Abstract
Purpose
Infection with human immunodeficiency virus (HIV) is associated with an increased risk of low bone mass and fractures. European guidance advocates screening using the FRAX tool at diagnosis, on initiation of antiretroviral therapy and biannually thereafter in order to decide the need for DXA scanning. This cross-sectional study evaluates the performance of FRAX and compares its sensitivity and specifity with that of another screening tool, peripheral forearm DXA.
Methods
HIV-infected men with varying exposure to anti-retroviral therapies were recruited. FRAX scores were calculated for all participants and everybody underwent peripheral forearm DXA scanning. Femoral neck and lumbar spine BMD was acquired on an Hologic QDR machine by an assessor blinded to the results of the FRAX and pDXA.
Results
168 men (median age 45 years) were recruited with a median duration since HIV diagnosis of 74 months. In total, 21% of subjects had osteoporosis and 38% osteopenia on DXA. Using a pDXA screening threshold of T<−0.9, sensitivity was high (91%) in defining those with osteoporosis on axial DXA but with poorer specificity (33%). Alternately using a threshold of T<−2.7 reduced sensitivity (34%) with an increase in specificity (91%). FRAX with HIV included as a secondary risk factor had poor sensitivity (31%) and specificity (75%) for osteoporosis.
Conclusion
In this setting, neither peripheral DXA scanning nor FRAX were sensitive and specific for osteoporosis on DXA and neither was performance much improved by using both screening tools. Prospective studies with fracture as an outcome are required in HIV.
Keywords: HIV, anti-retroviral therapies, FRAX, p-DXA, screening
BACKGROUND
HIV is a global pandemic, affecting an estimated 35 million people worldwide. The majority of people infected with HIV are in Sub-Saharan Africa and other countries that are economically deprived. Untreated, HIV causes severe immunodeficiency and susceptibility to opportunistic infections and malignancies. However, the advent of anti-retroviral therapies used in combination (cART) has produced dramatic improvement in the survival rates of people infected by HIV and the life expectancy of treated subjects is now thought to have normalised(1). However, as cART-treated patients increase in number, there has been growing recognition of the burden of non-AIDS morbidities including osteoporosis and fractures(2-6). An estimated 60% of HIV-infected patients have osteopenia and 10-15% have osteoporosis(7). Given its ubiquity, HIV could become a major cause of secondary osteoporosis and cost-effective strategies for identification and treatment of those at risk are required.
The World Health Organisation defined osteoporosis on the basis of bone mineral densitometry (BMD) measured at the lumbar spine and femoral neck using dual-energy X-ray absorptiometry (DXA)(8). However, DXA is a relatively scarce resource, especially in resource-poor countries. European and British HIV Association guidelines (9, 10) recommend that HIV-infected patients aged > 40 years should undergo regular risk assessment for low bone mass. The tool advocated is the FRAX algorithm (11) which the guidelines have suggested can be used to define those who should undergo axial bone mineral density (BMD) assessment. The guidelines also recommend that physicians should consider using HIV as a ‘secondary cause of osteoporosis’ in the FRAX algorithm. To date, use of FRAX in HIV-infected populations has not been validated in practice and indeed, the results of two studies have suggested that FRAX had poor sensitivity for BMD(12, 13).
Peripheral DXA (pDXA) is a screening tool for osteoporosis, which is portable, involves very low levels of radiation and correlates well with BMD at other sites(14). In other populations, it has been shown to be an effective screening tool and a good predictor of hip fracture (15). However it has not been evaluated in HIV patients. The aim of this investigation was to compare the utility of FRAX with that of pDXA at the distal forearm in stratifying those patients who should undergo gold-standard DXA.
METHODS
A consecutive cross-sectional sample of attendees at a UK Teaching Hospital HIV outpatient clinic was recruited May-August 2008. Patients were eligible if they were male, aged ≥ 18 years and had been diagnosed with HIV infection. Patients were excluded if they were unable to give written, informed consent or were current participators in other research studies and if they had undergone diagnostic DEXA scanning within the last 12 months. Patients were purposively sampled to represent a range of exposures to cART, including: cART naïve; a group recently exposed for the first time to cART (< 3years) and those exposed to longer term cART.
