistulizing CD | 1 (4.5%) |
CD: Crohn’s disease; UC: ulcerative colitis
The 13 patients treated for chronic refractory pouchitis had a median age of 38.0 years (95% CI 21.0–69.0) and five patients were female (38.5%). The median duration since proctocolectomy and pouch formation was 102.5 months (95% CI 16.0–288.0), and the duration of chronic pouchitis was 16.0 months (95% CI 4.0–216.0). All 13 patients (100%) had previously received antibiotics such as ciprofloxacin and metronidazole; and 11 patients (84.6%) had received topical steroids. Other reported prior treatments for pouchitis were probiotics such as VSL#3 (a mixture of eight different bacteria) or Mutaflor® (E. coli Nissle) (four patients, 30.8%), mesalazine (six patients, 46.2%) and biologics such as infliximab (five patients, 38.5%). None of these patients had a history of Clostridium difficile infection. At baseline, prior to initiation of alicaforsen, the median number of daily stools was 9.0 (95% CI 6.0–15.0) and the PDAI clinical subscore was 4.0 (95% CI 3.0–6.0). The median non-validated endoscopy disease activity score (as described in the methods section) was 4.0 (95% CI 3.0–5.0), indicating moderate-to-severe disease activity. Fecal calprotectin was only measured in three patients with chronic pouchitis prior to initiation with alicaforsen. The patient demographics and disease activity before alicaforsen therapy are shown in Table 2.
Table 2.
Demographic data of patient with chronic pouchitis
| Age (median in years) | 38.0 (95% CI 21.0–69.0) |
| Gender (male/female) | 8/5 (61.5%/38.5%) |
| Duration of pouchitis (median in months) | 102.5 (95% CI 16.0–288.0) |
| Duration of pouchitis (median in months) | 16.0 (95% CI 4.0–216.0) |
| Number of daily stools (median) | 9.0 (95% CI 6.0–15.0) |
| PDAI clinical subscore (median) | 4.0 (95% CI 3.0–6.0) |
PDAI: Pouchitis Disease Activity Index
Overall study outcome
All 13 patients with chronic refractory pouchitis were treated with an enema formulation of 240 mg alicaforsen in the evening, for 6 weeks total (median 42 doses, range 42–84). Two patients (15.4%) were re-treated with a 6-week course of alicaforsen for a second time, one due to a clinical relapse and the other due to a slightly increased stool frequency. No significant side effects were reported during or after treatment, and no patient had to discontinue therapy early. In 10 patients, a prior pouchitis therapy, such as antibiotics, steroids and anti-TNF was stopped. One patient continued with mesalazine, one had a 1-week overlap of topical steroids and alicaforsen, and in one patient the anti-TNF therapy with infliximab was continued.
All 13 patients were seen at least at one follow-up visit and they were re-evaluated within 2–3 months after initiation of alicaforsen. We had 11 of the 13 patients (84.6%) showing a clinical improvement based on symptom severity (stool frequency or PDAI clinical subscore) and interpretation by the responsible clinician. Only two patients (15.4%) showed neither a clinical nor an endoscopic response. The median number of daily stools was reduced from 9.0 (95% CI 6.0–15.0) to 6.0 (95% CI 4.0–12.5), while the PDAI clinical subscore decreased from 4.0 (95% CI 3.0–6.0) to 1.0 (95% CI 0.0–5.0). Both reductions were statistically significant (p = 0.003 and p = 0.005, respectively). Moreover, the median non-validated endoscopic disease activity score was significantly reduced from 4.0 (95% CI 3.0–5.0) to 2.0 (95% CI 0.0–4.0), with two applicable endoscopies (before versus after treatment) in 10 out of 13 patients (76.9%), indicating an improvement from a moderate-to-severe to a mild-to-moderate pouchitis disease activity (p = 0.017). All these reductions were still significant after the exclusion of the one patient who had received a combination therapy of infliximab and alicaforsen.
