Abstract
Honey bees (Apis mellifera) are important pollinators and their health is threatened worldwide by persistent exposure to a wide range of factors including pesticides, poor nutrition, and pathogens. Nosema ceranae is a ubiquitous microsporidian associated with high colony mortality. We used lab micro-colonies of honey bees and video analyses to track the effects of N. ceranae infection and exposure on a range of individual and social behaviours in young adult bees. We provide detailed data showing that N. ceranae infection significantly accelerated the age polyethism of young bees, causing them to exhibit behaviours typical of older bees. Bees with high N. ceranae spore counts had significantly increased walking rates and decreased attraction to queen mandibular pheromone. Infected bees also exhibited higher rates of trophallaxis (food exchange), potentially reflecting parasite manipulation to increase colony infection. However, reduction in queen contacts could help bees limit the spread of infection. Such accelerated age polyethism may provide a form of behavioural immunity, particularly if it is elicited by a wide variety of pathogens.
The Western honey bee, Apis mellifera, is a key global pollinator of cultivated and native plants, but the managed bee population has experienced concerning declines1. A variety of pathogens and parasites, xenobiotics and other environmental stressors leading to nutritional deficiencies, and their interactions contribute to high annual colony losses1. The microsporidian parasite Nosema ceranae has drawn particular attention, in part because it is so widespread2,3,4 and is often associated with colony losses5,6. Although its exact role in recent colony losses has been debated3,6, N. ceranae is now one of the most globally prevalent honey bee pathogens. Nosema sp. primarily contributes to poor colony health, rendering colonies more susceptible to other stressors7. In combination with other factors, such as pesticides, Nosema infection can reduce colony survival8,9.
Nosema ceranae is a spore forming fungus that lives as an obligate intracellular pathogen and exists outside the host cell as metabolically inactive spores10. It infects and reproduces inside epithelial cells of the midgut, but has also been showed to infect other tissues11,12. Spores are produced and released into the environment when an infected bee defecates. These spores are a source of infection for other bees, following a typical faecal-oral transmission pathway13. However N. ceranae may also be spread orally, through trophallaxis14, be transmitted through cleaning and grooming4, and sexually15.
Nosema ceranae infection can reduce bee longevity and leads to gut tissue degeneration6 and nutritional stress16. However, Nosema infection can also alter age polyethism, the natural progression of tasks that honey bees perform as they grow older. During the first 12–15 days of adult life, workers typically carry out nest activities such as cleaning, brood and queen tending, comb building and food handling. Ventilation and guard duty behaviours peak at around 18 days of age and the final task, foraging, typically peaks at day 2317. Bees infected with Nosema tend to forage precociously13,18,19 and a preliminary study suggested that other activities typically performed by very young bees are similarly time-shifted forward20. Recently, Natsopoulou et al.21 demonstrated that starting at 4 days of age, N. ceranae accelerated the temporal polyethism schedule, that infected bees displayed increased hyperactivity and foraging-related tasks but without reducing host behavioural repertoire. However, it is unclear how Nosema infection alters the normal progression of age polyethism in young bees.
An intriguing question is whether behavioural shifts caused by Nosema sp. infection benefit the parasite or the host. The parasite may manipulate host behaviour. Infected bees gravitate towards the higher temperatures of the inner hive, an area of high bee density and could thereby enhance parasite spread22. However, the host has defences. In addition to their innate immunity, honey bees exhibit behavioural immunity23 through actions such as hygienic removal of diseased bees24 and allogrooming25. Other behaviours that limit the spread of disease, such as precocious foraging (a form of self-removal26) could also reduce disease spread inside a colony. Finally, behaviours that limit exposure of the queen should protect the colony. The queen is an interaction centre and focal point of colony life. She is primarily attended by young adults that are particularly drawn to her queen mandibular pheromone (QMP)27. Do bees infected with Nosema attempt to enhance colony behavioural immunity or reduce disease spread?
We therefore tested the hypothesis that Nosema infection will accelerate the age polyethism of young bees and decrease contact between infected workers and the queen. To study age polyethism, we used a group of bees of the same age (a single cohort colony) that we could track through the first two weeks of life. Because we wished to repeatedly track the behaviours of all bees inside the colony, we video recorded micro-colonies of caged honey bees maintained in the lab, each micro-colony provided with a QMP lure to simulate queen presence (Fig. 1). Such pheromone presentation is highly effective, can maintain colony queen-right behaviours, and is used by beekeepers to create small nuclei28,29. We measured the most commonly exhibited behaviours, including attraction to QMP, as a measure of worker interaction with the queen.
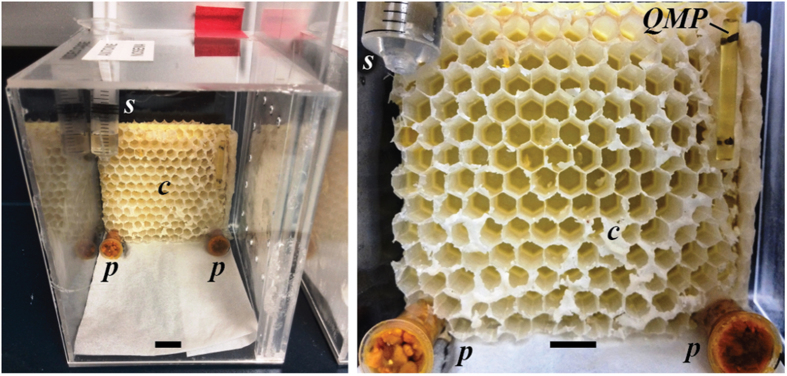
Figure 1. Detail of a video observation cage showing the comb = c, pollen = p, sucrose solution = s, and Queen Mandibular Pheromone = QMP.
The horizontal scale bars indicate 1 cm. The video observation window has been removed in these photos for a clearer image. Combs originally contained honey stores and some brood, but were carefully cleaned and sterilized before use (see Methods).
Results
We used bees from eight different colonies to conduct eight paired-treatment trials consisting of individually labeled 1-day old adult bees monitored for two weeks (364 bees in total). Bees were fed sucrose solution with the equivalent dose of 30,000 N. ceranae spores/bee (Nosema group) or sterile sucrose solution (control group). We measured day of death, midgut spore count at the end of the trial, and behaviours (Table 1) throughout the trial.