Participants completed a questionnaire for FRAX calculation (personal and family history of fracture, smoking and alcohol use, exposure to oral glucocorticoids, hypogonadism, renal and/or liver impairment). Subjects gave permission for extraction of demographic, HIV parameters, cART regimen and validation of comorbidities from the department database and clinic notes. BMD was measured at the non-dominant forearm using pDXA (PIXI, Lunar Corp., Madison, Wisconsin, USA) with version 2.2 software using the standardised region of interest of the distal one-third of the forearm. DXA scans of the lumbar spine and femoral neck were acquired using a Hologic QDR 4500C (Hologic, Inc., Waltham, Massachusetts, USA) by an assessor blinded to the results of the FRAX and pDXA. For subjects aged ≥ 50 years, BMD was evaluated using WHO T-scores. For subjects aged < 50 years, age-adjusted Z-scores were calculated. Osteopenia for this study was defined by a T-score <−1.0 and > −2.5 (aged >50 years) or Z-score <−1.0 and >−2.0 (aged <50 years). Osteoporosis was defined by a T-score <−2.5 (aged > 50 years) or Z-score <−2.0 (age < 50 years).
The study was approved by the Northern and Yorkshire Research Ethics Committee, UK (Ref: 08/H0903/13). All participants provided written, informed consent.
Statistical Analysis
Data were analysed in SPSS® version 19 for Macintosh. Correlations between the different BMD measurements were measured using Pearson’s correlation coefficients. The gold standard was a diagnosis of osteoporosis on the HOLOGIC DXA at either the femoral neck or the lumbar spine. The optimum pDXA forearm T-score threshold for discriminating osteoporosis at either femoral neck or the lumbar spine was assessed using receiver operator characteristic curves (ROC). FRAX scores were computed for 10-year risk of any osteoporotic fracture and 10-year risk of a hip fracture for all study participants and then re-calculated using HIV as a secondary cause of osteoporosis. The results were translated into >7.5% or <7.5% ten-year risk of major osteoporotic fracture in addition to the National Osteoporosis Guideline Group (NOGG) intervention thresholds of low, intermediate (scan) and high (treat) risk(11). The discriminatory capabilities of each of pDXA at defined thresholds and FRAX, with and without HIV as a secondary risk factor, were compared.
RESULTS
One hundred and sixty-eight HIV-infected eligible men were recruited. The median age of participants was 45 (IQR 38-51, range 20-85) years, 97% were Caucasian and 96% acquired HIV through sexual transmission (mostly men who have sex with men (MSM)) (Table 1). These characteristics were not significantly different from those of the entire cohort of outpatient attendees at this centre (data not shown). The mean body mass index of participants was 25 kg/m2 (95% confidence interval 24.5-25.7), 45% were current smokers, 31% consumed more than 21 units of alcohol/week and 6% had been diagnosed with or treated for hypogonadism.
Table 1. Demographic, gonadal state, fracture history and prevalence of low bone mass.
