Abstract
Background
Recruitment of individuals into clinical trials is a critical step in completing studies. Reports examining the effectiveness of different recruitment strategies, and specifically in infertile couples, are limited.
Methods
We investigated recruitment methods used in two NIH sponsored trials, Pregnancy in Polycystic Ovary Syndrome (PPCOS II) and Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS), and examined which strategies yielded the greatest number of participants completing the trials.
Results
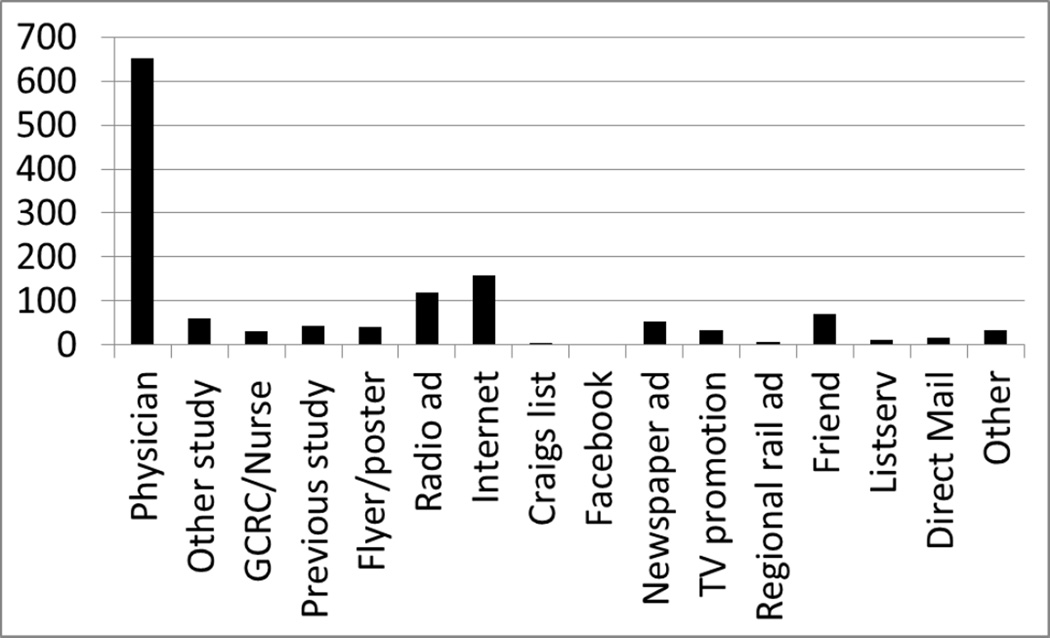
3683 couples were eligible for screening. 1650 participants were randomized and 1339 completed the trials. 750 women were randomized in PPCOS II; 212 of the participants who completed the trial were referred by physicians. Participants recruited from radio ads (84/750) and the internet (81/750) resulted in similar rates of trial completion in PPCOS II. 900 participants were randomized in AMIGOS. 440 participants who completed the trial were referred to the study by physicians. The next most successful method in AMIGOS was use of the internet, achieving 78 completed participants. Radio ads proved the most successful strategy in both trials for participants who earned <$50,000 annually. Radio ads were most successful in enrolling white patients in PPCOS II and black patients in AMIGOS. Seven ancillary Clinical Research Scientist Training (CREST) sites enrolled 324 of the participants who completed the trials.
Conclusions
Physician referral was the most successful recruitment strategy. Radio ads and the internet were the next most successful strategies, particularly for women of limited income. Ancillary clinical sites were important for overall recruitment.
