Abstract
Objectives
We evaluated clinical outcomes associated with ESA use in LVAD-supported patients.
Background
Use of erythropoiesis stimulating agents (ESAs) in patients with left ventricular assist devices (LVADs) may minimize blood transfusions and decrease allosensitization. ESAs increase thrombotic events which is concerning as LVADs are sensitive to pump thrombosis (PT).
Methods
We retrospectively reviewed 221 patients at our center who received a HeartMate II® LVAD between 1/2009 and 6/2013. Patients were divided into those who received ESAs during index admission (n = 121) and those who did not (n = 100). Suspected PT was defined as evidence of thrombus in the LVAD or severe hemolysis (LDH > 1,000 mg/dL or plasma free hemoglobin > 40mg/dL). Outcomes were compared between cohorts using inverse probability-weighted analyses.
Results
During a mean follow-up of 14.2 ± 11.9 months, suspected PT occurred in 37 patients (ESA 23%, no-ESA 12%; P =0.03). The ESA cohort received ESAs 13.9 ± 60.9 days after LVAD implantation. At 180-days, event-free rates for suspected PT were ESA 78.6% vs. no-ESA 94.5% (P < 0.001). ESA use had higher rates of suspected PT (HR 2.35, 95% CI 1.38-4.00; P = 0.002). For every 100 unit increase in cumulative ESA dosage, the hazard of suspected PT increased by 10% (HR 1.10, 95% 1.04-1.16; P < 0.001). After inverse probability weighting, ESA use was associated with a significantly higher rate of all-cause mortality (HR 1.62, 95% 1.12-2.33; P = 0.01).
Conclusions
ESA use in LVAD patients is associated with higher rates of suspected PT.
Keywords: Erythropoiesis stimulating agent, left ventricular assist device, thrombosis
Introduction
Anemia is an independent predictor of morbidity and mortality in patients with heart failure (HF) (1-6). The estimated prevalence of anemia in HF patients depends upon the severity of failure and can range from 20 to 80 percent in those with New York Heart Association class III to IV symptoms (7,8). A comparable rate has been reported in HF patients receiving left ventricular assist device (LVAD) support. In one retrospective study, anemia was present in almost half of 65 patients after implantation of LVAD and was associated with higher rates of all-cause mortality despite improvement in end-organ perfusion (9). Mechanisms for the development of anemia in patients with LVAD support are multifactorial but often involve a chronic inflammatory state created by the immune system reacting to LVAD biomaterial (10-12). The inflammatory milieu that ensues may suppress erythropoiesis(9) by inhibiting production of erythropoietin (EPO) and diminishing its effectiveness similar to the pathophysiology of anemia of chronic disease (10). It has been demonstrated that anemic LVAD patients have lower than expected circulating erythropoietin levels (9). This has led some clinicians to treat LVAD-supported patients with erythropoiesis stimulating agents (ESAs).
ESAs are ideal for patients destined for transplantation, as ESA treatment increases hemoglobin levels, thereby reducing the need for transfusions and subsequent risk of developing anti-HLA antibodies (13-16). Allosensitization most commonly results from previous blood transfusions and can complicate procurement of an appropriate donor heart, delaying time until transplantation (17). Post transplant, allosensitization may lead to higher rates of organ rejection and allograft vasculopathy (17).
These potential benefits of ESAs in LVAD-supported patients are offset by potential risks. Use of ESAs may cause thrombotic and vascular events in patients with malignancies, chronic kidney disease, end-stage renal disease, and systolic heart failure (18-22). Pump thrombosis can manifest as severe hemolysis with pump failure and hemodynamic collapse. No studies to date have evaluated thrombotic outcomes with use of ESAs in patients receiving LVAD support. In this retrospective dual-cohort study, we describe clinical outcomes associated with use of ESAs in patients with HF and LVAD support.
Methods
Study Population
We retrospectively identified 264 patients who underwent implantation of a HeartMate II® (Thoratec Corp., Pleasanton, CA) LVAD between 1/2009 and 6/2013 at our center. Of the 264 patients, 30 patients were excluded because they required extracorporeal membrane oxygenation support or right ventricular assist device support post operatively. Nine patients were excluded as this was their second LVAD during the inclusion period and 4 patients were excluded because they followed up at other institutions. The final cohort included 221 patients and was divided into those who received an ESA (epoetin or darbepoetin) versus those who did not. All ESAs were converted to darbepoetin equivalents using a fixed conversion ratio of 225 international units (IU) epoetin to 1 microgram (mcg) darbepoetin per the manufacturer’s suggestion.(23,24) Patient characteristics, the cumulative dosage of darbepoetin or epoetin, and clinical outcomes were obtained through review of the medical record. All data were collected and managed using REDCap, an electronic data capture tool hosted by our institution (25). The study was approved by our Institutional Review Board.