| Total | |
|---|---|
| Demographic | n=168 |
| Age /years (median (IQR)) | 45 (38-51) |
| Caucasian (%) | 163 (97) |
| MSM (%) | 162 (96) |
| BMI /kg/m2 (mean (95% CI)) | 25 (24.5-25.7) |
| BMI <18 (%) | 2 (1) |
| Current smoker (%) | 76 (45) |
| Alcohol >21units (%) | 52 (31) |
| IDU (%) | 3 (2) |
| History of hypogonadism (%) | 10 (6) |
| No regular exercise | 59 (35) |
|
| |
| HIV parameters | |
| Time since diagnosis/ months (median (IQR)) | 74 (34-149) |
| CDC stage A (%) | 99 (59) |
| B (%) | 39 (23) |
| C (%) | 30 (18) |
| VL/copies/mL (mean (95%CI)) | 14146 (5121-23171) |
| VL undetectable (<40 copies/mL (%)) | 118 (70) |
| CD4 count /cells/mm3 (median (IQR)) | 565 (415-735) |
| CD4 <200 cells/mm3 (%) | 6 (4) |
| cART exposure / weeks (median (IQR)) | 157 (4-522) |
| Current PI use (%) | 45 (27) |
| Current NNRTI use (%) | 71 (42) |
| Current TDF use (%) | 75 (45) |
|
| |
| Clinical hypogonadism (free testosterone <90pmol/l (%)) | 2 (1) |
|
| |
| BMD /g/cm2 (mean (95% CI)) | |
| Total hip | 0.95 (0.93-0.96) |
| Lumbar spine | 0.96 (0.94-0.98) |
|
| |
| T score < −2.5 or Z score < −2.0 (%) | 35 (21) |
Note: cART= combined anti-retroviral therapy; BMI= body mass index; IVDU= intravenous drug user; VL= viral load; PI= protease inhibitor; NNRTI= non-nucleoside reverse transcriptase inhibitor; TDF= tenofovir; BMD= bone mineral density
At study entry the mean time from participants’ diagnosis with HIV infection was 74 months (IQR 34-149). Thirty-seven subjects were cART naïve; 46 had been exposed to cART recently (<3 years) and 85 had received longer-term cART (median duration 157 weeks (IQR 4-544)). 27% were taking cART that included a protease inhibitor, 42% were taking a non-nucleoside reverse transcriptase inhibitor and 45% were receiving the nucleotide reverse transcriptase inhibitor tenofovir. 4% of participants had a CD4 count < 200 cells/mm3 and 70% had an undetectable viral load (< 40 copies/mL). According to the gold standard DXA, 21% of study participants were osteoporotic (T score < −2.5 or Z score < −2.0) at the lumbar spine (nobody at the femoral neck). 38% of participants had osteopenia (T score <−1.0 or Z score < −1.0) which affected one or both sites.
Peripheral DEXA of the non-dominant forearm (pDXA)
Forearm bone mineral density measured at the non-dominant forearm with p-DXA was highly significantly correlated with bone mineral density at all sites measured using the Hologic DXA (p<0.0001). The correlation coefficients were highest between p-DXA and BMD at the hip and forearm: total hip (correlation coefficient 0.624), femoral neck (correlation coefficient 0.624), non-dominant forearm (correlation coefficient 0.695) and spine (correlation coefficient 0.485).
The AUC of the ROC for forearm T score to demonstrate osteoporosis was 0.71. Using variable thresholds of T-score for the pDXA, sensitivity and specificity for a diagnosis of osteoporosis were assessed. A T score threshold < −0.9 on pDXA resulted in a sensitivity for detection of osteoporosis of 91 % (32/35) with a specificity of 33% (44/133). The positive predictive value (PPV) was 26% (32/121). Only three subjects with osteoporosis would not have been detected by pDXA, giving a negative predictive value (NPV) of 94% (44/47). Alternately a threshold of <−2.7 resulted in a higher specificity of 91% (121/133) at the expense of a lower sensitivity of 34% (12/35), with a PPV of 50% (12/24) and a NPV of 84% (121/144).
FRAX scores
FRAX scores were computed (with and without HIV as a secondary risk factor) for 10-year risk of any osteoporotic fracture and 10-year risk of a hip fracture for all study participants. The sensitivity and specificity for predicting those with/without osteoporosis, osteopenia and normal BMD using the NOGG intervention threshold (intermediate and high) were compared (Table 2). Addition of HIV as a secondary cause improved sensitivity of the FRAX scores as a screening tool to identify osteoporosis and osteopenia from 23 and 13 % respectively to 31 and 23% respectively. However, this change resulted in lower specificity of FRAX to correctly identify those without osteoporosis from 88% down to 75%.
Table 2. Sensitivity and Specificity of the FRAX Algorithm to Identify Human Immunodeficiency Virus (HIV)–Infected Patients with bone mineral density that is normal, osteopenic or osteoporotic according to the ‘gold standard’ DXA.