Keywords: Clinical trial, recruitment, subjects
Introduction
Recruitment of participants into clinical trials is a costly and often challenging step in the successful completion of studies. Protracted or insufficient recruitment may result in increased total study costs if enrollment must be extended, or low quality data if the enrollment goal is not met. A recent study at a large academic medical center reported that one-third of all clinical trials terminated between 2005 and 2009 had low enrollment and that these low-enrolling studies cost the institution almost $1 million annually (1). Given the critical need for the highest levels of medical evidence to inform practice in reproductive medicine, and considering the substantial number of clinical trials that risk sacrificing their scientific benefit due to poor enrollment, we sought to identify successful recruitment strategies in two recently completed randomized controlled trials (RCTs) performed by the National Institute of Child Health and Human Development (NICHD) Cooperative Reproductive Medicine Network (RMN) in infertile couples. The Pregnancy in Polycystic Ovary Syndrome II (PPCOS II) and the Assessment of Multiple Intrauterine Gestation from Ovarian Stimulation (AMIGOS) trials were conducted through the National Institutes of Health (NIH) funded multicenter RMN collaborative sites, located throughout the United States. We studied the methods utilized in these two large NIH sponsored clinical trials, which collected the same recruitment information in a prospective manner, to identify effective and efficient recruitment strategies for randomized controlled trials involving infertile couples.
Methods
The progress of women who were referred or self-referred to 7 primary and 7 ancillary clinical sites of the NICHD’s Cooperative RMN was tracked prospectively by the Data Coordinating Center (DCC). Enrollment for PPCOS II and AMIGOS clinical trials was conducted from 2009 through 2012. PPCOS II was a 20 week prospective, multicenter, double blinded, two-armed, randomized trial of clomiphene citrate (CC) and letrozole for the treatment of infertility in women with polycystic ovary syndrome. Details of the trial design and its primary outcomes have been reported (2,3). Its primary aim was to compare the safety and efficacy of CC, a selective estrogen receptor modulator and letrozole, an aromatase inhibitor, in achieving live birth in infertile women with polycystic ovary syndrome (PCOS). Three thousand three hundred and fifty eight women were referred as potential participants for the trial. One thousand fifty four women underwent screening and seven hundred fifty infertile women between 18 and 40 years of age with PCOS were randomized. The AMIGOS trial was a randomized, partially blinded, three-armed trial that examined whether treatment of couples with unexplained infertility with letrozole results in a lower rate of multiple gestations compared with ovarian stimulation with CC or gonadotropins, without significantly impairing the live birth rate. Details of the trial design have been reported (4). Three thousand seven hundred twenty seven women were referred and nine hundred infertile women (300 per treatment arm), ages 18–40 years, were enrolled. The overall goal of the inclusion/exclusion criteria was to identify a population of ovulatory infertile women with a normal uterine cavity, at least one patent tube and a male partner with motile sperm count of at least 5 million in the ejaculate. The participants were treated with ovarian stimulation with letrozole, CC, or gonadotropins; all were in combination with intrauterine insemination.
Researchers recruited subjects from individual gynecology and infertility practices, faculty/resident continuity clinics, and referring physicians. Physician referral included referral from the investigator’s own practice and external physician referrals. Investigators were encouraged to meet with a local university and hospital public relations representatives and local media to plan a news release about the study, and be available for any newspaper, radio, or TV stories. Local advertisements included posted flyers and paid advertisements on local radio stations, direct mailing and newspapers. Contact was made with infertility support groups. The trials were registered at Clinicaltrials.gov and details were posted on the RMN website. Each enrollment site publicized the study on their local practice and hospital websites. Many sites used internet postings such as Facebook or Craigslist. The RMN maintains a Recruitment Committee for all of its trials and this committee held monthly teleconferences to review enrollment and foster discussion of recruitment strategies to optimize the flow of participants to each site and maintain timely recruitment goals. Sites freely shared advertising and promotional materials to facilitate recruitment. Each recruitment site was permitted to advertise and register patients using approaches that were best suited for their academic center and region. Efforts were made to reach minority patients. One site (University of Texas) with a large Hispanic population translated consents into Spanish and employed Spanish speaking staff to facilitate recruitment of Hispanic patients into the trials. A variety of recruitment strategies were utilized to reach a more diverse patient population, including regional rail ads, grocery cart ads, and movie ads.
