Abstract
Several fluorinated derivatives of the anti-HIV maturation agent bevirimat (1) were synthesized and evaluated for anti-HIV replication activity. The modified positions were the C-2, C-3, C-28, and C-30 positions, either directly on the betulinic acid (2) skeleton or in the attached side chains. Compound 18, which has a trifluoromethyl group added to C-30 of its isopropenyl group, exhibited similar potency to 1 against HIV-1NL4-3. In total, our current studies support our prior conclusion that C-30 allylic modification is unlikely to be a pharmacophore for anti-HIV activity, but could be a meaningful route to manipulate other properties of 2-related compounds.
Keywords: Fluorinated betulinic acid derivatives, Bevirimat, Anti-HIV activity
HIV-1 infection affects more than 30 million people worldwide, and its treatment remains a serious problem due to the emergence of drug-resistant HIV strains and deleterious side effects. Thus, the discovery and development of new drug candidates with novel anti HIV mechanisms remain important to solve the problems of this disease.
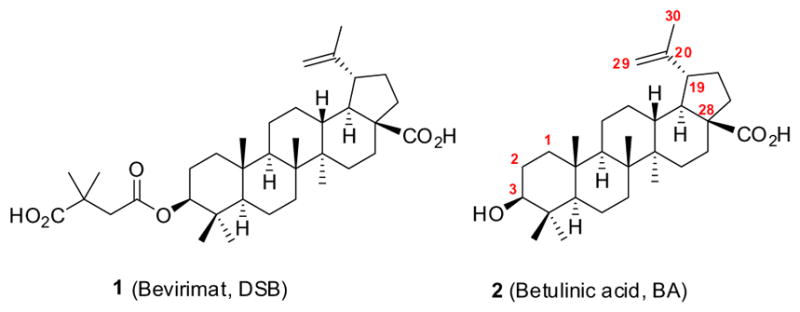
Bevirimat (DSB, 1 in Fig. 1), a triterpene natural product derivative, represents a promising class of anti-HIV agents with a novel mechanism. It inhibits HIV-1 maturation by blocking the cleavage of p25 to functional p24, resulting in the production of noninfectious HIV-1 particles. In recent years, efforts to improve the anti-HIV activity of 1 have focused on modifications at the C-3 and C-28 positions, particularly, insertion of side chains containing various functional groups, such as anhydrides, amino acids, and N-heterocycles.1 Correspondingly, success was achieved by the synthesis of new compounds with better EC50 data than 1 itself. However, improving the anti-HIV potency is not necessarily the sole driving force for modification of 1. For instance, structural changes can help define pharmacophores related to biological profile as well as potency. Also, the possibility of creating a new lead compound with action at a different HIV process/enzyme is always significant and meaningful in the field of anti-HIV research. Consequently, alteration of the activity or biological profile of a preclinical drug candidate can often be regarded as an important strategy in drug design.
Figure 1.
Fluorinated drugs and drug candidates based on natural products are present in many therapeutic classes. Fluorine has unique physical properties, such as strong electronegativity, small atomic size, and low polarizability of the C—F bond, and can mimic either a hydrogen or hydroxy group.2–7 A recent literature review extensively discussed fluorine’s effects on conformation, pKa, intrinsic potency, membrane permeability, metabolic pathways, and pharmacokinetic properties, concluding that its incorporation into a molecule can be significantly important in medicinal chemistry and the design of valuable future drugs.8 Thus, the introduction of one or more fluorine atoms into the structure of 1 could dramatically influence the lipophilicity, conformational flexibility, metabolic stability, or other properties, which could be useful in modulating the biological activity or pharmaceutical profile of 1. With this consideration in mind, herein, we report the synthesis of fluorinated derivatives of 1 and evaluation of their anti-HIV activities.