Table 1. List of observed and recorded behaviours and their definitions.
| Behaviour | Description |
|---|---|
| Trophallaxis | Nest mate exchange of food. The receiver extends its proboscis into the donor’s mouthparts; the donor opens its mouthparts, and regurgitates food. |
| Walking | A bee walking around on the comb |
| QMP attraction | A bee interacting with the QMP lure by contact with antennae or tongue |
| Antennating | Antennal contact, head to head between two bees, with no food exchange |
| Allo-grooming | A bee running a nest mate’s body parts through its mandibles |
| Auto-grooming | A bee running its own body parts through its mandibles |
| Grooming dance | A bee stands and vibrates her whole body dorso-laterally. Sometimes body vibration is mixed with brief bouts of self-grooming |
| Shaking signal | Nest mate vigorously and rhythmically shaking her body dorso-ventrally while gripping another bee or the comb33 |
| Fanning | Flapping wings while standing in hive |
| Cell inspecting | Momentary insertion of the anterior portion of the head into an empty cell |
| Visiting sugar | A bee imbibing the sugar solution |
| Visiting pollen | A bee consuming pollen |
| Standing | A bee standing stationary on the comb |
These were the most 13 commonly observed behaviours. References for these definitions are provided, as appropriate.
No difference in survival between Nosema and control groups
Although there was slightly higher mortality in the Nosema exposed group (11% died by the end of 15 days as compared to 6% for the control group), mortality was not significantly different between these groups (treatment factor: L-R χ21 = 0.26, P = 0.61). There was no effect of spore count upon adult death (L-R χ21 = 0.46, P = 0.50), no significant interaction of treatment*spore count (L-R χ21 = 0.26, P = 0.61), and no colony effect (L-R χ27 = 12.48, P = 0.09).
Nosema treatment altered bee behaviours
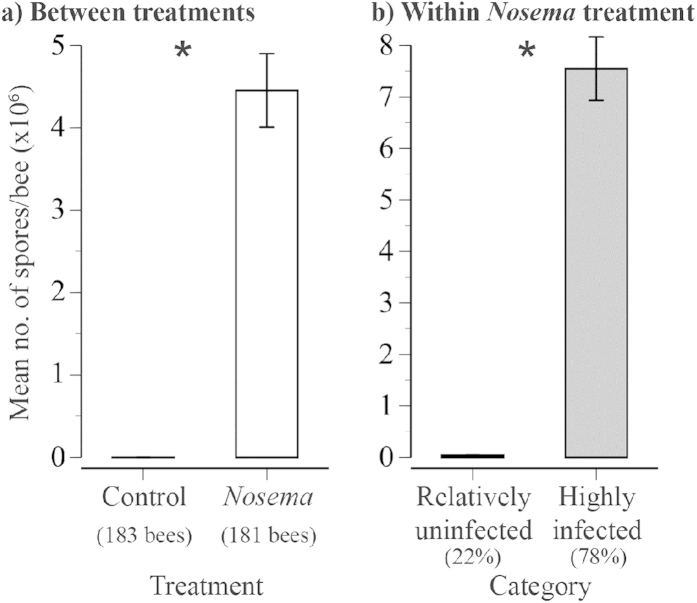
On average, Nosema-exposed bees were 21,935-fold more infected (Kruskal-Wallis test, χ12 = 256.2, P < 0.0001) than control bees. Only 1.5% of control bees had spores, and these were minimally infected (Fig. 2).
Figure 2.
Effect of Nosema treatment upon the mean number of midgut spores upon adult death (a) between treatments (sample size shown) and (b) within the Nosema treatment (% within each category shown). Asterisks indicate significant differences.
Multiple behaviours changed over time in both treatment groups. Detailed statistics are presented in Table 2. There was a significant effect of day for trophallaxis, antennating, allo-grooming, auto-grooming, grooming dancing, shaking signaling, cell inspecting, pollen visiting, and standing (P ≤ 0.004). In general, these behaviours tended to decrease over time, with the exception of walking, which increased over time.
Table 2. Statistical results of control versus Nosema-treated bees for all the recorded behaviours.
| Behaviour | Model Fit (R2) | Treatment |
Day |
Treatment*Day |
Colony Effect (%) | |||
|---|---|---|---|---|---|---|---|---|
| F 1, 333 | P-value | F 1, 4102 | P-value | F 1, 4102 | P-value | |||
| Trophallaxis | 0.161 | 7.701 | 0.006 | 190.896 | <0.001 (−) | 4.362 | 0.037 | 8.2 |
| Walking | 0.491 | 1.219 | 0.270 | 2041.778 | <0.001 (+) | 29.001 | <0.001 | 17.7 |
| QMP attraction | 0.262 | 1.039 | 0.309 | 3.681 | 0.055 | 5.902 | 0.015 | 4.9 |
| Antennating | 0.128 | 0.780 | 0.378 | 28.872 | <0.001 (−) | 0.114 | 0.736 | 7.0 |
| Allo-grooming | 0.079 | 2.413 | 0.121 | 14.619 | <0.001 (−) | 0.001 | 0.978 | 3.7 |
| Auto-grooming | 0.305 | <0.001 | 0.987 | 189.574 | <0.001 (−) | 2.607 | 0.106 | 16.6 |
| Grooming dance | 0.042 | 0.681 | 0.410 | 11.728 | <0.001 (−) | 2.622 | 0.105 | 0.9 |
| Shaking signal | 0.167 | 0.005 | 0.941 | 8.068 | 0.004 (−) | 0.430 | 0.512 | 1.3 |
| Fanning | 0.078 | 0.170 | 0.681 | 1.815 | 0.178 | 0.692 | 0.405 | 1.2 |
| Cell inspecting | 0.268 | 2.714 | 0.100 | 48.800 | <0.001 (−) | 1.413 | 0.235 | 17.3 |
| Visiting sugar | 0.052 | 0.649 | 0.421 | 0.698 | 0.404 | 1.654 | 0.199 | 1.7 |
| Visiting pollen | 0.115 | 0.893 | 0.345 | 189.385 | <0.001 (−) | 0.193 | 0.660 | 5.1 |
| Standing | 0.268 | 0.005 | 0.945 | 51.013 | <0.001 (−) | 1.314 | 0.252 | 5.5 |
Plus/minus signs indicate the direction of the changes from day 2 through to 14.
Bees in the Nosema treatment group exhibited a significantly higher level of food exchange (trophallaxis, P = 0.006) than bees in the control group (Fig. 3a). There was no significant interaction of treatment*day (P = 0.037, NSSB). Over the entire trial, Nosema-treated bees exhibited 1.4-fold higher levels of trophallaxis than control bees. Treatment was not significant for any other scored behaviour (F1, 333 ≤ 2.714, P ≥ 0.100, Table 2). Nosema treatment increased average trophallaxis per bee (ANOVAnon-RM: F1,332 = 7.23, P = 0.008).
Figure 3. Comparisons between the behaviours of bees in the control and Nosema treatments.