| Basis of FRAX score, fracture risk | No. of patients with osteopenia (n=64) | No. of patients with osteoporosis (n=35) | No. of patients with normal BMD (n=69) |
|---|---|---|---|
| FRAX score computed using CRFs only | |||
| Above intermediate IT | 8 | 8 | 8 |
| Below intermediate IT | 56 | 27 | 61 |
| Sensitivity | 13% | 23% | - |
| Specificity | - | - | 88% |
|
| |||
| FRAX scores computed using CRFs and HIV infection as a secondary cause of Osteoporosis | |||
| Above intermediate IT | 18 | 11 | 15 |
| Below intermediate IT | 46 | 24 | 54 |
| Sensitivity | 28% | 31% | - |
| Specificity | - | - | 78% |
NOTE. Results of a 2×2 contingency analysis to evaluate the sensitivity and specificity of the Fracture Risk Assessment Tool (FRAX) algorithm, computed on the basis of classic risk factors (CRFs) alone and re-computed considering HIV infection as a secondary cause of low bone mass. Dual-Energy X-ray Absorptiometry (DXA). IT: intervention threshold (defined by the National Osteoporosis Guideline Group).
The AUC of the ROC for 10-year major osteoporotic fracture risk to identify osteoporosis was lower than the pDXA ROC AUC, 0.55 and 0.55 with and with out HIV-infection as a secondary risk factor and 0.59 and 0.61 for hip fracture risk. The ROC derived threshold of > 7.5% 10-year risk of any major osteoporotic fracture using HIV as a secondary risk factor would miss less osteoporosis than the NOGG intervention threshold, with a sensitivity of 31% (11/35) and a comparable specificity to identify those with out osteoporosis of 86% (114/133) (Figure 1).
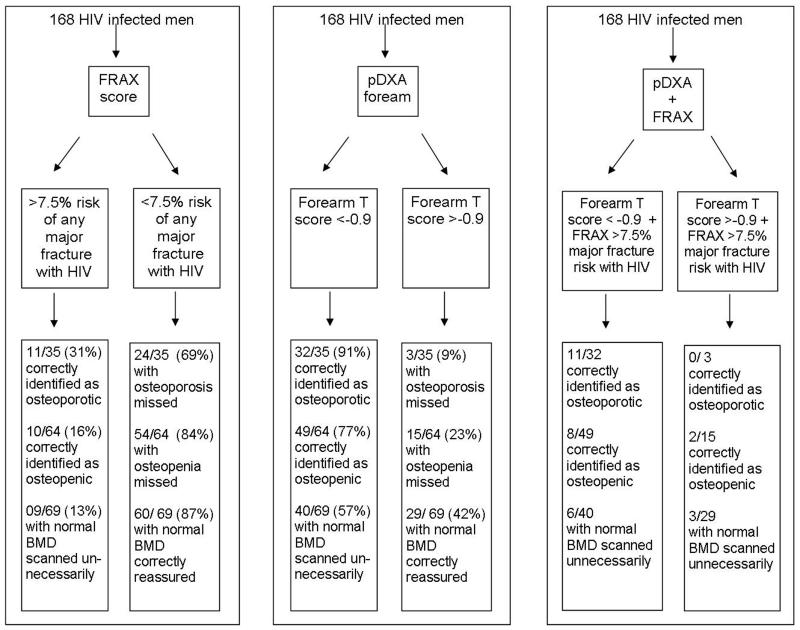
Figure 1. Schematic performance of FRAX scores and p-DXA forearm in predicting the need for gold-standard axial DEXA and identification of osteoporosis and osteopenia.
FRAX and BMD
Figure 1 compares the use of both FRAX scores and pDXA to identify those individuals with osteoporosis by DXA. Using the high sensitivity, high NPV forearm pDXA stratification threshold of T <−0.9, of the 133 men with out osteoporosis, 44 (33%) would have avoided axial-DXA scans however 40/69 (57%) men with normal BMD would be scanned. These forty men who would be sent for DXA after a ‘false positive’ pDXA could be reduced if FRAX was applied as 34/40 (90%) of these would have had a reassuring FRAX score. The three individuals with osteoporosis ‘missed’ by pDXA would not have been identified by FRAX score of >7.5%.
Interestingly if the >7.5% 10-year major fracture FRAX score was used in the risk assessment in addition to pDXA, the number of men diagnosed with low bone mass would remain unchanged at eleven people with osteoporosis and two fewer people with osteopenia would have been sent for gold standard DXA.