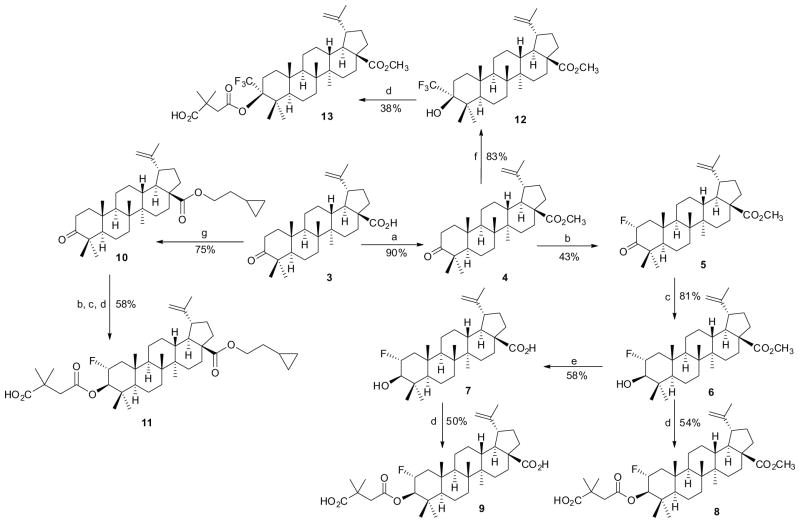
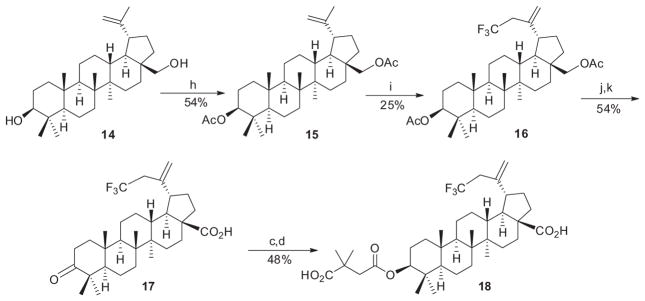
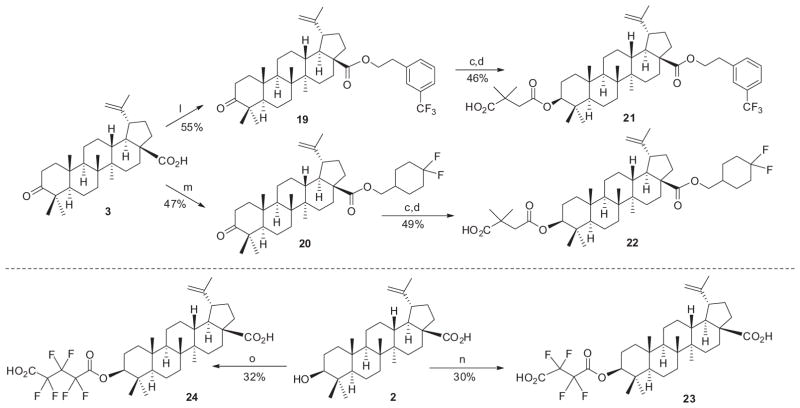
Readily modifiable positions of the betulinic acid (BA, 2) scaffold include the C-2, C-3, C-28, and C-19 positions. We introduced fluorine atoms either directly at C-2 or C-3 as shown in Scheme 1 or indirectly in the C-29, or C-3 and C-28 side chains of 2 as shown in Schemes 2 and 3 (see Supplementary data for the detailed synthetic procedures). Therefore, we could evaluate the effects of fluorine atoms in several different positions.
Scheme 1.
Reagents and conditions: (a) CH3I, K2CO3, DMF, 40 °C; (b) LDA, NFSI, THF, −78 °C–rt; (c) NaBH4, EtOH/THF, rt; (d) 2,2-dimethylsuccinic anhydride, DMAP, pyridine, 120 °C; (e) LiI, DMF, 140 °C; (f) CF3Si(CH3)3, Bu4N+F−, THF, rt; then 6 M HCl; (g) (CO)2Cl2, CH2Cl2, rt; then 2-cyclopropylethanol, Et3N, CH2Cl2, rt.
Scheme 2.
Reagents and conditions: (h) Ac2O, pyridine, CH2Cl2, rt; (i) S-(trifluoromethyl)diarylsulfonium salt, CuTc, 2,4,6-collidine, DMA, 40 °C; (j) 2 N NaOH, THF/EtOH, rt; (k) CrO3/H2SO4, acetone, 0–5 °C.
Scheme 3.