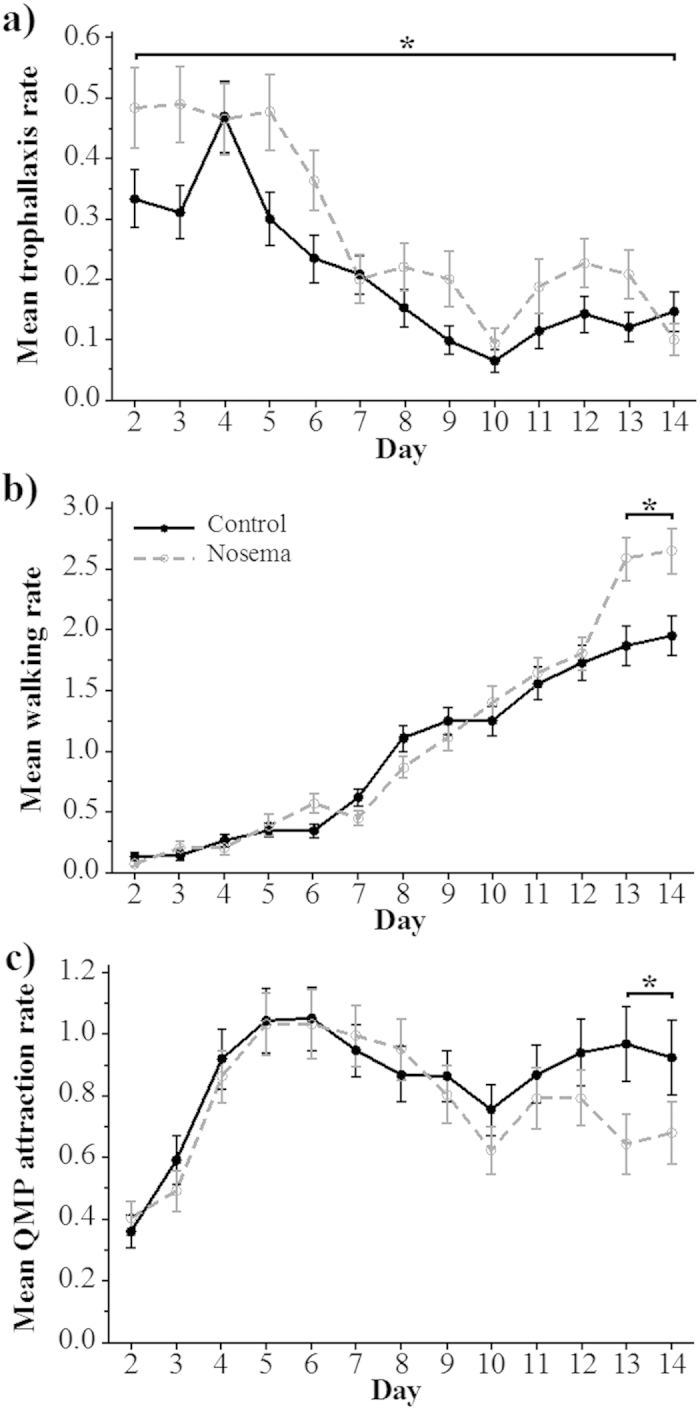
Mean daily rates (standard error bars shown) for (a) trophallaxis, (b) walking and (c) QMP attraction behaviours in control and Nosema-treated groups of honey bees. Significant contrast test differences are indicated with an asterisk.
There was a significant treatment*day interaction for walking (P < 0.001) and QMP attraction (P = 0.015). During the last two days of the trial (Fig. 3b), walking significantly increased by 1.4-fold (contrast test: F1, 1790 = 43.45, P < 0.001). Nosema treatment increased average walking per bee (ANOVAnon-RM: F1,332 = 14.96, P = 0.0001).
QMP attraction significantly decreased by 0.7-fold (contrast test: F1, 618 = 6.41, P = 0.011, Fig. 3c) in the Nosema-treated bees relative to the control bees. Nosema treatment increased average queen attraction per bee (ANOVAnon-RM: F1,332 = 5.94, P = 0.015).
Relatively uninfected bees within the Nosema treatment behaved differently
Bees were group-fed an average dose of 30,000 spores at the start of the trial. Thus, bees with 30,000 spores or less were either uninfected and had retained the fed spores in their midguts or were very weakly infected. We therefore call these bees “relatively uninfected”. Within the Nosema treatment group, 22% of bees were relatively uninfected. Bees with more than 30,000 spores at the end of the trial were considered highly infected. We collected video data on 181 bees in the Nosema treatment. Of these bees, 141 were highly infected (6,282,252 ± 6,684,402 spores/bee) and 40 were relatively uninfected (7,625 ± 10,252 spores/bee) upon adult death.
There was a significant effect of day upon trophallaxis, walking, auto-grooming, grooming dancing, shaking, signaling, cell inspecting, pollen visiting, and standing (P ≤ 0.021). Detailed statistical results are in Table 3. For these behaviours, there was a tendency for the behavioural rates to decrease between days 2 to 14, except for walking, which increased.
Table 3. Statistical results of comparisons between bees with ≤30,000 Nosema spores and those with >30,000 spores for all the recorded behaviours.
| Behaviour | Model Fit (R2) | Infection Level |
Day |
Infection Level*Day |
Colony Effect (%) | |||
|---|---|---|---|---|---|---|---|---|
| F 1, 176 | P-value | F 1, 2170 | P-value | F 1, 2170 | P-value | |||
| Trophallaxis | 0.172 | 1.697 | 0.194 | 39.339 | <0.001 (−) | 10.577 | 0.001 | 7.3 |
| Walking | 0.479 | 2.335 | 0.128 | 699.919 | <0.001 (+) | 26.783 | <0.001 | 13.8 |
| QMP attraction | 0.263 | 0.477 | 0.491 | 2.320 | 0.128 | 5.984 | 0.014 | 6.2 |
| Antennating | 0.159 | 0.117 | 0.733 | 11.331 | <0.001 (−) | 0.710 | 0.400 | 9.1 |
| Allo-grooming | 0.097 | 1.493 | 0.223 | 2.145 | 0.143 | 0.117 | 0.732 | 8.5 |
| Auto-grooming | 0.306 | 0.880 | 0.349 | 101.576 | <0.001 (−) | 1.000 | 0.317 | 17.9 |
| Grooming dance | 0.040 | 0.081 | 0.776 | 5.305 | 0.021 (−) | 0.645 | 0.422 | 1.3 |
| Shaking signal | 0.078 | 0.059 | 0.808 | 7.340 | 0.007 (−) | 0.595 | 0.440 | 1.3 |
| Fanning | 0.075 | 0.501 | 0.480 | 0.228 | 0.633 | 2.704 | 0.100 | 1.2 |
| Cell inspecting | 0.306 | 0.000 | 0.990 | 15.296 | <0.001 (−) | 2.899 | 0.089 | 20.0 |
| Visiting sugar | 0.046 | 2.616 | 0.108 | 0.064 | 0.800 | 0.282 | 0.595 | 2.0 |
| Visiting pollen | 0.112 | 0.657 | 0.419 | 39.341 | <0.001 (−) | 5.354 | 0.021 | 6.4 |
| Standing | 0.263 | 0.310 | 0.578 | 29.556 | <0.001 (−) | 8.289 | 0.004 | 5.4 |
Plus/minus signs indicate the direction of the changes from day 2 through to 14.