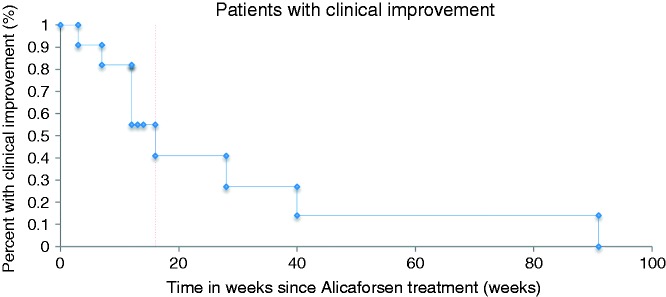
Only in four patients (30.8%) were two correlating biopsies available with inflammatory activity graded by the Moskowitz score. In these, the histological disease activity did not show any changes before versus after the treatment (Table 3 and Figure 1). In 9 of the 11 patients (81.8%) with clinical improvement after alicaforsen therapy a relapse was observed, with a median time from clinical improvement to relapse of 16 weeks (95% CI 9.0–23.0) (Figure 2). Two patients showed a sustained clinical improvement for more than nine months; however, one of them was retreated with alicaforsen after six months. The individual patient’s disease course is summarized in Table 4.
Table 3.
Clinical, endoscopic and histologic treatment outcome
| Before treatment (median) | After treatment (median) | p-value | |
|---|---|---|---|
| Daily stools n = 13 | 9.0 (95% CI 6.0–15.0) | 6.0 (95% CI 4.0–12.5) | 0.003 |
| PDAI clinical sub-score n = 12 | 4.0 (95% CI 3.0–6.0) | 1.0 (95% CI 0.0–5.0) | 0.005 |
| Endoscopic disease activity n = 10 | 4.0 (95% CI 3.0–5.0) | 2.0 (95% CI 0.0–4.0) | 0.017 |
| Histological disease activity (Moskowitz score) n = 4 | 5.0 (95% CI 3.0–8.0) | 5.0 (95% CI 3.0–7.0) | 0.564 |
PDAI: Pouchitis Disease Activity Index
Figure 1.
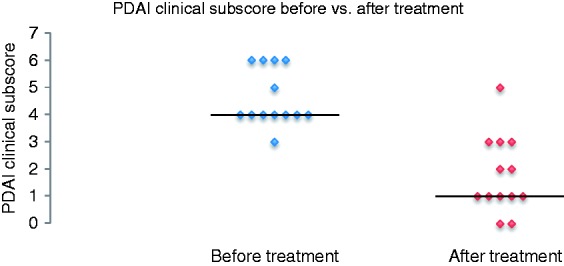
PDAI clinical subscore before versus after treatment.
PDAI: Pouchitis Disease Activity Index
Figure 2.
Durability of response (Kaplan Meier Curve).
Table 4.
Synopsis of all patients treated with alicaforsen for chronic pouchitis
| n | Gender (M/F) | Age (yrs) | Duration of pouch (months) | Duration of pouchitis (months) | Duration of alicaforsen treatment of 250 mg daily (weeks) | Daily stools before treatment (mean n) | Daily stools after treatment (mean n) | PDAIa clinical sub-score before treatment | PDAIa clinical sub-score after treatment | Fecal calprotectin before treatment (µg/g) | Fecal calprotectin after treatment (µg/g) | Endoscopy before treatment (Moskowitz Score2 if applicable) | Endoscopy after treatment (Moskowitz Score2 if applicable) | Durability of Response (weeks) | Clinical Improvement | Relapse | ADE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1c | M | 21 | 16 | 4 | 6 | 7 | 4 | 4 | 0 | 4549 | N.A. | N.A. | N.A. | 16 | Yes | yes | No |
| 2 | M | 69 | 288 | 18 | 6 | 7.5 | 7.5 | 3 | 3 | NA | 216 | Moderate pouchitis | Moderate pouchitis | 0 | No | No | |
| 3 | M | 23 | 41 | 13 | 6 | 8 | NA | 6 | 0 | NA | N.A. | Severe pouchitis | No significant pouchitis | 13+ | Yes | No | No |