Reagents and conditions: (l) (CO)2Cl2, CH2Cl2, rt; then 3-(trifluoromethyl)phenethyl alcohol, Et3N, CH2Cl2, rt; (m) (CO)2Cl2, CH2Cl2, rt; then 4,4-difluorocyclohexane methanol, Et3N, CH2Cl2, rt; (n) tetrafluorosuccinic anhydride, CH2Cl2, 0 °C–rt; (o) hexafluoroglutaric anhydride, CH2Cl2, 0 °C–rt.
Firstly, as shown in Scheme 1, the CO2H group of betulinic acid (3) was protected with CH3I to obtain the ester 4. Then, compound 4 was treated with lithium diisopropylamide (LDA) and N-fluorobenzenesulfonimide (NFSI) at −78 °C, resulting in the introduction of one fluorine atom at the C-2 α-position (5) in 43% yield. Similarly, if the CO2H group was esterified with 2-cyclopropyl ethanol, one fluorine atom was also introduced easily from the α-face at the C-2 position at −78 °C. In addition, when 4 was treated with CF3Si(CH3)3, a CF3 functional group was inserted at the C-3 position (12). Furthermore, application of classical organic synthetic methods including reduction by NaBH4, esterification with 2,2-dimethylsuccinic anhydride, or removal of a methyl ester with LiI, the desired fluorinated products 8, 9, 11, and 13 were obtained.
Secondly, the fluorinated modification of the isopropenyl group on C-19 is shown as Scheme 2. Betulin (14) was protected as its diacetate 15. Then, a CF3 group was added at the C-30 position in 25% yield by treating 15 with S-(trifluoromethyl)diarylsulfonium salt using copper thiophenecarboxylate (CuTC) as catalyst to produce the fluorinated key intermediate 16. The desired product 18 was obtained in a four-step sequence, deprotection of 16 with 2 N NaOH in THF/EtOH, oxidization with CrO3/H2SO4, reduction with NaBH4, and finally, esterification with 2,2-dimethylsuccinic anhydride.
Thirdly, fluorine atoms were introduced at various positions in the side chains on the BA C-3 and C-28 positions as shown in Scheme 3. Compounds 21 and 22, in which the C-28 side chain terminates in a trifluoromethylphenyl and difluorocyclohexyl group, respectively, were synthesized easily via esterification of 3 with 3-(trifluoromethyl)phenethyl alcohol or 4,4-difluorocyclohexane methanol, respectively. Fluorine atoms were incorporated in the C-3 side chain by esterifying 2 directly with tetrafluorosuccinic or hexafluoroglutaric anhydride to provide 23 and 24, respectively.
All of the synthesized new fluorinated compounds (8, 9, 11, 13, 18, 21–24) were evaluated for anti HIV activity by determining inhibition of NL4-3 virus replication in MT4 lymphocytes.9 Table 1 shows the anti-HIV results for the tested compounds, with the lead compound 1 as a positive control.
Table 1.
Anti-HIV data for fluorinated target compounds
| Compd | EC50 (μM) NL4–3 | # of tests | CC50 (μM) MT4 |
|---|---|---|---|
| 8 | >16 | 4 | >16 |
| 9 | >17 | 4 | >17 |
| 11 | 1.1 ± 0.28 | 3 | >6 |
| 13 | >6 | 3 | >6 |
| 18 | 0.097 ± 0.025 | 3 | >6 |
| 21 | 3.0 ± 0.49 | 3 | >5 |
| 22 | 0.31 ± 0.067 | 3 | >6 |
| 23 | >6 | 1 | >6 |
| 24 | >6 | 1 | >6 |
| 1 (Bevirmat, DSB) | 0.075 ± 0.0155 | 4 | >17 |
Note: For compounds 8 and 9, the highest concentration tested was 17 μM (10 μg/mL). For the remaining compounds 11, 13, 18, and 21–24, the highest concentration tested was 6 μM (4 μg/mL).