Relatively uninfected bees exchanged food less often with nest mates (trophallaxis, P = 0.001) as compared to infected bees. The relatively uninfected bees also walked less (P < 0.001) and had higher QMP attraction (P = 0.014). Upon closer inspection, the relatively uninfected bees exhibited decreased trophallaxis during the first week of the trial (contrast test: days 2–8, F1, 350 = 8.220, P = 0.004, Fig. 4a). Towards the end of the trial, these relatively uninfected bees also walked less (contrast test: days 8–14, F1, 125 = 15.000, P < 0.001, Fig. 4b) and had higher QMP attraction (contrast test: days 13–14, F1, 749 = 6.720, P = 0.009, Fig. 4c) than highly infected bees.
Figure 4. Comparisons between uninfected bees and infected bees in the Nosema treatment.
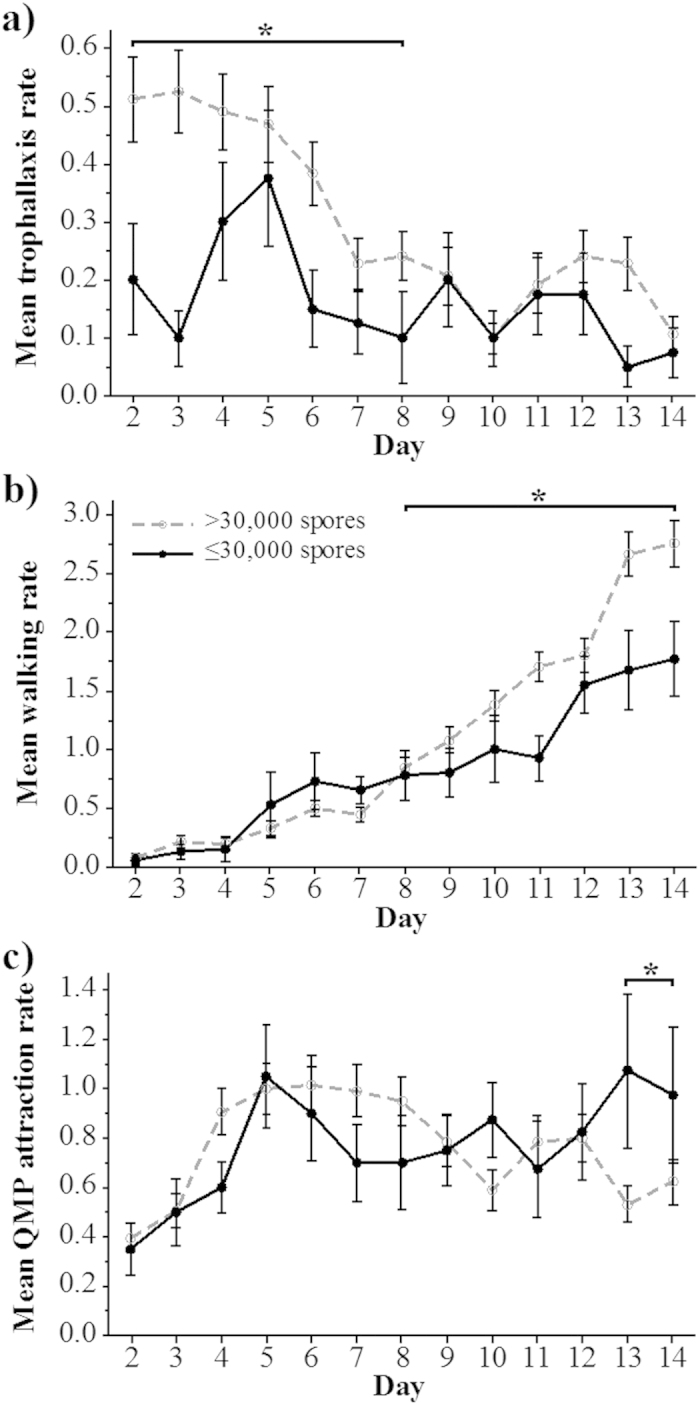
Mean daily rates (standard error bars shown) for (a) trophallaxis, (b) walking and (c) QMP attraction behaviours in relatively uninfected bees with ≤30,000 spores and infected bees with >30,000 spores. An asterisk indicates significant contrast test differences.
We found significant interactions (Table 3) between infection level and day for visiting pollen (P = 0.021) and standing (P = 0.004), but there were no consistent overall trends and no significant contrast differences over any contiguous set of days for these behaviours (P > 0.025).
Discussion
Nosema ceranae is a major disease that also accelerates honey bee age polyethism. We provide detailed data showing that young worker behavioural development accelerates as a result of N. ceranae infection. We found evidence for two accelerated behaviours: 12 days after exposure, walking significantly increased and attraction to Queen Mandibular Pheromone (QMP) decreased as compared to control bees. This change in QMP attraction could limit queen exposure to infection, which typically reaches a peak 10–12 days after first exposure30. Bees in the Nosema-treated group also exhibited significantly higher rates of trophallaxis than control bees, perhaps reflecting increased hunger and food begging16,31. Within the Nosema-treated bees, highly infected bees (>30,000 spores/bee) exhibited the same behavioural changes: increased walking and decreased QMP attraction towards the end of the trial and higher overall rates of trophallaxis.
Workers in our micro-colonies exhibited other bee behaviours, including communication signals such as the grooming dance, which elicits grooming from receivers32, and the shaking signal, which helps to reallocate colony labour33. Production of these signals significantly decreased over time (Table 2), but was not affected by Nosema treatment. Similar results were observed in a study by Natsopoulou et al.21, who observed bees beginning at four days of adult age and found that they retained a wide behavioural repertoire under parasitic stress.
McDonnell et al.34 used observation colonies but did not find behavioural differences between Nosema-infected and healthy bees. However, this study observed randomly selected bees within each treatment group for 15 min per trial. In contrast, our results show that longer-term observations (144 min per trial equally spaced over 12 days) are evidently needed to reveal Nosema effects on age polyethism, as also demonstrated by Natsopoulou et al.21.
