Abstract
Background
Our previous pilot studies aimed to examine the role of hydrogen sulfide (H2S) in the generation of endothelial progenitor cells led to an unexpected result, i.e., H2S promoted the differentiation of certain hematopoietic stem/progenitor cells in the bone marrow. This gave rise to an idea that H2S might promote hematopoiesis.
Methods
To test this idea, a mice model of myelosuppression and cultured fetal liver cells were used to examine the role of H2S in hematopoiesis.
Results
H2S promoted the generation of megakaryocytes, increased platelet levels, ameliorate entorrhagia, and improved survival. These H2S effects were blocked in both in vivo and in vitro models with thrombopoietin (TPO) receptor knockout mice (c-mpl−/− mice). In contrast, H2S promoted megakaryocytes/platelets generation in both in vivo and in vitro models with TPO knockout mice (TPO−/− mice).
Conclusions
H2S is a novel promoter for megakaryopoiesis by acting on the TPO receptors but not TPO to generate megakaryocytes/platelets.
Electronic supplementary material
The online version of this article (doi:10.1186/s13045-016-0244-7) contains supplementary material, which is available to authorized users.
Keywords: Hydrogen sulfide, Myelosuppression, Hematopoiesis
Background
Serious myelosuppression is one of the fatal diseases manifested as a suppression on all types of hematopoietic cells and remains as one of the most difficult diseases to treat to date. Myelosuppression is either primary including aplastic anemia (AA) and myelodysplastic syndrome (MDS) or secondary to various disease status including chemotherapy and accidental/therapeutic radiation exposure [1–3]. Among the severe complications of myelosuppression, thrombocytopenia is immediately life threatening. Platelet transfusion is currently applied to treat severe thrombocytopenia; however, it has various side effects being common with the transfusion of blood components, and the endogenous generation of platelet is not ameliorated with such therapy [4, 5]. A more etiological treatment is the administration of recombinant thrombopoietin (TPO) which exerts significant effects in promoting platelet generation; however, it also has some side effects such as induction of autoantibodies due to the cross-reactions with endogenous TPO [4, 6, 7]. Since the effects of TPO receptor agonists are mild [6, 8, 9], exploration for new approaches to treat severe myelosuppression has potential translational values.
TPO has been established as a main cytokine to promote the generation of megakaryocytes and platelets by acting on its receptor, c-mpl [10–12]. The most established role of TPO is to promote the generation and differentiation of the hematopoietic stem cells in the bone marrow by acting on the TPO receptors, particularly the megakaryocytic stem/progenitor cells differentiated to megakaryocytes [13–15]. The TPO receptors are also found in the leukocytic and erythrocytic hematopoietic stem cells to be involved in the generation of leukocytes and erythrocytes in adulthood bone marrow [14, 16, 17].
Hydrogen sulfide (H2S) is endogenously generated in mammals via the H2S-generating enzymes cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST) [18–20]. H2S was considered as a metabolite without any physiological roles at the very start. The first piece of evidence reflecting a physiological role of H2S was found in organ bath experiments where an H2S donor, sodium hydrosulfide (NaHS), caused relaxation of the isolated vessels [21]. In the following years, H2S has been found to regulate the function of the cardiovascular system [22–24], the nervous system [25–27], the endocrine system [28, 29], and the immune system [30–32], among which the cardiovascular role of H2S is the most well established, including a regulation of vascular tone [33, 34] and remodeling [35–37], a protection against ischemia/reperfusion injury of the cardiomyocytes and myocardium [38, 39], regulation of ion channels [22, 40], and promotion of angiogenesis [41–43]. To date, any potential role of H2S in hematopoiesis is unknown.
Here, we report a novel protective effect of the gas molecule, H2S, against fatal myelosuppression being dependent on c-mpl. This is an unexpected finding in our studies investigating the role of endothelial progenitor cells in the proangiogenic effects of H2S which we found previously [41, 44, 45]. Though our data showed that the endothelial progenitor cells were not involved in these H2S effects, these pilot experiments revealed a novel promoting effect of H2S on hematopoiesis. This prompts us to propose a hypothesis that H2S may ameliorate myelosuppression.
In the present study, an H2S donor (NaHS) was applied to treat fatal myelosuppression in a mice model exposed to radiation and this is the first effort to examine the role of the gas transmitter H2S on hematopoiesis.
Results
H2S promotes megakaryocytes/platelets generation, alleviates bleeding, and improves survival in WT mice with radiation-induced myelosuppression
Exposure to radiation caused significant arrest of the bone marrow evidenced with a decrease in platelet levels in days 7, 14 and 21 in a mice model. The eliminating effect of radiation on circulating platelets peaked at days 7 and 14. Circulating platelet levels rebounded to control levels at day 28 and remained stable afterwards (Fig. 1a). Treatment with NaHS caused a significant increase in platelet levels at day 7 at dosages of 100 and 150 μmol kg−1 day−1 as compared with the vehicle-treated mice with radiation-induced myelosuppression (Fig. 1b). TPO treatment (15 μg kg−1) also increased platelet levels at day 7 (Fig. 1b). Morphological examination showed that NaHS treatment (100 μmol kg−1 day−1) significantly increased the number of megakaryocytes in the bone marrow of the mice exposed to radiation at day 7 (Fig. 1c). Bleeding time was approximately sixfold longer in the mice exposed to radiation at day 7, and NaHS treatment (100 μmol kg−1 day−1) significantly decreased bleeding time in the mice exposed to radiation (Fig. 1d). Likewise, fecal occult blood was significantly increased in the mice exposed to radiation as compared with those in the controls without exposure to radiation. NaHS treatment (100 μmol kg−1 day−1) significantly decreased fecal occult blood in the mice exposed to radiation (Fig. 1e). Plasma TPO levels were dramatically increased after radiation exposure; however, they were not changed by NaHS treatment (100 μmol kg−1 day−1) (Fig. 1f). Radiation exposure caused a mortality rate of 58 and 71 % at day 14 and day 21, respectively. Mortality did not further increase after day 28 in all groups. Survival was significantly increased in the mice treated with NaHS at a dosage of 100 μmol kg−1 day−1 at days 14, 21, 28, 35, and 42 after radiation exposure (P < 0.05 vs the vehicle-treated group) (Fig. 1g). TPO treatment (15 μg kg−1) also caused a significant increase in survival (Fig. 1g). On the other hand, endogenous H2S generation might be decreased after radiation exposure, since we found decreased plasma H2S levels after radiation exposure at day 7 (Fig. 1h), whereas NaHS treatment (100 μmol kg−1 day−1) recovered plasma H2S to a level comparable to that in the mice without radiation exposure (Fig. 1h).
Fig. 1.