Procedural Technique
The HeartMate II® Left ventricular Assist system has been described previously.(26,27) All patients in this cohort were implanted through a median sternotomy using cardiopulmonary bypass. Following implant, patients were monitored in the cardiothoracic intensive care unit for complications and received transfusion of blood products at the discretion of the care team. Anticoagulation and antiplatelet protocols changed over time and are shown in the Online Supplementary Appendix Table S1.
Follow-up and Clinical Outcomes
Follow-up was assessed for every patient by review of the medical record. The primary outcome of the study was suspected pump thrombosis at 180 days. Although mean follow up duration was 14.2 months, events occurring within 180 days were of particular interest as this encompasses just over 5 half-lives of darbepoetin (half-life 22-27 days) (24) and thus a majority of medication related events. Secondary outcomes included rates of stroke and all-cause mortality. Suspected pump thrombosis was defined as direct observation of obstructive thrombus in the pump or conduit post pump exchange or severe hemolysis. Severe hemolysis was defined as lactate dehydrogenase (LDH) level greater than 1,000 mg/dL (4 times the upper limit of normal for the study institution’s laboratory) or plasma free hemoglobin level greater than 40 mg/dL with symptoms of decompensated HF in the absence of a kinked inflow or outflow cannulae (28,29). A LDH level 2.5 times the upper limit of normal is 78% sensitive and 97% specific for detection of device thrombosis, and a value 5 times the upper limit of normal has a sensitivity of 100% and a specificity of 92.5%.(30,31) Hemolysis was not present if a patient’s LDH was greater than 1,000 mg/dL prior to LVAD implant but continually decreased after the operation, or if the elevated LDH level was felt to be due to sepsis with multiorgan failure.
Statistical Analysis
Categorical variables, including the number of hemolysis or pump thrombus events, were compared using Fisher’s exact tests. Continuous variables were compared with Student’s two sample t-tests. For non-normal and ordinal variables, the median (min, max) were reported and a Kruskal-Wallis test was conducted to compare cohorts.
Time until suspected pump thrombosis was evaluated through Kaplan Meier (KM) analysis and curves were compared with the Wilcoxon tests. The start time was the device implant date and event-free patients were censored due to death, transplant, pump replacement, or at last follow-up, whichever occurred earliest. In the Cox proportional hazards model, inclusion into the ESA cohort was treated as a time-dependent variable to account for differences between implant date and initial use of ESA. Using logistic regression, inverse probability of treatment propensity scores were created for ESA to balance groups when conducting comparisons. The variables used to create the propensity scores were age, gender, body mass index, atrial fibrillation, creatinine on admission, creatinine clearance on admission, hemoglobin on admission, diabetes mellitus, coronary artery disease, history of intra-cardiac thrombus, bridge to transplant strategy, INTERMACS profile, presence of bacteremia, platelet count on discharge, bridged with heparin or bivalirudin post operatively, any positive anti-PF4 during index admission, and number of red blood cell units transfused during index hospitalization. Sensitivity analyses were conducted to control for an observed increase in pump thrombosis over time (Online Supplementary Appendix S1).(28)
A two-sided P-value <0.05 was considered significant. The p-values presented do not adjust for multiple comparisons. All statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
Most patients were male (81%), classified as INTERMACS profile 2, and bridge-to-transplant approach was used for 68% of the 221 patients (Table 1). The ESA cohort was more likely to have had bacteremia during index hospitalization than the control cohort (13% vs. 4%, P = 0.02). There were no significant between-cohort differences in rates of intra-cardiac thrombus, creatinine clearance, hemoglobin level (on admission, day before implant, or on discharge), or units of packed red blood cells transfused (ESA: 9.0 units, IQR 4.0-20.0 vs. no-ESA 9.0 units, IQR 4.0 to 15.0; P = 0.23). Covariate balance was achieved after propensity inverse weighting as shown in Figure S1 (Online Supplementary Appendix).
Table 1.