Because our goal was to conduct detailed, long-term observations of behavioural changes, we used single-cohort micro-colonies so that we could repeatedly measure behaviours over time. While laboratory assays rarely offer a complete representation of behaviours in the natural environment, they allow for manipulations and repeatability seldom matched by field conditions. A primary advantage of our approach is that it allowed us to observe all bees within a micro-colony over an extended period. A disadvantage is that natural colonies are normally much larger and have bees of different ages performing different tasks. However, the single-cohort colony approach has proven valuable for understanding age polyethism in honey bees35, and using micro-colonies facilitated more thorough behavioral observations. Although it is possible to track a large number of bee movements over time in a largely automated way36, this technology, to date, does not automatically measure most of the detailed behaviours that we commonly observed.
Previous studies have shown that micro-colonies can be used to determine the effects of pathogens on honey bee health and these results can correspond to results with full-size colonies37. Our approach was sufficient to show decreases in queen attraction, matching the preliminary results of a study using Nosema-infected bees studied in full colonies38. Moreover, recent research21,39 demonstrated that workers infected with Nosema significantly decreased their attraction towards a living honey bee queen.
In our full experiment, the micro-colonies did not contain brood, and thus we could not monitor nursing behaviours that would be typical of very young bees. However, we conducted a preliminary trial with more realistic conditions: micro-colonies containing brood and adults of different ages (Supplemental Information, SI). We changed our procedures for the full experiment because of issues with this design, mainly poor brood survival, but the data showed the same trends: bees fed Nosema significantly increased trophallaxis and walking (Fig. S1). In this preliminary trial, QMP attraction was not videotaped, although chance observations of such attraction led us to reposition the QMP lure and monitoring attraction in our full experiment.
In a typical honey bee colony, workers have some behavioural flexibility and can perform multiple tasks at any given age17. However, the first 2 to 3 weeks are generally spent tending the queen and the brood and maintaining the nest17. Queen tending then decreases as workers age40. The endocrine factor, juvenile hormone41 increases as workers age13,42 and is a primary regulator of these behavioural changes42. Goblirsch et al.13 showed that young workers infected with Nosema sp. had higher JH titres than uninfected bees and initiated premature foraging. McQuillan et al.42 demonstrated that young workers treated with JH became less attracted to a QMP lure. We observed the same behaviour in our Nosema-infected bees. This decrease in QMP attraction may have resulted from an increased level of JH. Attraction to the QMP lure, a proxy for queen-tending, decreased by 0.7-fold (Nosema vs. control group) and 0.6-fold (within the Nosema group) in infected bees during the last two days of the trial. Similarly, Wang and Moeller38 reported a 0.2-fold decrease in queen attendance in 6–10 day old bees infected with Nosema in observation colonies.
We measured 1.4-fold increases in walking from bees that were 8–14 days old (Figs 3b and 4b). This observation is also consistent with accelerated age polyethism. Johnson40 showed that middle-aged bees (14–18 days old) naturally performed 6-fold more walking than nurse bees (4–8 days old). Increases in walking also correspond to a general increase in activity reported in Nosema-infected bees21,38. Accelerated age polyethism can also be the result of other types of demographic stressors, leading to positive feedbacks that could drive the rapid depopulation of a colony43.
Trophallaxis was significantly higher in Nosema-group bees as compared to control bees throughout the 14-day trial and was also higher in infected as compared to relatively infected bees within the Nosema group during the first 8 days of the trial. Interestingly, this difference was evident almost immediately, beginning at day 2 (equivalent to 24 hours post-exposure after the consumption of the full 30,000 spore dose). What accounts for this rapid effect? Most studies on the effect of N. ceranae on bee physiology or behaviour tend to begin data collection at least one week post-exposure13,22,31,44,45. Although mature N. ceranae spores are not detectable until 4 days post-infection, the proliferating vegetative stage is already quite active30. In cell culture, this vegetative stage begins 16 hours after inoculation and the first spores can be detected after 48 hours46. Thus, the early infection stage could have caused the increase in trophallaxis that we observed.
These changes in behaviour could serve as host defense mechanisms, result from parasite manipulation, or both47. With respect to host defense, diseased individuals that accelerate their age polyethism will more rapidly become foragers38. In consequence, they will spend more time outside the nest and potentially reduce disease transmission within the nest48. For example, immune-suppressed honey bees avoid social contact with healthy nest mates26. Reduced contact with the queen could also help limit the spread of infection, given that the queen is a nexus of colony contacts. Alternatively, Nosema may be manipulating its host to increase transmission49. Infected bees prefer the higher temperatures of the inner hive, benefiting pathogen transmission22. Increased trophallaxis (Figs 3a and 4a) could increase the spread of infection, particularly if spores are orally transmitted14. The actual roles of these behaviours in benefiting or harming the colony are unclear. Future studies testing the efficacy of self-removal for reducing infection spread and the extent of Nosema transmission through trophallaxis would be beneficial. However, our results add to growing evidence that N. ceranae infection prematurely accelerates honey bee age polyethism, thereby perturbing a delicate balance that is important to the correct allocation of colony tasks.
Methods
Video observation cages
The experimental cages were custom made (12 × 8 × 12 cm) of transparent acrylic with a sliding plastic door pierced with holes for ventilation and a hole in the top through which we inserted a 5 ml syringe containing sucrose solution to feed the bees (Fig. 1). Inside, we placed an empty, sterile honey bee comb with its back against the cage so that bees could only access the side facing the video camera. The camera viewing window was removable so that it could be swapped out for a clean window. Each cage was provided with ad-libitum 50% (1.8 M) sterile sucrose solution and two 1.5 ml centrifuge tubes, each with 1 g of a pollen mixture: 90% (w/w) irradiated corbicular pollen (Mann Lake, Hackensack, Minnesota, USA) mixed with tap water50. Each cage was also fitted with a half strip of a queen mandibular pheromone (QMP) lure (Bee Boost, PheroTech Inc., Delta, Canada) to simulate the presence of a queen. All cages with bees were kept inside a dark 33 °C and 70% humidity incubator. To ensure initially sterile conditions, we soaked all combs, cages, feeding syringes, and pollen tubes in a 10% bleach solution for at least 30 min, followed by soaking in and multiple rinses with deionized water. All items were then sterilized for 1 hr with ultraviolet light inside an AirClean 600 sterile laminar flow hood (www.BioExpress.com) and then dried inside this hood for at least 24 hrs.