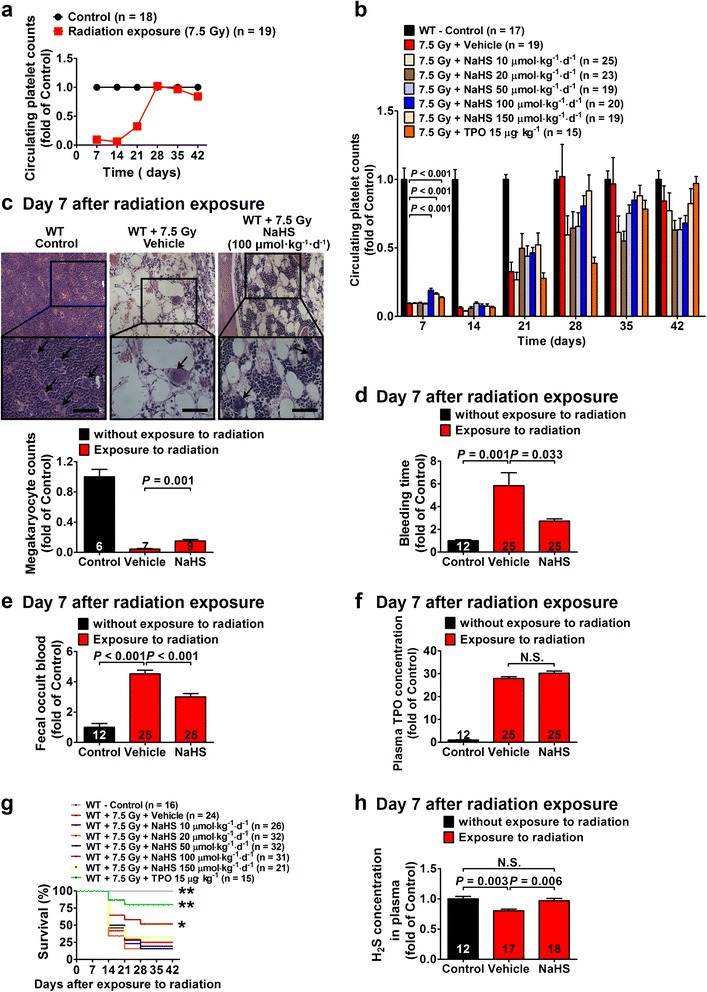
H2S treatment promotes platelet generation, alleviates bleeding, and improves survival in WT mice with radiation-induced myelosuppression. a Time course of circulating platelet counts after exposure to radiation of 137Cs at a single dose of 7.5 Gy. b NaHS treatment caused a significant increase in circulating platelet levels at 100 and 150 μmol kg−1 day−1 on day 7 after radiation exposure. TPO treatment also provided a similar amelioration at 15 μg kg−1. c Morphological examination showed a significant decrease in the number of megakaryocytes (black arrow) in the bone marrow of WT mice exposed to radiation, whereas NaHS (100 μmol kg−1 day−1) significantly increased the number of megakaryocytes as compared with the vehicle-treated group. Scale bar = 250 μm. d Bleeding time was prolonged after exposure to radiation, and bleeding was ameliorated by NaHS treatment (100 μmol kg−1 day−1) as compared with that of vehicle-treated WT mice. e Likewise, fecal occult blood was significantly increased after exposure to radiation and NaHS treatment (100 μmol kg−1 day−1) caused a significant decrease in fecal occult blood in mice exposed to radiation. f Plasma TPO concentrations were increased in WT mice on day 7 after radiation exposure where NaHS treatment (100 μmol kg−1 day−1) did not change TPO concentrations. g Radiation exposure resulted in a significant decrease in survival as compared with that of the mice without radiation exposure. Both NaHS (100 μmol kg−1 day−1) and TPO (15 μg kg−1) treatment significantly improved survival in these mice exposed to radiation. *P < 0.05, **P < 0.01 versus vehicle-treated mice exposed to radiation, log-rank test. h Plasma H2S levels were decreased in mice exposed to radiation, and this decrease was recovered by administration of NaHS (100 μmol kg−1 day−1). Data in the graphs are means ± SEM. P values less than 0.05 represent statistical significance
H2S promotes differentiation of the hematopoietic stem cells into megakaryocytes
To investigate if the abovementioned in vivo effect of H2S resulted from a stimulation on the hematopoietic stem cells, the potential effect of H2S on the differentiation of the hematopoietic stem cells into megakaryocytes was examined with flow cytometry where CD61 was used as the surface molecular marker for the megakaryocyte (Fig. 2a). These cells were further examined with confocal microscopy with double-staining using fluorescent-labeled antibodies against CD41 (green) and CD61 (red) where these two markers were co-localized on the cell membrane (Fig. 2c). This is in accordance with the literature that both CD41 and CD61 are expressed on the cell membrane of mature megakaryocytes. Since the hematopoietic stem cells are originally generated in fetal liver at the 11.5 day during the embryonic period (E11.5) and then expand at E14.5 before migration to the bone marrow [46, 47], we harvested the E14 fetal liver cells which were rich in hematopoietic stem cells to examine the in vitro effect of H2S in the present study. NaHS treatment concentration dependently promoted differentiation of cultured fetal liver cells into the megakaryocytes at 10, 20, 50, and 100 μmol L−1 as examined with flow cytometry (Fig. 2a). Platelet generation was also increased in the cultured fetal liver cells treated with NaHS at concentrations of 50 and 100 μmol L−1 examined with flow cytometry defined as CD41-positive and 7-AAD-negative signals, respectively (Fig. 2b). H2S-induced increase in megakaryocyte generation at a concentration of 100 μmol L−1 was even higher than that induced by TPO at 10 ng mL−1 (Fig. 2a), whereas TPO treatment (10 ng mL−1) showed a more significant effect in promoting platelet generation than NaHS treatment (100 μmol L−1) (Fig. 2b). Scanning electron microscopy showed the morphology of the megakaryocytes where pseudopod formation (blue arrow) and membrane blebbing (yellow arrow head) were observed in the cells treated with NaHS (Fig. 2d).
Fig. 2.