Baseline Clinical Characteristics for the Study Population
| No ESA (N = 121 patients) | ESA (N = 100 patients) | p-value | |
|---|---|---|---|
|
| |||
| Age (years) | 57.10 ± 12.29 | 55.62 ± 11.79 | 0.37 |
| Male | 95 (79%) | 85 (85%) | 0.23 |
| Caucasian | 87 (72%) | 76 (76%) | 0.54 |
| BMI (kg/m2) | 28.50 ± 6.35 | 28.95 ± 5.30 | 0.57 |
| Medical history | |||
| Atrial fibrillation | 54 (45%) | 45 (45%) | 1.00 |
| Current smoker | 69 (57%) | 54 (54%) | 0.68 |
| CAD | 61 (50%) | 50 (50%) | 1.00 |
| DM | 52 (43%) | 47 (47%) | 0.59 |
| INTERMACS profile | 2.0 (1.0, 2.0) | 2.0 (1.0, 2.0) | 0.76 |
| Intracardiac thrombus | 18 (15%) | 11 (11%) | 0.43 |
| Bridge to transplant | 79 (65%) | 71 (71%) | 0.39 |
| PRBC units transfused | 9.0 (4.0, 15.0) | 9.0 (4.0, 20.0) | 0.23 |
| Labs on admission | |||
| Creatinine (mg/dL) | 1.4 (1.1, 2.0) | 1.5 (1.2, 2.3) | 0.09 |
| CrCl (mL/min) | 72.4 ± 34.2 | 71.5 ± 40.4 | 0.86 |
| Hgb (g/dL) | 12.03 ± 1.96 | 11.75 ± 1.96 | 0.30 |
| Serum Iron (μg/dL) | 43.5 (30.5, 60.5) | 40.5 (31.0, 65.0) | 0.88 |
| Ferritin (ng/mL) | 139.5 (53.0, 331.5) | 171.0 (80.0, 301.0) | 0.56 |
| TIBC (μg/dL) | 305.8 ± 85.48 | 318.26 ± 85.39 | 0.42 |
| TSAT (%) | 15.0 (10.0, 21.0) | 14.5 (10.0, 20.0) | 0.83 |
| Labs on discharge | |||
| CrCl (mL/min) | 100.9 ± 44.9 | 101.9 ± 58.7 | 0.90 |
| Hgb (g/dL) | 9.56 ± 1.01 | 9.52 ± 1.07 | 0.82 |
| INR | 2.18 ± 0.54 | 2.16 ± 0.58 | 0.78 |
| Postop anticoagulation | 102 (84%) | 77 (77%) | 0.17 |
| Aspirin on discharge | 107 (96%) | 92 (98%) | 0.69 |
| Aspirin dose | 0.47 | ||
| 325 mg daily | 44 (41%) | 43 (47%) | |
| 81 mg daily | 63 (59%) | 49 (53%) | |
| Coumadin on discharge | 107 (96%) | 86 (91%) | 0.15 |
| Year of implant | <0.01 | ||
| 2009 | 33 (27%) | 8 (8%) | |
| 2010 | 22 (18%) | 22 (22%) | |
| 2011 | 9 (7%) | 34 (34%) | |
| 2012 | 30 (25%) | 33 (33%) | |
| 2013 | 27 (22%) | 3 (3%) | |
Values are shown as absolute numbers (percentages), mean ± SD, or median (IQR). ESA = erythropoietin stimulating agent; BMI = body mass index; CAD = coronary artery disease; DM = diabetes mellitus; PRBC = packed red blood cell; CrCl = creatinine clearance; Hgb = hemoglobin; TIBC = total iron binding capacity; TSAT = transferring saturation; INR = international normalized ratio.
Suspected Pump Thrombosis
Clinical follow-up was available for every patient with a mean follow-up period of 14.2 ± 11.9 months. The proportion of suspected pump thrombosis was significantly higher with use of ESAs compared with no ESAs (23% vs. 12%, P = 0.03). Kaplan-Meier estimates of freedom from suspected pump thrombosis (Figure 1A) were significantly higher in patients who did not receive ESAs compared with patients who received ESAs (Wilcoxon, P = 0.004). Event-free rates are shown in the Online Supplementary Appendix Table S2. At 180 days, the event-free rates were 78.6% in the ESA cohort versus 94.5% in the no-ESA cohort (P < 0.001) and this effect persisted up to 1-year (P = 0.024) but was not found at 2 years (P = 0.06). ESA use was associated with higher rates of suspected pump thrombosis when compared with no-ESA use (HR 2.35, 95% 1.38-4.00; P = 0.002) (Table 2).
Figure 1.