Another recruitment strategy utilized in the trial was adding ancillary study recruitment sites. In December 2010, RMN principal investigators began mentoring Clinical Research/Reproductive Scientist Training (CREST Scholars to begin recruitment and implementation of RMN trials. CREST is a one-year clinical research program supported by the NICHD, the Clinical Research Training Program (CRTP) at Duke University School of Medicine and the American Society for Reproductive Medicine (ASRM). The primary goal of adding the CREST sites was to enable the scholars to gain greater experience with clinical trials while also serving to augment recruitment to the studies as this increased the number of gynecology and infertility practices from which to draw potential participants.
Each of the RMN and CREST sites reported to the DCC the total number of prescreened participants, number of ineligible participants at prescreening or those not interested in proceeding with screening, and reasons for ineligibility. Recruitment methods used were tabulated prospectively, as well as how many subjects were eligible for screening from each recruitment method used. The prescreening data and enrollment reports collected by the DCC from each site were compiled and are the basis for this report. Demographics were recorded for each subject and were tracked for each recruitment method. This report limits the analysis to those recruitment methods that had >50 recruited/enrolled patients (physician referral, other study coordinator referral, radio station, and internet); chi-square and Fisher's exact tests were used to compare recruitment methods for race, income and education. A questionnaire was sent to each site inquiring about site specific costs for each of the recruitment methods utilized. Completion of the study by a subject was defined as completion of the pregnancy test at the fifth cycle in the PPCOS trial and at the fourth cycle in the AMIGOS trial for women who did not conceive during the trial, or by the occurrence of pregnancy during the treatment phase in either of the trials.
Results
A total of 7085 couples were referred to both trials. Three thousand six hundred fifty of these couples met eligibility criteria for screening. One thousand three hundred thirty nine couples completed the trials (Tables 1 and 2). The most common reason for screening ineligibility was loss of contact or failure of the participant to return phone messages. At most recruitment sites physician referrals, both from investigators’ practices and outside referrals, resulted in the greatest number of randomized participants. Data collection for the trials did not specify how many referrals came from each type of physician referral. Seven hundred seventy six couples were randomized and six hundred fifty two participants completed the study out of one thousand three hundred and five women referred from physicians. The next most successful methods of recruitment were through radio ads (120 completed participants) and the internet; including hospital and practice websites (159 completed participants). Referrals from friends, general clinical research centers, nurses, or other study coordinators resulted in a smaller numbers of referred patients, but of the patients referred from these sources > 75% completed the study. Social media sites, including Facebook and Craigslist, were less successful, leading to only 4 participants who completed the studies. Enrollment in PPCOS II began prior to the AMIGOS study enrollment and was completed in 34.6 months. Enrollment in AMIGOS was completed in 25.9 months.
Table 1.
Number of patients eligible for screening/ Number of patients referred to trials
| PPCOS II | AMIGOS | |
|---|---|---|
| Physician | 443/633 | 629/943 |
| Other study | 112/166 | 34/64 |
| GCRC/Nurse | 35/70 | 18/47 |
| Previous study patient | 34/54 | 49/82 |
| Flyer/poster | 70/134 | 37/76 |
| Radio ad | 248/508 | 201/343 |
| Internet | 54/111 | 14/68 |
| Craigslist | 50/97 | 6/20 |
| 5/7 | 1/3 | |
| Newspaper ad | 48/108 | 49/83 |
| TV promotion | 192/318 | 143/340 |
| Regional rail ad | 22/22 | 2/6 |
| Friend | 78/120 | 87/163 |
| Listserv | 107/190 | 64/442 |
| Direct mail | 31/58 | 73/228 |
| Other | 63/164 | 34/64 |
Table 2.