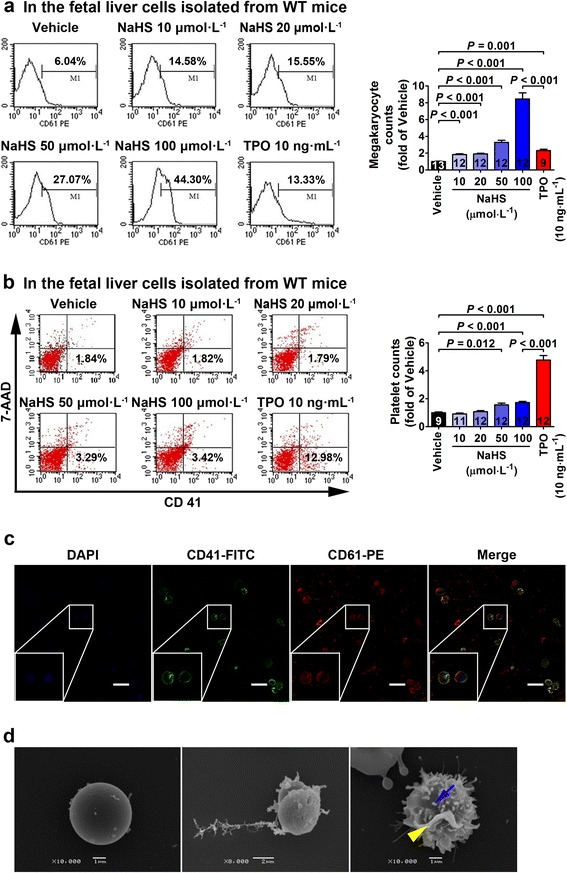
H2S treatment promotes generation of megakaryocytes/platelets in cultured fetal liver cells. a NaHS treatment caused a concentration-dependent increase in megakaryocytes which were identified as CD61-positive cells using flow cytometry in fetal liver cells isolated from WT mice. b Platelets were identified as CD41-positive and 7-AAD-negative signals using flow cytometry. Platelet counts were significantly increased with NaHS treatment at 50 and 100 μmol L−1 in the culture medium of fetal liver cells. TPO treatment (10 ng mL−1) also increased platelet counts. c Morphology of megakaryocytes in cultured fetal liver cells were shown with a confocal fluorescence microscopy with staining of DAPI, CD41-FITC, and CD61-PE. Scale bar = 50 μm. d Scanning electron microscopy revealed pseudopod formation (blue arrow) and membrane blebbing (yellow arrow) in the megakaryocytes identified in cultured fetal liver cells treated with NaHS (100 μmol L−1). Data in the graphs are means ± SEM. P values less than 0.05 represent statistical significance
c-mpl is required for H2S to promote megakaryocytes/platelets generation and improve survival
To further clarify how H2S can promote megakaryocytes generation and provide protection against fatal myelosuppression, some knockout mice models were applied. TPO has a pivotal role in the generation of megakaryocytes and platelets by acting on its receptor, c-mpl [10–12]. The data showed that platelet count was decreased by about 80 % in c-mpl−/− mice as compared with that in wild-type (WT) mice (Fig. 3a). NaHS treatment (20 μmol kg−1 day−1) for 14 days caused an increase in circulating platelet levels in WT mice but not in c-mpl−/− mice (Fig. 3a). Likewise, NaHS treatment promoted the generation of megakaryocytes in the fetal liver cells isolated from WT mice at concentrations ranging from 10 to 100 μmol L−1 (Fig. 2a) but not in the fetal liver cells isolated from the c-mpl−/− mice (Fig. 3b). Circulating platelet levels were significantly decreased in c-mpl−/− mice as compared with those of the WT mice. Radiation exposure caused significant decrease in circulating platelet levels in both WT and c-mpl−/− mice, and this decrease was partially recovered with NaHS treatment (100 μmol kg−1 day−1) in WT mice but not in c-mpl−/− mice (Fig. 3c). Likewise, NaHS treatment increased megakaryocyte levels in the bone marrow in WT mice exposed to radiation (Fig. 1c) but not in those of the c-mpl−/− mice (Fig. 3d). Bleeding time (Fig. 3e) and fecal occult blood (Fig. 3f) were not ameliorated by NaHS treatment (100 μmol kg−1 day−1) in the c-mpl−/− mice exposed to radiation at day 7, though these parameters were improved in the WT mice by NaHS treatment (100 μmol kg−1 day−1) (Fig. 1d, e). Finally, NaHS treatment (100 μmol kg−1 day−1) improved survival after radiation exposure in WT mice (Fig. 1g) but not in c-mpl−/− mice (Fig. 3g).
Fig. 3.
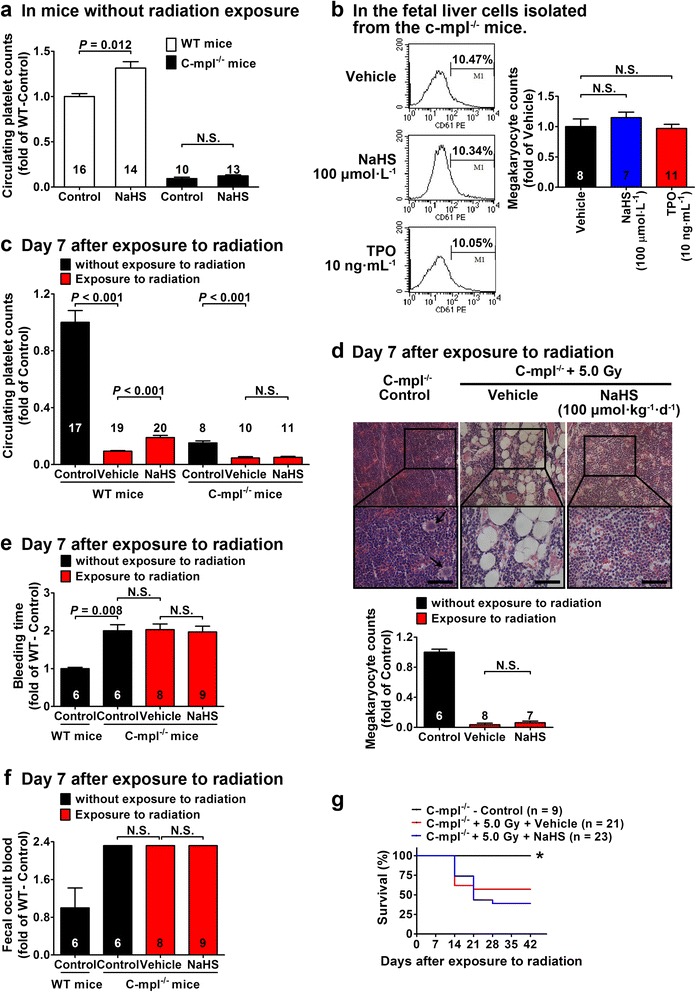
H2S-induced promotion of megakaryocytes/platelets generation and improvement of survival are blunted in c-mpl−/− mice. a Without radiation exposure, NaHS treatment (20 μmol kg−1 day−1) for 14 days caused an increase in circulating platelet levels in WT mice but not in c-mpl−/− mice. b Flow cytometry showed that NaHS treatment (100 μmol L−1) failed to increase the number of megakaryocytes identified as CD61-positive cells in cultured fetal liver cells isolated from c-mpl−/− mice. c Circulating platelets were significantly decreased in both WT and c-mpl−/− mice exposed to radiation at day 7. NaHS treatment (100 μmol kg−1 day−1) increased platelet levels in WT mice but not in c-mpl−/− mice. d Morphological examination of the bone marrow showed that NaHS treatment (100 μmol kg−1 day−1) failed to increase the number of megakaryocytes (black marrows) in c-mpl−/− mice. Scale bar = 250 μm. e Bleeding was not ameliorated with NaHS treatment (100 μmol kg−1 day−1) in c-mpl−/− mice exposed to radiation at day 7. f Likewise, fecal occult blood was not ameliorated with NaHS treatment (100 μmol kg−1 day−1) in c-mpl−/− mice exposed to radiation at day 7. g NaHS treatment (100 μmol kg−1 day−1) failed to improve survival in c-mpl−/− mice exposed to radiation as compared to vehicle-treated mice. *P < 0.05 versus vehicle-treated mice exposed to radiation, log-rank test. Data in the graphs are means ± SEM. P values less than 0.05 represent statistical significance
