Abstract
Cardiovascular disease (CVD) is the leading cause of global deaths, with the majority occurring in low- and middle-income countries (LMIC). The primary and secondary prevention of CVD is suboptimal throughout the world, but the evidence-practice gaps are much more pronounced in LMIC. Barriers at the patient, health-care provider, and health system level prevent the implementation of optimal primary and secondary prevention. Identification of the particular barriers that exist in resource-constrained settings is necessary to inform effective strategies to reduce the identified evidence-practice gaps. Furthermore, targeting modifiable factors that contribute most significantly to the global burden of CVD, including tobacco use, hypertension, and secondary prevention for CVD will lead to the biggest gains in mortality reduction. We review a select number of novel, resource-efficient strategies to reduce premature mortality from CVD, including: (1) effective measures for tobacco control; (2) implementation of simplified screening and management algorithms for those with or at risk of CVD, (3) increasing the availability and affordability of simplified and cost-effective treatment regimens including combination CVD preventive drug therapy, and (4) simplified delivery of health care through task-sharing (non-physician health workers) and optimizing self-management (treatment supporters). Developing and deploying systems of care that address barriers related to the above, will lead to substantial reductions in CVD and related mortality.
Keywords: health system, cardiovascular disease, ischemic heart disease, stroke
Introduction
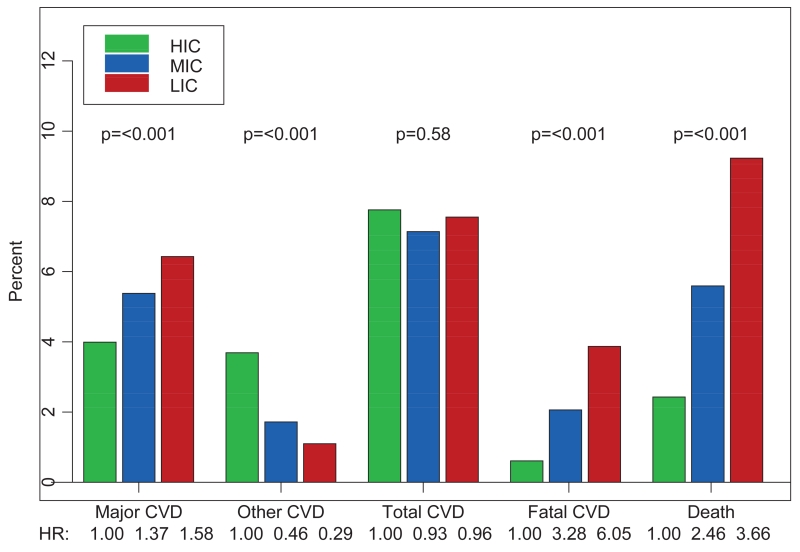
The 2013 Global Burden of Disease study showed that, despite a 39% decrease in age-specific death rates, global deaths from cardiovascular disease (CVD) have risen by 41% between 1990 and 2013 1. Ischemic heart disease has risen to become the leading cause of global deaths, up from fourth position in 1990 (41% increase), and stroke has risen to third position from fifth (50% increase) 1. As outlined in Figure 1, the incidence of major and fatal CVD events is lowest in high income countries (HIC) 2. It is predicted that, by 2020, nearly three quarters of the global mortality and 80% of the CVD burden (as measured by disability life years lost) will occur in low and middle income countries (LMIC), where 85% of the world’s population now live 2, 3. These changes have been driven largely by population growth and ageing 1, 3, 4.
Figure 1.
Cardiovascular disease (CVD) events and death by country income strata2.
HIC have experienced large reductions in the incidence and mortality of CVD, with mortality declines averaging 50% since the 1970s, and as much as 75% in countries such as the United Kingdom, United States, and Finland 5, 6. The precise contribution of different factors varies among countries, in part reflecting differences in the initial pattern of risk factors and strategies employed, but they include healthier diets, lower rates of smoking, improved management of risk factors such as hypertension, better management of acute CVD events, and greater use of secondary prevention 5, 7. Despite these impressive gains in HIC, morbidity and mortality associated with cardiovascular diseases remain very high, particularly among poor countries and in marginalized populations 8.
In September 2011, the United Nations made a political declaration to adopt a global target of a 25% reduction in premature (< 70 years) mortality from CVD, cancer, diabetes, and chronic respiratory disease by 2025, which subsequently translated into a non-communicable disease (NCD) action plan 9. This action plan has designated eight indicators to measure progress towards the overall “25 × 25” goal, including: (1) decreasing raised blood pressure, (2) decreasing tobacco use, (3) increasing physical inactivity, (4) decreasing sodium intake, (5) decreasing the harmful use of alcohol, (6) halting the rise of diabetes and obesity, (7) improving access to drug therapy and counselling to prevent CVD, and (8) increasing availability of the affordable basic technologies and essential medicines to manage NCDs 10. However, none of these targets will likely be met unless the many existing barriers to evidence-based and efficient CVD management are addressed. This review focuses on ischemic heart disease and stroke, along with related risk factors, given these two conditions are the leading contributors to the global burden of CVD 1. This paper reviews the current gaps in the prevention and management of CVD compared to the evidence (evidence-practice gaps), barriers to closing evidence-practice gaps, and targets for resource-effective interventions. Finally, we review a select number of novel, resource-efficient population, individual, and health system strategies to overcome identified barriers.
Evidence-practice gaps in the prevention and management of CVD
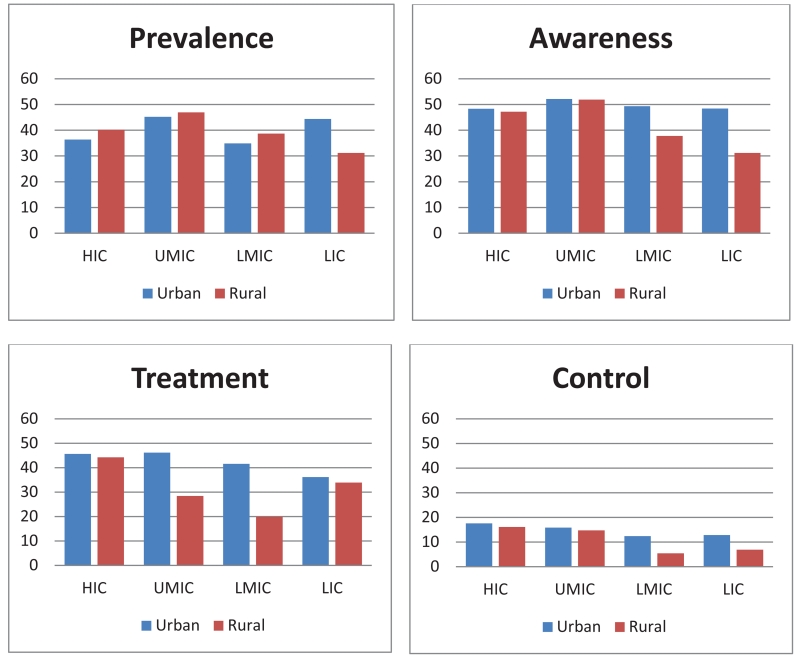
The extent of evidence-practice gaps in the prevention and management of CVD can be demonstrated by data from the Prospective Urban Rural Epidemiology (PURE) study, a longitudinal cohort study being conducted in 630 urban and rural communities from 17 high-, middle-, and low-income countries, with expansion underway to include 800 communities in 25 countries 8. Unbiased and systematic, community screening of individuals was undertaken to achieve a representative sample of adults aged 35-70 years 8. Standardized questionnaires, physical measurements including blood pressure, and laboratory investigations were completed to capture baseline demographics, medication use and clinical events 8. Data from PURE demonstrate that the management of hypertension is suboptimal throughout the world, but especially in LMIC (Figure 2) 11. First, approximately half of PURE participants who have hypertension are unaware of their diagnosis. Second, while the majority of individuals who are aware of their diagnosis of hypertension receive treatment, only a minority (< 20%) achieves adequate blood pressure control, defined as a systolic blood pressure (SBP) less than 140 mmHg and a diastolic blood pressure less than 90 mmHg. Third, most participants (77%) who receive blood pressure lowering treatment use only one medication 11; however, it is widely acknowledged that at least two antihypertensive medications are needed to control blood pressure 12. Gaps in hypertension awareness, treatment, and control are evident in all countries within the PURE study, but larger gaps are present in LMIC 11. These data demonstrate the need for broader and more efficient blood pressure screening coupled with more effective blood pressure control. Similar evidence-practice gaps are evident in the management of lipids, with suboptimal use of statins8. Many of those with known CVD continue to be exposed to major risk factors, with 19% continuing to smoke, with only 35% participating in high levels of physical activity, and only 39% following a healthy diet, with the greatest problem, again, in low-income countries (LIC) 13. Given the consistent evidence-practice gaps in the management of CVD risk across multiple risk factors, there is a need for comprehensive programs to tackle the structural determinants of CVD, case finding and, where shown to be effective, risk factor screening for CVD prevention and management.
Figure 2.
Prevalence, awareness, treatment, and control of hypertension by country economic status11.
