Abstract
Signal transducer and activator of transcription 3 (STAT3) signaling is constantly activated in human melanoma, and promotes melanoma metastasis. The dietary flavonoid apigenin is a bioactive compound that possesses low toxicity and exerts anti-metastatic activity in melanoma. However, the anti-metastasis mechanism of apigenin has not been fully elucidated. In the present study, we showed that apigenin suppressed murine melanoma B16F10 cell lung metastasis in mice, and inhibited cell migration and invasion in human and murine melanoma cells. Further study indicated that apigenin effectively suppressed STAT3 phosphorylation, decreased STAT3 nuclear localization and inhibited STAT3 transcriptional activity. Apigenin also down-regulated STAT3 target genes MMP-2, MMP-9, VEGF and Twist1, which are involved in cell migration and invasion. More importantly, overexpression of STAT3 or Twist1 partially reversed apigenin-impaired cell migration and invasion. Our data not only reveal a novel anti-metastasis mechanism of apigenin but also support the notion that STAT3 is an attractive and promising target for melanoma treatment.
Melanoma is the most serious type of skin cancer because it is highly aggressive and can spread earlier and more quickly than other skin cancers. It represents only 4% of the skin cancer cases, but it accounts for more than 80% of all skin cancer-related deaths1. Surgical resection can only cure early diagnosed primary melanoma2. For the advanced melanoma, current therapeutics such as BRAF inhibitors, cytotoxic T lymphocyte-associated antigen 4 antibody and interleukin-2 biological therapy show different toxicities and side-effects3,4,5. Resistance to the BRAF targeted therapy in melanoma has also been reported, and has become a major problem for BRAF inhibitors6. Therefore, novel targeted therapies with low toxicity are urgently needed.
Transcription factor STAT3 (signal transducer and activator of transcription 3) is constitutively activated in 50–90% of melanomas7. Activation of STAT3 promotes transcription of many genes that involve in melanoma metastasis7. Examinations show that activation of STAT3 in human melanoma promotes brain metastasis, and increases the expressions of vascular endothelial growth factor (VEGF) and matrix metalloproteinase-2 (MMP-2)8. Constitutive STAT3 activation has been reported to up-regulate VEGF expression and stimulate melanoma tumor angiogenesis9. In addition, a wide array of aberrated oncogenes/angiogenic proteins such as Src10 and JAK211 converges on STAT3. Therefore, targeting STAT3 assaults melanoma on multiple fronts by suppressing the oncogenic potentials due to upstream and downstream aberrations12. Indeed, studies show that inhibiting STAT3 signaling in melanoma tumor models prevents metastasis8,13 and inhibits angiogenesis14.
Accumulating evidences from observational and prospective studies indicate that natural compounds present in the human diet or as supplements may substantially alter the natural history of carcinogenesis15. In recent years, there has been growing interest in the role of nutrition in melanoma chemoprevention16. Apigenin is a natural dietary flavonoid that has multiple biological functions such as anti-inflammatory and anti-oxidant properties17. Moreover, mounting epidemiological evidence suggests that intake of apigenin and other flavonoids reduces risk of cancers and this dietary intervention is negatively associated with the cancer recurrence17. Early in 1997, it has been found that apigenin inhibited ultraviolet light-induced skin carcinogenesis in mice18. Subsequent studies also suggest the anti-melanoma effects of apigenin, which include inhibition of melanoma metastasis19,20. Some of the cellular targets for apigenin have been identified21, including STAT3 signaling22,23. However, whether apigenin affects STAT3 signaling in melanoma metastasis has not been elucidated. In the present study, we examined the involvement of the STAT3 signaling pathway in the anti-metastatic effect of apigenin in melanoma. Two human melanoma cell lines A375 and G361 with constitutive activation of STAT324, together with a murine melanoma cell line B16F10 that widely used in lung metastasis study25 were employed in this study.
Results
Apigenin inhibited melanoma B16F10 cell lung metastasis
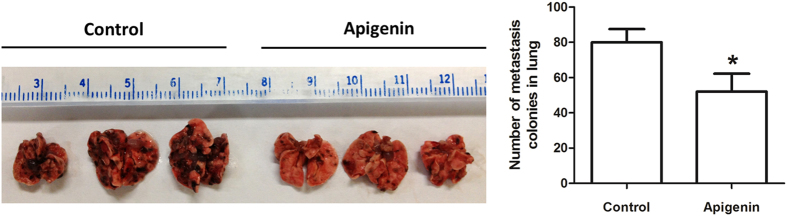
We first determined the in vivo anti-metastatic effect of apigenin in an experimental lung metastasis model. B16F10 melanoma cells were injected into the lateral tail vein of C57BL/6 mice. Then these mice were treated by intragastric administration with either vehicle (0.5% CMC-Na solution) or apigenin (150 mg/kg)26. We found that the apigenin-treated mice had significant fewer metastatic nodules (Fig. 1) when compared to the vehicle control group, suggesting apigenin inhibits the metastasis potential of B16F10 melanoma cells in vivo in our mouse model. This finding is in agreement with a previous report20.
Figure 1. Apigenin inhibited murine melanoma B16F10 cell lung metastasis.
B16F10 melanoma cells were injected into the tail vein of the C57BL/6 mice. These mice then received intragastric administration of vehicle or apigenin (150 mg/kg/day) for 24 consecutive days. Lung metastasis of B16F10 melanoma cells in the mouse model (upper) and the metastasis nodules number in the lungs (bottom) were shown. Data were mean ± SD, n = 8, *P < 0.05.
Apigenin impaired the migratory and invasive abilities of melanoma cells
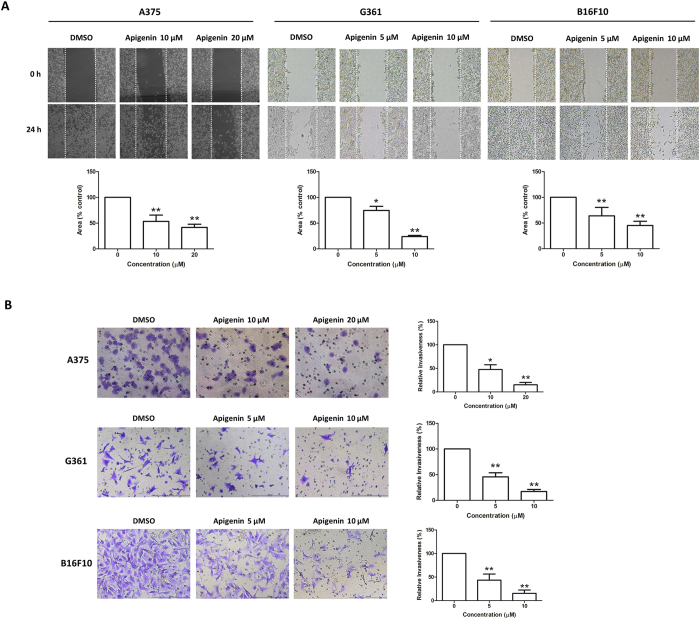
Apigenin has been shown to inhibit tumor cell migration and invasion in various types of cancers27,28, including mouse melanoma B16-BL6 cells19; however, its effects in human melanoma cells remain unknown. We therefore examined the effects of apigenin on migratory and invasive capacities of melanoma cells by using the wound healing assay and Transwell invasion assay, respectively. As shown in Fig. 2, apigenin (10 and 20 μM) significantly inhibited A375 cell motility and invasion in dose-dependent manners. It was reported that the migratory ability of A375 was about two times higher than that of G361 cells29. In agreement with this, we also found that the migratory and invasive capacities of G361 were much lower than A375 cells. Nevertheless, apigenin (5 and 10 μM) showed significant inhibitory effects on G361 cell migration and invasion. Apigenin (5 and 10 μM) also dose-dependently inhibited B16F10 cell migration and invasion. These data suggest that apigenin inhibits melanoma cells migration and invasion. Under the same culture condition, apigenin did not apparently affect cell viability (see Supplementary Fig. 1).