Number of participants who completed PPCOS II and AMIGOS trials by recruitment method
The CREST sites added four clinical sites to the PPCOS II trial and enrolled 70 of the 750 participants, and added six clinical sites to the AMIGOS trial that enrolled 254 of the 900 participants. Thus, addition of clinical recruitment sites through CREST was an important component of overall recruitment. CREST sites used the same recruitment modalities as the RMN sites.
Demographics
Demographic characteristics (e.g., race, ethnicity, education and income) for both trials are listed in Table 3. The AMIGOS trial had overall more highly educated participants with higher reported incomes than participants in PPCOS II.
Table 3.
Demographics of participants
| PPCOSII (N=750) | AMIGOS (N=900) | P value* | |
|---|---|---|---|
| Age (years), mean±SD | 28.9±4.3 | 32.2±4.2 | <0.001 |
| Ethnicity | |||
| Hispanic | 17.1% | 10.4% | <0.001 |
| Race | |||
| White | 78.7% | 80.2% | 0.002 |
| Black | 13.3% | 9.3% | |
| Asian | 3.2% | 6.6% | |
| American Indian | 0.9% | 1.1% | |
| Native Hawaiian | 0.3% | 0.0% | |
| Mixed race | 3.6% | 2.8% | |
| Education | <0.001 | ||
| High school | 23.1% | 8.1% | |
| College | 65.5% | 65.4% | |
| Graduate school | 11.5% | 26.4% | |
| Income | <0.001 | ||
| <$50,000 | 40.0% | 16.9% | |
| $50–100,000 | 40.5% | 44.3% | |
| >$100,000 | 5.2% | 21.2% | |
| Wish to not answer | 14.3% | 17.6% |
P values were calculated with the use of the chi-square test or Fisher’s exact test for categorical data and the Wilcoxon rank-sum test for continuous data
In both trials, income and race were significantly related to the success of the recruitment methods. PPCOS II patients earning <$50,000 had a significantly higher (and those earning >50,000 had a significantly lower) rate of recruitment from radio ads than from other recruitment methods. AMIGOS patients with an income <$50,000 had a significantly higher rate of recruitment from radio ads than from other recruitment methods. An evaluation of the association of race and recruitment method yielded different results for each trial. In PPCOS II, white patients had a significantly higher (and black patients had a significant lower) rate of recruitment from radio ads than from other recruitment methods. In AMIGOS, black patients had a significantly higher (and other race groups had a significantly lower) rate of recruitment from radio ads than from other recruitment methods. Educational level was not related to recruitment methodology in PPCOS II (p=0.14 for recruitment methods vs. education). In AMIGOS, patients with a high school degree had a significantly higher rate of recruitment/enrollment from radio ads; those with college degree had a significantly higher rate of recruitment/enrollment from friends; and those with a graduate degree had a significantly higher rate of recruitment/enrollment from physician referral than from other recruitment methods.
Costs
Physician referral, the most effective strategy, was free at all but one site that paid a total of $183 for mailings to referring physicians. Advertising costs varied greatly by site. Many sites were able to combine advertising simultaneously for the two trials as a means of cost reduction. The most expensive advertising method was radio, ranging cumulatively from $5,660 to $35,280 per site depending on frequency and air time. Print media ads, such as newspaper and magazine, ranged from $164 to $8,609 per site. The least expensive type of advertising was use of hospital and practice websites that were free to all the clinical sites. Social media sites were free of charge, but less effective at recruiting and enrolling participants for these two trials. The monetary costs of recruitment methods at all sites for PPCOS II and AMIGOS are listed in Table 4. The exact cost of each recruitment method to the RMN grant is difficult to quantify as many sites combined other resources, such as marketing for their practice, to cover the cost of advertising.
Table 4.