There are also important, modifiable differences in the acute management of patients with CVD between HIC and LMIC. For instance, patients from HIC with a previous acute myocardial infarction are more likely to receive evidence-based medications, including aspirin, clopidogrel, and statin, at discharge (odds ratio [OR] = 2.3, 95% confidence interval [CI] 1.2, 4.5) and are more likely to receive percutaneous coronary intervention (OR= 19.7, 95% CI 10.5, 37.0), as compared to LMIC 14.
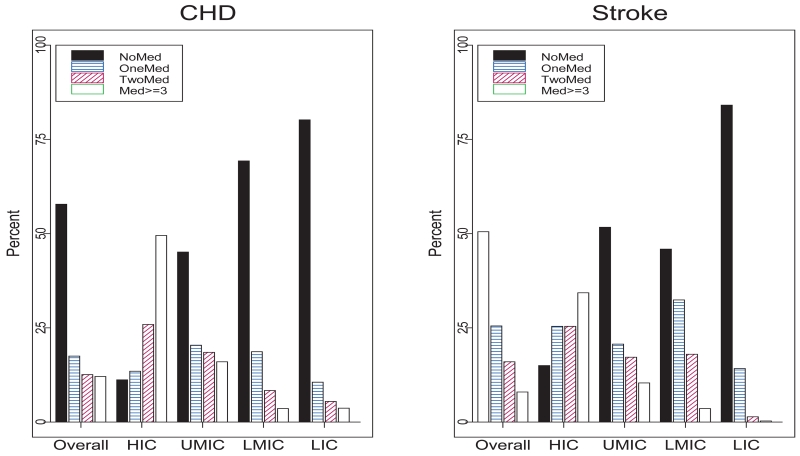
The PURE study also found sub-optimal secondary prevention drug therapy in patients with established CVD (Figure 3) 8. More than 75% of participants in LIC with prior ischemic heart disease or stroke were not taking any medications for secondary prevention of CVD, compared with less than 10% among participants in HIC 8. Similar gaps have been identified by other research. For example, a 2005 World Health Organization-led study of patients visiting health care facilities in LMIC found that, of those with ischemic heart disease, only 81% had been prescribed aspirin, 48% beta-blockers, 40% angiotensin-converting enzyme (ACE) inhibitors, and 30% statins. Even lower medication rates were demonstrated among patients with cerebrovascular disease 15. Collectively, these studies and others demonstrate the global magnitude of the evidence-practice gaps in the prevention and management of CVD, particularly in LMIC 16, 17.
Figure 3.
Number of drugs taken by individuals with previous stroke or ischemic heart disease by country economic status.8 *CVD-cardiovascular disease, LMIC-low and middle income countries, LIC-low income countries, HIC-high income countries, MI-myocardial infarction, HF-heart failure, IHD-ischemic heart disease
Barriers to evidence-based and efficient CVD management
Closing these evidence-practice gaps in the prevention and management of CVD requires overcoming barriers that prevent the uptake of best practices. Systematic evaluations of barriers to effective CVD care in LMIC have received relatively little attention from researchers to date, but this is now changing 18, 19. For example, Khatib and colleagues performed a systematic review of barriers to hypertension awareness, treatment, and follow-up by evaluating both quantitative and qualitative reports. This review outlined that barriers occur at the patient, health-care provider, and health system or policy level (Table 1) 18, 20, 21, 22 Further research is required to elucidate the way in which these barriers combine and how they can be overcome, using methods that allow the researcher to determine which changes are necessary and which are sufficient to bring about desired changes 23.
Table 1.
Barriers to evidence-based cardiovascular disease health care at the patient, health-care provider and health system/policy level, prioritized by country income strata.
| Barrier | Example | Country Income Strata |
|---|---|---|
| Patient 18: | ||
| Availability, access, and costs (External) | Lack of health insurance | Both HIC and LMIC |
| Knowledge (Internal) | Asymptomatic thus question need for ongoing treatment | Both HIC and LMIC |
| Beliefs (Internal) | Alternative/traditional medicine | LMIC > HIC |
| Memory (Internal) | Affects adherence to recommended therapies | Both HIC and LMIC |
| Side effects to medications (Internal) | Whether real or perceived (myalgia, cough, etc.) | Both HIC and LMIC |
| Health Care Provider 18, 21: | ||
| Knowledge | Familiarity and awareness of management options | LMIC: Standards of education, ongoing options for continued medical education |
| Attitudes | Lack of agreement with guidelines, outcome expectancy, self-efficacy, motivation and treatment inertia | LMIC: Conflicting opinions regarding alternative medicine |
| Behavior | External or environmental factors limiting management (time, resources, reimbursement) | LMIC>HIC |
| Health System/Policy 22: | ||
| Health Care Financing System | Low priority in national budgets: competing political agendas (military, other medical conditions such as HIV, etc.). Limited universal health care coverage. | LMIC>HIC |
| Medical Products and Technologies | Lacking infrastructures for stocking pharmacies with evidence-based generic medications. Poor affordability of essential medications, even when they are generic. | LMIC>HIC |
| Leadership/Governance | Low Priority for cardiovascular disease prevention: lack of effective screening programs, smoking cessation programs, safe environments for exercise, high costs for healthy foods | Both HIC and LMIC |
| Health Workforce | Limited number of adequately trained physicians and health care professionals | LMIC>HIC |
| Health Information System and Research | Limited health system infrastructures to ensure monitoring of health determinants, performance and health status | LMIC>HIC |
| Service Delivery | Efficient delivery of effective and safe interventions | Both HIC and LMIC |
HIC=high-income countries; LMIC=low- and middle-income countries.
While some barriers to optimal CVD management can be found in many countries at varying income levels, some barriers are specific to certain contexts. It must also be noted that barriers to primordial prevention overlap with barriers identified primarily at the patient and health system level 24. Ideal cardiovascular primordial prevention targets include never smoking, healthy diet, adequate physical activity, optimal blood pressure, glucose, and lipids, and a normal body mass index 24. Barriers to achieving such targets are often related to limited patient knowledge, motivation, and an unfavorable environment that is not supported by appropriate health policy and government leadership that promote cardiovascular health in younger populations.
Patient-Level Barriers
There are two important barrier categories at the patient level that impact best practice with respect to CVD prevention and management. First, internal barriers that prevent optimal adherence to medications and lifestyle modification have been well documented 25. These barriers include: lack of awareness of their condition (i.e., hypertension or a need for secondary prevention), reluctance to take medications for conditions that are asymptomatic or which cause side-effects, and difficulties in remembering to adhere to treatment regimens, especially if they are complex. It has been found that some patients harbor beliefs that pills are unnatural and that taking pills reminds them they are “unhealthy” 25. In some LMIC, medication use and adherence may be negatively impacted by beliefs derived from alternative or traditional medicine, which sees disease literally as “dis-ease”, or discomfort and that medicines should only be used during acute illness, rather than prevention.26 The second type of patient-level barriers are external including: lack of availability of appropriate health care resources, lack of the necessary financial resources to access care, either directly (e.g., purchasing medications) or related costs (e.g., transportation costs or fees to consult health practitioners) 27.
Health-Care Provider-level Barriers
At the health-care provider level, barriers have been classified according to knowledge, attitudes, and behavior 21. Knowledge barriers include: limitations relating to the large volume of health information that health care professionals must synthesize, inadequate training of health care providers in the evidence-based management of CVD and related risk, and inappropriate risk stratification 21, 28. Attitude barriers include unwillingness to accept evidence-based guidelines, lack of motivation to change current practice patterns, described as inertia, and beliefs that the guidelines are difficult to implement and/or will not result in the desired outcomes 21. Behavioral barriers include environmental and external factors that prevent behavior change, thus limiting best practices. Some examples include: limited time and resources to implement guideline recommendations, coupled with misaligned financial incentives. Low health worker to patient ratios in LMIC reduce the time available to see and counsel patients 29. The complexity of guidelines has been described as another factor that can affect behavior change 28, 30-32. Current guidelines for the diagnosis and management of cardiovascular risk and disease are complex and may be impractical to implement in resource-constrained settings 33-36. For instance, the US Joint National Committee (JNC) 8 outlines a detailed evidence-based guideline for the identification and management of individuals with hypertension 36. However, this algorithm is complex and varies according to age, co-morbidities, and race 36. Furthermore, it includes three separate drug treatment titration strategies, which involve up to 6 steps and require many visits to a clinic to diagnose hypertension, initiate treatment, and ultimately to control blood pressure at recommended levels. Such features limit the use of guidelines by primary care physicians in the HIC where they are developed and are even less practical in LMIC 21.
In HIC, even when primary care physicians are supported by multifaceted quality improvement interventions comprising automated risk assessment, computerized decision support, and audit and feedback tools to improve guideline implementation, it has been difficult to demonstrate improvements in processes and outcomes 37. Even if such complex interventions were effective, their implementation within infrastructures seen in LMIC can be challenging.