Figure 2. Apigenin inhibited melanoma cell migration and invasion.
(A) A single scratch was created in the confluent monolayer of A375, G361 or B16F10 cells. The scratch was photographed at 0 h and 24 h after treatment with the indicated concentrations of apigenin (upper) and relative migrated areas (bottom) were analyzed by Image J software. (B) A375, G361 or B16F10 cells were treated with indicated concentrations of apigenin for 16 h, and the invasive capacity was determined by the Transwell invasion assay. Representative photographs of invasive cells (left) and quantification of invasive cells (right) were shown. Data were mean ± SD from three independent experiments, *P < 0.05 and **P < 0.01.
Apigenin reduced constitutive STAT3 phosphorylation, suppressed STAT3 nuclear localization and STAT3-luciferase reporter activity in melanoma cells
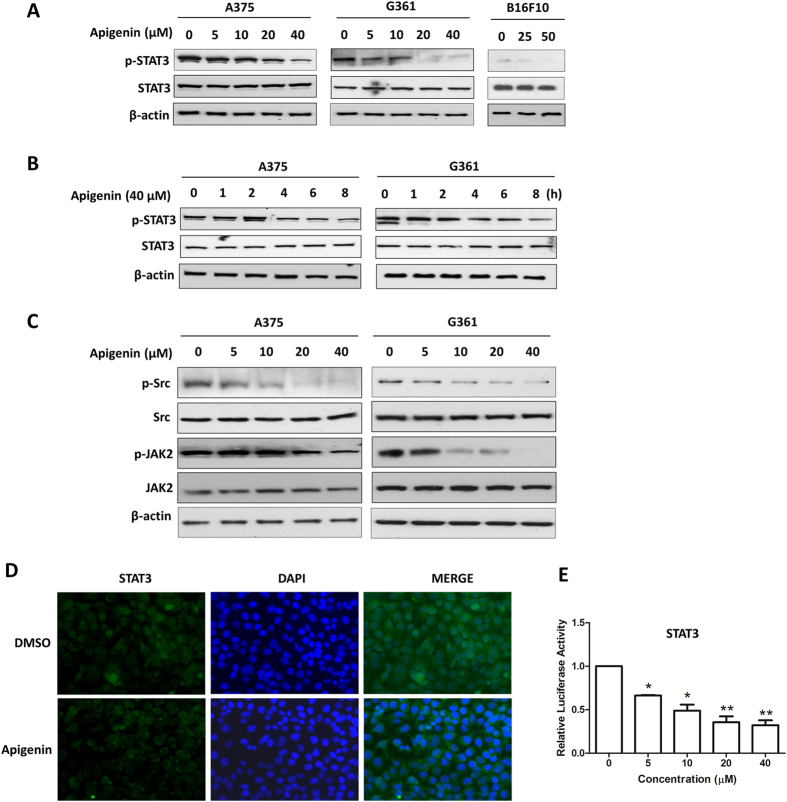
Constitutive activation of the STAT3 signaling pathway is well recognized to play a critical role in human melanoma development and progression by promoting cancer cell growth, survival, migration and invasion7. Therefore, we examined if apigenin inhibited the constitutive phosphorylation/activation of STAT3. As shown in Fig. 3A, in melanoma A375, G361 and B16F10 cells, the phosphorylation of STAT3 at the tyrosine 705 (Tyr705) site was dose-dependently inhibited by apigenin (Fig. 3A). When treated melanoma cells with 40 μM apigenin for various durations, the expression levels of phospho-STAT3 were decreased at time dependent manners (Fig. 3B); while apigenin did not affect the total STAT3 protein expressions (Fig. 3A,B) at the same concentration. These findings strongly suggest that apigenin effectively suppresses the constitutive activation of STAT3 in melanoma cells.
Figure 3. Apigenin reduced constitutive STAT3 phosphorylation, suppressed STAT3 nuclear localization and STAT3-luciferase reporter activity in melanoma cells.
Cells were treated with apigenin (A) at different concentrations for 24 h or (B) at 40 μM for different time periods as indicated. Then the expression levels of STAT3 and phospho-STAT3 were determined by the Western blot analysis. (C) Cells were treated with apigenin at various concentrations for 8 h and then the expressions of phospho-JAK2, JAK2, phospho-Src and Src were examined by Western blotting. (D) A375 cells were treated with 20 μM apigenin for 8 h, and then analyzed for the intracellular distribution of STAT3 by immunocytochemistry. (E) A375 cells were transfected with a STAT3-luciferase reporter plasmid together with a PRL-CMV construct for 12 h, followed by treatment with indicated concentrations of apigenin for another 12 h, and then the STAT3 transcriptional activity was measured by the dual-luciferase reporter assay. Data were shown as mean ± SD from three independent experiments, *P < 0.05 and **P < 0.01.
STAT3 has been reported to be activated by tyrosine kinases of the Janus family (JAKs) and Src kinase families at tyrosine 705 (Tyr705) site30. So we wondered if apigenin inhibited the activation of JAK2 and Src. As shown in Fig. 3C, apigenin dose-dependently suppressed the constitutive phosphorylation of JAK2 and Src in these two cell types. The expression levels of total JAK2 and Src kinase were not affected by apigenin treatment under the same condition. These results suggest that inhibition of STAT3 activation by apigenin is, at least in part, attributed to the reduction of the phosphorylation of JAK2 and Src in melanoma cells.
Tyrosine phosphorylation of STAT3 at Tyr705 initiates STAT3 dimerization and nuclear translocation. So we examined whether apigenin inhibited the nuclear translocation of STAT3. As shown in Fig. 3D, apigenin treatment obviously reduced STAT3 nuclear localization. STAT3 is a transcription factor. Inhibition of its nuclear localization will impede its binding on gene promoters and the subsequent gene transcriptional activities. To examine whether apigenin affected STAT3-mediated transcriptional activity, a STAT3-luciferase reporter construct (4xM67 pTATA TK-Luc) harboring four copies of STAT3 binding sites was transfected into A375 cells. Then these cells were treated with indicated concentrations of apigenin for 12 h before measuring the transcriptional activity by luciferase assay. As shown in Fig. 3E, apigenin significantly inhibited the STAT3-luciferase reporter activity in a dose-dependent manner. These results further confirm the inhibitory effect of apigenin on STAT3 signaling.