DISCUSSION
We present the first study to evaluate pDXA as a pre-screening tool among HIV-infected men for defining those who should have gold-standard DXA and compare its utility with that of the FRAX algorithm. We found that neither tool performed optimally on an individual level or when used together.
A threshold of T-score <−0.9 on pDXA would be more sensitive than FRAX for osteoporosis but would result in more individuals having a DXA scan and falsely reassure 9% of those with osteoporosis. The 91% sensitivity is particularly noteworthy given than none of the 20 subjects with osteoporosis were affected at the femoral neck. Since the relative proportion of cortical and trabecular bone in the vertebrae is different from that in the distal forearm and femoral neck, it is reassuring that only 3/35 of those with vertebral osteoporosis would have been ‘missed’ screening with pDXA. As suggested by EACS, FRAX has optimal sensitivity when HIV is included as a secondary risk factor but, even then, its use results in considerably fewer gold-standard scans at a cost of falsely reassuring 69% of those with osteoporosis.
We, and others, have shown that pDXA correlated well with DXA, particularly at appendicular sites(15). The advantages of pDXA include its speed, cheapness and portability combined with very low doses of radiation. However, our results suggest that, with this performance, its use will not save the costs of many gold standard scans.
The FRAX tool and other fracture risk assessment tools have shifted a paradigm in osteoporosis management. Previously, risk assessment was based on the comparison of an individual’s BMD with the young adult mean in order to define those at highest risk of fracture but absolute fracture risk was not quantified and considerable numbers of people sustained osteoporotic fractures with ‘normal’ BMD. Using the FRAX tool allows a clinician to weigh up risk factors as they apply to an individual in order to define those who can be reassured, those who have intermediate risk and should attend for DXA scanning and those for whom the absolute fracture risk is sufficiently high that intervention is indicated. FRAX has been validated in many populations but not for those aged < 40 years and nor has its validity been established in HIV populations. Validation in HIV has been hindered by the fact that many of the HIV-infected patients worldwide are still relatively young (the cohort studied here have a median age of 47 years) and the rates of osteoporotic fracture in HIV patients have not yet therefore been quantified. HIV patients may be at relatively higher risk of osteoporotic fracture given that they have multiple co-existing pathologies and therapies with complex effects on bone health. Indeed, there is a growing body of evidence to suggest that this is the case (2-6) and the results of the largest of these studies suggested that crude rates of all fractures may be as much as doubled in the HIV cases as compared with uninfected controls (2). However, until the fracture risk levels in HIV are established, the results of this study, along with those of others (12, 13), suggest that FRAX does not perform well in identifying those who should have the gold standard test for osteoporosis.
The results of this study need to be considered alongside a number of limitations. The population studied here was typical of those attending this centre: male, Caucasian and mostly infected through sexual transmission. At many centres, this demographic would be atypical and it may be that the results presented here are not generalizable to more heterogeneous HIV populations and/or women with HIV. The age range in this study was wide: 20-85 years but 29% were aged <40 years. As stated, FRAX has not been validated for individuals younger than 40 years and BMD typically increases until aged 25 years when peak bone mass is attained and then remains relatively stable for approximately 10 years before bone loss commences.
In summary, this study suggests that, using a theshold of T <−0.9, p-DXA has better sensitivity for osteoporosis than FRAX but this is achieved at the cost of low specificity. Clearly the true performance of these tools can only be assessed with prospective trials in which fracture is the outcome. However, for now, clinicians should consider DXA for those HIV patients with greatest risk of osteoporosis.
Acknowledgements
This study was funded by a British HIV Association Research Grant.
We are grateful to the patients who participated in this study.
We would like to acknowledge Dr D Churchill, Dr D Richardson, Dr D Pao, Dr D Maitland, Dr C Bell, Dr G Dean, Dr K Nambiar, Dr C Iwuji, Dr Z Warwick, Dr J Whetham, Dr C Robertson, Dr S Soni and Miss E. Nixon for their assistance in recruiting patients.
We would like to acknowledge Miss N Perry, HIV Research Manager, for her support and advice regarding study protocol and ethical approval; Ms S Walker, research nurse and the Clinical Investigation and Research Unit, Brighton and Sussex University NHS Hospitals Trust, for their support with data collection.