The role of TPO in H2S-induced promotion on megakaryocytes/platelets generation
On the other hand, TPO is the main cytokine to promote the production of megakaryocytes and platelets [11, 48, 49]. Though plasma TPO levels were not increased by H2S treatment in WT mice exposed to radiation (Fig. 1f), the role of TPO in mediating the hematopoietic effects of H2S was still worthy to be investigated. Therefore, the potential hematopoietic effects of H2S were also examined in TPO−/− mice with or without radiation exposure and in the fetal liver cells isolated from the TPO−/− mice. TPO knockout caused about 90 % reduction in circulating platelet levels in mice without radiation exposure (Fig. 4a). NaHS treatment (20 μmol kg−1 day−1) for 14 days caused about a 43 % increase in circulating platelet levels in TPO−/− mice (Fig. 4a). Megakaryocyte generation was promoted with NaHS treatment (100 μmol L−1) in fetal liver cells isolated from the TPO−/− mice, and this effect was more pronounced than that with exogenous TPO treatment (10 ng mL−1) (Fig. 4b). Radiation exposure caused a significant decrease in circulating platelet levels in both WT mice and TPO−/− mice (Fig. 4c). NaHS treatment (100 μmol kg−1 day−1) increased circulating platelet levels in both WT mice and TPO−/− mice exposed to radiation (Fig. 4c), whereas this H2S effect in TPO−/− mice was less pronounced than that in WT mice (Fig. 4d). However, NaHS treatment (100 μmol kg−1 day−1) did not cause a significant increase in megakaryocyte levels in the bone marrow of the TPO−/− mice exposed to radiation (Fig. 4e). Likewise, NaHS treatment (100 μmol kg−1 day−1) failed to ameliorate bleeding (Fig. 4f) and fecal occult blood (Fig. 4g) despite an increase in the circulating platelet levels in TPO−/− mice with radiation exposure and treated with NaHS treatment (100 μmol kg−1 day−1). As a final consequence, NaHS treatment (100 μmol kg−1 day−1) did not improve survival in TPO−/− mice exposed to radiation (Fig. 4h) although survival was improved in WT mice (Fig. 1g).
Fig. 4.
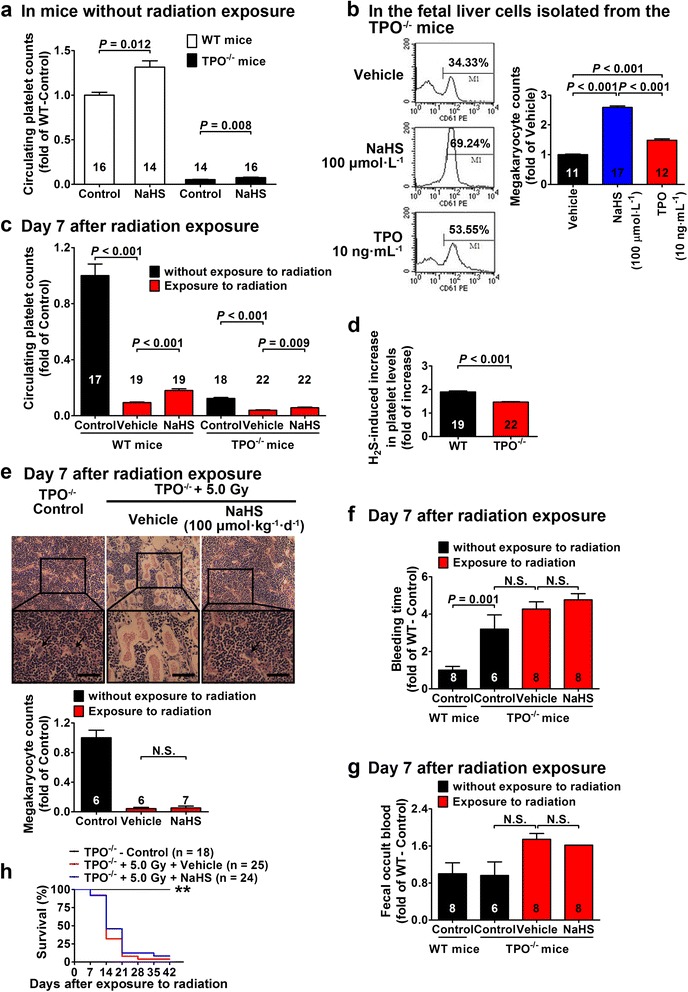
H2S promotes megakaryocytes/platelets generation but does not improve survival in TPO−/− mice. a Without radiation exposure, NaHS treatment (20 μmol kg−1 day−1) for 14 days caused an increase in circulating platelet levels in both WT and TPO−/− mice. b NaHS treatment (100 μmol L−1) increased the number of megakaryocytes identified as CD61-positive cells using flow cytometry in cultured fetal liver cells isolated from TPO−/− mice. c, d NaHS treatment (100 μmol kg−1 day−1) increased circulating platelet levels on day 7 after radiation exposure in both WT and TPO−/− mice (c). However, the effects of NaHS treatment (100 μmol kg−1 day−1) in increasing circulating platelet levels were blunted in TPO−/−mice (d). e Morphological examination of the bone marrow showed that NaHS treatment (100 μmol kg−1 day−1) did not increase the number of megakaryocytes (black arrows) in the TPO−/− mice exposed to radiation. Scale bar = 250 μm. f Bleeding was not ameliorated with NaHS treatment (100 μmol kg−1 day−1) in the TPO−/− mice exposed to radiation at day 7. g Likewise, fecal occult blood was not ameliorated with NaHS treatment (100 μmol kg−1 day−1) in the TPO−/− mice exposed to radiation at day 7. h Survival was not improved with NaHS treatment (100 μmol kg−1 day−1) in TPO−/− mice exposed to radiation. **P < 0.01 versus vehicle-treated mice exposed to radiation, log-rank test. Data in the graphs are means ± SEM. P values less than 0.05 represent statistical significance
Discussion
This is the first time that the gas transmitter H2S has been found to protect against fatal myelosuppression with improvement in bone marrow morphology, circulating platelet levels, and final survival. The increased survival in H2S-treated mice may be ascribed to the decreased internal bleeding. This idea was supported by less bleeding time and fecal occult blood found in these H2S-treated mice. Moreover, these effects were found at physiologically relevant dosages of H2S since the treatment just recovered the decrease in plasma H2S levels in radiation-exposed mice. Therefore, these findings have potential translational values and may be further explored for new approaches to treat fatal diseases resulting from severe myelosuppression.