Apigenin regulated the expressions of STAT3 target genes involved in cell migration and invasion
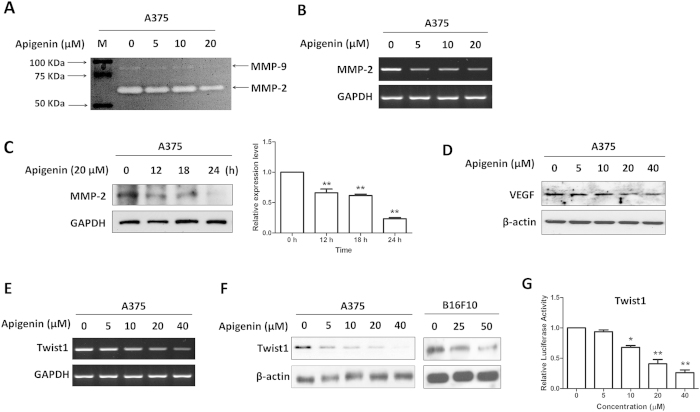
We next examined if apigenin affected STAT3 target genes in human melanoma cells. MMP-2 and MMP-9 are the target genes of STAT3 involving in cell migration and invasion8,13. As shown in Fig. 4A, apigenin inhibited the gelatinase activities of MMP-2 and MMP-9 in A375 cells. Apigenin also dose-dependently reduced the mRNA expression level of MMP-2 (Fig. 4B) and decreased its protein level in a time dependent manner (Fig. 4C), while the mRNA level of MMP-9 was too low to be detected. VEGF, another STAT3 target gene9, is one of the most potent mediators of tumor angiogenesis and metastasis. Apigenin also dose-dependently reduced the protein level of VEGF (Fig. 4D).
Figure 4. Apigenin regulated the expression of STAT3 target genes.
Cells were treated with various concentrations of apigenin for 24 h, and then (A) the enzymatic activities of MMP-2 and MMP-9 were determined by gelatinase zymography, (B) the mRNA expression level of MMP-2 was measured by RT-PCR, the protein levels of (C) MMP-2 and (D) VEGF were determined by immunoblotting, the relative protein level of MMP-2 was analyzed by Image J software and shown as mean ± S.D., **P < 0.01. (E) Twsit1 mRNA expression level and (F) Twist1 protein expression level were determined by RT-PCR and Western blotting, respectively. (G) A375 cells were transfected with a Twist1 promoter construct together with a PRL-CMV construct for 12 h, and then exposed to various concentrations of apigenin for another 12 h. The Twist1 transcriptional activity was measured by the dual-luciferase reporter assay. Data were shown as mean ± SD from three independent experiments, *P < 0.05 and **P < 0.01.
Twist1 is a transcription factor that frequently expressed in a wide array of human cancers including melanoma. It correlates with aggressive, invasive and metastatic lesions31. It was reported that constitutive activation of STAT3 can significantly activate the Twist1 promoter32. We found that apigenin reduced the Twist1 mRNA (Fig. 4E) and protein (Fig. 4F) levels in dose-dependent manners. To ascertain whether the decreased Twist1 mRNA expression was due to an inhibition of the Twist1 promoter activity, we generated a Twist1-promoter reporter construct (Twist1-Luc) and performed the luciferase assay. Data showed that apigenin reduced Twist1 promoter activity in a dose-dependent manner (Fig. 4G).
Apigenin treatment partially reversed epithelial-to-mesenchymal transition (EMT)
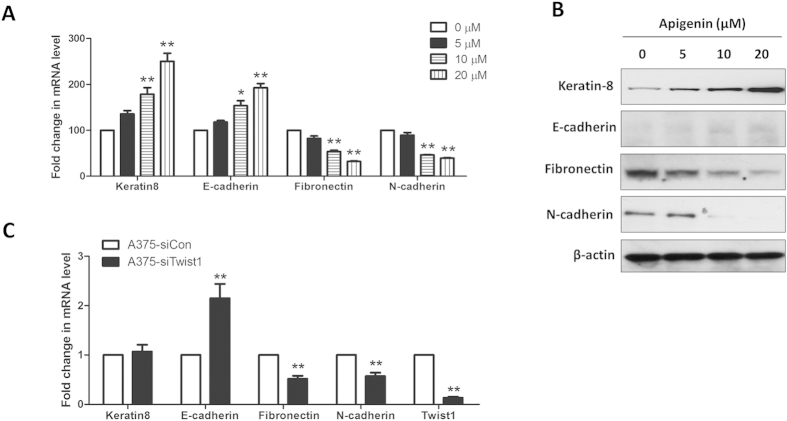
Twist1 has also been reported to induce epithelial-to-mesenchymal transition (EMT)33. EMT is a biological process that allows epithelial cells to undergo multiple biochemical changes that enable them to assume a mesenchymal cell phenotype. This process usually includes enhanced migratory and invasive capacities34. Tumors derived from epithelial cells can become more motile and invasive by acquiring characteristics of mesenchymal cells35. Since apigenin inhibited the expression level and promoter activity of Twist1 in melanoma A375 cells, we wondered if apigenin reversed the EMT in A375 cells. Real-time PCR analysis demonstrated that apigenin treatment dose-dependently increased the mRNA expression levels of epithelial markers keratin 8 and E-cadherin, while decreased the levels of mesenchymal markers fibronectin and N-cadherin (Fig. 5A). Western blot analysis also showed that apigenin caused partial reversal of EMT in melanoma cells, as evidenced by upregulation of keratin 8 and E-cadherin, and downregulation of fibronectin and N-cadherin (Fig. 5B). The partial reversal of EMT in melanoma cells after apigenin treatment may due to a reduction in Twist1 expression. Because in the Twist1-siRNA transfected cells (A375-siTwist1), mRNA levels of fibronectin and N-cadherin were reduced and that of an epithelial marker E-cadherin was increased (Fig. 5C). These data suggest that apigenin treatment or reduced expression of Twist1 regulates the expressions of the EMT factors and hence partially reverses the EMT in melanoma cells.
Figure 5. Apigenin treatment partially reversed EMT in A375 cells.
(A) A375 cells were treated with indicated concentrations of apigenin for 24 h, and the mRNA expression levels of several EMT markers were determined by real-time PCR. Data were shown as mean ± SD from three independent experiments, *P < 0.05 and **P < 0.01. (B) After apigenin treatment for 24 h, the protein expression levels of EMT markers in A375 cells were determined by Western blotting. (C) A375 cells were transfected with a Twist1-specific siRNA (si-Twist1) or a control siRNA (si-Con) for 24 h, the mRNA expression levels of several EMT markers were determined by real-time PCR. Data were shown as mean ± SD from three independent experiments, **P < 0.01.