Footnotes
Conflict of interest
Dr Charlotte-Eve Short, Dr Simon Shaw, Professor Martin Fisher, Dr Yvonne Gilleece and Dr Karen Walker-Bone declare that they have no conflict of interest
Previous presentation
Part of this work was presented at the 16th International Conference on Retroviruses and Opportunistic Infections, Montreal, Canada, 2009. In Program and Abstracts 16th CROI [abstract 753].
References
- 1.Ray M, Logan R, Sterne JA, Hernandez-Diaz S, Robins JM, Sabin C, Bansi L, van Sighem A, de Wolf F, Costagliola D, Lanoy E, Bucher HC, von Wyl V, Esteve A, Casbona J, del Amo J, Moreno S, Justice A, Goulet J, Lodi S, Phillips A, Seng R, Meyer L, Perez-Hoyos S, Garcia de Olalla P, Hernan MA. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collin F, Duval X, Le Moing V, Piroth L, Al Kaied F, Massip P, Villes V, Chene G, Raffi F. Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. AIDS. 2009;23:1021–1024. doi: 10.1097/QAD.0b013e3283292195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mundy LM, Youk AO, McComsey GA, Bowlin SJ. Overall benefit of antiretroviral treatment on the risk of fracture in HIV: nested case-control analysis in a health-insured population. AIDS. 2012;26:1073–1082. doi: 10.1097/QAD.0b013e328351997f. [DOI] [PubMed] [Google Scholar]
- 5.Yin MT, Zhang CA, McMahon DJ, Ferris DC, Irani D, Colon I, Cremers S, Shane E. Higher rates of bone loss in postmenopausal HIV-infected women: a longitudinal study. J Clin Endocrinol Metab. 2012;97:554–562. doi: 10.1210/jc.2011-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedimo R, Tebas P. Increased fracture risk with HIV infection--a growing concern. Nat Rev Endocrinol. 2013;9:260–261. doi: 10.1038/nrendo.2013.62. [DOI] [PubMed] [Google Scholar]
- 7.Brown Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 8.WHO (WHO Technical Report Series 843).Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. 1994 [PubMed]
- 9.Asboe D, Aitken C, Boffito M, Booth C, Cane P, Fakoya A, Geretti AM, Kelleher P, Mackie N, Muir D, Murphy G, Orkin C, Post F, Rooney G, Sabin C, Sherr L, Smit E, Tong W, Ustianowski A, Valappil M, Walsh J, Williams M, Yirrell D. British HIV Association guidelines for the routine investigation and monitoring of adult HIV-1-infected individuals 2011. HIV Med. 2011;13:1–44. doi: 10.1111/j.1468-1293.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 10.EACS Guidelines: Clinical Management and Treatment of HIV Infected Adults in Europe. 2009.
- 11.Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A. Case finding for the management of osteoporosis with FRAX--assessment and intervention thresholds for the UK. Osteoporos Int. 2008;19:1395–1408. doi: 10.1007/s00198-008-0712-1. [DOI] [PubMed] [Google Scholar]
- 12.Gazzola L, Comi L, Savoldi A, Tagliabue L, Del Sole A, Pietrogrande L, Bini T, d'Arminio Monforte A, Marchetti G. Use of the FRAX equation as first-line screening of bone metabolism alteration in the HIV-infected population. J Infect Dis. 2010;202:330–331. doi: 10.1086/653584. author reply 331-332. [DOI] [PubMed] [Google Scholar]
- 13.Calmy A, Fux CA, Norris R, Vallier N, Delhumeau C, Samaras K, Hesse K, Hirschel B, Cooper DA, Carr A. Low bone mineral density, renal dysfunction, and fracture risk in HIV infection: a cross-sectional study. J Infect Dis. 2009;200:1746–1754. doi: 10.1086/644785. [DOI] [PubMed] [Google Scholar]
- 14.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 15.Miller PD, Siris ES, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Chen YT, Berger ML, Santora AC, Sherwood LM. Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: evidence from the National Osteoporosis Risk Assessment. J Bone Miner Res. 2002;17:2222–2230. doi: 10.1359/jbmr.2002.17.12.2222. [DOI] [PubMed] [Google Scholar]