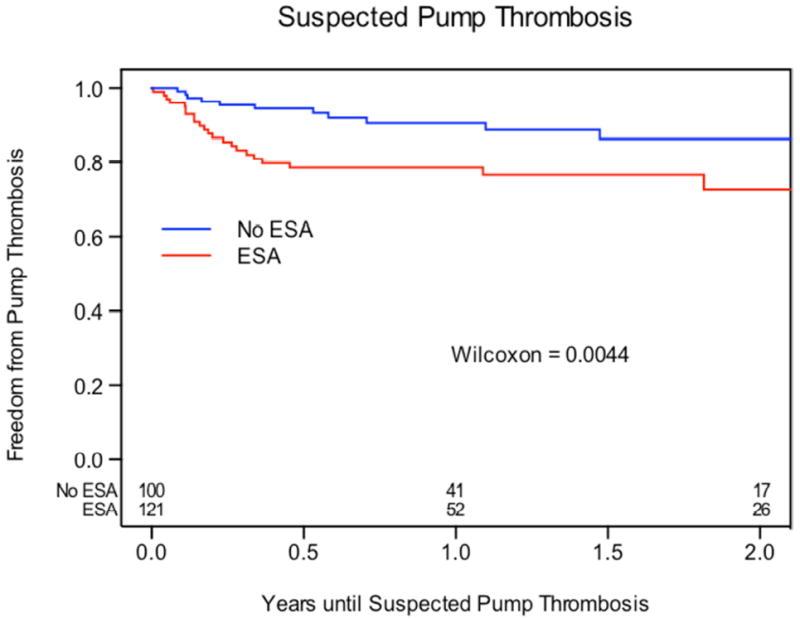
A: Freedom from Suspected Pump Thrombosis. Kaplan-Meier analysis of freedom from suspected pump thrombosis between patients who received ESAs and those who did not receive ESAs.
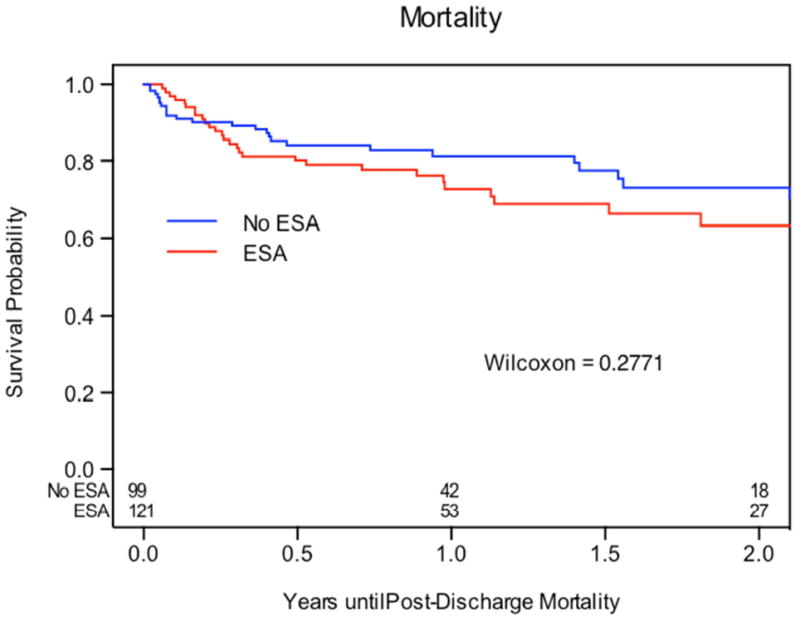
B: Freedom from All-cause Mortality. Kaplan-Meier analysis of freedom from all-cause mortality between patients who received ESAs and those who did not receive ESAs.
Table 2.
Clinical Outcomes and Hazard Ratios Before and After Inverse Weighting for Patients Receiving LVAD Support with and without Use of ESAs (n = 221)
| Before Inverse Weighting | After Inverse Weighting | |||
|---|---|---|---|---|
| HR (95% CI) | p- value | HR (95% CI) | p- value | |
| Suspected Pump Thrombosis | 2.26 (1.15-4.40) | 0.002 | 2.35 (1.38-4.00) | 0.002 |
| All-cause Mortality | 1.56 (0.93-2.62) | 0.09 | 1.62 (1.12-2.33) | 0.01 |
| Stroke | 0.59 (0.26-1.31) | 0.19 | 0.55 (0.30-1.02) | 0.06 |
| Ischemic stroke | 0.59 (0.20-1.74) | 0.34 | 0.51 (0.21-1.24) | 0.14 |
All results based on Cox proportional hazards models. HR = hazards ratio. LVAD = left ventricular assist device. ESA = erythropoietin stimulating agent.
Patients in the ESA cohort were initiated on darbepoetin (n = 89; median dose 200 mcg) or epoetin (n = 11; median dose 40,000 Units) an average of 13.8 days after LVAD implantation for an average of 2.2 doses of ESA. The mean time between the first and last dose of ESA was 17 days. In the ESA cohort, the median total ESA dose equivalent for patients with suspected pump thrombosis was 300 mcg compared with 200 mcg for those without an event (P = 0.06). A significant association between cumulative ESA dosage and the primary endpoint was observed (Online Supplementary Appendix Table S3). For every 100 unit increase in equivalent ESA dosage, the hazard of suspected pump thrombosis increased by 10% (HR 1.10, 95% 1.04-1.16; P < 0.001). The average hemoglobin of patients in both ESA and no-ESA cohorts who had pump thrombosis was 10.1 ± 1.7 g/Dl. No significant differences in admission or discharge creatinine clearance, hemoglobin, or INR were observed between those in the ESA cohort with versus without the primary endpoint (Online Supplementary Appendix Table S4). Additionally, the incidence of suspected pump thrombosis has increased over time since 2009 as shown in Table S5 (Online Supplementary Appendix).