| 4 | F | 49 | 61 | 57 | 6 | 9 | 6 | 6 | 2 | 448 | 219 | N.A. | No significant pouchitis | 28 | Yes | Yes | No |
| 5 | M | 32 | 20 | 17 | 6 | 12.5 | 12.5 | 5 | 5 | 511 | N.A. | Moderate to severe pouchitis | Moderate to severe pouchitis | 0 | No | No | |
| 6 | M | 57 | 168 | 16 | 6 | 11 | 9 | 4 | 3 | NA | N.A. | Moderate to severe pouchitis | Moderate pouchitis Moskowitz 5 | 3 | Yes | Yes | No |
| 7 | F | 59 | 192 | 192 | 6 | 8 | 5 | 4 | 1 | NA | N.A. | Moderate to severe pouchitis Moskowitz 3 | Mild pouchitis | 12 | Yes | Yes | No |
| 8 | M | 24 | NA | 6 | 6 | 15 | 8 | 4 | 1 | NA | N.A. | Moderate Pouchitis Moskowitz4 | Mild pouchitis | 12 | Yes | Yes | No |
| 9 | M | 38 | 24 | 8 | 6 | 11 | 8 | 4 | 2 | NA | N.A. | Severe pouchitis Moskowitz 8 | Moderate to severe pouchitis | 12 | Yes | yes | No |
| 10 | F | 35 | 240 | 9 | 6 | 8 | 5 | 4 | 1 | NA | N.A. | Moderate pouchitis Moskowitz 4 | Mild pouchitis Moskowitz 4 | 14+ | Yes | No | No |
| 11 | F | 64 | 216 | 216 | 6 | 6 | 4.5 | 4 | 3 | NA | N.A. | Moderate pouchitis Moskowitz 5 | Moderate pouchitis Moskowitz 6 | 7 | Yes | Yes | No |
| 12 | M | 27 | 16 | 9 | 2x6 | 10 | 4.5 | 6 | 1 | NA | N.A. | Moderate pouchitis Moskowitz 6 | N.A. | 40 | Yes | Yes | No |
| 13 | F | 54 | 144 | 108 | 6 | 12 | 6 | 6 | 1 | NA | N.A. | Moderate to severe pouchitis Moskowitz 6 | Mild to moderate pouchitis Moskowitz 7 | 91 | Yes | Yes | No |
Discussion
This retrospective case series analyzes the efficacy of alicaforsen as a potential therapeutic alternative in chronic refractory pouchitis that was non-responsive to other treatments, such as topical corticosteroids, mesalazine or antibiotics. After 2–3 months, patients treated with alicaforsen as a once-daily 240 mg enema formulation for 6 weeks showed significant reduction in clinical disease activity (as assessed by symptom severity) and endoscopic disease activity. We found that 11 out of 13 patients achieved clinical improvement, indicating a success rate of 84.6%; however, in 9 of these 11 (81.8%) patients, a clinical and/or endoscopic relapse was observed. The median time from the clinical improvement to relapse was 16 weeks (95% CI 9.0–23.0).
Published data on alicaforsen in refractory pouchitis is very limited. Only one open-label study with a limited study population of 12 patients has been conducted so far that shows symptom improvement, but no significant changes in histological disease activity.13 Alicaforsen is shown to be well tolerated, with no serious side effects. Our findings of a significant symptomatic improvement, but no histological changes are consistent with the open-label study for alicaforsen in pouchitis.