In the embryonic period, hematopoiesis mainly occurs in the liver where the hematopoietic stem cells were differentiated into various types of blood cells [50–52]. Here, we show a novel protective role of H2S in fatal myelosuppression, i.e., H2S ameliorates internal bleeding and improves survival by promoting generation of megakaryocytes and platelets. Future works are required to further validate this hypothesis using multiple approaches including bone marrow transplantation experiments.
We found here that the hematopoietic effects of H2S were blunted in the c-mpl−/− mice exposed to radiation and also in the fetal liver cells isolated from the c-mpl−/− mice. These data suggest that the TPO receptors are essential for H2S to promote hematopoiesis. In the adult stage, the TPO receptors are expressed on different maturation stages of megakaryocyte and early hematopoietic stem/progenitor cells in the bone marrow [53–55]. The predominant role of the TPO receptors is to mediate the signals to induce proliferation and differentiation of megakaryocyte progenitor cells to generate megakaryocytes and platelets [10–12]. In addition, since the TPO receptors are also expressed on the hematopoietic stem/progenitor cells, it is also involved in the expansion of erythroid, granulocyte-macrophage, and megakaryocytic progenitor cells [14, 16, 17]. Although there are also some evidence showing expression of the TPO receptors in the tissues outside of the bone marrow, e.g., in placenta, fetal liver, fetal blood, cord blood, and peripheral blood, bone marrow is the main place of hematopoiesis in adulthood [51, 52, 56]. In this context, if the hematopoietic effect of H2S is dependent on the TPO receptors, these H2S effects are mediated via the TPO receptors located in the hematopoietic stem/progenitor cells in the bone marrow. Indeed, the absence of more direct evidence using bone marrow transplantation models is a limitation of our current study.
The mechanisms about how H2S would activate the TPO receptors are unknown. How a molecule as small as H2S targets its “receptors” is one of the most challenging questions in the field. Our previous studies aim to find that the “receptor” of H2S shows that VEGFR2 serves as a direct target molecule for H2S to induce angiogenesis. The study further shows that H2S targets a specific molecular switch, i.e., the Cys1024-Cys1045 motif, to regulate the conformation and function of VEGFR2 by sulfur-sulfur nucleophilic attack [44]. This suggests a new mechanism beyond the typical docking between a ligand and its receptor. Some theories of atomic biology involving molecular orbitals are involved in this H2S-induced regulation of VEGFR2. Interestingly, the TPO receptors and VEGFR2 belong to the receptor tyrosine kinase family. Some members of this kinase family, e.g., EGFR, have been recently found to contain an intracellular kinase domain similar to that of the VEGFR2 also with a molecular switch labile to H2S regulation [57]. This gives rise to an idea that the TPO receptors might also contain a similar intracellular kinase domain with a molecular switch sensitive to H2S regulation. Indeed, much future works are required to examine this hypothesis.
In TPO−/− mice exposed to radiation, H2S also failed to ameliorate internal hemorrhage and improve survival despite a significant increase in circulating platelet levels. In addition, H2S promoted megakaryocytes/platelets generation in cultured fetal liver cells isolated from TPO−/− mice but not in c-mpl−/− mice. These data suggest an essential role of the TPO receptor but not TPO for H2S to promote hematopoiesis. The question is why H2S increased platelet levels in TPO−/− mice without ameliorating bleeding and subsequent mortality? This discrepancy might be ascribed to a significantly decreased survival in TPO−/− mice treated with vehicle as compared with that in c-mpl−/− mice (P < 0.01, Additional file 1: Figure S1). Myelosuppression might be too severe to be recovered by H2S in these TPO−/− mice, whereas the TPO receptors were present in the cells isolated from the TPO−/− mice and might serve as a target molecule for H2S to promote hematopoiesis.
In addition, H2S also increased circulating leukocytes in WT mice exposed to radiation at day 14 though erythrocytes were not increased (Additional file 2: Figure S2). Our present data are not sufficient to clarify whether such an improvement in circulating leukocyte counting contributes to an increase in survival in mice exposed to radiation due to an improvement in the immune system.
Conclusions
Taken together, treatment for fatal myelosuppression is proven a difficult challenge in medicine. Here, we report a novel protective role of H2S in a mice model of fatal myelosuppression. This finding has potential translational values which could be further explored to develop new approaches to treat fatal myelosuppression. Moreover, experiments using the models with knockout of the TPO receptors or TPO itself indicate that the hematopoietic effect of H2S is dependent on the TPO receptors but not on TPO. In addition, the endogenous generation of H2S was reduced in the myelosuppression model and a recovery of the H2S levels was associated with a protection against radiation exposure. These findings shed some light to uncovering a new mechanism to regulate hematopoiesis by a gas transmitter, H2S.
Methods
Animals
Wild-type C57BL/6 mice (WT mice) were purchased from the Department of Laboratory Animal Science of Fudan University and acclimatized for at least 2 weeks before experiments. c-mpl is the receptor of TPO; the c-mpl knockout mice (c-mpl−/− mice) were provided by The Jackson Laboratory (Bar Harbor, ME, USA) with an 80 % decrease in the number of platelets in comparison to that of wild-type C57BL/6 mice [58, 59] (the strain name of these c-mpl−/− mice is C57BL/6J-Mplhlb219/J with the stock number 005124). Likewise, TPO-deficient mice (TPO−/− mice) have a >80 % decrease in their platelets [59, 60]. The embryos of TPO−/− mice were gifts kindly provided by Dr. Fred de Sauvage (Genentech Inc., USA) and developed into mice through the Embryo Recovery Service of The Jackson Laboratory (these TPO−/−mice were produced by targeted mutation on the background of C57BL/6J mice; for more details, see de Sauvage [60]). Both TPO−/− and c-mpl−/− mice were hybridized with WT mice and then their offspring were genotyped according to the protocol provided by The Jackson Laboratory. Homozygote animals were used in the experiments while heterozygote mice were maintained for breeding. Only female mice were used in the experiments. Mice were given free access to food and water while housed in a clean environment with appropriate temperature and humidity. At the time of experiments, all mice were 6–8 weeks old weighting 18–21 g. Considering that in the experiment mice will die or suffer severe sickness in the process, we set at least 10 mice per group at the beginning and repeated the experiment if necessary. To ensure the reliability of results, mice were randomly allocated to each group. All procedures were approved by the Institutional Animal Care and Use Committee, Fudan University Shanghai Medical College (20120302-098).
Irradiation-induced myelosuppression
The Cs-137 instrument (Gammacell-40, MDS Nordion, CA) was used as the γ-ray irradiation source in this study. Six- to 8-week-old mice were either exposed to 7.5 Gy (WT mice) or 5.0 Gy (TPO−/− and c-mpl−/− mice) cesium (137Cs)-emitted radiation for total body irradiation (TBI) to build up the myelosuppression model. The TPO−/− and c-mpl−/− mice were more sensitive to radiation exposure, and the mortality was too high to perform subsequent experiments in these mice. We performed a pilot study to explore the intensity of radiation exposure in these mice to cause radiation-induced myelosuppression with a mortality within the range that enables the subsequent experiments to be performed. The results showed 5.0 Gy as the radiation intensity for the TPO−/− and c-mpl−/− mice.