Secondary Outcomes
Kaplan-Meier estimate of freedom from all-cause mortality (Wilcoxon P = 0.289) was not significantly different between ESA and no-ESA cohorts (Figure 1B). After inverse weighting however, ESA use was associated with a significantly higher rate of all-cause mortality (HR 1.62, 95% 1.12-2.33; P = 0.01). Thirty of the thirty-seven patients (81%) who developed the primary outcome required pump exchange or transplant within 90 days, or expired. Only three of the thirty-seven patients survived for greater than 1 year with their index LVAD and had resolution of hemolysis with stronger anticoagulants (two with IV heparin and eptifibatide and one with higher INR target).
Kaplan-Meier estimates of freedom from stroke (Wilcoxon P = 0.16) and ischemic stroke (Wilcoxon P = 0.52) were not significantly different between ESA and no-ESA cohorts (Online Supplementary Appendix Figure S2-3). Freedom from stroke at 180 days was 94.1% in the ESA group versus 88.8% no-ESA group, P = 0.19 and freedom from ischemic stroke at 180 days was ESA 94.1% versus no-ESA 93.3%, P = 0.82).
Results of the primary sensitivity analysis, which controlled for a reported increase in rates of suspected pump thrombosis after 5/1/2011, were similar to those obtained in the primary analysis and the association between ESA use and suspected pump thrombosis remained significant (HR 2.33, 95% 1.37-3.97; P = 0.002). Additional sensitivity analysis excluding devices implanted prior to March 2010 (incorporation date of sealed inflow conduit and outflow graft) continued to demonstrate a significant association, though of lower magnitude (HR 1.98, 95% CI 1.10-3.57; P = 0.024), between ESA use and suspected pump thrombosis, while all-cause mortality was no longer significant. Results from sensitivity analyses are shown in the Online Supplementary Appendix Tables S6-8.
Discussion
The results of our study suggest that use of ESAs in patients receiving LVAD support is associated with a significant increase in rates of suspected pump thrombosis and all-cause mortality.
It is unclear whether anemia independently contributes to increased mortality in advanced heart failure or simply acts as a marker of disease severity. Studies have suggested that increasing hemoglobin levels with ESAs may improve functional status.(32) Little is known about outcomes with use of ESAs in patients receiving LVAD support. The recently published RED-HF trial randomized 2278 patients with HF to darbepoetin or placebo and failed to show reduction in death or hospitalization for worsening heart failure and had increased rates of thromboembolic events at 28 months of follow-up (21). No conclusions can be made about use of ESAs in patients receiving LVAD support based on the seven patients with LVADs enrolled in that trial. Our study is the first to show a significantly higher rate of thrombotic complications with use of ESAs in patients receiving LVAD support and is consistent with the findings of Swedberg et al (21).
Mechanisms for development of pump thrombosis in patients receiving ESAs are likely multifactorial and may involve a combination of hyperviscosity (presumably due to polycythemia), thrombocytosis, platelet hyperactivity, and activation of blood coagulation in the setting of a prothrombotic milieu (33). Studies of ESA use in patients with chronic kidney disease (CKD) suggest a risk of mortality and thrombotic events associated with targeting levels of hemoglobin > 13 g/dL (20,34). Patients experiencing the primary event in our study did not have supraphysiologic or even normal hemoglobin levels at the time of suspected pump thrombosis. Although it is unclear which factor predominantly contributed to suspected pump thrombosis in these patients, hyperviscosity likely played a smaller role as patients on average had hemoglobin levels between 10 to 11 mg/dL and were therapeutically anticoagulated at the time of the primary event. Perhaps platelet hyperactivity in combination with the prothrombotic state propagated by LVAD support induced pump thrombosis in our patients. An additional potential pathway would be an effect of ESAs on von Willebrand Factor availability in these patients, though little is known in this regard.