Estimated cost of recruitment method for PPCOS II and AMIGOS.
| Recruitment method | Estimated cost (US$) |
|---|---|
| Local newspaper/magazine ad | 22200.59 |
| Radio ad | 83778.00 |
| Direct mail | 17060.00 |
| Movie ad | 4665.00 |
| Flyer/poster | 1744.97 |
| Trial X | 1600.00 |
| Grocery cart ad | 1555.00 |
| Placemat ad | 750.00 |
Discussion
Despite the cost and critical part recruitment plays in completing a clinical trial, there are limited data documenting effective strategies. A recent Cochrane systematic review quantified the effects of strategies to improve recruitment of participants in 45 trials. Unfortunately, most of the interventions differed between studies, making comparisons difficult (5). Prior studies in primary care trials reported enhanced recruiting through repeated promotion delivered by locally based principal investigators well known to their medical community (6,7). A similar method of repeated promotion was employed by the RMN sites using radio advertisements that were aired at recurring intervals. Frequent updates on the progress of the recruitment and the value of the trial to patients were highlighted in these studies. The single most effective strategy resulting in the greatest number of eligible participants in both RMN trials was physician referrals of patients to the study. These referrals came from both local referring doctors as well as the investigators’ own clinical practice. This strategy however, may limit access to only those women who have insurance coverage. This avenue of referral may increase as more people obtain medical insurance coverage.
A study by Krushe at al (8), evaluating effective recruitment methods in a psychiatric study, reported minimal success with referrals from general practitioners. Referrals to trials may prove to be most effective in specialties that have close relationships between general providers and subspecialists, as is common in fertility treatment practice. This strategy may be relatively unique to reproductive medicine fertility treatment trials and may not be as generalizable to other areas of medicine.
Radio ads and TV promotion were also effective strategies for attracting eligible participants, particularly in patients with lower incomes. Radio ads proved the most successful strategy in both trials for participants who earned <$50,000 annually. One site with high recruitment from radio ads initially tried an easy listening station but had a more robust response after changing to a country music station and airing the ads to coincide with the time of a local rodeo event. Another site aired radio ads to coincide with a local NASCAR auto race. These strategies may have reached a wider audience and played a role to help target the patients from lower income brackets. There was no clear recruitment strategy that was uniformly more successful in recruiting patients from minority populations for both trials. Radio ads were most successful in enrolling white participants in PPCOS II and black participants in AMIGOS. Sites with high recruitment from radio and internet sites (hospital and practice websites) used this approach in both trials. Two sites, one in Detroit, MI and one in San Antonio, TX, had the highest recruitment from radio and TV ads and had low numbers of physician referrals. This may be related to the patient base and catchment area of each clinical site.
Using financial incentives facilitates recruitment (9,10). Neither RMN trial directly paid a stipend to participants. However, the cost of the diagnostic evaluation of both female and male partners and the fertility treatments were paid by the study, resulting in substantial indirect financial incentives to the participants. The PPCOS II and AMIGOS trials paid several thousand dollars towards the cost of diagnostic tests and monitoring per patient. The study medications were free to patients. Many infertile patients do not have insurance coverage for these costly tests and treatments and therefore participation in these trials offered a significant incentive even without direct stipends. The strongest incentive for recruitment is the yearning often felt by infertile patients who want to have children but cannot afford the infertility testing or treatment.
A business marketing model for recruitment has been advocated which emphasizes “buy in” from the public and collaborators (11) and provides frequent positive reinforcement for sites recruiting well. These business-type practices were implicitly conducted in both trials during the bimonthly teleconferences between RMN sites and the DCC in which monthly enrollment data and effective recruitment strategies were shared. Sites recruiting well were acknowledged during the teleconferences by the DCC and a quarterly certificate was presented to the site that had the highest recruitment rates for each trial.