As in all other studies conducted so far, no serious adverse events were reported. Moreover, we did not observe any side effect of the enema formulation, suggesting both a safe and a well-tolerated drug. The latter is especially noteworthy, as several patients and also physicians appear to be somewhat reluctant to administer topical treatment options in UC, despite their well-established and often superior efficacy, compared to their oral counterparts.19
In pouchitis, an exclusively distal inflammatory affection of the intestine, the benefit of a topical treatment option may be even more distinctive, due to a higher local drug concentration, as proposed by Yacyshyn et al.10 and observed by Van Deventer et al.11 and Miner et al.12 Therefore, it is plausible to assume that a better response rate may be achieved, compared to an intravenous formulation. Recently, the topical delivery of an active compound to the intestinal mucosa via the lumen (in contrast to the majority of treatment options that act via the blood stream) has gained traction in the development of new drugs in IBD, as illustrated for instance by budesonide MMX20 and mongersen. Of note, the latter is also an antisense oligonucleotide against Smad7, and it represents one of the most promising new therapeutic targets in CD, as suggested by the results of a Phase II study.21
The high rates of clinical response may have been due to a relatively deferred follow-up visit with the first re-assessment being after 2–3 months, emphasizing the possible disease-modifying effect of alicaforsen; however, a prolonged clinical response was only observed in two patients, with a durability of response of >40 weeks. In most patients, a relapse occurred with a median clinical improvement to relapse time of 16 weeks. Interestingly, one patient with a second 6-week course of alicaforsen due to a slightly increased stool frequency after 6 months showed the second-longest durability of response, with 40 weeks. In addition, one other patient with a second alicaforsen cycle after relapse showed a second clinical improvement, for 12 weeks. Thus, maintenance therapy or repeated ad hoc treatment with alicaforsen may have led to longer sustained response rates and fewer relapses. In this respect, the early days of anti-TNF treatment come to mind. Initially, an episodic treatment was common practice; whereas subsequently, it was realized that in the vast majority of patients, a maintenance therapy is of need. However, given the above-mentioned evidence for a prolonged remission and a possible disease-modifying effect of alicaforsen, a repeated ad hoc treatment on an as-needed basis may be favored over maintenance therapy, and appears to be plausible in chronic refractory pouchitis that is responsive to ICAM-1 inhibition. The latter sheds light on the therapeutic potential of other available or upcoming agents targeting leucocyte migration, such as vedolizumab.22,23
A limitation of our case series certainly is the retrospective nature of this study; and therefore, the lack of controls and blinding. Furthermore, two non-validated and non-standardized assessment scores were used for stool frequency and endoscopic disease activity. Thus, endoscopic evaluation may have been subjectively influenced by the responsible clinician. The validated PDAI was only used as its clinical subscore, due to the lack of PDAI assessment of endoscopy and histology. Unfortunately, only four complete sets of histology before and after treatment were available. In addition, the improvement and success rate was not standardized. The study population was limited; however, it even exceeded the only conducted study with alicaforsen in chronic pouchitis, with its 12 patients.
In conclusion, alicaforsen may be efficacious in inducing clinical and endoscopic improvements in chronic refractory pouchitis that is non-responsive to other agents; and may be a promising treatment alternative in those patients, due to the limitation of other treatment options. Moreover, it seems to be very well tolerated and safe, with no reported side effects. Given the high proportion of relapse, one 6-week course of alicaforsen may not be sufficient, and repeated courses may lead to higher and longer sustained response rates, with fewer relapses; however, further studies, especially randomized placebo-controlled trials, are needed to answer these questions.
Acknowledgement
We would like to thank Marlise Grossenbacher for creating a database for all the alicaforsen patients from Bern, Switzerland.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
GR and FS are members of the international advisory board of Atlantic healthcare.
References