Health System-level Barriers
The World Health Organization Health Systems Framework can help classify barriers at the health system level 22. It comprises six building blocks: (1) leadership/governance, (2) health care financing, (3) health workforce, (4) medical products and technologies, (5) information and research, and (6) service delivery (Table 1) 22. Examples of barriers related to these building blocks include limited numbers of adequately trained physicians and allied health professionals, lack of financing or pre-payment mechanisms to reduce point of service costs, and payment structures that disincentivize prevention 38. Access to affordable generic evidence-based CVD medications is frequently a much greater problem in LMIC than HIC, with statins and ACE inhibitors often not being available in many hospitals, health clinics, and community pharmacies in LMIC.39 Even when available, these evidence-based medications are often unaffordable in LMIC.39 For example, while the cost of CVD medications (statins, ACE inhibitors, beta blockers and aspirin collectively) is less than 1% of the average monthly income in HIC, in rural India, the cost of a statin or an ACE inhibitor approaches 50% and 20%, respectively, of a household’s median monthly income.39
Barriers relating to health care financing can be seen in countries at all income levels, but are more pronounced in LMIC. Typically 1-2% of a much smaller gross domestic product is committed by governments to health expenditures in LMIC compared with around 12% on average in HIC 40. Moreover, in LMIC a much greater share of health expenditure is out of pocket, leading to catastrophic health spending and distress financing, which affects the poor and uninsured disproportionately 41. This microeconomic burden is exacerbated by the limited progress that many LMICs have made towards universal health coverage 42. While several MICs including, India (lower-middle income) and Brazil, China, and South Africa (upper-middle income) are making progress, significant challenges have been identified, including unwillingness or inability to collect the necessary funds, which can be coupled with a lack of political will 42. Many LMIC have also made less progress in developing leadership and governance, as exemplified by generally weak tobacco control policies, reflecting low priorities, limited capacity to develop and implement healthy public policies, as well as corruption and susceptibility of governments to pressure from international tobacco corporations 43, 44.
Identification of the particular barriers at the patient-, health-care provider-, and health system-level that exist in resource-constrained settings is necessary to inform effective strategies to reduce the identified evidence-practice gaps 45.
Targets for resource effective strategies
Just as it is important to understand the barriers that impede the uptake of the evidence-based management of CVD, it is important to understand the modifiable factors that contribute most significantly to the global burden of CVD. It has long been recognized that the greatest health gains will be achieved by shifting the overall distribution of risk factors. Where the goal is to reduce exposure to hazardous products, be they tobacco, alcohol or energy dense food, this requires a combination of actions on price, availability, and marketing at population levels, something examined in more detail below. However, such a strategy should be complemented by one designed to identify and manage those with the ability to benefit from more interventions targeted at specific high risk strata, especially as they relate to metabolic risk factors.
Identification of high-risk individuals can be informed by research such as the INTERHEART study, which included 28,000 people in 52 countries 46, and the INTERSTROKE study, with 26,000 participants from 33 countries 47. These studies identified the most important modifiable risk factors for ischemic heart disease and stroke worldwide. Smoking, lipids, hypertension, and diabetes, account for 80% of the population attributable risk for both ischemic heart disease and stroke 46. Among these, reducing the first three has been demonstrated clearly to reduce major adverse cardiac events. First, tobacco kills approximately 6 million people and causes more than half a trillion dollars of economic damage each year 43. Tobacco control using a population wide strategy is acknowledged to be important and cost-effective. Second, the Cholesterol Treatment Trialists’ Collaboration meta-analysis of >90,000 people in 14 RCTs of statins showed that for each 1 mmol/L reduction in low-density lipoprotein (LDL) over 5 years there was 12% reduction in relative risk for total mortality, 23% for major CVD events, 26% for myocardial infarction, 24% for coronary revascularizations, 17% for strokes and 21% for major vascular events 48. Effects were seen even in those at low risk and those with low average LDL levels 48. Third, the Blood Pressure Lowering Treatment Trialists’ Collaboration meta-analysis, with >160,000 participants in 29 trials, showed that lowering SBP by 5 mmHg over 4 to 5 years with most drugs reduced the risk of ischemic heart disease by 20%, stroke by 28% and major CVD events by 22%, but this benefit is most clear in those with SBP >140 mmHg 49. Thus, a strategy that focuses on population (for tobacco) and individual measures (blood pressure and lipid reduction in high risk populations or those with elevated levels) can yield very large benefits in terms of reducing CVD.
The PURE study (Table 2) indicates that over 4-year follow-up, 20% of the CVD events that occur are in the 5% of individuals who have a history of previous myocardial infarction (MI) or stroke. Furthermore, 66% of events derive from the 41% of individuals with hypertension (those with a history of hypertension or those with SBP >140 mmHg on a measurement at a single time). It was also noted that 29% of CVD events occur in the 21% who are current smokers 50 . Collectively 81% of major CV events (CV deaths, myocardial infarction, stroke, and hospitalizations for heart failure) occur in the 55% of the population who are either smokers, have hypertension, have a history of CVD, or a combination thereof. While diabetes is undoubtedly an important risk factor and has been associated with a hazard ratio of 2.32 (95% CI 2.11-2.56) for death from vascular causes 51, its addition to tobacco, hypertension and prior CVD only increases future CVD events from 81% to 84% 50. Given the overlap in CVD risk factors in patients with diabetes, the evidence for managing blood sugars beyond aggressive management of tobacco use, blood pressure control, and secondary prevention is less clear. A 30% reduction in tobacco use, 30% improved hypertension control and 30% improvement in secondary prevention over 10-15 years can collectively reduce incident CVD events by 25% globally 12. In addition, in-hospital and post-discharge use of aspirin, statins, ACE inhibitors, and beta blockers can reduce case-fatality rates from acute coronary syndromes by over 80% 12. Therefore, collectively, these measures could have an important impact on global CVD mortality and are the basis of the CVD Road Maps developed by the World Heart Federation 50, 52-54. Similar findings have been described in a US population, where 87-92% of patients experiencing a fatal or non-fatal myocardial infarction had at least one antecedent major CV risk factor 55. Approaching risk assessment with these three factors in mind, tobacco use, hypertension, and documented CVD, makes screening for those who should be targets for primary and secondary prevention potentially simple and inexpensive. While adding lipids improves the discrimination of CVD risk assessment, these investigations may be cost-prohibitive in resource-constrained settings. Adding a statin, without lipid assessment, in those with documented CVD, self-reported diabetes, or a high risk non-lab-based risk score, has been estimated to be a cost-effective strategy 46, 56.
Table 2.
Number (%) of major CVD events over 4 year follow-up for the different sub-groups in the Population Urban Rural Epidemiology (PURE) study (n=152 609), highlighting the majority of events occur in current smokers, those with hypertension, or documented history of CVD.50
| Baseline Condition | Number in PURE cohort with a condition at baseline (%) | Follow-up Major CVD Events N = 3,488 (2.2%) |
|---|---|---|
| Cardiovascular disease | 7,743 (5.1) | 673 (19.3) |
| Hypertension (history or blood pressure >140/90 mmHg) | 62,034 (40.7) | 2,317 (66.4) |
| Current smoker | 31,397 (20.6) | 1,021 (29.4) |
| CVD, hypertension, and/or current smoker | 84,078 (55) | 2,822 (80.9) |
| Diabetes (history or FPG >7mmol) | 16,071 (10.5) | 905 (26.0) |
| CVD, hypertension, smoker and/or diabetes | 88,326 (57.9) | 2,929 (84.0) |
CVD=cardiovascular disease FPG=fasting blood glucose
Resource effective strategies to prevent and manage CVD
Strategies to prevent and manage CVD should be based on (1) evidence of benefit and cost effectiveness, (2) feasibility of implementing such strategies in settings with different levels of resources, (3) ability to scale and sustain them, and (4) sociopolitical acceptability 57. The following examples illustrate resource-effective and evidence-based interventions to address (A) population health, (B) primary and secondary prevention, and (C) deficiencies in health systems to prevent and manage CVD. Table 3 summarizes the quality of the evidence supporting the strategies outlined below.
Table 3.
The evidence supporting the major resource effective strategies to prevent and treat cardiovascular disease.
| Resource Effective Strategies | Level of Evidence* | References |
|---|---|---|
| Tobacco prevention and control policies (WHO MPOWER) | A | 58-60 |
| Improving price, availability and marketing of healthy foods | C | 61-65 |
| Simplified CVD-risk screening and management algorithms | B | 66 |
| Availability of combination therapy for CVD | A | 12, 67-71 |
| Task-sharing with NPHWs and treatment supporters | B | 72-78 |
Level-A body of evidence = multiple populations have been evaluated, or data are derived from multiple randomized clinical trials or meta-analyses. A level-B body of evidence = limited populations have been evaluated, or data are derived from a single randomized trial or nonrandomized trial. A level-C body of evidence = very limited populations have been evaluated or that only the consensus opinions of experts, case studies, or standards of care support the recommendation.
WHO=World Health organization, CVD=cardiovascular disease, NPHWs=non-physician health workers
Population health strategies
Tobacco prevention and control policies
Despite a reduction in cigarette smoking rates in HIC over the last 3 decades, global sales of cigarettes continue to rise due to increased consumption in LMIC 79. This requires a two-pronged strategy. In the short term, the most effective way to reduce tobacco-related health concerns is to help current tobacco users quit because the majority of the tobacco-related morbidity and mortality in the next two or three decades will arise from those over the age of 30 years old who currently use tobacco. However, in the longer term, over the next 30 to 60 years, it will be necessary to prevent adolescents and young adults from initiating tobacco use. The World Health Organization has identified six evidence-based tobacco control strategies, known as “MPOWER” (Table 4) 58, that address both approaches. The MPOWER strategies correspond to provisions included in the World Health Organization Framework Convention on Tobacco Control (FCTC), which outlines policy measures for tobacco control (Table 5) 59. It has been estimated that full implementation of the FCTC in 23 LMIC could avert over 5 million deaths over a decade 60. The measures set out in the FCTC are considered cost-effective for reducing tobacco use and would cost only US$ 0.14 in China to US$ 0.49 in Russia per person per year 57. However, despite the FCTC having been signed by almost all countries, ratification of the FCTC lags behind, and tobacco marketing remains widespread in LMIC 80-82. This demonstrates the need for countries to not only promulgate but to also enforce laws that control tobacco marketing. Unfortunately, as countries such as Uruguay and Jamaica have discovered, those enlightened politicians seeking to implement comprehensive tobacco control face challenges from powerful transnational tobacco corporations exploiting international trade agreements.83
Table 4.