Expanding clinical sites by utilizing the CREST Scholar sites helped to support timely recruitment for both RCTs. As this was not done in a systematic fashion, it is difficult to estimate its full impact on the recruitment. CREST sites were initially chosen based on the size of their clinical practice and access to the patient populations of interest to the RMN among respondents to an informal survey of past and current CREST Scholars. By the time the AMIGOS trial was underway, an effort was made to involve CREST sites that were particularly active in treating large numbers of women with unexplained infertility. The large proportion of total participants in AMIGOS who came from a CREST site underscores the value of adding recruitment venues whenever possible to enhance participant flow and avoid flagging enrollment.
The primary limitation of this study is that while the enrollment and recruitment data were collected prospectively in a uniform manner for each site, the cost of each recruitment method was collected retrospectively. Cost data was collected through a questionnaire after completion of the trials. The true costs of each recruitment method was difficult to ascertain as many of the sites incorporated study advertising into practice marketing materials. Sites also had cost overlap between PPCOS II and AMIGOS by using the same ads or flyers for both studies. The other consideration of the findings is the great variability among sites regarding which strategy was most successful for the site’s local patient population. For example, radio ads were highly effective in a minority of the sites. This may limit the generalizability of the study’s findings. A significant limitation was the source of physician referrals in our data base was not collected prospectively. Many investigators recruited well from within their own practices while other investigators relied on outside referrals and required more advertising for recruitment.
The use of social media through Craigslist and Facebook as a recruitment tool for both RMN trials was not an effective method in recruiting patients; furthermore, very few patients who responded to these methods completed the trials. This finding differs from several publications identifying social media as a successful method of recruiting patients into clinical trials. Most reports using social media as a recruitment tool for trials are targeting a relatively young adult population which differs from the population in the RMN trials. However, some studies with a similar target of reproductive age reported higher recruitment rates with social media methods. For example, Lohse (12) reported a 17% response rate and 11% completion rate for women age 8–45 recruited into a nutrition study through social media networks. Shere et al (13) reported a 12-fold increase in recruitment of women (mean age 31 years old) into a periconception study after implementation of social media methods. One important distinction between the RMN trials and many of the published trials which report greater success with social media recruitment is that the RMN trials required frequent office visits. In general, the studies that have reported success with social media recruitment involved online questionnaires (12–14).
Conclusion
The PPCOS II and AMIGOS trials successfully completed enrollment close to the anticipated recruitment period by using multiple strategies and employing frequent communication and encouragement between study sites and the DCC. Expanding the number of clinical sites helped recruit more participants in a timely manner. Physician referrals, hospital and practice websites were less costly methods and physician referral was the single most successful method of recruitment for most of the RMN sites. Radio ads had more variable effectiveness across sites in terms of numbers of participants recruited, but still proved to be a relatively successful method, particularly in sites that had fewer physician referrals. Compared to other recruitment techniques, use of social media sites were less effective at recruiting participants at all of the sites, but had minimal costs.
Acknowledgments