Nosema spore preparation
Nosema ceranae spores were originally obtained from infected A. cerana workers in Thailand and fed to A. mellifera workers in La Jolla, California. Spore-producing bees were not fed pollen, only pure 55% (w/w) sucrose solution to ensure that gut contents consisted mainly of spores. To obtain spores, we dissected out adult honey bee midguts, homogenized them in sterile double distilled water (ddH20), and vacuum-filtered them through Fisherbrand P8 filter paper (Fisher Scientific, Pittsburgh, Pennsylvania, USA) with 20–25 μm pores. We collected the filtrate in microcentrifuge tubes that we centrifuged (Eppendorf 5415D centrifuge, Hauppauge, New York, USA) at 9279 g (Relative Centrifugal Force) for 15 min51,52. We then removed the supernatant and re-suspended the pellet in sterile ddH20 water. This procedure resulted in fairly pure spore preparations as determined with a microscope. We measured spore concentrations with a haemocytometer in a compound microscope53,54. We infected new bees and extracted fresh spores for each trial.
We sequenced the DNA of our Nosema spores to determine its species identity. To obtain the DNA, we froze spore stock with liquid nitrogen and crushed the spores with a pestle before DNA extraction with a Bioneer Accuprep Genomic DNA extraction kit. We used primer pairs NoscRNAPol-F2 (AGCAAGAGACGTTTCTGGTACCTCA) and NoscRNAPol-R2 (CCTTCACGACCACCCATGGCA)55. The resulting spore DNA was then amplified with PCR and sequenced using standard methods55. Cross-checking with Genbank sequences confirmed that we were infecting our bees with N. ceranae.
Treatment of bees
These studies were conducted at UCSD, La Jolla, California. USA. We used nine colonies, a different colony for our preliminary trial (N = 108 bees, see SI) and each of our eight full experiment trials (N = 400 bees). New package colonies were obtained in the spring and treated with fumagilin dicyclohexylammonium (Fumagilin-B) in 25 mg/l of 2.0 sucrose solution (3.8 l/colony). After this initial treatment, we did not use the colonies for 60 days to allow the antibiotic to dissipate (method of Milbrath et al.56, detailed information on generating Nosema-free colonies from author Z. Huang). Huang et al.57 analyzed fumagillin residues and calculated that N. ceranae could hyperproliferate 2 to 5.5 months after treatment cessation. A 60-day waiting period yielded uninfected bees that could become highly infected with N. ceranae in our study (Fig. 2) and in Milbrath et al.56.
For each trial, a frame of Nosema-free capped brood from one of the research colonies was collected 1–2 days before brood emergence and incubated in the lab at 33 °C and 70% humidity. We randomly selected 50 bees upon emergence, individually numbered them with bee tags attached with cyanoacrylate adhesive (Queen Marking Kit, www.beeworks.com) and divided them into two cages, a control and a Nosema test cage (25 bees per cage). Each trial consisted of one control and one Nosema cage running in parallel. The control treatment bees were only fed sterile 50% sucrose solution. The Nosema treatment bees were group fed 750,000 Nosema spores in a 1 ml solution of 50% sucrose, equivalent to 30,000 spores per bee. On average, the bees consumed these 30,000 spores in 4.1 ± 1.2 days and were fed sterile sucrose solution, like the control cage, for the remainder of the experiment.
Behavioural analysis
Each trial covered the first 15 days of adult bee life. Every 4 hrs (00:00, 04:00, 08:00, 12:00, 16:00, and 20:00), we video-recorded (H.264 Network DVR, www.cibsecurity.com) the bees for 30 min. For each cage, we used a CCD camera (Sony 1/3 Varifocal Camera, 700 TVL, 976 × 494 pixels) with infra-red LED lights (850 nm) that are invisible to bees58 to illuminate the bees in the dark incubator. To eliminate LED reflections, we placed each camera directly against the camera-viewing window of the cage. Subsequently the behaviours were scored (Table 1) by manual inspections of the videos during the first and the last minute of each 30 min recording period and summed to create behaviour counts for that 30 min period. Each bee could perform multiple behaviours within each 1-min observation interval. Behaviours typically lasted a few seconds and thus performing one behaviour did not prevent the bee from performing other behaviours. We focused on the 13 most common behaviours that we observed. Video data transcribers were extensively trained as a group to record the behaviours, and their work was verified before they were allowed to collect data on their own.
Inclusion of data for analyses
We excluded three bees from the behavioural analysis of control trials (1.5% of control bees) because they had Nosema spores. These bees were minimally infected compared to Nosema group bees (Fig. 2), and their infection levels (2.7 ± 1.2 spores per bee haemocytometer count) may have arisen from subsequent contamination. In total, we analyzed the behaviours of 183 bees in the control group and 181 bees in the Nosema group (78% of Nosema group bees had >30,000 spores and 22% had ≤30,000 spores per bee, the average dose fed to these bees).
Statistical analyses
We log-transformed all behavioural measures and used Repeated-Measures Analysis of Variance (ANOVA), REML algorithm (JMP Pro v11.2.0 software), with bee identity as a repeated measure and treatment (control or Nosema), day and the interaction treatment*day as fixed effects. In addition, each trial consisted of two cages (one control and one Nosema cage). Cage identity was therefore incorporated in our models through the inclusion of colony and treatment. We initially tested time of day in our models, but excluded this variable from our final models59 because it was not significant for any behaviors (P ≥ 0.22).
For behaviours that showed changes over time, we applied post-hoc contrast tests to test for treatment differences. In our initial models, we included observer as a random factor, but found that it accounted for <1% of model variances. This indicates that observer bias was fairly low, and we therefore did not include observer as a factor in our final models.
Measurement of the Nosema spore counts in the bees exposed to N. ceranae spores, revealed different levels at the end of the experiment in spite of all bees sharing the same sugar solution with the same concentration of Nosema spores. To determine if this variable level of infection corresponded to bee behaviours, we divided our data into two subgroups: bees that showed elevated spore levels upon death, a sign of active infection, and a second group, for those bees that retained less than 30,000 spores, a sign of being uninfected or less infected, ran the same model, and applied contrast tests as appropriate. In these models, incorporating colony as a random effect included the effects of trial and cage because each trial was conducted with a different colony and consisted of one cage of bees.
Because we analyzed the data twice (control vs. Nosema treatment and infected vs. uninfected within the Nosema treatment, we applied a sequential Bonferroni correction60 with k = 2, yielding an adjusted alpha value of 0.0253, to correct for potential type I statistical error. Tests were only considered significant if P < 0.0253. Tests that fail this corrected alpha are denoted as NSSB. To explore the effects of Nosema infection in detail, we measured 13 different behavioural responses (Table 1) to our treatment. However, we had no a priori expectations of how these behaviours would change. Moreover, a Bonferroni k that is the sum of all these behavioural measures would not be appropriate because the Bonferroni correction is too conservative for large k, is susceptible to type II statistical errors, and inhibits detailed analyses of the phenomena being studied60,61. We therefore followed the procedures suggested by Moran60 in presenting our analyses and applied the k = 2 Bonferroni correction as a compromise between the problems of type I and type II statistical error.