Limited data exist regarding the dose-response relationship between ESAs and adverse vascular outcomes. The best evidence to date comes from a meta-regression performed by Koulouridis et al. that included 31 trials and showed a significant association between rates of stroke and thrombotic events and 3-month cumulative mean ESA dose independent of hemoglobin level.(35) A major limitation of this analysis was that numerous assumptions and transformations were required to harmonize results from individual trials. Also, it is difficult to extrapolate findings to the LVAD population as patients in the previous study had chronic kidney disease and received ESAs for at least 3 months. Findings from our retrospective study are consistent with the conclusions made by Koulouridis et al. and provide further evidence that a cumulative dose-response relationship likely exists between ESAs and thrombotic complications. In a trial performed by Weltert et al., 320 patients undergoing coronary artery bypass were randomized to a cumulative erythropoietin dose of 52,000 IU (cumulative darbepoetin equivalent of 260 mcg) versus standard of care.(16) The trial found a significant reduction in transfusion rate and increase in hemoglobin levels with no adverse events. However, the mean follow-up period was only 45 days and thus might have missed late thrombotic complications. Patients in our study were treated with similar doses of ESAs (median cumulative darbepoetin equivalent dose 200 mcg) and the intrinsic effects of higher ESA doses were associated with suspected pump thrombosis. It is also possible those receiving the highest cumulative doses of ESAs were severely ill and anemic for longer periods of time and other factors contributed to their development of suspected pump thrombosis. In addition the dosage effect seen in our study leaves the possibility of acceptable safety with transient low dose ESA usage.
Several studies have suggested increased mortality with use of ESAs especially when higher levels of hemoglobin are achieved.(36,37) However, patients in our study who received ESAs had significantly higher rates of all-cause mortality compared with those who did not receive ESAs despite achieving low-normal levels of hemoglobin. We believe the increased mortality rate is a result of the primary outcome as several studies demonstrate poor outcomes with pump thrombosis.(28) In our study, those who developed suspected pump thrombosis had very poor outcomes with only three of the thirty-seven surviving beyond 1 year with their index LVAD. A majority of patients with suspected pump thrombosis required either pump exchange or transplantation in keeping with findings from other studies (28).
Contrary to other studies, ESA use in our study was not associated with higher rates of stroke (20). It is possible that subjects in the ESA cohort were more likely to manifest thrombotic events as pump thrombosis and were thus censored before any strokes occurred. Additionally, it is likely that the use of ESAs as a time dependent variable leads to an underestimation of overall stroke in the ESA group. No event could be attributed to the ESA group until they had received a dose of ESA. Since the first dose of ESA was given an average of 14 days after implant, any perioperative neurologic event in this group would not be included. However, the control group would include any perioperative events, likely increasing the prevalence of stroke. Finally, it is likely that any patient who had a stroke would not have received ESAs given that they are known to increase rates of stroke (20).
Our data support recent analyses performed by INTERMACs and others which reveal a small but statistically significant increase in rates of pump thrombosis in the past few years (Online Supplementary Appendix Table S5).(28,38) Our analysis demonstrated a nearly 16.9% incidence of suspected pump thrombosis over a median follow-up of 14.2 months. This result is in line with what Starling et al reported a rate of confirmed plus suspected pump thrombosis of 11.3% at one year and 18.3% at two year follow up (28). The observed increase might be attributable to patient-level factors such as gastrointestinal bleeding, infection, and inadequate anticoagulation as well as an evolution in patient selection for LVAD therapy since FDA approval for Destination Therapy in January 2010. In addition, protocols for post-operative bridging anticoagulation as well as outpatient anticoagulation targets changed during the study period, which is difficult to account for. Increased clinician awareness and screening for pump thrombosis have also been occurring over time and may explain the observed increase in incidence of suspected pump thrombosis. Lastly, variations in device design and manufacturing of the HeartMate II® device (such as sealing the outflow graft and inflow conduit) might also account for the rise in pump thrombosis. To account for the potential contribution of concurrent device related changes, a series of sensitivity analyses were conducted. The association between ESA use and suspected pump thrombosis persisted throughout the sensitivity analysis, though the effect was reduced when time was included in the model with HR decreasing from 2.35 to 1.98 (p =0.024).
When interpreting the results of our study, several limitations must be considered. The study is retrospective and non-randomized. It is possible that patients perceived to be at increased risk of adverse outcomes were more likely to receive ESAs. We attempted to mitigate selection bias by using propensity scores and inverse weighted analyses. However, it is possible there were unmeasured confounders contributing to differences in clinical outcomes observed between the two cohorts. In particular, INTERMACS profiles were used to control for severity of illness, but even amongst INTERMACS profile 1 patients there is often a wide range of expected mortality. It is possible the INTERMACS profile 1 patients who received ESAs had greater degrees of multiorgan dysfunction and shock than the control cohort. The Heartmate II risk score is better at predicting mortality than INTERMACS profile, however, the data available to us did not allow this calculation for all patients (39). Additionally, our secondary endpoints should be interpreted with caution as no adjustments were made for multiple comparisons, thereby increasing the chances of a type I error. Finally, length of stay (LOS) was not included in our propensity score model as our institutional data has shown LOS has decreased over time. The decision not to include LOS was made a priori as it was felt the decrease in LOS over time has more to do with clinician familiarity with the device as opposed to patient characteristics and severity of illness. However, it is possible that LOS may represent a more ill population that is at higher risk for suspected pump thrombosis (39).