Supported by the National Institutes of Health (NIH)/ Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grant 5R25HD075737. Also supported by U10 HD39005 (to M.P.D.), U10 HD38992 (to R.S.L.), U10 HD27049 (to C.C.), U10 HD38998 (to W.D.S and R.A.), HD055944 (to P.C.), U10 HD055936 (to G.C.), U10 U54-HD29834 (to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core of the Specialized Cooperative Centers Program in Reproduction and Infertility Research) and by the National Center for Research Resources and the National Center for Advancing Translational Sciences through an NIH grant (UL1 TR000127) to Pennsylvania State University. This research also was made possible by the funding by American Recovery and Reinvestment Act (U10HD039005-0851 to M.P.D., U10HD055925-02W1 to H.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kitterman DR, Cheng SK, Dilts DM, Orwoll Eric S. The Prevalence and Economic Impact of Low-Enrolling Clinical Studies at an Academic Medical Center. Acad Med. 2011;86(11):1360–1366. doi: 10.1097/ACM.0b013e3182306440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legro RS, Kunselman AR, Brzyski RG, Casson PR, Diamond MP, Schlaff WD, Christman GM, Coutifaris C, Taylor HS, Eisenberg E, Santoro N, Zhang H. NICHD Reproductive Medicine Network The Pregnancy in Polycystic Ovary Syndrome II (PPCOS II) trial: rationale and design of a double-blind randomized trial of clomiphene citrate and letrozole for the treatment of infertility in women with polycystic ovary syndrome. Contemp Clin Trials. 2012 May;33(3):470–481. doi: 10.1016/j.cct.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, Christman GM, Huang H, Yan Q, Alvero R, Haisenleder DJ, Barnhart KT, Bates GW, Usadi R, Lucidi S, Baker V, Trussell JC, Krawetz SA, Snyder P, Ohl D, Santoro N, Eisenberg E, Zhang H NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014 Jul 10;371(2):119–129. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond MP, Mitwally M, Casper R, Ager J, Legro RS, Brzyski R, Casson P, Eisenberg E, Zhang H NICHD Cooperative Reproductive Medicine Network. Estimating rates of multiple gestation pregnancies: sample size calculation from the assessment of multiple intrauterine gestations from ovarian stimulation (AMIGOS) trial Contemp. Clin Trials. 2011 Nov;32(6):902–908. doi: 10.1016/j.cct.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treweek S, Mitchell E, Pitkethly M, Cook J, Kjeldstrom M, Johansen M, Taskila TK, Sullivan F, F Wilson S, Jackson C, Jones R, Lockhart P Cochrane Methodology Review Group. Strategies to improve recruitment to randomized controlled trials. Cochrane Database of Syst. 2011 doi: 10.1002/14651858.MR000013.pub5. Rev 10. [DOI] [PubMed] [Google Scholar]
- 6.Prout H, Butler C, Kinnersley P, Robling M, Hood K, Tudor-Jones R. A qualitative evaluation of implementing a randomized controlled trial in general practice. Fam Pract. 2003;20:675–681. doi: 10.1093/fampra/cmg609. [DOI] [PubMed] [Google Scholar]
- 7.McKinstry B, Hammersley V, Daly F, Sullivan F. Recruitment and retention in a multicentre randomised controlled trial in Bell's palsy: A case study. BMC Med Res Methodol. 2007;7:15. doi: 10.1186/1471-2288-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krusche A, Rudolf von Rohr I, Muse K, Duggan D, Crane C, Williams JM. An evaluation of the effectiveness of recruitment methods: The staying well after depression randomized controlled trial. Clin Trials. 2014;11(2):141–149. doi: 10.1177/1740774514521905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Free C, Hoile E, Robertson S, Knight R. Three controlled trials of interventions to increase recruitment to randomized controlled trial of mobile phone based smoking cessation support. Clin Trials. 2010;7:265–273. doi: 10.1177/1740774510367687. [DOI] [PubMed] [Google Scholar]
- 10.Bentley JP, Thacker PG. The influences of risk and monetary payment on the research participation decision making process. J Med Ethics. 2004;30:293–298. doi: 10.1136/jme.2002.001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald AM, Treweek S, Shakur H, Free C, Knight R, Speed C, Campbell MK. Using a business model approach and marketing techniques for recruitment to clinical trials. Clin Trials. 12:74. doi: 10.1186/1745-6215-12-74. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohse B. Facebook is an effective strategy to recruit low-income women to online nutrition education. J Nutr Educ Behav. 2013 Jan-Feb;45(1):69–76. doi: 10.1016/j.jneb.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Shere M, Zhao XY, Koren G. The role of social media in recruiting for clinical trials in pregnancy. PLoS One. 2014 Mar 26;9(3):e92744. doi: 10.1371/journal.pone.0092744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonard A, Hutchesson M, Patterson A, Chalmers K, Collins C. Recruitment and retention of young women into nutrition research studies: practical considerations. Trials. 2014;15:23. doi: 10.1186/1745-6215-15-23. PMCID: PMC3901327. [DOI] [PMC free article] [PubMed] [Google Scholar]