Overexpression of Twist1 abrogated apigenin-mediated inhibitory effects on melanoma cell migration and invasion
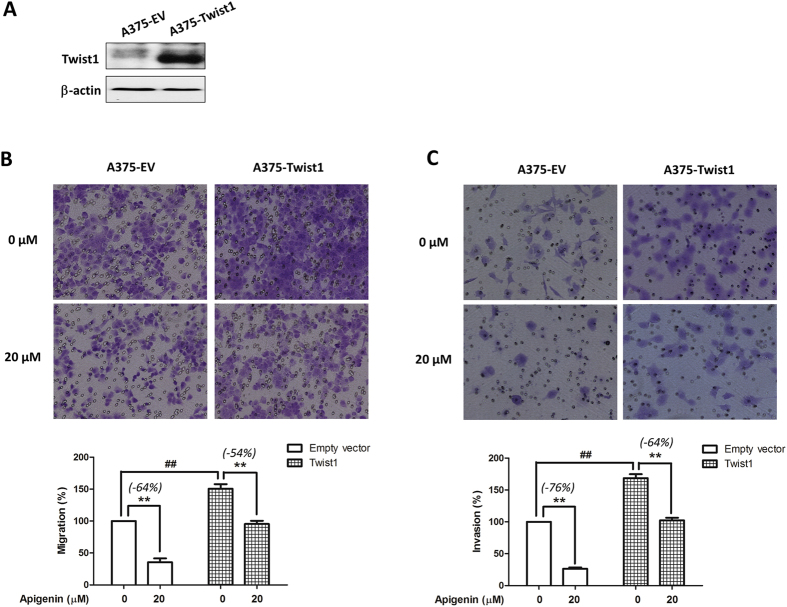
To further investigate if Twist1 involved in apigenin-mediated migration and invasion inhibition in melanoma cells, we overexpressed Twist1 in A375 cells by transient transfection of a Twist1-expressing construct pcDNA3.1-Twist1. After 24 h transfection, the expression of Twist1 was increased remarkably (Fig. 6A). Moreover, cells that transfected with Twist1 construct showed a significant increase in the migratory (Fig. 6B) and invasive (Fig. 6C) abilities as compared with cells that were transfected with the empty vector (##P < 0.01). In Twist1 overexpressing cells, apigenin-mediated cell migration inhibition was partially reduced from 64% to 54% (Fig. 6E), and cell invasion inhibition was reduced from 76% to 64% (Fig. 6F). All these data suggest that the inhibitory effects of apigenin on melanoma migration and invasion are due to, at least in part, the reduction of Twist1 expression.
Figure 6. Overexpression of Twist1 abrogated apigenin-mediated migration and invasion inhibitions in A375 cells.
A375 cells were transiently transfected with an empty vector or a Twist1-expressing construct for 24 h, and then (A) the expression level of Twist1 in A375-Twist1 cells and A375-EV cells were examined by immunoblotting. (B,C) A375-EV or A375-Twist1 cells were incubated with 20 μM apigenin for 16 h, and (B) cell migratory and (C) invasive abilities were measured by the Transwell chamber assay. Representative photographs of migrated or invasive cells (upper) and quantification of these cells (bottom) were shown. Data were mean ± SD from three independent experiments, ##P < 0.01 and **P < 0.01.
Ectopic expression of STAT3 rescued apigenin-mediated melanoma cell migration and invasion, and reduced the apigenin-mediated Twist 1 inhibition
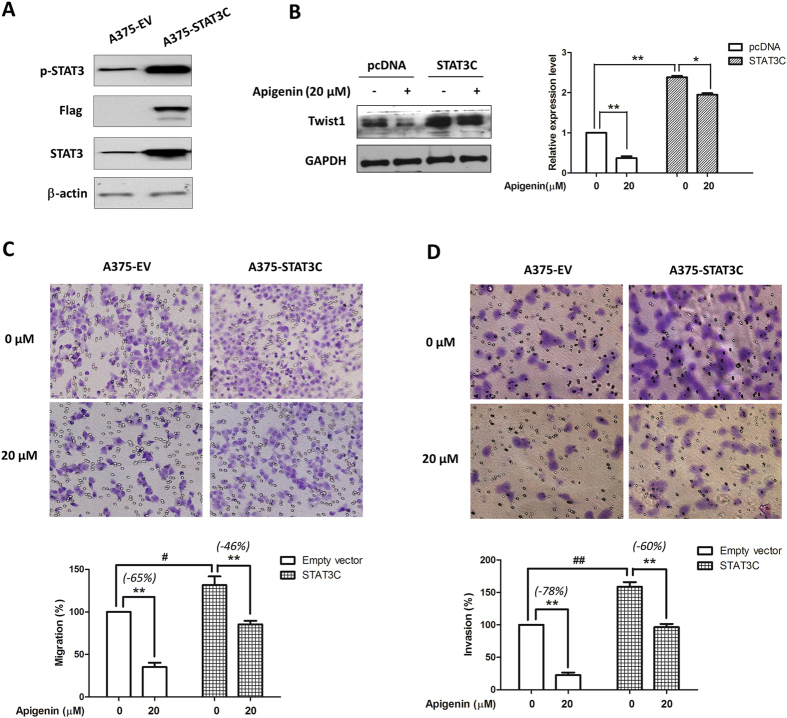
To further confirm if STAT3 inhibition was crucial in apigenin-mediated anti-migratory and anti-invasive activities, A375 cells were transiently transfected with a STAT3C-expressing construct before apigenin treatments. Western blotting data showed that transient transfect of the STAT3C-expressing construct in A375 cells resulted in a remarkable increase in STAT3 and p-STAT3 expressions (Fig. 7A). As expected, overexpression of STAT3 in A375 cells apparently increased the cell migratory (Fig. 7C) and invasive (Fig. 7D) abilities. And in STAT3C-expressing A375 cells, the inhibitory effects of apigenin on cell migration and invasion were inhibited from 65% to 46% and from 78% to 60%, respectively.
Figure 7. Overexpression of STAT3C reversed apigenin-mediated inhibition on cell migration and invasion, and reduced the apigenin-mediated Twist 1 inhibition.
(A) A375 cells were transiently transfected with an empty vector or a STA3TC-expressing construct for 24 h, and then lysed for the Western blot analysis by using antibodies specific to p-STAT3, Flag, STAT3 and β-actin. (B) After transfection, cells were treated with apigenin for 24 h, and the effects of apigenin on Twist1 were determined by immunoblotting. The relative expression levels were analyzed by Image J software and shown as mean ± S.D., *P < 0.05, **P < 0.01. (C) Cell migratory and (D) invasive capacities were measured by the Transwell chamber assay. Representative photographs of migrated or invasive cells (upper) and quantification of these cells (bottom) were shown. Data were mean ± SD from three independent experiments, #P < 0.05 ##P < 0.01 and **P < 0.01.
We also found that Twist1, a downstream target of STAT332, was increased in STAT3-overexpressed A375 cells, and the apigenin-mediated Twist1 inhibition was also partially reversed (Fig. 7B). Moreover, the overexpression of STAT3 and hence the increased expression of Twist1 reduced the apigenin-mediated EMT suppression (see Supplementary Fig. 2).
Taken together, we suggest that inhibition of STAT3 activation is critical in apigenin-afforded anti-metastatic activity in melanoma.