Platelet counts
Platelet counts were measured by automatic blood cell analyzer (XN-1000, Sysmex, Kobe, Japan). About 80–100 μL peripheral blood was obtained from the angular vein of individual mice then pumped into a 1.5-mL Eppendorf tube coated with ethylenediaminetetraacetic acid (EDTA) and mixed immediately. For platelet counting, whole blood was used without any dilution. During all experiments, every single mouse was sampled at 7-day intervals. Frequency and timing of sampling of each individual mouse was identical in all types of mice in this study. In this part, the operator of the automatic blood analyzer was blinded to the sample groups. Results were excluded in cases where there was clotting in the sample.
Bleeding time test
The tails of mice were placed horizontally then amputated about 3 mm from the tip and immersed into saline solution pre-warmed and maintained at 37 °C. The time from severing to cessation of bleeding was recorded as bleeding time, and the longest observation time was 20 min to avoid over bleeding which frequently occurred in the TPO−/− and c-mpl−/− mice. Researchers were blinded to the sample groups.
Fecal occult blood test
A fecal occult blood test kit (Baso, Zhuhai, China) was used to examine occult gastrointestinal bleeding according to protocols provided by the manufacturer. Briefly, 20–30 mg feces were smeared on the window of the test card then dripped with a drop of developer A (pyramidon). After sufficient penetration, a drop of developer B (alcohol and hydrogen peroxide) was dripped and then the results were read by comparing with a color card within 2 min. Researchers were blinded to the sample groups.
Bone marrow megakaryocytes counts
The femur and tibia were collected from some mice sacrificed at day 7 during the experimental period. After fixing in 10 % formaldehyde and dehydrated, the bone samples were embedded into paraffin, sliced, and stained with hematoxylin and eosin. The morphology of the bone marrow was assessed via light microscopy (Leica DMLB, Leica, Germany), and megakaryocytes were counted by an observer blinded to the sample groups. For WT mice, no less than 10 visions were observed in each sample, whereas for TPO−/− and c-mpl−/− mice the megakaryocytes were counted throughout the whole slice of the samples.
Cell culture
Mouse fetal livers were collected at E14, cut to pieces, digested with 0.125 % Tryspin (Life, Thermo fisher scientific, Waltham, MA, USA), and then filtered successively through 200-mesh (pore size, 74 μm) and 400-mesh (pore size, 37 μm) stainless steel screens. The yielded cells were cultured in RPMI1640 (Gibco, Thermo Fisher scientific, Waltham, MA, USA) supplemented with 10 % FBS (HyClone, Logan, Utah, USA) and incubated at 37 °C in 5 % CO2.
Flow cytometry
Cultured fetal liver cells were stained with PE-CD61 (104308, 1:100, Biolegend, San Diego, CA), FITC-CD41 (13903, 1:200, BioLegend, San Diego, CA), or 7-AAD viability staining solution (420403, 1:20, BioLegend, San Diego, CA) according to instructions provided by the manufacturer. Briefly, about one million cells were collected and resuspended in 100 μL phosphate-buffered saline (PBS) and then stained with respective antibodies according to its recommended usage for 30 min at 4 °C. Then the cells were centrifuged at 1000 rpm for 5 min, and the supernatant was discarded. Then the cells were washed in 1 mL PBS twice before being resuspended in 500 μL PBS. FACS analysis was performed with a flow cytometer (FACSCalibur, BD, USA). More than 10,000 cells were acquired and analyzed by CELLQuest. For each experiment of this part, fetal liver cells isolated from at least three individual mice were used and for at least three repetitions for each group. The operator of the flow cytometer was blinded to the sample groups.
Administration of H2S and TPO
NaHS (Sigma-Aldrich, St. Louis, MO, USA) was applied as a donor of H2S [45] in the present study. For the in vivo administration, NaHS and recombinant murine TPO (R&D, NASDAQ, USA) were freshly diluted in sterile saline or PBS for each administration. A single dose of TPO (15 μg kg−1) was given by intraperitoneal injection after radiation exposure whereas NaHS was injected once a day during the experimental period. For in vitro experiments, recombinant murine TPO (PeproTech, NJ, USA) was freshly diluted with cell culture medium and added to the cultured cells with a single dose of 10 ng mL−1 given at the first day of the experiments. NaHS was diluted with cell culture medium and added to the cultured cells every 8 h.
Scanning electron microscopy
Specimen were first double fixed with 2.5 % glutaraldehyde and 1 % osmium tetroxide and then gradiently dehydrated with ethanol followed by dehydration in HCP-2 critical point dryer (Haitchi, Tokyo, Japan) with liquid CO2. The dehydrated specimen was coated with gold palladium and then observed in a JSM-6360LV scanning electron microscope (JEOL, Tokyo, Japan).
Confocal microscopy
Cultured fetal liver cells were stained with PE-CD61 (12-0611, 1:40, eBioscience, San Diego, CA), FITC-CD41 (11-0411, 1:200, eBioscience, San Diego, CA), and the DAPI staining solution (C1006, 100 μL, Beyotime, China) and then examined with a confocal laser scanning microscope LSM 710 (Zeiss, Jena, Germany).
Measurement of plasma H2S
Plasma H2S levels in some mice were measured according to a method previously described [61]. The concentrations were determined using the standard curve generated with a standard NaHS solution.
Measurement of plasma TPO
Plasma TPO levels in some mice were measured using the Mouse Thrombopoietin Quantiken ELISA Kit (R&D, NASDAQ, USA) according to the manufacturer’s instructions.
Statistical analysis
Results were expressed as the mean ± standard error. Unless specifically stated, the data show normal distribution. For two groups, independent-sample t test was used. For more than two groups, statistical analysis was performed with one-way ANOVA followed by LSD if the variance is homogeneous or Tamhane’s T2 test when the variance is not homogeneous. For the fecal occult blood assay, the data were transformed in rank cases first and then analyzed with one-way ANOVA as above. Comparisons of mortality were performed with Kaplan-Meier survival curves, in which log-rank test was used to evaluate differences in survival. P < 0.05 was considered as statistically significant.
Acknowledgements
The authors thank Dr. Fred de Sauvage (Genentech Inc., San Francisco, CA) for providing the embryo of TPO−/− mice. This work was supported by grants from the National Natural Science Foundation of China (81230003), the Ministry of Science and Technology (2012ZX09501001-001-002) of China, the Science and Technology Commission of Shanghai Municipality (12JC1400700), and a key laboratory program of the Education Commission of Shanghai Municipality (ZDSYS14005).