A repeated-measures analysis is suited to the nature of our experimental design, but as an alternative (but not independent) analysis, we simply averaged the behaviors of each bee and compared the effects of treatment upon three behaviors over relevant time spans (see Figs 3 and 4): trophallaxis (days 2–8), walking (days 13–14), and queen attendance (days 13–14). For these analyses, we used ANOVA with treatment (control vs. Nosema) as a fixed effect and colony as a random effect. We denote these analyses as ANOVAnon-RM.
We tested survival using a proportional hazards fit model, including colony and spore count upon adult death as a factor. The data were right-censored because all trials terminated when bees were 15 days old. We report our results25 as mean ± 1 standard deviation.
Additional Information
How to cite this article: Lecocq, A. et al. Parasite infection accelerates age polyethism in young honey bees. Sci. Rep. 6, 22042; doi: 10.1038/srep22042 (2016).
Supplementary Material
Acknowledgments
We would like to thank the anonymous reviewers whose comments improved our manuscript, Ian Abramson for his statistical advice, Jacalyn Ho for conducting and analyzing the preliminary experiment, and the volunteers who spent many hours collecting and analysing the data: Lindsay Goldasich, Neil Srinivas and Cheryl Patel.
Footnotes
Author Contributions A.L., A.J., P.K. and J.N. conceived the experiments, A.L. conducted the experiments, A.L. and J.N. analysed the results. All authors reviewed the manuscript.
References
- vanEngelsdorp D. & Meixner M. D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 103, S80–S95, 10.1016/j.jip.2009.06.011 (2010). [DOI] [PubMed] [Google Scholar]
- Klee J. et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 96, 1–10, 10.1016/j.jip.2007.02.014 (2007). [DOI] [PubMed] [Google Scholar]
- Fries I. Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 103, S73–S79, 10.1016/j.jip.2009.06.017 (2010). [DOI] [PubMed] [Google Scholar]
- Higes M., Martín-Hernández R. & Meana A. Nosema ceranae in Europe: an emergent type C nosemosis. Apidologie 41, 375–392 (2010). [Google Scholar]
- Higes M. et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ.l Microbiol. 10, 2659–2669 (2008). [DOI] [PubMed] [Google Scholar]
- Higes M., Meana A., Bartolome C., Botias C. & Martin-Hernandez R. Nosema ceranae (Microsporidia), a controversial 21st century honey bee pathogen. Environ. Microbiol. Rep. 5, 17–29, 10.1111/1758-2229.12024 (2013). [DOI] [PubMed] [Google Scholar]
- Paxton R. J. Does infection by Nosema ceranae cause “Colony Collapse Disorder” in honey bees (Apis mellifera)? J. Apicult. Res. 49, 80–84, 10.3896/IBRA.1.49.1.11 (2010). [DOI] [Google Scholar]
- Aufauvre J. et al. Parasite-insecticide interactions: a case study of Nosema ceranae and fipronil synergy on honeybee. Scientific Reports 2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidau C. et al. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PloS ONE 6, e21550 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigliardi E. & Sacchi L. Cell biology and invasion of the microsporidia. Microbes Infect. 3, 373–379 (2001). [DOI] [PubMed] [Google Scholar]
- Chen Y. et al. Asymmetrical coexistence of Nosema ceranae and Nosema apis in honey bees. J. Invertebr. Pathol. 101, 204–209, 10.1016/j.jip.2009.05.012 (2009). [DOI] [PubMed] [Google Scholar]
- Gisder S. et al. Five-year cohort study of Nosema spp. in Germany: does climate shape virulence and assertiveness of Nosema ceranae? Appl. Environ. Microb. 76, 3032–3038 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goblirsch M., Huang Z. Y. & Spivak M. Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PloS ONE 8, e58165, 10.1371/journal.pone.0058165 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. L. The honey bee parasite Nosema ceranae: transmissible via food exchange. PloS ONE 7, e43319 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K. E. et al. The cost of promiscuity: sexual transmission of Nosema microsporidian parasites in polyandrous honey bees. Scientific Reports 5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayack C. & Naug D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 100, 185–188 (2009). [DOI] [PubMed] [Google Scholar]
- Winston M. L. The biology of the honey bee. (Harvard University Press, 1991). [Google Scholar]
- Hassanein M. H. The influence of infection with Nosema apis on the activities and longevity of the worker honeybee. Ann. Appl. Biol. 40, 418–423, 10.1111/j.1744-7348.1953.tb01093.x (1953). [DOI] [Google Scholar]
- Dussaubat C. et al. Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PloS ONE 7, 10.1371/journal.pone.0037017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. & Moeller F. Histological comparisons of the development of hypopharyngeal glands in healthy and Nosema-infected worker honey bees. J. Invertebr. Pathol. 14, 135–142 (1969). [Google Scholar]
- Natsopoulou M. E., McMahon D. P. & Paxton R. J. Parasites modulate within-colony activity and accelerate the temporal polyethism schedule of a social insect, the honey bee. Behav. Ecol. Sociobiol. 10.1007/s00265-015-2019-5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J., Kessler B., Mayack C. & Naug D. Behavioural fever in infected honeybees: parasitic manipulation or coincidental benefit? Parasitology 137, 1487–1491, 10.1017/S0031182010000235 (2010). [DOI] [PubMed] [Google Scholar]
- Evans J. D. & Spivak M. Socialized medicine: Individual and communal disease barriers in honey bees. J. Invertebr. Pathol. 103, Supplement S62–S72, http://dx.doi.org/10.1016/j.jip.2009.06.019 (2010). [DOI] [PubMed] [Google Scholar]
- Rothenbuhler W. C. Behavior genetics of nest cleaning in honey bees. Responses of F1 and backcross generations to disease-killed brood. American Zoologist 4, 111–123 (1964). [DOI] [PubMed] [Google Scholar]
- Peng Y. S., Fang Y. Z., Xu S. Y. & Ge L. S. The resistance mechanism of the Asian honey bee, Apis cerana Fabr, to an ectoparasitic mite, Varroa jacobsoni Oudemans. J. Invertebr. Pathol. 49, 54–60, 10.1016/0022-2011(87)90125-x (1987). [DOI] [Google Scholar]
- Rueppell O., Hayworth M. K. & Ross N. P. Altruistic self-removal of health-compromised honey bee workers from their hive. J. Evol. Biol. 23, 1538–1546, 10.1111/j.1420-9101.2010.02022.x (2010). [DOI] [PubMed] [Google Scholar]