Discussions
Our research group is interested in the possible beneficial biological effects of natural compounds on malignant diseases, especially on melanoma36,37,38. In this study, we demonstrated the anti-metastatic effect of apigenin, a natural dietary compound that commonly presented in human diet, in melanoma cells and in animals. Our data showed that apigenin inhibited murine melanoma B16F10 cell lung metastasis in an animal model. Apigenin also exerted anti-migratory and anti-invasive activities in cultured B16F10 cells and human melanoma A375 cells. These results are in agreement with previous studies19,20.
Apigenin is generally non-toxic unless it is intraperitoneally injected with a high dose39. Indeed, apigenin in particular gains interest compared with other structurally related flavonoids because of its low intrinsic toxicity and its striking effect on cancer cells versus normal cells40. The average intake of flavonoids including apigenin, quercetin, kaempferol, myricetin and luteolin ranges from 6 mg/day in Finland to 64 mg/day in Japan17. Apigenin is emerging as one of the key nutraceuticals21. Our in vivo study also suggests a low toxicity of apigenin because we did not observe any apparent significant difference in body weights between apigenin-treated mice and control mice (see Supplementary Fig. 3).
The major problem for using apigenin as a chemopreventer is its poor oral bioavailability41. In 2005, Gradolatto et al. showed that oral intake a single dose of radio-labeled apigenin in rats resulted in 51% recovery of radioactivity in urine, 12% in feces, 1.2% in blood, 0.4% in the kidneys, 9.4% in the intestine, 1.2% in liver and 24.8% in the rest of the body within 10 days. The radioactivity did not appear in blood until 24 h after oral apigenin intake. The kinetics of apigenin in blood exhibited a relatively high elimination half-time of 91.8 h compared to other dietary flavonoids. These data suggest that although the bioavailability of apigenin is limited, the slow absorption and slow elimination properties may lead to possible accumulation of apigenin in the tissues to effectively impart its chemopreventive effects42. Indeed, in our study, oral administration with apigenin for 24 consecutive days significantly inhibited melanoma lung metastasis in mice. Daily intragastric injection of apigenin has also been reported to inhibit the progression and metastasis of ovarian cancer and prostate cancer in animal models26,43. Thus, daily oral intake of apigenin is able to prevent tumor progression and metastasis. Meanwhile, to improve the dissolution rate and increase the bioavailability of apigenin, several formulation techniques for apigenin have been investigated, including nanocrystals44, liposome45, and carbon nanopowder solid dispersion46. The solubility and bioavailability of apigenin have been significantly enhanced by these strategies. Taken together, apigenin is a promising anti-cancer agent.
The mechanistic study clearly showed that apigenin inhibited STAT3 activation, and moreover, cell migratory and invasive inhibitory effects of apigenin could be rescued by STAT3C (a constitutive active form of STAT3) over-expression. These data strongly suggest that inactivation of STAT3 signaling by apigenin may serve as an effective approach for melanoma treatment. After phosphorylation at Tyr-705, STAT3 dimerizes and translocates into the nucleus which in turn binds to the specific DNA response element in the promoter regions of target genes, leading to transcriptional activation. Therefore, inhibition of STAT3 phosphorylation may result in decreased STAT3 nuclear localization and transcriptional activity. In good agreement with this, our results indeed showed that STAT3 nuclear localization and transcriptional activity were substantially reduced by apigenin treatment. Constitutive STAT3 activation has been linked to the development of various cancer types. The suppression of constitutive STAT3 activation by apigenin in melanoma cells raises a possibility that apigenin may also inhibit the constitutively activated STAT3 in other cancer types, thereby providing a rationale for its use to treat other cancers. It is interesting to note that apigenin has been recently found to suppress STAT3 signaling in several types of cancer cells22,23.
Aberrant STAT3 activation is predominantly due to persistent tyrosine receptor kinase (Tyr) phosphorylation signals emanating from dysregulated upstream Tyr kinases, including Src and JAK230. Our results showed that expressions of both phospho-JAK2 and phospho-Src were decreased in A375 and G361 cells. On the other hand, numerous protein tyrosine phosphatases (PTPs) such as SHP-1, SHP-2, TC-PTP and PTP-1D have been implicated in STAT3 signaling, and inhibition of STAT3 activation has also been linked to the induction of PTPs by some anticancer agents47. Whether PTPs are involved in apigenin-mediated STAT3 inactivation in cancer cells including melanoma cells remains unknown and deserves further investigation.
We showed that apigenin impeded A375 melanoma cell migration and invasion. MMP-2 and MMP-9 are collagenases which favor tumor growth and invasion by digesting the extracellular matrix surrounding the tumor tissue. Strong MMP-2 expression is prevalent in primary and metastatic melanomas as compared to normal and dysplastic nevi, and is associated with worse survival of melanoma patients48. High serum level of MMP-9 is associated with rapid progression in patients with metastatic melanoma49. Moreover, it has been reported that overexpression of STAT3 enhances the invasiveness in less-invasive melanoma cells through increasing MMP-2 expression and activity; while inactivation of STAT3 remarkably impairs the invasive ability of invasive melanoma cells through decreasing MMP-2 expression and activity13. In this regard, it is plausible to postulate that apigenin inhibited melanoma invasiveness by decreasing MMP-2 activity and expression through suppressing the constitutively active STAT3. Apigenin also decreased the activity of MMP-9. However, the basal expression level of MMP-9 was very low, which was in line with the others’ studies8.
The development of metastasis has been shown to depend on the development of an adequate blood supply through angiogenesis50. VEGF is the most potent pro-angiogenic stimulus that plays critical role in tumor angiogenesis and metastasis. In melanoma patient, high serum VEGF values are associated with shorter disease-free survival as compared with lower values51. STAT3 has been reported to directly participate in regulating transcription of the VEGF gene9. Inhibition of STAT3 signaling suppresses Src- and IL-6-medaited VEGF up-regulation as well as tumor angiogenesis9,52. In our study, the expression of VEGF in melanoma cells was decreased by apigenin treatment, suggesting that the anti-metastatic effect of apigenin on melanoma is associated with VEGF inhibition. Besides promotes tumor metastasis, STAT3 also participates in the immune evasion process through up-regulation of immunosuppressive factors and down-regulation of pro-inflammatory cytokines and pro-inflammatory chemokines53. VEGF is one of the immunosuppression factors54. Whether apigenin enhances the anti-melanoma immune responses through STAT3/VEGF inhibition needs to be further studied.
We also demonstrated that apigenin treatment reduced the expression of Twist1, another target gene of STAT3, which is generally overexpressed in melanoma55. However, the role of Twist1 in melanoma is less studied. It is reported that Twist1 promoted melanoma metastasis and growth, which was accompanied by the up-regulation of several vascular growth factors and receptors, including VEGF and MMP-155,56. MMP-2 and MMP-9 can also be regulated by Twist1 and involved in Twist1-induced promotion of metastasis57,58. Both VEGF and MMPs are the molecular targets of STAT3. In this study, we suggest that Twist1 and STAT3 activation contribute to melanoma migratory and invasive abilities because overexpression of Twist1 or STAT3 enhanced cell migratory and invasive abilities, and also reversed apigenin-mediated melanoma migration and invasion inhibition.