The WHO MPOWER measures for tobacco reduction 58.
| The 6 WHO MPOWER measures are: |
|---|
| Monitor tobacco use and prevention policies |
| Protect people from tobacco use |
| Offer help to quit tobacco use |
| Warn about the dangers of tobacco |
| Enforce bans on tobacco advertising, promotion and sponsorship |
| Raise taxes on tobacco |
Table 5.
Core provisions of the World Health Organization Framework Convention on Tobacco Control 59
| Core Demand Reduction Provisions | Price and tax measures to reduce the demand for tobacco |
Non-price measures to reduce the demand for tobacco:
|
|
| Core Supply Reduction Provisions | Control illicit trade in tobacco products |
| Ban sales to and by minors | |
| Provision of support for economically viable alternative activities |
Dietary policies
Data from HIC countries suggest that increased fruits and vegetables, reduced saturated fats, and elimination of trans fats in the diet are associated with lower risk of ischemic heart disease 61, 62. However, similar data are not widely available from LMIC. Consequently, large studies conducted in LMIC, where diet patterns substantially differ from HIC, are urgently needed. Until such studies are available, as with tobacco control, population-based strategies are the most effective measures 63, with a focus on price, availability, and marketing. Unfortunately, healthy foods are often unaffordable, in part because fruits and vegetables are very expensive in LMIC 64, 65 (Mente unpublished 2015), and in part because of agricultural subsidies for products such as high fructose corn syrup in the HIC 84. Again, as with tobacco, the spread of energy dense food is being driven by trade liberalization 85.
Individual strategies for the prevention and management of CVD
Simplified CVD- risk screening and management algorithms
Given the limitations of current diagnostic and management algorithms for patients at risk of CVD, simple cost-effective strategies are required to tackle the three modifiable risk factors (i.e., hypertension, smoking, and secondary prevention) that are found in 55% of the population over the age of 35 years old and account for 81% of major CVD events in the PURE study (Table 2). Unfortunately, as noted above, many guidelines for hypertension detection and management recommend that multiple elevated blood pressure readings should be documented before initiating treatment, and some recommend multiple investigations to assess CVD and medication-related complications 33-36. These recommendations can create barriers to early initiation of effective treatments. The PURE study data show that approximately 90% of individuals with an initial SBP >160 mmHg (Stage 2 hypertension), based on an average of 3 measures on one occasion, had a sustained high SBP (>140/90 mmHg) on repeat visits within one year, so meeting existing criteria for hypertension (Table 6) 66 (Yusuf, unpublished 2015). Furthermore, 74% of patients with a single SBP between 140-159 mmHg and another cardiac risk factor or documented CVD had an SBP of > 140 mmHg on follow-up visit. These data support a simplified screening process (e.g., initiate antihypertensive treatments in those with an SBP > 160 mmHg on a single occasion) to diagnose and initiate treatment for hypertension.
Table 6.
Proportion of participants with no history of hypertension but with systolic blood pressure >140 mmHg on repeat visit in the PURE study population based on various criteria at enrollment (Yusuf, unpublished PURE Data, 2015).
| Population at baseline visit | Baseline (N) | SBP > 140 mmHg at 1 year follow-up visit (N, %) |
|---|---|---|
| SBP > 180 mmHg | 686 | 634, 92.4% |
| SBP > 160 mmHg | 2512 | 2263, 90.1% |
| SBP >160 mmHg and history of MI, stroke/TIA, angina, participant reported-DM | 223 | 203, 91.0% |
| SBP >160 mmHg and no history of MI, stroke/TIA, angina, self-DM | 2289 | 2059, 90.0% |
| SBP 140-159 mmHg and history of MI, stroke/TIA, angina, self-DM | 576 | 424, 73.6% |
| SBP 140-159 mmHg and no history of MI, stroke/TIA, angina, self-DM | 6124 | 4072, 66.5% |
| SBP <140 mmHg | 29007 | 6538, 22.5% |
SBP=systolic blood pressure MI=myocardial infarction, TIA=transient ischemic attack, DM=diabetes mellitus. Note BP was measured using an Omron automated device with 3 readings obtained during a single visit .The average of the 3 readings is used.
Diagnostic and management strategies that can be adopted without expensive risk stratification tests, as recommended by the World Health Organization, can be cost-effective 86. Thus, the strategy recommended by the World Health Organization incorporates the use of basic technologies (stethoscopes, sphygmomanometers, blood glucose test strips etc.), simple non-lab-based risk assessment scores relevant to the population being assessed, and a core list of recommended generic CVD medications (Table 6) 86. This simplified approach to cardiovascular risk assessment and management may help reduce current evidence-practice gaps by targeting and simplifying the approach to modifiable risk factors including hypertension, smoking, and secondary prevention of CVD, including the management of dyslipidemia. Furthermore, this simplified approach can overcome many commonly identified barriers, including time constraints, cost, complexity, and confusion with existing guidelines.
Resource efficient management of acute presentations of CVD
The significant reduction of CVD mortality in HIC by 50% to 75% since the 1970s has been attributed to different factors, including better management of acute ischemic heart disease events 5-7. While the widespread implementation of primary percutaneous coronary intervention for acute myocardial infarctions or a thrombolytic program for acute strokes (with computed tomography scans within 4 hours of symptom onset) may not feasible or resource-efficient options in many LMICs, other acute interventions are needed. The use of aspirin and streptokinase for the acute, in-hospital management of ST-elevation myocardial infarction is considered to be cost-effective and could avert 335,000 DALYs among patients 30-69 years old in LMIC 87. Furthermore, clopidogrel, beta-blockers, ACE inhibitors, diuretics, and statins for the management of acute coronary syndromes, stroke, and acute heart failure are considered essential medicines by the World Health Organization (Table 7).88 Ensuring these evidence-based medications are available post-myocardial infarction, keeping the costs to the patient low through the elimination of co-payments results in improved medication adherence and rates of first major vascular events, without increasing overall health costs 89.
Table 7.
Core list of medications required for implementing essential cardiovascular disease prevention interventions in primary care, based on the World Health Organization List of essential medicines 88.
| Medication | Indication |
|---|---|
| Aspirin | Primary and secondary prevention (IHD and ischemic stroke) |
| Clopidogrel | Secondary prevention (IHD and ischemic stroke) |
| Thiazide diuretic | Hypertension |
| Calcium channel blocker | Hypertension |
| Statin | Primary and secondary prevention (IHD and ischemic stroke) |
| Angiotensin converting enzyme (ACE) inhibitor | Hypertension, primary and secondary prevention (IHD and ischemic stroke), heart failure |
| Beta-blocker | Hypertension, secondary prevention (IHD), heart failure |
| Furosemide | Heart failure |
| Spironolactone | Hypertension, heart failure, secondary prevention (IHD) |
| Isosorbide dinitrate, glyceryl trinitrate | IHD |
| Glibenclamide | Diabetes mellitus |
| Metformin | Diabetes mellitus |
| Insulin | Diabetes mellitus |
IHD=ischemic heart disease.
Expanding management options by appropriate and affordable combination therapy for CVD
Fixed-dose combination drug therapy has transformed the treatment of infectious diseases (e.g., human immunodeficiency virus [HIV] and tuberculosis) 90. In principle, the same strategy could be applied to CVD risk management 12. Current strategies require multiple pills for the adequate treatment of CVD risk factors, which often leads to low adherence and poor clinical effectiveness 91. Since a single drug is frequently insufficient to control blood pressure, control can be better achieved using fixed-dose combinations of blood pressure lowering drugs, an approach endorsed in European and US hypertension guidelines 34, 36, 92. Such a “polypill”, containing evidence-based medications including aspirin (for high risk patients and secondary prevention), statin, and blood pressure lowering drugs could result in a cumulative risk reduction of 75% in CVD events 93. Modelling based on the effect on risk factors observed in The Indian Polycap Study (TIPS) 1 and 2 study, such a preparation is estimated to reduce CHD risk by 62% and stroke risk by 48% in moderate-risk populations who are free of CVD 67, 68. A cross-over randomized controlled trial by Wald et al. indicated that, in individuals enrolled in a risk factor control program, the risk factor reduction achieved through use of a fixed-dose combination pill is of sufficient magnitude to result in a 60-70% relative risk reduction for CVD 69. The Use of a Multidrug Pill In Reducing cardiovascular Events (UMPIRE) trial has demonstrated that fixed-dose combination therapy significantly improves adherence compared to usual care with individual medications 70. Finally, a recent publication suggested that lifestyle modification combined with the Polycap could reduce CVD events by as much as 80%, as long as adherence is high (>90%) 12. A 2014 systematic review highlighted improved risk factor control and adherence with a fixed-dose combination cardiovascular medication as compared to placebo or a single drug 71. Ongoing randomized clinical trials evaluating the impact of a “polypill” on clinical events are underway 12. However, the evidence to date supports a combined approach including combination drug therapy, lifestyle modification, and adherence-improvement strategies.