- Urlacher E., Tarr I. S. & Mercer A. R. Social modulation of stress reactivity and learning in young worker honey bees. PloS ONE 9, e113630, 10.1371/journal.pone.0113630 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann K., Winston M. L., Wyborn M. H. & Slessor K. N. Effects of synthetic, honey bee (Hymenoptera: Apidae) queen mandibular-gland pheromone on workers in packages. J. Econ. Entomol. 83, 1271–1275 (1990). [Google Scholar]
- Winston M. L. & Slessor K. N. Applications of queen honey bee mandibular pheromone for beekeeping and crop pollination. Bee world 74, 111–128 (1993). [Google Scholar]
- Forsgren E. & Fries I. Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet. Parasitol. 170, 212–217, 10.1016/j.vetpar.2010.02.010 (2010). [DOI] [PubMed] [Google Scholar]
- Naug D. & Gibbs A. Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie 40, 595–599, 10.1051/apido/2009039 (2009). [DOI] [Google Scholar]
- Haydak M. H. The language of the honeybees. Am. Bee. J. 85, 2 (1945). [Google Scholar]
- Nieh J. C. The honey bee shaking signal: function and design of a modulatory communication signal. Behav. Ecol. Sociobiol. 42, 23–36 (1998). [Google Scholar]
- McDonnell C. M. et al. Ecto-and endoparasite induce similar chemical and brain neurogenomic responses in the honey bee (Apis mellifera). BMC ecology 13, 25 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.-Y. & Robinson G. E. Regulation of honey bee division of labor by colony age demography. Behav. Ecol. Sociobiol. 39, 147–158 (1996). [Google Scholar]
- Kimura T. et al. Development of a new method to track multiple honey bees with complex behaviors on a flat laboratory arena. PloS ONE 9, e84656, 10.1371/journal.pone.0084656 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brødsgaard C. J., Hansen H. & Ritter W. Progress of Paenibacillus larvae larvae infection in individually inoculated honey bee larvae reared singly in vitro, in micro colonies, or in full-size colonies. J. Apicult. Res. 39, 19–27 (2000). [Google Scholar]
- Wang D. I. & Moeller F. E. The division of labor and queen attendance behavior of Nosema-infected worker honey bees. J. Econ. Entomol. 63, 1539–1541 (1970). [Google Scholar]
- Lecocq A. et al. Parasite-mediated interactions between honey bee workers and a queen. PhD Dissertation. University of Copenhagen, Copenhagen, Denmark (2015). [Google Scholar]
- Johnson B. R. Within-nest temporal polyethism in the honey bee. Behav. Ecol. Sociobiol. 62, 777–784, 10.1007/s00265-007-0503-2 (2008). [DOI] [Google Scholar]
- Robinson G. E. & Vargo E. L. Juvenile hormone in adult eusocial Hymenoptera: gonadotropin and behavioral pacemaker. Arch. Insect. Biochem. 35, 559–583 (1997). [DOI] [PubMed] [Google Scholar]
- McQuillan H. J., Nakagawa S. & Mercer A. R. Juvenile hormone enhances aversive learning performance in 2-day old worker honey bees while reducing their attraction to queen mandibular pheromone. PloS ONE 9, 10, 10.1371/journal.pone.0112740 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C. J., Søvika E., Myerscough M. R. & Barron A. B. Rapid behavioral maturation accelerates failure of stressed honey bee colonies. P. Natl. Acad. Sci. USA 112(11), 3427–3432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralj J. & Fuchs S. Nosema sp. influences flight behavior of infected honey bee (Apis mellifera) foragers. Apidologie 41, 21–28 (2010). [Google Scholar]
- Alaux C. et al. Pathological effects of the microsporidium Nosema ceranae on honey bee queen physiology (Apis mellifera). J. Invertebr. Pathol. 106, 380–385, 10.1016/j.jip.2010.12.005 (2011). [DOI] [PubMed] [Google Scholar]
- Gisder S., Möckel N., Linde A. & Genersch E. A cell culture model for Nosema ceranae and Nosema apis allows new insights into the life cycle of these important honey bee‐pathogenic microsporidia. Environ. Microbiol. 13, 404–413 (2011). [DOI] [PubMed] [Google Scholar]
- Adamo S. A. Parasites: evolution’s neurobiologists. J. Exp. Biol. 216, 3–10 (2013). [DOI] [PubMed] [Google Scholar]
- Wilson-Rich N., Spivak M., Fefferman N. H. & Starks P. T. Genetic, individual, and group facilitation of disease resistance in insect societies. Annu. Rev. Entomol. 54, 405–423, 10.1146/annurev.ento.53.103106.093301 (2009). [DOI] [PubMed] [Google Scholar]
- Moore J. The behavior of parasitized animals. Bioscience, 89–96 (1995). [Google Scholar]
- Williams G. R. et al. Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apicult. Res. 52, 1–36 (2013). [Google Scholar]
- Webster T. C., Pomper K. W., Hunt G., Thacker E. M. & Jones S. C. Nosema apis infection in worker and queen Apis mellifera. Apidologie 35, 49–54, 10.1051/apido:2003063 (2004). [DOI] [Google Scholar]
- Eiri D. M., Suwannapong G., Endler M. & Nieh J. C. Nosema ceranae can infect honey bee larvae and reduces subsequent adult longevity. PloS ONE 10, 10.1371/journal.pone.0126330 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell G. E. Standard methods for counting Nosema spores. Amer. Bee. J. 110, 2 (1970). [Google Scholar]
- Human H. et al. Miscellaneous standard methods for Apis mellifera research. J. Apicult. Res. 52, 55, 10.3896/ibra.1.52.4.10 (2013). [DOI] [Google Scholar]
- Gisder S. & Genersch E. Molecular differentiation of Nosema apis and Nosema ceranae based on species–specific sequence differences in a protein coding gene. J. Invert. Pathol. 113.1, 1–6 (2013). [DOI] [PubMed] [Google Scholar]
- Milbrath M. O. et al. Comparative virulence and competition between Nosema apis and Nosema ceranae in honey bees (Apis mellifera). J. Invert. Pathol. 125, 9–15 (2015). [DOI] [PubMed] [Google Scholar]
- Huang W.-F., Solter L. F., Yau P. M. & Imai B. S. Nosema ceranae escapes Fumagillin control in honey bees. PLoS Pathogens 9(3), e1003185 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Frisch K. Bees: their vision, chemical senses, and language. (Cornell Univ. Press, 1964). [Google Scholar]
- Sokal R. R. & Rohlf F. J. Biometry: the principles and practice of statistics in biological research. 2nd edition (1981). [Google Scholar]
- Moran M. D. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos. 403–405 (2003). [Google Scholar]
- Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav. Ecol. 15.6, 1044–1045 (2004). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.