EMT has been widely recognized as an important mechanism for cancer progression. Our data suggest that apigenin treatment reduces the expression of Twist1 and regulates EMT factors. Recently, a study suggests that invasion promoted by Twist1 is not due to a paradigm EMT protein changes in melanoma59. We will further investigate if the changed expressions of EMT markers, due to apigenin treatment or knockdown of Twist1 by siRNA, contribute to the inhibition of invasion in the melanoma cell model.
In conclusion, our study shows that inhibition of the STAT3 signaling pathway contributes to the anti-metastatic effect of apigenin in melanoma. Together with the reported anti-proliferative activity and low toxicity property of this compound, we suggest that apigenin has a potential role in melanoma treatment/prevention.
Materials and Methods
Reagents
Antibodies against phospho-STAT3 (Tyr705), STAT3, phospho-JAK2 (Y1007/1008), JAK2, phospho-Src (Tyr 416), Src, Keratin and Fibronectin were obtained from Cell Signaling Biotechnology (Beverly, MA, USA). Antibodies against Twist1 and β-actin, and Twist1 small interfering RNA (Twist1-siRNA) were purchased from Santa Cruz Company. Goat anti-rabbit IgG, goat anti-mouse IgG and protein marker were supplied by Bio-Rad (Hercules, CA, USA). Antibody against N-cadherin was purchased from BD Biosciences (San Jose, CA, USA). Other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). Apigenin was obtained from Shanghai R&D Center for Standardization of Chinese Medicines (Shanghai, China, purity >99% as determined by HPLC). The stock solution of 100 mM apigenin was prepared in dimethyl sulfoxide (DMSO).
Cell culture
Human melanoma A375 and G361 cell lines, and murine melanoma B16F10 cells were purchased from the American Type Culture Collection (ATCC, USA) and maintained in high glucose Dulbecco’s modified Eagle’s medium (DMEM, GIBCO, USA) supplemented with 10% fetal bovine serum (FBS, GIBCO, USA) and 1% penicillin/streptomycin (P/S, GIBCO, USA) in a humidified 5% CO2 atmosphere at 37 °C.
Wound healing assay
Wounds were created by scratching the confluent cell monolayer using a plastic pipette tip, and any loose cellular debris or detached cells were removed by PBS wash. The cells were then refed with full DMEM medium containing DMSO or apigenin with mitomycin C (Sigma-Aldrich) to block mitosis. The gaps of the wounds were observed with phase contrast microscopy and digitally photographed under 100× magnifications. Each experiment was performed in triplicate.
Transwell migration assay
Transwell migration assay was performed using 6.5 mm diameter transwell cell culture chamber units (Costar, Cambridge, MA) with 8-μm pore size polycarbonate membranes. Briefly, cells (5 × 104) suspended in DMEM-0.1% BSA with apigenin or DMSO were plated in the upper chamber wells, while serum-starved NIH-3T3 culture medium was added to the lower chamber wells as chemoattractants. After incubation for 16 h at 37 °C, cells in upper wells were completely removed from the upper surface of the filters with cotton swabs. Cells that had migrated to the lower surface of the filters were fixed with methanol and stained with crystal violet. The migratory phenotypes were determined by counting the cells that migrated to the lower side of the filters in different fields under a microscope at 200×. Assays were repeated in triplicate.
Transwell invasion assay
Cell invasion was determined by using BD BioCoat™ Matrigel™ invasion chamber (BD Biosciences) according to the manufacturer’s instruction. In brief, 0.75 ml of serum-starved NIH-3T3 culture medium was added to the lower chamber wells as chemoattractants, and aliquots of 0.5 ml of cell suspensions consisting of 5 × 105 cells/ml in DMEM-0.1% BSA containing apigenin or DMSO were seeded on upper wells and allowed to invade for 16 h. The non-invading cells were removed by scrubbing with cotton swab and the cells on the lower surface of the membrane were stained with crystal violet. The invasive capacity was quantified by counting the cells that migrated to the lower side of the filters in different fields under a microscope at 200×. Assays were repeated in triplicate and data were expressed as the percentage of invasion in control.
Western blotting
Western blot analysis was performed as described previously60. Briefly, cells were harvested and lysed with the RIPA buffer (50 mM Tris-HCl, 1% NP-40, 0.35% sodium-deoxycholate, 150 mM NaCl, 1 mM EDTA, pH 7.4, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaF, 1 mM Na3VO4 and 10 μg/ml each of aprotinin, leupetin and pepstatin A). Equal amount of protein samples were separated by sodium dodecyl sulfate-poly-acrylamide gel electrophoresis (SDS-PAGE) and then electro-transferred onto nitrocellulose membranes (Amersham Biosciences, USA). After blocked with 5% milk in the TBST buffer for 1 h, membranes were subsequently probed with appropriate primary antibodies overnight at 4 °C. The membranes were then incubated with HRP-conjugated secondary antibodies. Immunoreactive bands were visualized using the ECL detection kit (Invitrogen, USA).
Semi-quantitative and real-time quantitative polymerase chain reaction analyses
Total RNA was extracted with Trizol reagent (Invitrogen, USA), and reverse-transcribed with oligo-dT using the M-MLV reverse transcriptase (Promega, USA) according to the manufacturer’s protocol. For semi-quantitative polymerase chain reaction (PCR), the resultant cDNA was subjected to 25–30 cycles of PCR amplification (denaturing at 95 °C for 30 s, annealing at 55–60 °C for 30 s, extension at 72 °C for 60 s). The PCR products were separated by electrophoresis on a 2% agarose gel and visualized with ethidium bromide staining. Quantitative real-time PCR was performed using SYBR green reaction mixture in the ABI 7500 fast real-time PCR system (Applied Biosystems, USA). The PCR conditions were one cycle at 55 °C for 2 min and at 95 °C for 10 min, followed by 40 cycles of amplification at 95 °C for 15 s and at 60 °C for 1 min. The fluorescent signals were detected using the ABI Prism 7500HT sequence detection system (Applied Biosystems, USA). The gene expression data were normalized to the endogenous control β-actin. The relative expression levels of genes were measured according to the formula 2−ΔΔCt, where ΔCt is the difference in threshold cycle values between the targets and β-actin, and ΔΔCt is the difference between the treatment and vehicle control groups. All samples were analyzed in triplicate.
Gelatin zymography
Cells were treated with apigenin or vehicle for 24 h and then cultured in serum-free medium for 24 h. Twelve hours later, the serum-free supernatants were harvested and concentrated using Centricon YM-10 concentrator (Millipore, USA). After the protein concentrations were determined, the samples were separated by SDS-PAGE in 10% polyacrylamide gel containing 0.1% gelatin under non-reducing conditions. Following electrophoresis, the gels were washed in renaturing buffer (50 mM Tris-HCl, pH 7.5, and 2.5% Triton X-100), and incubated overnight in developing buffer (50 mM Tris-HCl, pH 7.5, 5 mM CaCl2, 200 mM NaCl, 0.2% Brij-35) to allow the gelatinases to digest the gelatin structure. The gels were stained with 0.5% Coomassie Brillant Blue R-250 in 30% methanol and 10% acetic acid. The gelatinase activity was visible as clear bands on blue background.