Abbreviations
- AA
aplastic anemia
- EDTA
ethylenediaminetetraacetic
- H2S
hydrogen sulfide
- MDS
myelodysplastic syndrome
- NaHS
sodium hydrosulfide
- TBI
total body irradiation
- TPO
thrombopoietin
Additional files
Survival analysis of c-mpl −/− and TPO −/− mice after exposure to radiation and treated with vehicle. TPO−/− mice after exposure to 5.0 Gy 137Cs then treated with vehicle had a significantly decreased survival compared with that in c-mpl−/− mice .**P < 0.01, log-rank test. (PDF 44.1 kb)
H 2 S increases circulating leukocytes in WT mice exposed to radiation. (a) NaHS treatment did not change circulating erythrocytes at dosages of 10, 20, 50, 100, and 150 μmol kg−1 day−1 in WT mice exposed to radiation at day 7 and day 14. (b) NaHS treatment increased circulation leukocytes at a dosage of 100 μmol kg−1 day−1 in WT mice exposed to radiation at day 14 but not day 7. Data in the graphs are means ± SEM. P values less than 0.05 represent statistical significance. (PDF 311 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
H-DL and YC performed the experiments, A-JZ performed the experiments and drafted the manuscript, J-JX performed the pilot experiments, and Y-CZ designed the study and wrote the manuscript. All authors read and approved the final manuscript.
References
- 1.Henry MK, Lynch JT, Eapen AK, Quelle FW. DNA damage-induced cell-cycle arrest of hematopoietic cells is overridden by activation of the PI-3 kinase/Akt signaling pathway. Blood. 2001;98(3):834–41. doi: 10.1182/blood.V98.3.834. [DOI] [PubMed] [Google Scholar]
- 2.Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31(5):1319–39. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107(1):358–66. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Yang C, Xia Y, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98(12):3241–8. doi: 10.1182/blood.V98.12.3241. [DOI] [PubMed] [Google Scholar]
- 5.Schrezenmeier H, Seifried E. Buffy-coat-derived pooled platelet concentrates and apheresis platelet concentrates: which product type should be preferred? Vox Sang. 2010;99(1):1–15. doi: 10.1111/j.1423-0410.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Zheng L. The pharmacology and clinical application of thrombopoietin receptor agonists. Int J Hematol. 2014;100(6):529–39. doi: 10.1007/s12185-014-1660-5. [DOI] [PubMed] [Google Scholar]
- 7.Wormann B. Clinical indications for thrombopoietin and thrombopoietin-receptor agonists. Transfus Med Hemother. 2013;40(5):319–25. doi: 10.1159/000355006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stasi R. Eltrombopag: the discovery of a second generation thrombopoietin-receptor agonist. Expert Opin Drug Discov. 2009;4(1):85–93. doi: 10.1517/17460440802642484. [DOI] [PubMed] [Google Scholar]
- 9.Jeong JY, Levine MS, Abayasekara N, Berliner N, Laubach J, Vanasse GJ. The non-peptide thrombopoietin receptor agonist eltrombopag stimulates megakaryopoiesis in bone marrow cells from patients with relapsed multiple myeloma. J Hematol Oncol. 2015;8:37. doi: 10.1186/s13045-015-0136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Sauvage FJ, Hass PE, Spencer SD, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369(6481):533–8. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 11.Kaushansky K, Lok S, Holly RD, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369(6481):568–71. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- 12.Wendling F, Maraskovsky E, Debili N, et al. cMpl ligand is a humoral regulator of megakaryocytopoiesis. Nature. 1994;369(6481):571–4. doi: 10.1038/369571a0. [DOI] [PubMed] [Google Scholar]
- 13.Solar GP, Kerr WG, Zeigler FC, et al. Role of c-mpl in early hematopoiesis. Blood. 1998;92(1):4–10. [PubMed] [Google Scholar]
- 14.Kaushansky K, Lin N, Grossmann A, Humes J, Sprugel KH, Broudy VC. Thrombopoietin expands erythroid, granulocyte-macrophage, and megakaryocytic progenitor cells in normal and myelosuppressed mice. Exp Hematol. 1996;24(2):265–9. [PubMed] [Google Scholar]
- 15.Sitnicka E, Lin N, Priestley GV, et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood. 1996;87(12):4998–5005. [PubMed] [Google Scholar]
- 16.Kaushansky K, Broudy VC, Grossmann A, et al. Thrombopoietin expands erythroid progenitors, increases red cell production, and enhances erythroid recovery after myelosuppressive therapy. J Clin Invest. 1995;96(3):1683–7. doi: 10.1172/JCI118210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi M, Laver JH, Kato T, Miyazaki H, Ogawa M. Recombinant human thrombopoietin (Mpl ligand) enhances proliferation of erythroid progenitors. Blood. 1995;86(7):2494–9. [PubMed] [Google Scholar]
- 18.Predmore BL, Lefer DJ, Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal. 2012;17(1):119–40. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura H. Hydrogen sulfide and polysulfides as signaling molecules. Proc Jpn Acad Ser B Phys Biol Sci. 2015;91(4):131–59. doi: 10.2183/pjab.91.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson KR, Straub KD. The role of Hydrogen sulfide in Evolution and the Evolution of Hydrogen Sulfide in Metabolism and Signaling. Physiology (Bethesda) 2016;31(1):60–72. doi: 10.1152/physiol.00024.2015. [DOI] [PubMed] [Google Scholar]
- 21.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237(3):527–31. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 22.Ma SF, Luo Y, Ding YJ, et al. Hydrogen sulfide targets the Cys320/Cys529 motif in Kv4.2 to inhibit the Ito potassium channels in cardiomyocytes and regularizes fatal arrhythmia in myocardial infarction. Antioxid Redox Signal. 2015;23(2):129–47. doi: 10.1089/ars.2014.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagpure BV, Bian JS. Interaction of hydrogen sulfide with nitric oxide in the cardiovascular system. Oxid Med Cell Longev. 2016;2016:6904327. doi: 10.1155/2016/6904327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res. 2008;79(4):632–41. doi: 10.1093/cvr/cvn140. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Bereguiain MA, Samhan-Arias AK, Martin-Romero FJ, Gutierrez-Merino C. Hydrogen sulfide raises cytosolic calcium in neurons through activation of L-type Ca2+ channels. Antioxid Redox Signal. 2008;10(1):31–42. doi: 10.1089/ars.2007.1656. [DOI] [PubMed] [Google Scholar]
- 26.Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol. 2002;26(1):13–9. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- 27.Partlo LA, Sainsbury RS, Roth SH. Effects of repeated hydrogen sulphide (H2S) exposure on learning and memory in the adult rat. Neurotoxicology. 2001;22(2):177–89. doi: 10.1016/S0161-813X(01)00016-X. [DOI] [PubMed] [Google Scholar]