With one exception, the fluorinated compounds were much less active than the non-fluorinated lead compound 1. Five compounds (8, 9, 13, 23, and 24) did not show anti-HIV potency at the highest concentration tested. Indeed, the inactivity of 9, which differs structurally from 1 only by a fluorine rather than hydrogen at C-2, might suggest that a proton at C-2 could play a critical role for anti HIV activity. The importance of the dimethylsuccinyl C-3 ester group to the anti-HIV activity was confirmed by the inactivity of the fluorinated esters 23 and 24. Two compounds with fluorine in the C-28 ester side chain showed only weak anti-HIV activity with (4,4-difluorocyclohexyl)methyl (22) resulting in greater potency than trifluoromethylphenethyl (21). However, compounds 1 and 18, which has a trifluoromethyl group added to C-30 of the isopropenyl group of 1, exhibited similar potency against HIV-1NL4-3. Based on the data, this position was the only one among those tested to actually tolerate the addition of a fluorine atom. This finding was not unexpected, because prior studies had shown that certain changes in the isopropenyl group (e.g., saturation of the 20(29) double bond,10 thioether,11 or oxyether12 substitution at C-30) did not appreciably impact the anti-HIV-1 potency of derivatives of 2. Thus, our studies confirm the previous conclusion that C-30 allylic modification is unlikely to be a pharmacophore for anti-HIV activity, but could be a meaningful route to manipulate other properties of 2-related compounds.12
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) Grant AI33066 (K.H.L.).
Footnotes
Supplementary data (general methods, synthetic procedures, biological assay) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2015.11.029.
References and notes
- 1.See: Sun IC, Chen CH, Kashiwada Y, Wu JH, Wang HK, Lee KH. J Med Chem. 2002;45:4271. doi: 10.1021/jm020069c.Yu D, Sakurai Y, Chen CH, Chang FR, Huang L, Kashiwada Y, Lee KH. J Med Chem. 2006;49:5462. doi: 10.1021/jm0601912.Dang ZD, Lai WH, Qian K, Lee KH, Chen CH, Huang L. J Med Chem. 2009;52:7887. doi: 10.1021/jm9004253.Qian K, Kuo RY, Chen CH, Huang L, Morris-Natschke SL, Lee KH. J Med Chem. 2010;53:3133. doi: 10.1021/jm901782m.Qian K, Bori ID, Chen CH, Huang L, Lee KH. J Med Chem. 2012;55:8128. doi: 10.1021/jm301040s.Dang Z, Ho P, Zhu L, Qian K, Lee KH, Huang L, Chen CH. J Med Chem. 2013;56:2029. doi: 10.1021/jm3016969.
- 2.Nakano T, Makino M, Morizawa Y, Matsumura Y. Angew Chem, Int Ed Engl. 1996;35:1019. [Google Scholar]
- 3.O’Hagan D, Harper DB. J Fluorine Chem. 1999;100:127. [Google Scholar]
- 4.Murphy CD, Schaffrath C, O’Hagan D. Chemosphere. 2003;52:455. doi: 10.1016/S0045-6535(03)00191-7. [DOI] [PubMed] [Google Scholar]
- 5.Swinson J. Halocarbon Products Corp. Pharmaceuticals. 2005 [Google Scholar]
- 6.Isanbor C, O’Hagan D. J Fluorine Chem. 2006;127:303. [Google Scholar]
- 7.Wang J, Sanchez-Rosello M, Acena JL, del Pozo C, Sorochinsky AE, Fustero S, Soloshonok VA, Liu H. Chem Rev. 2014;114:2432. doi: 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- 8.Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA. J Med Chem. 2015 doi: 10.1021/acs.jmedchem.5b00258. http://dx.doi.org/10.1021/acs.jmedchem.5b00258. [DOI] [PubMed]
- 9.Asada Y, Sukemori A, Watanabe T, Malla KJ, Yoshikawa T, Li W, Kuang X, Koike K, Chen CH, Akiyama T, Qian K, Nakagawa-Goto K, Morris-Natschke SL, Lu Y, Lee KH. J Nat Prod. 2013;76:852. doi: 10.1021/np300815t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Lee KH. J Med Chem. 1996;39:1016. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- 11.Evers M, Poujade C, Soler F, Ribeill Y, James C, Lelievre Y, Gueguen JC, Reisdorf D, Morize J, Pauwels R, De Clercq E, Henin Y, Bousseau A, Mayaux JF, Le Pecq JB, Derett N. J Med Chem. 1996;39:1056. doi: 10.1021/jm950670t. [DOI] [PubMed] [Google Scholar]
- 12.Qian K, Yu D, Chen CH, Huang L, Morris-Natschke SL, Nitz TJ, Salzwdedl K, Reddick M, Allaway GP, Lee KH. J Med Chem. 2009;52:3248. doi: 10.1021/jm900136j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.