- 1.Mosli MH, Rivera-Nieves J, Feagan BG. T-cell trafficking and anti-adhesion strategies in inflammatory bowel disease: Current and future prospects. Drugs 2014; 74: 297–311. [DOI] [PubMed] [Google Scholar]
- 2.Cottone M, Renna S, Orlando A, et al. Medical management of Crohn's disease. Expert Opin Pharmacother 2011; 12: 2505–2525. [DOI] [PubMed] [Google Scholar]
- 3.Rivera-Nieves J, Gorfu G, Ley K. Leukocyte adhesion molecules in animal models of inflammatory bowel disease. Inflamm Bowel Dis 2008; 14: 1715–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dustin ML, Rothlein R, Bhan AK, et al. Induction by IL-1 and interferon-gamma: Tissue distribution, biochemistry and function of a natural adherence molecule (ICAM-1). J Immunol 1986; 137: 245–254. [PubMed] [Google Scholar]
- 5.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood 1994; 84: 2068–2101. [PubMed] [Google Scholar]
- 6.Barish CF. Alicaforsen therapy in inflammatory bowel disease. Expert Opin Biol Ther 2005; 5: 1387–1391. [DOI] [PubMed] [Google Scholar]
- 7.Gewirtz AT, Sitaraman S. Alicaforsen. Curr Opin Investig Drugs 2001; 2: 1401–1406. [PubMed] [Google Scholar]
- 8.Yacyshyn BR, Barish C, Goff J, et al. Dose ranging pharmacokinetic trial of high-dose alicaforsen (intercellular adhesion molecule-1 antisense oligodeoxynucleotide) (ISIS 2302) in active Crohn's disease. Aliment Pharmacol Ther 2002; 16: 1761–1770. [DOI] [PubMed] [Google Scholar]
- 9.Yacyshyn BR, Chey WY, Goff J, et al. Double blind, placebo-controlled trial of the remission inducing and steroid sparing properties of an ICAM-1 antisense oligodeoxynucleotide, alicaforsen (ISIS 2302), in active steroid-dependent Crohn's disease. Gut 2002; 51: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yacyshyn B, Chey WY, Wedel MK, et al. A randomized, double-masked, placebo-controlled study of alicaforsen, an antisense inhibitor of intercellular adhesion molecule 1, for the treatment of subjects with active Crohn's disease. Clin Gastroenterol Hepatol 2007; 5: 215–220. [DOI] [PubMed] [Google Scholar]
- 11.Van Deventer SJ, Wedel MK, Baker BF, et al. A Phase II dose-ranging, double-blind, placebo-controlled study of alicaforsen enema in subjects with acute exacerbation of mild-to-moderate left-sided ulcerative colitis. Aliment Pharmacol Ther 2006; 23: 1415–1425. [DOI] [PubMed] [Google Scholar]
- 12.Miner PB, Wedel MK, Xia S, et al. Safety and efficacy of two dose formulations of alicaforsen enema, compared with mesalazine enema, for treatment of mild-to-moderate left-sided ulcerative colitis: A randomized, double-blind, active-controlled trial. Aliment Pharmacol Ther 2006; 23: 1403–1413. [DOI] [PubMed] [Google Scholar]
- 13.Miner P, Wedel M, Bane B, et al. An enema formulation of alicaforsen, an antisense inhibitor of intercellular adhesion molecule-1, in the treatment of chronic, unremitting pouchitis. Aliment Pharmacol Ther 2004; 19: 281–286. [DOI] [PubMed] [Google Scholar]
- 14.Mahadevan U, Sandborn WJ. Diagnosis and management of pouchitis. Gastroenterology 2003; 124: 1636–1650. [DOI] [PubMed] [Google Scholar]
- 15.Sandborn WJ. Trends in inflammatory bowel disease 1996. In: McLeod RS Martin F, Sutherland LR, et al. (eds) Lancaster, PA: Kluwer Academic Publishers; 1997.
- 16.Patel RT, Pall AA, Adu D, et al. Circulating soluble adhesion molecules in inflammatory bowel disease. Eur J Gastroenterol Hepatol 1995; 7: 1037–1041. [DOI] [PubMed] [Google Scholar]
- 17.Sandborn WJ, Tremaine WJ, Batts KP, et al. Pouchitis after ileal pouch-anal anastomosis: A Pouchitis Disease Activity Index. Mayo Clin Proc 1994; 69: 409–415. [DOI] [PubMed] [Google Scholar]
- 18.Moskowitz RL, Shepherd NA, Nicholls RJ. An assessment of inflammation in the reservoir after restorative proctocolectomy with ileoanal ileal reservoir. Int J Colorectal Dis 1986; 1: 167–174. [DOI] [PubMed] [Google Scholar]
- 19.Ford AC, Khan KJ, Achkar J-P, et al. Efficacy of oral vs. topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: Systematic review and meta-analysis. Am J Gastroenterol 2012; 107: 167–176. [DOI] [PubMed] [Google Scholar]
- 20.Sandborn WJ, Travis S, Moro L, et al. Once-daily budesonide MMX extended-release tablets induce remission in patients with mild-to-moderate ulcerative colitis: Results from the CORE I study. Gastroenterology 2012; 143: 1218–1226. [DOI] [PubMed] [Google Scholar]
- 21.Monteleone G, Neurath M, Ardizzone S, et al. OP203 mongersen, an oral SMAD7 antisense oligonucleotide, in active Crohn’s disease. United Europ Gastroenterol J 2014; 2: A65–A65. [Google Scholar]
- 22.Lobaton T, Vermeire S, Van Assche G, et al. Review article: Anti-adhesion therapies for inflammatory bowel disease. Aliment Pharmacol Therapeut 2014; 39: 579–594. [DOI] [PubMed] [Google Scholar]
- 23.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]