Immunofluorescence staining for STAT3 localization
Cells were seeded on glass coverslips in 6-well plate overnight, and treated with apigenin or vehicle for 24 h. Cells were then fixed with 4% paraformaldehyde and permeablized with 0.5% Triton X-100 in PBS. After blocked with 5% bovine serum albumin (BSA) in PBS, the cells were incubated with an anti-STAT3 antibody diluted in PBS/2.5% BSA (1:500) overnight. Subsequently, the cells were washed with PBS followed by incubation with Cy2-conjugated anti-rabbit secondary antibody (Invitrogen, USA) diluted in 2.5% BSA/PBS (1:400) for 1 h. Cells were washed in PBS and counterstained with DAPI (Sigma-Aldrich, USA). Images of the cell signal were captured by a fluorescence microscope.
Plasmids
We generated Twist1 promoter reporter construct Twist1-Luc by cloning human Twist1 promoter −451 to +1 fragment into pGL3-basic luciferase vector at KpnI/XhoI sites. The full length Twist1 coding domain sequence was PCR amplified from pWZL Blast Twist1 ER construct (Addgene, USA) and inserted into pcDNA3.1 vector at BamHI/ECORI sites, yielding pcDNA3.1-Twist1. The STAT3 reporter construct 4xM67 pTATA TK-Luc and the constitutive activated STAT3 expression construct STAT3-C Flag pRc/CMV were obtained from Addgene.
Transfection
Transfection of plasmids and siRNAs into melanoma cells was conducted by using Lipofectamine 2000 (Invitrogen, USA). Cells were transfected with indicated siRNA or plasmids for 24 h or 48 h before functional assays were carried out.
Luciferase reporter assay
Cells were seeded in 24-well plates and co-transfected with the reporter construct (Twist1-Luc or 4xM67 pTATA TK-Luc) plus Renilla luciferase expression vector PRL-CMV (Promega, USA) by using lipofectamine 2000 reagent (Invitrogen, USA). At 12 h post-transfection, cells were treated with apigenin or vehicle (DMSO) for another 12 h. Then cells were harvested for luciferase reporter assay using the dual-luciferase reporter assay system (Promega, USA). Firefly luciferase values were normalized to Renilla luciferase values. All assays were performed in triplicate.
Experimental lung metastasis
C57BL/6 mice were obtained from The Laboratory Animal Services Centre, The Chinese University of Hong Kong. Lung metastasis of B16F10 mouse melanoma was done as described elsewhere61. Briefly, B16F10 melanoma cells were washed in PBS containing 5 mM EDTA, the cell number and viability were examined using trypan blue. Sample containing only single-cell suspension of >90% viability were suspended at 3 × 105 cells in 50 μl PBS and were injected into the tail vein of the C57BL/6 mice. These mice were then randomly divided into 2 groups and received intragastric administration of either 0.5% CMC-Na solution as the vehicle control or apigenin (150 mg/kg/day)26 for consecutive 24 days. After the treatment, mice were scarified and lungs were dissected. Metastasized colonies were counted using a dissecting microscope.
Statistical analysis
Data were summarized as mean ± SD. The significant difference between two groups was analyzed using the Student’s t-test.
Additional Information
How to cite this article: Cao, H.-H. et al. Inhibition of the STAT3 signaling pathway contributes to apigenin-mediated anti-metastatic effect in melanoma. Sci. Rep. 6, 21731; doi: 10.1038/srep21731 (2016).
Supplementary Material
Acknowledgments
This work is partially supported by grants HKBU 262512 from the Research Grants Council of Hong Kong, JCYJ20120829154222473 and JCYJ20140807091945050 from the Science, Technology and Innovation Commission of Shenzhen, HMRF 11122521 from Food and Health Bureau of Hong Kong, and FRG2/14-15/056, FRG2/15-16/020 from the Hong Kong Baptist University.
Footnotes
Author Contributions Z.Y., G.C. and H.M. supervised the entire project. Z.Y., J.C., H.C., H.K. and A.T. participated in study design. J.C., H.C. and H.K. performed the majority the experiments, analyzed data and drafted the manuscript. H.Y. contributed to the reagents. T.S., C.C., X.F., H.G. and T.L. supported several experiments. All authors read and approved the final manuscript.
References
- Orgaz J. L. & Sanz-Moreno V. Emerging molecular targets in melanoma invasion and metastasis. Pigment Cell Melanoma Res 26, 39–57, (2013). [DOI] [PubMed] [Google Scholar]
- DeSantis C. E. et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64, 252–271, (2014). [DOI] [PubMed] [Google Scholar]
- Hauschild A. et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380, 358–365, (2012). [DOI] [PubMed] [Google Scholar]
- Callahan M. K., Postow M. A. & Wolchok J. D. Immunomodulatory therapy for melanoma: ipilimumab and beyond. Clin Dermatol 31, 191–199, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poust J. C., Woolery J. E. & Green M. R. Management of toxicities associated with high-dose interleukin-2 and biochemotherapy. Anticancer Drugs 24, 1–13, (2013). [DOI] [PubMed] [Google Scholar]
- Sullivan R. J. & Flaherty K. T. Resistance to BRAF-targeted therapy in melanoma. Eur J Cancer 49, 1297–1304, (2013). [DOI] [PubMed] [Google Scholar]
- Kortylewski M., Jove R. & Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev 24, 315–327, (2005). [DOI] [PubMed] [Google Scholar]
- Xie T. X. et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res 66, 3188–3196, (2006). [DOI] [PubMed] [Google Scholar]
- Niu G. et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21, 2000–2008, (2002). [DOI] [PubMed] [Google Scholar]
- Niu G. et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 21, 7001–7010, (2002). [DOI] [PubMed] [Google Scholar]
- Liu L. et al. 6-Bromoindirubin-3′-oxime inhibits JAK/STAT3 signaling and induces apoptosis of human melanoma cells. Cancer Res 71, 3972–3979, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. & Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer 4, 97–105, (2004). [DOI] [PubMed] [Google Scholar]
- Xie T. X. et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 23, 3550–3560, (2004). [DOI] [PubMed] [Google Scholar]
- Kamran M. Z. & Gude R. P. Pentoxifylline inhibits melanoma tumor growth and angiogenesis by targeting STAT3 signaling pathway. Biomed Pharmacother 67, 399–405, (2013). [DOI] [PubMed] [Google Scholar]
- Martinez M. E., Marshall J. R. & Giovannucci E. Diet and cancer prevention: the roles of observation and experimentation. Nat Rev Cancer 8, 694–703, (2008). [DOI] [PubMed] [Google Scholar]
- Tong L. X. & Young L. C. Nutrition: the future of melanoma prevention? J Am Acad Dermatol 71, 151–160, (2014). [DOI] [PubMed] [Google Scholar]
- Shukla S. & Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res 27, 962–978, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birt D. F., Mitchell D., Gold B., Pour P. & Pinch H. C. Inhibition of ultraviolet light induced skin carcinogenesis in SKH-1 mice by apigenin, a plant flavonoid. Anticancer Res 17, 85–91 (1997). [PubMed] [Google Scholar]
- Caltagirone S. et al. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer 87, 595–600, (2000). [DOI] [PubMed] [Google Scholar]
- Piantelli M. et al. Flavonoids inhibit melanoma lung metastasis by impairing tumor cells endothelium interactions. J Cell Physiol 207, 23–29, (2006). [DOI] [PubMed] [Google Scholar]