In conclusion, ESA use after LVAD implantation is associated with significantly higher rates of suspected pump thrombosis in this analysis. Given the large observed magnitude of effect, dosage effect, persistence with sensitivity analysis, and consistency with evidence from other populations, these results remain concerning. Caution should be taken before administering ESAs to patients with continuous flow LVADs.
Supplementary Material
Acknowledgments
Funding Sources: This study was supported in part by research funds from the National Institutes of Health (NIH grant U10 HL110309, Heart Failure Network). No relationships with industry.
We gratefully acknowledge Douglas Mann, MD, for his valuable input throughout the manuscript writing process.
Abbreviations
- HF
heart failure
- LVAD
left ventricular assist device
- ESA
erythropoiesis stimulating agent(s)
- LDH
lactate dehydrogenase
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- HR
hazard ratio(s)
- IQR
interquartile range
- CKD
chronic kidney disease
- CAD
coronary artery disease
Footnotes
Disclosures
Gregory A. Ewald – Thoratec
Brian F. Gage – None
Ronald Jackups Jr. - None
Shane J. LaRue – None
Eric Novak - None
Jayendrakumar S. Patel – None
Sunil Prasad - None
David S. Raymer – None
Jerrica E. Shuster - None
Scott C. Silvestry Thoratec, HeartWare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Meara E, Clayton T, McEntegart MB, et al. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2006;113:986–94. doi: 10.1161/CIRCULATIONAHA.105.582577. [DOI] [PubMed] [Google Scholar]
- 2.Valeur N, Nielsen OW, McMurray JJ, Torp-Pedersen C, Kober L, Group TS. Anaemia is an independent predictor of mortality in patients with left ventricular systolic dysfunction following acute myocardial infarction. European journal of heart failure. 2006;8:577–84. doi: 10.1016/j.ejheart.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Felker GM, Adams KF, Jr, Gattis WA, O’Connor CM. Anemia as a risk factor and therapeutic target in heart failure. Journal of the American College of Cardiology. 2004;44:959–66. doi: 10.1016/j.jacc.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 4.Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. Journal of the American College of Cardiology. 2001;38:955–62. doi: 10.1016/s0735-1097(01)01470-x. [DOI] [PubMed] [Google Scholar]
- 5.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003;107:223–5. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 6.He SW, Wang LX. The impact of anemia on the prognosis of chronic heart failure: a meta-analysis and systemic review. Congestive heart failure. 2009;15:123–30. doi: 10.1111/j.1751-7133.2008.00030.x. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg DS, Wexler D, Blum M, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. Journal of the American College of Cardiology. 2000;35:1737–44. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- 8.Lindenfeld J. Prevalence of anemia and effects on mortality in patients with heart failure. American heart journal. 2005;149:391–401. doi: 10.1016/j.ahj.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Vrtovec B, Radovancevic R, Delgado RM, et al. Significance of anaemia in patients with advanced heart failure receiving long-term mechanical circulatory support. European journal of heart failure. 2009;11:1000–4. doi: 10.1093/eurjhf/hfp110. [DOI] [PubMed] [Google Scholar]
- 10.Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. 2005;20:83–90. doi: 10.1191/0267659105pf793oa. [DOI] [PubMed] [Google Scholar]
- 11.Pierce CN, Larson DF, Arabia FA, Copeland JG. Inflammatory mediated chronic anemia in patients supported with a mechanical circulatory assist device. The Journal of extra-corporeal technology. 2004;36:10–5. [PubMed] [Google Scholar]
- 12.Loebe M, Koster A, Sanger S, et al. Inflammatory response after implantation of a left ventricular assist device: comparison between the axial flow MicroMed DeBakey VAD and the pulsatile Novacor device. ASAIO journal. 2001;47:272–4. doi: 10.1097/00002480-200105000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Alghamdi AA, Albanna MJ, Guru V, Brister SJ. Does the use of erythropoietin reduce the risk of exposure to allogeneic blood transfusion in cardiac surgery? A systematic review and meta-analysis. Journal of cardiac surgery. 2006;21:320–6. doi: 10.1111/j.1540-8191.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- 14.Yazicioglu L, Eryilmaz S, Sirlak M, et al. Recombinant human erythropoietin administration in cardiac surgery. The Journal of thoracic and cardiovascular surgery. 2001;122:741–5. doi: 10.1067/mtc.2001.115426. [DOI] [PubMed] [Google Scholar]
- 15.Messmer K. Consensus statement: using epoetin alfa to decrease the risk of allogeneic blood transfusion in the surgical setting. Roundtable of Experts in Surgery Blood Management. Seminars in hematology. 1996;33:78–80. [PubMed] [Google Scholar]