The provision of affordable generic combination therapies containing evidence-based cardiovascular agents could simplify the management of CVD risk and address two of the identified modifiable targets, including hypertension and secondary prevention. Affordable combination drugs are currently unavailable in public health systems in most countries (except in some regions of India and a few Central American countries). This situation limits therapeutic options and complicates prescribing practices. Furthermore, there are increased costs to the patient and health system because more visits to health care providers are required to achieve adequate risk factor control. Implementation of a multi-drug strategy has been demonstrated to be cost-effective in LMIC 94-96. A regimen including a statin, aspirin and two-blood-pressure lowering medications to at risk populations in 23 LMIC over 10 years has been estimated to prevent 18 million deaths from CVD at a cost per head of US$1.08 ($0.75-1.40) 94. However, the true costs of the combined medications are higher than previously modelled (i.e., US$3.90-7.80 per month in India). This cost is still much less than the costs to patients for purchasing the individual components of the combined medications (i.e., US$28.4 in India) and would still be considered cost-effective 97.
Health System Strategies
Task sharing with non-physician health workers (NPHW), community health workers (CHW), and treatment supporters
Given the enormous burden of CVD (and the relative simplicity of identifying those with elevated blood pressure or those with known vascular disease) and limitations of current health systems, task sharing with NPHWs or CHWs may be an effective strategy to achieving the World Health Organization “25 × 25” goal. Experience in LMIC countries shows that elements of basic chronic disease management can be shifted to NPHW, often with improved outcomes 98. A systematic review of task-shifting for HIV care in Africa demonstrated that NPHWs offer cost-effective and high-quality care to a higher volume of patients than a physician-centered model 99. Furthermore, task-shifting has been shown to be a potentially effective and affordable strategy for improving access to healthcare for NCDs.72 Such a strategy is supported by the World Health Organization Task Shifting-Global Recommendations and Guidelines 100.
Task shifting or task-sharing has also been used successfully in HIC. It has been demonstrated that nurse-practitioner, nurse, or community health worker-led programs can improve blood pressure, lipids, and HbA1c 73-75, as well as the management of some chronic conditions 76. Not only would task sharing be of benefit in LMIC, but it may also be useful in high risk/low socioeconomic position areas in HIC due to the increased burden of CVD, vulnerable populations, and limited resources 3, 101. With the use of simplified algorithms for CVD assessment and management as outlined above, combined with appropriate physician oversight and readily available, fixed-dose combination cardiovascular medications, it is likely that task-sharing with NPHWs can be implemented reliably, safely, and effectively in diverse community health settings in LMIC 86, 102, 103. Yet, for this to succeed, there is a need to address those factors that prevent their widespread implementation, including concerns regarding safety, clear benefits in health outcomes, difficulties with staff retention, a need to re-engineer the health workforce that includes contextualized training of NPHWs to receive a core set of skills in the screening and management of CVD and policy limiting the NPHW’s ability to prescribe from a restricted list of medications. The latter is a significant limitation in many countries.72 Despite these barriers to implementation, NPHWs could be a cost-effective strategy to overcome identified barriers to best-practice and help achieve the World Health Organization “25 × 25” goal through improved screening of at risk individuals, counseling, and medical management.
As described above, patient adherence to guideline-recommended medications and lifestyle modification remain barriers to optimal care. However, mechanisms to improve adherence utilized in other chronic conditions (e.g., HIV treatment strategies in South Africa) are likely also applicable to CVD risk management. For example, the use of treatment supporters, defined as uncompensated, patient-nominated friends or family who help ensure optimal adherence to recommended therapies and clinic appointment schedules, has been demonstrated to be effective in patients with HIV 77. Treatment supporters, coached by NPHWs or health-care providers, could help promote an effective self-management program for patients with or at risk of CVD, thus proving to be an effective strategy in resource constrained settings 78.
The task-sharing and task-shifting roles, as described above, are only a few suggested components of a more comprehensive strategy towards widening the experience and training of health system personnel. Moving beyond primary-care physicians and specialists to nurses, pharmacists, NPHWs, CHWs, village volunteers, treatment supporters and community groups is essential to overcome the financial and human resource limitations that plague current health systems. Government policies that move towards universal health care, with coverage of essential medications, diagnostic tests, and interventions for CVD care are essential in minimizing inequities in health care.
Finally, while not specific to CVD, a comprehensive health system strategy will also consider the financing of health-care. Although many of the interventions and policies described in this paper will be cost saving, some will require additional revenue. Universal health coverage is now firmly on the international agenda, in particular as a result of its inclusion within the Sustainable Development Goals and the growing recognition of its contribution to international obligations on the right to health 104. Those advocating for improved prevention and treatment of CVD must therefore necessarily address the issue of health care financing, drawing on evidence such as that in a recent study of progress towards universal health coverage that highlighted the crucial role of effective systems of progressive taxation. 105
Finally, the inclusion of a target for NCDs in the Sustainable Development Goals has provided a powerful stimulus to measure and respond to the so far largely hidden burden of CVD in the poorest countries, in particular those being left behind, as countries such as Pakistan and Bangladesh move from low-income to lower-middle income status. The PURE Study does include two countries still classified in the World Bank’s LIC category, Tanzania and Zimbabwe, but further progress will be limited by the simple lack of research capacity, especially in rural areas, in many of these countries, an issue that should now be a priority for research funders106.
Summary
CVD accounts for the majority of NCD deaths around the world, with about 80% occurring in LMIC. A large body of evidence supports the findings in this review. However, there are gaps in knowledge, particularly relating to LMIC. Table 8 outlines the gaps in knowledge relating to resource effective strategies to prevent and treat CVD and suggested next steps to minimize these gaps. Using the evidence available to date, this review has demonstrated that significant evidence-practice gaps in the prevention and management of CVD exist, particularly in LMIC. Targeting key modifiable factors, including tobacco use, hypertension, and secondary prevention of CVD with resource-efficient strategies in at-risk populations within LMIC, coupled with population-level policies, could help achieve and even exceed the projected target of 25% reduction in premature mortality from CVD by 2025 in some countries. Strategies include: (1) effective measures for tobacco control; (2) implementation of simplified screening and management algorithms for those with or at risk of CVD, (3) increasing the availability and affordability of simplified and cost-effective treatment regimens including combination CVD preventive drug therapy, and (4) simplified delivery of health care through task-sharing (NPHWs) and optimizing self-management (treatment supporters).
Table 8.
Current gaps in knowledge relating to resource effective strategies to prevent and treat cardiovascular disease and suggested next steps.
| Gaps in Knowledge | Next Steps |
|---|---|
| A. Evidence-Practice Gaps | |
| Evidence-practice gaps relating to the prevention and management of CVD in LMIC. | Continued follow-up and further analyses of data from the PURE study and other longitudinal cohort studies in LMICs, expansion and deepening of country/community based registries, such as those in the INDEPTH Network 107, strengthening of vital registration systems, and design and implementation of intervention studies in LMIC. |
| B. Barriers to Care | |
| Barriers to evidence-based and efficient CVD prevention and management in LMIC. | Systematic assessments of barriers to care at the patient, health care provider, and health system level in different countries to identify the important contextual factors and to inform resource effective strategies to prevent and manage CVD. |
| C. Interventions | |
| Evidence supporting the impact of dietary policies on CVD health in LMIC. | Large-scale studies conducted in LMIC, including descriptive, etiological, and interventional, where diet patterns substantially differ from HIC, complemented with research on the food environment and food policy, including the effects of trade liberalization. |
| Strategies to support the implementation of tobacco prevention and control policies (i.e., FCTC). | Promulgation and enforcement of laws that control tobacco cultivation, manufacturing, trade, distribution, marketing, taxation, and treatment. |
| Impact of contextually appropriate, simple, and cost-effective risk stratification and management tools on community-based populations. | Large scale studies across low-, middle-, and high-income countries to evaluate the implementation of WHO-recommended diagnostic and management strategies. |
| Clinical benefit of fixed-dose combination therapy for the prevention and management of CVD. | Randomized clinical trials evaluating the impact of a “polypill” on clinical events. |
| Appropriate clinical responsibilities of NPHWs in the prevention and management of CVD. | Large randomized controlled trials evaluating the effectiveness and efficiency of NPHWs and LHWs in the prevention and management of CVD, with additional studies evaluating the safety of NPHWs prescribing first-line CVD evidenced-based medications. |
CVD=cardiovascular disease, LMIC-low and middle income countries, PURE=Prospective Urban Rural Epidemiology Study, FCTC= Framework Convention on Tobacco Control, LHWs=lay health workers, NPHWs= non-physician health workers, WHO=World Health Organization.
Supplementary Material
Acknowledgments
Funding Sources: None.
Footnotes
Conflict of Interest Disclosures: SY conducts research on single-pill, fixed-dose cardiovascular combination pharmacotherapy for which the Population Health Research Institute has received grant support from the Canadian Institute for Health Research, the Heart and Stroke Foundation Canada, Wellcome Trust, Cadila Pharmaceuticals Ltd and Astra Zeneca. MDH receives support from the World Heart Federation for its Emerging Leaders program, which is supported by AstraZeneca, Boehringer Ingelheim, and Bupa.