- Arango D. et al. Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Proc Natl Acad Sci USA 110, E2153–2162, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H. S. et al. Induction of caspase-dependent apoptosis by apigenin by inhibiting STAT3 signaling in HER2-overexpressing MDA-MB-453 breast cancer cells. Anticancer Res 34, 2869–2882, (2014). [PubMed] [Google Scholar]
- Ruela-de-Sousa R. R. et al. Cytotoxicity of apigenin on leukemia cell lines: implications for prevention and therapy. Cell Death Dis 1, e19, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. et al. A novel 7-bromoindirubin with potent anticancer activity suppresses survival of human melanoma cells associated with inhibition of STAT3 and Akt signaling. Cancer Biol Ther 13, 1255–1261, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon L. G., Kuttan R. & Kuttan G. Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer Lett 95, 221–225 (1995). [DOI] [PubMed] [Google Scholar]
- He J. et al. Oral administration of apigenin inhibits metastasis through AKT/P70S6K1/MMP-9 pathway in orthotopic ovarian tumor model. Int J Mol Sci 13, 7271–7282, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng C. J. & Yen G. C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev 31, 323–351, (2012). [DOI] [PubMed] [Google Scholar]
- Clere N., Faure S., Martinez M. C. & Andriantsitohaina R. Anticancer properties of flavonoids: roles in various stages of carcinogenesis. Cardiovasc Hematol Agents Med Chem 9, 62–77, (2011). [DOI] [PubMed] [Google Scholar]
- Yamazaki H. et al. Overexpression of the miR-34 family suppresses invasive growth of malignant melanoma with the wild-type p53 gene. Exp Ther Med 3, 793–796, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Zaid Siddiquee K. & Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res 18, 254–267, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastid J., Ciancia C., Puisieux A. & Ansieau S. Role of twist proteins in cancer progression. Atlas Genet Cytogenet Oncol Haematol 14, 898–907 (2010). [Google Scholar]
- Lo H. W. et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res 67, 9066–9076, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. & Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 118, 277–279, (2004). [DOI] [PubMed] [Google Scholar]
- Kalluri R. & Weinberg R. A. The basics of epithelial-mesenchymal transition. J Clin Invest 119, 1420–1428, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky D. C. Epithelial-mesenchymal transition. J Cell Sci 118, 4325–4326, (2005). [DOI] [PubMed] [Google Scholar]
- Cao H. H. et al. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem Pharmacol 87, 424–434, (2014). [DOI] [PubMed] [Google Scholar]
- Fu X. Q. et al. Inhibition of STAT3 signalling contributes to the antimelanoma action of atractylenolide II. Exp Dermatol 23, 855–857, (2014). [DOI] [PubMed] [Google Scholar]
- Tse A. K. et al. The herbal compound cryptotanshinone restores sensitivity in cancer cells that are resistant to the tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem 288, 29923–29933, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Mishra S. K., Noel S., Sharma S. & Rath S. K. Acute exposure of apigenin induces hepatotoxicity in Swiss mice. PLoS One 7, e31964, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Afaq F. & Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun 287, 914–920, (2001). [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu D., Huang Y., Gao Y. & Qian S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int J Pharm 436, 311–317, (2012). [DOI] [PubMed] [Google Scholar]
- Gradolatto A. et al. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab Dispos 33, 49–54, (2005). [DOI] [PubMed] [Google Scholar]
- Shukla S. et al. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis 35, 452–460, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Huang Y., Liu D., Gao Y. & Qian S. Preparation of apigenin nanocrystals using supercritical antisolvent process for dissolution and bioavailability enhancement. Eur J Pharm Sci 48, 740–747, (2013). [DOI] [PubMed] [Google Scholar]
- Arsic I. et al. Preparation of novel apigenin-enriched, liposomal and non-liposomal, antiinflammatory topical formulations as substitutes for corticosteroid therapy. Phytother Res 25, 228–233, (2011). [DOI] [PubMed] [Google Scholar]
- Ding S. M. et al. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int J Nanomedicine 9, 2327–2333, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S., Pandey M. K., Yadav V. R. & Aggarwal B. B. Gambogic acid inhibits STAT3 phosphorylation through activation of protein tyrosine phosphatase SHP-1: potential role in proliferation and apoptosis. Cancer Prev Res (Phila) 4, 1084–1094, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rotte A., Martinka M. & Li G. MMP2 expression is a prognostic marker for primary melanoma patients. Cell Oncol (Dordr) 35, 207–216, (2012). [DOI] [PubMed] [Google Scholar]
- Nikkola J. et al. High serum levels of matrix metalloproteinase-9 and matrix metalloproteinase-1 are associated with rapid progression in patients with metastatic melanoma. Clin Cancer Res 11, 5158–5166, (2005). [DOI] [PubMed] [Google Scholar]
- Zetter B. R. Angiogenesis and tumor metastasis. Annu Rev Med 49, 407–424, (1998). [DOI] [PubMed] [Google Scholar]
- Ascierto P. A. et al. Prognostic value of serum VEGF in melanoma patients: a pilot study. Anticancer Res 24, 4255–4258 (2004). [PubMed] [Google Scholar]
- Wei L. H. et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 22, 1517–1527, (2003). [DOI] [PubMed] [Google Scholar]
- Yu H., Pardoll D. & Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9, 798–809, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Kortylewski M. & Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7, 41–51, (2007). [DOI] [PubMed] [Google Scholar]
- Weiss M. B. et al. TWIST1 is an ERK1/2 effector that promotes invasion and regulates MMP-1 expression in human melanoma cells. Cancer Res 72, 6382–6392, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Roth J. M., Brooks P., Ibrahim S. & Karpatkin S. Twist is required for thrombin-induced tumor angiogenesis and growth. Cancer Res 68, 4296–4302, (2008). [DOI] [PubMed] [Google Scholar]
- Singh S., Mak I. W., Handa D. & Ghert M. The Role of TWIST in Angiogenesis and Cell Migration in Giant Cell Tumor of Bone. Advances in Biology 2014 (2014). [Google Scholar]
- Yu L. et al. TWIST expression in hypopharyngeal cancer and the mechanism of TWIST-induced promotion of metastasis. Oncol Rep 27, 416–422, (2012). [DOI] [PubMed] [Google Scholar]
- Puisieux A., Valsesia-Wittmann S. & Ansieau S. A twist for survival and cancer progression. Br J Cancer 94, 13–17, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y. et al. Effects of sesquiterpenes isolated from largehead atractylodes rhizome on growth, migration, and differentiation of B16 melanoma cells. Integr Cancer Ther 10, 92–100, (2011). [DOI] [PubMed] [Google Scholar]
- Xie Q. et al. Recombinant adenovirus snake venom cystatin inhibits the growth, invasion, and metastasis of B16F10 cells in vitro and in vivo. Melanoma Res 23, 444–451, (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.