- 16.Weltert L, D’Alessandro S, Nardella S, et al. Preoperative very short-term, high-dose erythropoietin administration diminishes blood transfusion rate in off-pump coronary artery bypass: a randomized blind controlled study. The Journal of thoracic and cardiovascular surgery. 2010;139:621–6. doi: 10.1016/j.jtcvs.2009.10.012. discussion 626-7. [DOI] [PubMed] [Google Scholar]
- 17.Velez M, Johnson MR. Management of allosensitized cardiac transplant candidates. Transplantation reviews. 2009;23:235–47. doi: 10.1016/j.trre.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant MD, Piper M, Bohlius J, et al. Epoetin and Darbepoetin for Managing Anemia in Patients Undergoing Cancer Treatment: Comparative Effectiveness Update. Rockville (MD): 2013. [PubMed] [Google Scholar]
- 19.Tonia T, Mettler A, Robert N, et al. Erythropoietin or darbepoetin for patients with cancer. The Cochrane database of systematic reviews. 2012;12:CD003407. doi: 10.1002/14651858.CD003407.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. The New England journal of medicine. 2009;361:2019–32. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 21.Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. The New England journal of medicine. 2013;368:1210–9. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Struble K Public Health Advisory: erythropoiesis-stimulating agents (ESAs). U.S Food and Drug Administration. 2009 [Google Scholar]
- 23.Glaspy J, Jadeja JS, Justice G, et al. A dose-finding and safety study of novel erythropoiesis stimulating protein (NESP) for the treatment of anaemia in patients receiving multicycle chemotherapy. British journal of cancer. 2001;84(Suppl 1):17–23. doi: 10.1054/bjoc.2001.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aranesp(R). [package insert] Thousand Oaks, CA: Amgen Pharmaceuticals Inc; 2010. [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke DJ, Burke E, Parsaie F, et al. The Heartmate II: design and development of a fully sealed axial flow left ventricular assist system. Artificial organs. 2001;25:380–5. doi: 10.1046/j.1525-1594.2001.06770.x. [DOI] [PubMed] [Google Scholar]
- 27.Griffith BP, Kormos RL, Borovetz HS, et al. HeartMate II left ventricular assist system: from concept to first clinical use. The Annals of thoracic surgery. 2001;71:S116–20. doi: 10.1016/s0003-4975(00)02639-4. discussion S114-6. [DOI] [PubMed] [Google Scholar]
- 28.Starling RC, Moazami N, Silvestry SC, et al. Unexpected Abrupt Increase in Left Ventricular Assist Device Thrombosis. The New England journal of medicine. 2013 doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. The Journal of Heart and Lung Transplantation. 2013;32:667–670. doi: 10.1016/j.healun.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Uriel N, Morrison KA, Garan AR, et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. Journal of the American College of Cardiology. 2012;60:1764–75. doi: 10.1016/j.jacc.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah P, Mehta VM, Cowger JA, Aaronson KD, Pagani FD. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013 doi: 10.1016/j.healun.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Ngo K, Kotecha D, Walters JA, et al. Erythropoiesis-stimulating agents for anaemia in chronic heart failure patients. The Cochrane database of systematic reviews. 2010:CD007613. doi: 10.1002/14651858.CD007613.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lippi G, Franchini M, Favaloro EJ. Thrombotic complications of erythropoiesis-stimulating agents. Seminars in thrombosis and hemostasis. 2010;36:537–49. doi: 10.1055/s-0030-1255448. [DOI] [PubMed] [Google Scholar]
- 34.Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. Journal of the American Society of Nephrology. JASN. 2005;16:2180–9. doi: 10.1681/ASN.2004121039. [DOI] [PubMed] [Google Scholar]
- 35.Koulouridis I, Alfayez M, Trikalinos TA, Balk EM, Jaber BL. Dose of erythropoiesis-stimulating agents and adverse outcomes in CKD: a metaregression analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61:44–56. doi: 10.1053/j.ajkd.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. The New England journal of medicine. 1998;339:584–90. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 37.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA : the journal of the American Medical Association. 2008;299:914–24. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 38.Kirklin JK, Naftel DC, Kormos RL, et al. Intermacs Analysis of Pump Thrombosis in The Heartmate II Left Ventricular Assist Device. The Journal of Heart and Lung Transplantation. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Cowger J, Sundareswaran K, Rogers JG, et al. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. Journal of the American College of Cardiology. 2013;61:313–21. doi: 10.1016/j.jacc.2012.09.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