References
- 1.Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, Naghavi M, Mensah GA, Murray CJ. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372:1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, Bo J, Lou Q, Lu F, Liu T, Yu L, Zhang S, Mony P, Swaminathan S, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear S, Anand S, Wielgosz A, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Yusoff K, Ismail N, Iqbal R, Rahman O, Rosengren A, Yusufali A, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Oguz A, McQueen M, McKee M, Dagenais G, Investigators P Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371:818–827. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part i: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 4.Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, Murray CJ, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: The global burden of disease 2010 study. Circulation. 2014;129:1493–1501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in u.S. Deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 6.Mirzaei M, Truswell AS, Taylor R, Leeder SR. Coronary heart disease epidemics: Not all the same. Heart. 2009;95:740–746. doi: 10.1136/hrt.2008.154856. [DOI] [PubMed] [Google Scholar]
- 7.Vartiainen E, Puska P, Pekkanen J, Tuomilehto J, Jousilahti P. Changes in risk factors explain changes in mortality from ischaemic heart disease in finland. Bmj. 1994;309:23–27. doi: 10.1136/bmj.309.6946.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, Gupta R, Kelishadi R, Iqbal R, Avezum A, Kruger A, Kutty R, Lanas F, Lisheng L, Wei L, Lopez-Jaramillo P, Oguz A, Rahman O, Swidan H, Yusoff K, Zatonski W, Rosengren A, Teo KK, Prospective Urban Rural Epidemiology Study I Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the pure study): A prospective epidemiological survey. Lancet. 2011;378:1231–1243. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 9.Mamudu HM, Yang JS, Novotny TE. Un resolution on the prevention and control of non-communicable diseases: An opportunity for global action. Glob Public Health. 2011;6:347–353. doi: 10.1080/17441692.2011.574230. [DOI] [PubMed] [Google Scholar]
- 10.2008-2013 Action plan for the global strategy for the prevention and control of noncommunicable diseases. World Health Organization; 2009. [Google Scholar]
- 11.Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R, Kazmi K, Lanas F, Wei L, Lopez-Jaramillo P, Fanghong L, Ismail NH, Puoane T, Rosengren A, Szuba A, Temizhan A, Wielgosz A, Yusuf R, Yusufali A, McKee M, Liu L, Mony P, Yusuf S, investigators PS Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–968. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 12.Working Group on the Summit on Combination Therapy for CVD. Yusuf S, Attaran A, Bosch J, Joseph P, Lonn E, McCready T, Mente A, Nieuwlaat R, Pais P, Rodgers A, Schwalm JD, Smith R, Teo K, Xavier D. Combination pharmacotherapy to prevent cardiovascular disease: Present status and challenges. Eur Heart J. 2014;35:353–364. doi: 10.1093/eurheartj/eht407. [DOI] [PubMed] [Google Scholar]
- 13.Teo K, Lear S, Islam S, Mony P, Dehghan M, Li W, Rosengren A, Lopez-Jaramillo P, Diaz R, Oliveira G, Miskan M, Rangarajan S, Iqbal R, Ilow R, Puone T, Bahonar A, Gulec S, Darwish EA, Lanas F, Vijaykumar K, Rahman O, Chifamba J, Hou Y, Li N, Yusuf S, Investigators P Prevalence of a healthy lifestyle among individuals with cardiovascular disease in high-, middle- and low-income countries: The prospective urban rural epidemiology (pure) study. JAMA. 2013;309:1613–1621. doi: 10.1001/jama.2013.3519. [DOI] [PubMed] [Google Scholar]
- 14.Shimony A, Grandi SM, Pilote L, Joseph L, O’Loughlin J, Paradis G, Rinfret S, Sarrafzadegan N, Adamjee N, Yadav R, Gamra H, Diodati JG, Eisenberg MJ, Investigators Z Utilization of evidence-based therapy for acute coronary syndrome in high-income and low/middle-income countries. Am J Cardiol. 2014;113:793–797. doi: 10.1016/j.amjcard.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Mendis S, Abegunde D, Yusuf S, Ebrahim S, Shaper G, Ghannem H, Shengelia B. Who study on prevention of recurrences of myocardial infarction and stroke (who-premise) Bull World Health Organ. 2005;83:820–829. [PMC free article] [PubMed] [Google Scholar]
- 16.Shah NS, Huffman MD, Ning H, Lloyd-Jones DM. Trends in vascular risk factor treatment and control in us stroke survivors: The national health and nutrition examination surveys (1999-2010) Circ Cardiovasc Qual Outcomes. 2013;6:270–277. doi: 10.1161/CIRCOUTCOMES.113.000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloomfield GS, Baldridge A, Agarwal A, Huffman MD, Colantonio LD, Bahiru E, Ajay VS, Prabhakaran P, Lewison G, Prabhakaran D. Disparities in cardiovascular research output and citations from 52 african countries: A time-trend, bibliometric analysis (1999-2008) J Am Heart Assoc. 2015;4:e001606. doi: 10.1161/JAHA.114.001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khatib R, Schwalm JD, Yusuf S, Haynes RB, McKee M, Khan M, Nieuwlaat R. Patient and healthcare provider barriers to hypertension awareness, treatment and follow up: A systematic review and meta-analysis of qualitative and quantitative studies. PloS one. 2014;9:e84238. doi: 10.1371/journal.pone.0084238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieuwlaat R, Schwalm JD, Khatib R, Yusuf S. Why are we failing to implement effective therapies in cardiovascular disease? Eur Heart J. 2013;34:1262–1269. doi: 10.1093/eurheartj/ehs481. [DOI] [PubMed] [Google Scholar]
- 20.Grimshaw J, Eccles MP. Knowledge translation of research findings. Institute for Health Economics; Alberta: 2008. Chapter 2. [Google Scholar]
- 21.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 22.Who . Everybody’s business: Strengthnening heath systems to improve health outcomes. WHO’s Framework for Action. 2007. [Google Scholar]
- 23.Glaser BGSA. The constant comparative method of qualitative analysis. Social problems. 1965;12:436–445. [Google Scholar]
- 24.Stuart-Shor EM, Berra KA, Kamau MW, Kumanyika SK. Behavioral strategies for cardiovascular risk reduction in diverse and underserved racial/ethnic groups. Circulation. 2012;125:171–184. doi: 10.1161/CIRCULATIONAHA.110.968495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbaum L. Beyond belief--how people feel about taking medications for heart disease. N Engl J Med. 2015;372:183–187. doi: 10.1056/NEJMms1409015. [DOI] [PubMed] [Google Scholar]
- 26.Risso-Gill I, Balabanova D, Majid F, Ng KK, Yusoff K, Mustapha F, Kuhlbrandt C, Nieuwlaat R, Schwalm JD, McCready T, Teo KK, Yusuf S, McKee M. Understanding the modifiable health systems barriers to hypertension management in malaysia: A multi-method health systems appraisal approach. BMC Health Serv Res. 2015;15:254. doi: 10.1186/s12913-015-0916-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: Clinical applications. JAMA. 2002;288:2880–2883. doi: 10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- 28.Bagnall AJ, Yan AT, Yan RT, Lee CH, Tan M, Baer C, Polasek P, Fitchett DH, Langer A, Goodman SG. Optimal medical therapy for non-st-segment-elevation acute coronary syndromes: Exploring why physicians do not prescribe evidence-based treatment and why patients discontinue medications after discharge. Circ Cardiovasc Qual Outcomes. 2010;3:530–537. doi: 10.1161/CIRCOUTCOMES.109.919415. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Evans T, Anand S, Boufford JI, Brown H, Chowdhury M, Cueto M, Dare L, Dussault G, Elzinga G, Fee E, Habte D, Hanvoravongchai P, Jacobs M, Kurowski C, Michael S, Pablos-Mendez A, Sewankambo N, Solimano G, Stilwell B, de Waal A, Wibulpolprasert S. Human resources for health: Overcoming the crisis. Lancet. 2004;364:1984–1990. doi: 10.1016/S0140-6736(04)17482-5. [DOI] [PubMed] [Google Scholar]
- 30.McAlister FA, Campbell NR, Zarnke K, Levine M, Graham ID. The management of hypertension in canada: A review of current guidelines, their shortcomings and implications for the future. CMAJ. 2001;164:517–522. [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC, Tu JV, Ko DT, Alter DA. Factors associated with the use of evidence-based therapies after discharge among elderly patients with myocardial infarction. CMAJ. 2008;179:901–908. doi: 10.1503/cmaj.080295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham IM, Stewart M, Hertog MG, Cardiovascular Round Table Task F Factors impeding the implementation of cardiovascular prevention guidelines: Findings from a survey conducted by the european society of cardiology. Eur J Cardiovasc Prev Rehabil. 2006;13:839–845. doi: 10.1097/01.hjr.0000219112.02544.24. [DOI] [PubMed] [Google Scholar]