- 28.Ali MY, Whiteman M, Low CM, Moore PK. Hydrogen sulphide reduces insulin secretion from HIT-T15 cells by a KATP channel-dependent pathway. J Endocrinol. 2007;195(1):105–12. doi: 10.1677/JOE-07-0184. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko Y, Kimura Y, Kimura H, Niki I. L-cysteine inhibits insulin release from the pancreatic beta-cell: possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes. 2006;55(5):1391–7. doi: 10.2337/db05-1082. [DOI] [PubMed] [Google Scholar]
- 30.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. Faseb J. 2006;20(12):2118–20. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Bhatia M, Moore PK. Hydrogen sulphide--a novel mediator of inflammation? Curr Opin Pharmacol. 2006;6(2):125–9. doi: 10.1016/j.coph.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Bhatia M, Zhu YZ, et al. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. Faseb J. 2005;19(9):1196–8. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 33.Dunn WR, Alexander SP, Ralevic V, Roberts RE. Effects of hydrogen sulphide in smooth muscle. Pharmacol Ther. 2016;158:101–13. doi: 10.1016/j.pharmthera.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Denizalti M, Bozkurt TE, Akpulat U, Sahin-Erdemli I, Abacioglu N. The vasorelaxant effect of hydrogen sulfide is enhanced in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 2011;383(5):509–17. doi: 10.1007/s00210-011-0601-6. [DOI] [PubMed] [Google Scholar]
- 35.Bir SC, Kolluru GK, McCarthy P, et al. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc. 2012;1(5):e4093. doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qipshidze N, Metreveli N, Mishra PK, Lominadze D, Tyagi SC. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int J Biol Sci. 2012;8(4):430–41. doi: 10.7150/ijbs.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lilyanna S, Peh MT, Liew OW, et al. GYY4137 attenuates remodeling, preserves cardiac function and modulates the natriuretic peptide response to ischemia. J Mol Cell Cardiol. 2015;87:27–37. doi: 10.1016/j.yjmcc.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 38.Ji Y, Pang QF, Xu G, Wang L, Wang JK, Zeng YM. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol. 2008;587(1-3):1–7. doi: 10.1016/j.ejphar.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 39.Sivarajah A, Collino M, Yasin M, et al. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock. 2009;31(3):267–74. doi: 10.1097/SHK.0b013e318180ff89. [DOI] [PubMed] [Google Scholar]
- 40.Moustafa A, Habara Y. Hydrogen sulfide: a novel gaseous signaling molecule and intracellular Ca2+ regulator in rat parotid acinar cells. Am J Physiol Cell Physiol. 2015;309(7):C480–90. doi: 10.1152/ajpcell.00147.2015. [DOI] [PubMed] [Google Scholar]
- 41.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76(1):29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 42.Wang MJ, Cai WJ, Zhu YC. Mechanisms of angiogenesis: role of hydrogen sulphide. Clin Exp Pharmacol Physiol. 2010;37(7):764–71. doi: 10.1111/j.1440-1681.2010.05371.x. [DOI] [PubMed] [Google Scholar]
- 43.Papapetropoulos A, Pyriochou A, Altaany Z, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009;106(51):21972–7. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao BB, Liu SY, Zhang CC, et al. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal. 2013;19(5):448–64. doi: 10.1089/ars.2012.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, Zhu YC. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal. 2010;12(9):1065–77. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 46.Lessard J, Faubert A, Sauvageau G. Genetic programs regulating HSC specification, maintenance and expansion. Oncogene. 2004;23(43):7199–209. doi: 10.1038/sj.onc.1207940. [DOI] [PubMed] [Google Scholar]
- 47.Schulze H. Culture of murine megakaryocytes and platelets from fetal liver and bone marrow. Methods Mol Biol. 2012;788:193–203. doi: 10.1007/978-1-61779-307-3_14. [DOI] [PubMed] [Google Scholar]
- 48.Chang Y, Bluteau D, Debili N, Vainchenker W. From hematopoietic stem cells to platelets. J Thromb Haemost. 2007;5(Suppl 1):318–27. doi: 10.1111/j.1538-7836.2007.02472.x. [DOI] [PubMed] [Google Scholar]
- 49.Kaushansky K, Broudy VC, Lin N, et al. Thrombopoietin, the Mp1 ligand, is essential for full megakaryocyte development. Proc Natl Acad Sci U S A. 1995;92(8):3234–8. doi: 10.1073/pnas.92.8.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116(10):2808–16. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mirshekar-Syahkal B, Fitch SR, Ottersbach K. Concise review: from greenhouse to garden: the changing soil of the hematopoietic stem cell microenvironment during development. Stem Cells. 2014;32(7):1691–700. doi: 10.1002/stem.1680. [DOI] [PubMed] [Google Scholar]
- 52.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631–44. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong-Feng Z, Ting L, Yong Z, Cheng C, Xi Z, Pei-Yan K. The TPO/c-MPL pathway in the bone marrow may protect leukemia cells from chemotherapy in AML patients. Pathol Oncol Res. 2014;20(2):309–17. doi: 10.1007/s12253-013-9696-z. [DOI] [PubMed] [Google Scholar]
- 54.Matsumura I, Kanakura Y, Kato T, et al. Growth response of acute myeloblastic leukemia cells to recombinant human thrombopoietin. Blood. 1995;86(2):703–9. [PubMed] [Google Scholar]
- 55.Ninos JM, Jefferies LC, Cogle CR, Kerr WG. The thrombopoietin receptor, c-Mpl, is a selective surface marker for human hematopoietic stem cells. J Transl Med. 2006;4:9. doi: 10.1186/1479-5876-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vigon I, Mornon JP, Cocault L, et al. Molecular cloning and characterization of MPL, the human homolog of the v-mpl oncogene: identification of a member of the hematopoietic growth factor receptor superfamily. Proc Natl Acad Sci U S A. 1992;89(12):5640–4. doi: 10.1073/pnas.89.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ge SN, Zhao MM, Wu DD, et al. Hydrogen sulfide targets EGFR Cys797/Cys798 residues to induce Na(+)/K(+)-ATPase endocytosis and inhibition in renal tubular epithelial cells and increase sodium excretion in chronic salt-loaded rats. Antioxid Redox Signal. 2014;21(15):2061–82. doi: 10.1089/ars.2013.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan ER, Lavender H, Li G, Haviernik P, Bunting KD, Adams MD. An ENU-induced recessive mutation in Mpl leads to thrombocytopenia with overdominance. Exp Hematol. 2009;37(2):276–84. doi: 10.1016/j.exphem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murone M, Carpenter DA, de Sauvage FJ. Hematopoietic deficiencies in c-mpl and TPO knockout mice. Stem Cells. 1998;16(1):1–6. doi: 10.1002/stem.160001. [DOI] [PubMed] [Google Scholar]
- 60.de Sauvage FJ, Carver-Moore K, Luoh SM, et al. Physiological regulation of early and late stages of megakaryocytopoiesis by thrombopoietin. J Exp Med. 1996;183(2):651–6. doi: 10.1084/jem.183.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med. 2011;50(9):1021–31. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