- 33.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr., Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, Smith SC, Jr., Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Nishimura R, Ohman EM, Page RL, Stevenson WG, Tarkington LG, Yancy CW, American College of Cardiology F, American Heart A 2010 accf/aha guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Kjeldsen SE, Erdine S, Narkiewicz K, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Cifkova R, Dominiczak A, Fagard R, Heagerty AM, Laurent S, Lindholm LH, Mancia G, Manolis A, Nilsson PM, Redon J, Schmieder RE, Struijker-Boudier HA, Viigimaa M, Filippatos G, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Kiowski W, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Viigimaa M, Waeber B, Williams B, Zamorano JL, The task force for the management of arterial hypertension of the European Society of H. The task force for the management of arterial hypertension of the European Society of C 2007 guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the european society of hypertension (esh) and of the european society of cardiology (esc) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 35.Campbell NR, Poirier L, Tremblay G, Lindsay P, Reid D, Tobe SW, Canadian Hypertension Education P Canadian hypertension education program: The science supporting new 2011 chep recommendations with an emphasis on health advocacy and knowledge translation. Can J Cardiol. 2011;27:407–414. doi: 10.1016/j.cjca.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 36.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr., Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr., Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 37.Peiris D, Usherwood T, Panaretto K, Harris M, Hunt J, Redfern J, Zwar N, Colagiuri S, Hayman N, Lo S, Patel B, Lyford M, MacMahon S, Neal B, Sullivan D, Cass A, Jackson R, Patel A. Effect of a computer-guided, quality improvement program for cardiovascular disease risk management in primary health care: The treatment of cardiovascular risk using electronic decision support cluster-randomized trial. Circ Cardiovasc Qual Outcomes. 2015;8:87–95. doi: 10.1161/CIRCOUTCOMES.114.001235. [DOI] [PubMed] [Google Scholar]
- 38.Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med. 2004;19:638–645. doi: 10.1111/j.1525-1497.2004.30516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khatib R, McKee M, Shannon H, Chow C, Rangarajan S, Teo K, Wei L, Mony P, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear SA, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Yusoff K, Ismail N, Kazmi K, Rahman O, Rosengren A, Monsef N, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Temizhan A, Dagenais G, Gafni A, Yusuf S, investigators Ps Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: An analysis of the pure study data. Lancet. 2016;387:61–69. doi: 10.1016/S0140-6736(15)00469-9. [DOI] [PubMed] [Google Scholar]
- 40.World development indicators: Health systems (internet) The World Bank; 2014. http://wdi.worldbank.org/table/2.15. [Google Scholar]
- 41.Huffman MD, Rao KD, Pichon-Riviere A, Zhao D, Harikrishnan S, Ramaiya K, Ajay VS, Goenka S, Calcagno JI, Caporale JE, Niu S, Li Y, Liu J, Thankappan KR, Daivadanam M, van Esch J, Murphy A, Moran AE, Gaziano TA, Suhrcke M, Reddy KS, Leeder S, Prabhakaran D. A cross-sectional study of the microeconomic impact of cardiovascular disease hospitalization in four low- and middle-income countries. PloS one. 2011;6:e20821. doi: 10.1371/journal.pone.0020821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marten R, McIntyre D, Travassos C, Shishkin S, Longde W, Reddy S, Vega J. An assessment of progress towards universal health coverage in brazil, russia, india, china, and south africa (brics) Lancet. 2014;384:2164–2171. doi: 10.1016/S0140-6736(14)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Who report on the global tobacco epidemic, 2013. The World Health Organization; Geneva: 2013. [Google Scholar]
- 44.Bogdanovica I, McNeill A, Murray R, Britton J. What factors influence smoking prevalence and smoke free policy enactment across the european union member states. PloS one. 2011;6:e23889. doi: 10.1371/journal.pone.0023889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker R, Camosso-Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp S, Robertson N. Tailored interventions to overcome identified barriers to change: Effects on professional practice and health care outcomes. Cochrane Database Systematic Rev. 2010:CD005470. doi: 10.1002/14651858.CD005470.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, Investigators IS Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the interheart study): Case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 47.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez-Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S, investigators I Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the interstroke study): A case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 48.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists C Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 49.Turnbull F, Blood Pressure Lowering Treatment Trialists C Effects of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 50.Perel P, Avezum A, Huffman M, Pais P, Rodgers A, Vedanthan R, Wood D, Yusuf S. Reducing premature cardiovascular morbidity and mortality in people with atherosclerotic vascular disease: The world heart federation roadmap for secondary prevention of cardiovascular disease. Global Heart. 2015;10:99–110. doi: 10.1016/j.gheart.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Emerging Risk Factors C. Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perel P, Bianco E, Poulter N, Prabhakaran D, Pais P, Ralston J, Wood D, Yusuf S. Reducing premature cardiovascular mortality by 2025: The world heart federation roadmap. Global heart. 2015;10:97–98. doi: 10.1016/j.gheart.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Perel P, Bianco E, Poulter N, Prabhakaran D, Pais P, Ralston J, Wood D, Yusuf S. Adapting the world heart federation roadmaps at the national level: Next steps and conclusions. Global heart. 2015;10:135–136. doi: 10.1016/j.gheart.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Yusuf S, Perel P, Wood D, Narula J. Reducing cardiovascular disease globally: The world heart federation’s roadmaps. Global heart. 2015;10:93–95. doi: 10.1016/j.gheart.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB, Wilson PW. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290:891–897. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 56.Pandya A, Weinstein MC, Salomon JA, Cutler D, Gaziano TA. Who needs laboratories and who needs statins?: Comparative and cost-effectiveness analyses of non-laboratory-based, laboratory-based, and staged primary cardiovascular disease screening guidelines. Circ Cardiovasc Qual Outcomes. 2014;7:25–32. doi: 10.1161/CIRCOUTCOMES.113.000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, Baugh V, Bekedam H, Billo N, Casswell S, Cecchini M, Colagiuri R, Colagiuri S, Collins T, Ebrahim S, Engelgau M, Galea G, Gaziano T, Geneau R, Haines A, Hospedales J, Jha P, Keeling A, Leeder S, Lincoln P, McKee M, Mackay J, Magnusson R, Moodie R, Mwatsama M, Nishtar S, Norrving B, Patterson D, Piot P, Ralston J, Rani M, Reddy KS, Sassi F, Sheron N, Stuckler D, Suh I, Torode J, Varghese C, Watt J, Lancet NCDAG. Alliance NCD Priority actions for the non-communicable disease crisis. Lancet. 2011;377:1438–1447. doi: 10.1016/S0140-6736(11)60393-0. [DOI] [PubMed] [Google Scholar]
- 58.M-power in action-defeating the global tobacco epidemic. World Health Organization; Geneva: 2013. [Google Scholar]
- 59.Who framework convention on tobacco control. World Health Organization; Geneva: 2003. [Google Scholar]
- 60.Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: Health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370:2044–2053. doi: 10.1016/S0140-6736(07)61698-5. [DOI] [PubMed] [Google Scholar]
- 61.American Heart Association Nutrition C. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: A scientific statement from the american heart association nutrition committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 62.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA, Investigators PS Primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 63.Cecchini M, Sassi F, Lauer JA, Lee YY, Guajardo-Barron V, Chisholm D. Tackling of unhealthy diets, physical inactivity, and obesity: Health effects and cost-effectiveness. Lancet. 2010;376:1775–1784. doi: 10.1016/S0140-6736(10)61514-0. [DOI] [PubMed] [Google Scholar]
- 64.Jones HA, Charlton KE. A cross-sectional analysis of the cost and affordability of achieving recommended intakes of non-starchy fruits and vegetables in the capital of vanuatu. BMC public health. 2015;15:301. doi: 10.1186/s12889-015-1644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Temple NJ, Steyn NP. The cost of a healthy diet: A south african perspective. Nutrition. 2011;27:505–508. doi: 10.1016/j.nut.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Markovic N, Olomu IN, Bunker CH, Huston SL, Ukoli FA, Kuller LH. Adequacy of a single visit for classification of hypertensive status in a nigerian civil servant population. Int J Epidemiol. 1994;23:723–729. doi: 10.1093/ije/23.4.723. [DOI] [PubMed] [Google Scholar]
- 67.Indian Polycap S. Yusuf S, Pais P, Afzal R, Xavier D, Teo K, Eikelboom J, Sigamani A, Mohan V, Gupta R, Thomas N. Effects of a polypill (polycap) on risk factors in middle-aged individuals without cardiovascular disease (tips): A phase ii, double-blind, randomised trial. Lancet. 2009;373:1341–1351. doi: 10.1016/S0140-6736(09)60611-5. [DOI] [PubMed] [Google Scholar]
- 68.Yusuf S, Pais P, Sigamani A, Xavier D, Afzal R, Gao P, Teo KK. Comparison of risk factor reduction and tolerability of a full-dose polypill (with potassium) versus low-dose polypill (polycap) in individuals at high risk of cardiovascular diseases: The second indian polycap study (tips-2) investigators. Circ Cardiovasc Quality Outcomes. 2012;5:463–471. doi: 10.1161/CIRCOUTCOMES.111.963637. [DOI] [PubMed] [Google Scholar]
- 69.Wald DS, Morris JK, Wald NJ. Randomized polypill crossover trial in people aged 50 and over. PLoS One. 2012;7:e41297. doi: 10.1371/journal.pone.0041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thom S, Poulter N, Field J, Patel A, Prabhakaran D, Stanton A, Grobbee DE, Bots ML, Reddy KS, Cidambi R, Bompoint S, Billot L, Rodgers A, Group UC Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of cvd: The umpire randomized clinical trial. JAMA. 2013;310:918–929. doi: 10.1001/jama.2013.277064. [DOI] [PubMed] [Google Scholar]
- 71.de Cates AN, Farr MR, Wright N, Jarvis MC, Rees K, Ebrahim S, Huffman MD. Fixed-dose combination therapy for the prevention of cardiovascular disease. Cochrane Database Systematic Rev. 2014;4:CD009868. doi: 10.1002/14651858.CD009868.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joshi R, Alim M, Kengne AP, Jan S, Maulik PK, Peiris D, Patel AA. Task shifting for non-communicable disease management in low and middle income countries - a systematic review. PloS one. 2014;9:e103754. doi: 10.1371/journal.pone.0103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allen JK, Dennison-Himmelfarb CR, Szanton SL, Bone L, Hill MN, Levine DM, West M, Barlow A, Lewis-Boyer L, Donnelly-Strozzo M, Curtis C, Anderson K. Community outreach and cardiovascular health (coach) trial: A randomized, controlled trial of nurse practitioner/community health worker cardiovascular disease risk reduction in urban community health centers. Circ Cardiovasc Qual Outcomes. 2011;4:595–602. doi: 10.1161/CIRCOUTCOMES.111.961573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clark CE, Smith LF, Taylor RS, Campbell JL. Nurse led interventions to improve control of blood pressure in people with hypertension: Systematic review and meta-analysis. BMJ. 2010;341:c3995. doi: 10.1136/bmj.c3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shaffer J, Wexler LF. Reducing low-density lipoprotein cholesterol levels in an ambulatory care system. Results of a multidisciplinary collaborative practice lipid clinic compared with traditional physician-based care. Arch Intern Med. 1995;155:2330–2335. [PubMed] [Google Scholar]
- 76.Task shifting to tackle health worker shortages : The world health report – Working together for health. World Health Organization; Geneva: [accessed 27 April 2007]. 2006. http://www.who.int/whr/2006/en. [Google Scholar]
- 77.Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, Rachlis B, Wu P, Cooper C, Thabane L, Wilson K, Guyatt GH, Bangsberg DR. Adherence to antiretroviral therapy in sub-saharan africa and north america: A meta-analysis. JAMA. 2006;296:679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 78.Bosworth HB, Powers BJ, Oddone EZ. Patient self-management support: Novel strategies in hypertension and heart disease. Cardiol Clinics. 2010;28:655–663. doi: 10.1016/j.ccl.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJ, Gakidou E. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 80.Mackay J, Crofton J. Tobacco and the developing world. Br Med Bull. 1996;52:206–221. doi: 10.1093/oxfordjournals.bmb.a011527. [DOI] [PubMed] [Google Scholar]
- 81.Gilmore AB, Fooks G, Drope J, Bialous SA, Jackson RR. Exposing and addressing tobacco industry conduct in low-income and middle-income countries. Lancet. 2015;385:1029–1043. doi: 10.1016/S0140-6736(15)60312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Savell EGA, Sims M, Mony PK, Teo K, Yusoff K, Lear SA, Seron P, Ismail NH, Calik KBT, Rosengren A, Bahonar A, Kumar R, Vijayakumar K, Kruger A, Swidan H, Gupta R, Igumbor E, Afridi A, Rahman O, Chifamba J, Zatonska K, Mohan V, Deepa M, Lopez-Jaramillo P, Avezum A, Poirier P, Orlandini A, Li W, McKee M, Rangarajan S, Yusuf S, Chow CK. The global tobacco marketing environment: Data from 462 communities across 16 countries from the environmental profile of a community’s health (epoch) study. Bull WHO. 2015;93:851–861. doi: 10.2471/BLT.15.155846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andrew Russell MW, Mamudu Hadii. A chilling example? Uruguay, philip morris international, and who’s framework convention on tobacco control. Med Anthropol Q. 2015;29:256–277. doi: 10.1111/maq.12141. [DOI] [PubMed] [Google Scholar]
- 84.Fields S. The fat of the land: Do agricultural subsidies foster poor health? Environ Health Perspect. 2004;112:A820–823. doi: 10.1289/ehp.112-a820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stuckler D, McKee M, Ebrahim S, Basu S. Manufacturing epidemics: The role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS Med. 2012;9:e1001235. doi: 10.1371/journal.pmed.1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Who package for essential non-communicable (pen) disease intervention for primary care in low-resource settings. 2010. [Google Scholar]
- 87.Megiddo I, Chatterjee S, Nandi A, Laxminarayan R. Cost-effectiveness of treatment and secondary prevention of acute myocardial infarction in india: A modeling study. Global Heart. 2014;9:391–398. e393. doi: 10.1016/j.gheart.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 88.Who model list of essential medicines. Apr, 2015. [Google Scholar]
- 89.Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, Reisman L, Fernandes J, Spettell C, Lee JL, Levin R, Brennan T, Shrank WH. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088–2097. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- 90.Volberding PA, Deeks SG. Antiretroviral therapy and management of hiv infection. Lancet. 2010;376:49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- 91.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 92.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ, Joint National Committee on Prevention DE. Treatment of High Blood Pressure National Heart L, Blood I, National High Blood Pressure Education Program Coordinating C. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 93.Yusuf S. Two decades of progress in preventing vascular disease. Lancet. 2002;360:2–3. doi: 10.1016/S0140-6736(02)09358-3. [DOI] [PubMed] [Google Scholar]
- 94.Lim SS, Gaziano TA, Gakidou E, Reddy KS, Farzadfar F, Lozano R, Rodgers A. Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: Health effects and costs. Lancet. 2007;370:2054–2062. doi: 10.1016/S0140-6736(07)61699-7. [DOI] [PubMed] [Google Scholar]
- 95.Ortegon M, Lim S, Chisholm D, Mendis S. Cost effectiveness of strategies to combat cardiovascular disease, diabetes, and tobacco use in sub-saharan africa and south east asia: Mathematical modelling study. Bmj. 2012;344:e607. doi: 10.1136/bmj.e607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaziano TA, Opie LH, Weinstein MC. Cardiovascular disease prevention with a multidrug regimen in the developing world: A cost-effectiveness analysis. Lancet. 2006;368:679–686. doi: 10.1016/S0140-6736(06)69252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khatib RMM, Shannon H, Chow C, Rangarajan S, Teo K, Wei L, Mony P, Gupta R, Kumar R, Vijavakumar K, Lear S, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Yusoff K, Ismail N, Kazmi K, Rahman O, Rosengren A, Monsef N, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Temizhan A, Dagenais G, Gafni A, Yusuf S, PURE study investigators Availability and affordability of cardiovascular disease medicines and their impact on use: Comparison across high, middle, and low-income countries. Lancet. 2016;387:61–69. doi: 10.1016/S0140-6736(15)00469-9. [DOI] [PubMed] [Google Scholar]
- 98.Ahmed T, Jakaria SM. Community-based skilled birth attendants in bangladesh: Attending deliveries at home. Reprod Health Matters. 2009;17:45–50. doi: 10.1016/S0968-8080(09)33446-1. [DOI] [PubMed] [Google Scholar]
- 99.Callaghan M, Ford N, Schneider H. A systematic review of task- shifting for hiv treatment and care in africa. Hum Resour Health. 2010;8:8. doi: 10.1186/1478-4491-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Task-shifting: Rational redistribution of tasks among health workforce teams: Global recommendations and guidelines. World Health Organization; Geneva: 2008. [Google Scholar]
- 101.Lee DS, Chiu M, Manuel DG, Tu K, Wang X, Austin PC, Mattern MY, Mitiku TF, Svenson LW, Putnam W, Flanagan WM, Tu JV, Canadian Cardiovascular Outcomes Research T Trends in risk factors for cardiovascular disease in canada: Temporal, socio-demographic and geographic factors. CMAJ. 2009;181:E55–66. doi: 10.1503/cmaj.081629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.WHO cvd-risk management package for low – and medium-resource settings. World Health Organization; Geneva: 2002. [Google Scholar]
- 103.Abegunde DO, Shengelia B, Luyten A, Cameron A, Celletti F, Nishtar S, Pandurangi V, Mendis S. Can non-physician health-care workers assess and manage cardiovascular risk in primary care? Bull World Health Organ. 2007;85:432–440. doi: 10.2471/BLT.06.032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sridhar D, McKee M, Ooms G, Beiersmann C, Friedman E, Gouda H, Hill P, Jahn A. Universal health coverage and the right to health: From legal principle to post-2015 indicators. Int J Health Serv. 2015;45:495–506. doi: 10.1177/0020731415584554. [DOI] [PubMed] [Google Scholar]
- 105.Reeves A, Gourtsoyannis Y, Basu S, McCoy D, McKee M, Stuckler D. Financing universal health coverage--effects of alternative tax structures on public health systems: Cross-national modelling in 89 low-income and middle-income countries. Lancet. 2015;386:274–280. doi: 10.1016/S0140-6736(15)60574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McKee M, Stuckler D, Basu S. Where there is no health research: What can be done to fill the global gaps in health research? PLoS Med. 2012;9:e1001209. doi: 10.1371/journal.pmed.1001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ngom P, Binka FN, Phillips JF, Pence B, Macleod B. Demographic surveillance and health equity in sub-saharan africa. Health Policy Plan. 2001;16:337–344. doi: 10.1093/heapol/16.4.337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.