Abstract
IL-31, a member of the IL-6 protein family, is one of the latest additions to the list of T-cell-derived cytokines. Th2 cells are regarded as a main source of IL-31, which is produced in response to stimulation by IL-4. Because the development of Th9 cells also requires IL-4 as a polarizing cytokine, the present study investigates IL-31 production in human Th9 cells compared to Th2 cells. We found that, although Th9 cells were able to release IL-31 during the first weeks of in vitro polarization, no IL-31 was detected in Th9 cultures after a final re-stimulation in the absence of polarizing cytokines. We further show that TGF-β, which is required to obtain Th9 cells in vitro, potently inhibits the release of IL-31 from Th2 cells, whereas IL-33, a cytokine associated with Th2-mediated inflammation, synergizes with IL-4 in inducing IL-31 secretion. To analyze the molecular mechanisms underlying the induction of IL-31, electrophoretic mobility shift assays, reporter gene assays and siRNA-based silencing experiments were carried out. We show that STAT6 and NF-κB are central players in mediating IL-31 expression induced by IL-4/IL-33. In addition, we identified a novel NF-κB-binding element within the Il31 promoter that mediates the enhancing effects of IL-33 on IL-4/STAT6-induced IL-31 expression in human Th2 cells.
Taken together, this study shows that IL-4 is essential for the production of IL-31, whereas TGF-β significantly suppresses IL-31 expression at the mRNA and protein levels. As a consequence, in vitro-polarized Th2 cells, but not Th9 cells, are able to release IL-31.
Introduction
CD4+ helper T (Th) cells are crucial players in orchestrating adaptive immune responses to various infectious agents. Depending on the type of presented antigen, naïve CD4+ T cells develop into several distinct subsets that can be distinguished by their function and their unique cytokine profile (1, 2). More than 25 years ago, the first two subsets, termed Th1 and Th2, were described by Coffman and Mosmann (3). Th1 cells predominantly secrete IFN-γ and are important for protective immune responses to intracellular bacterial and viral infection. In contrast, Th2 cells contribute to the defense against helminthic parasites and are key players in allergic inflammation. Th2 cells are characterized by the production of IL-4, IL-5 and IL-13, which promote IgE/eosinophil-mediated immune responses. More recently, two further subsets, known as Th17 cells and Treg cells, were described. Through the production of IL-17 (IL-17A), IL-17F, IL-22, and IL-26, Th17 cells control immune responses to extracellular bacteria and fungi (4). In addition, naïve CD4+ T cells can be induced to differentiate into inducible regulatory T cells (iTreg), which are characterized by the production of IL-10 and TGF-β. Together with naturally occurring regulatory T cells, these cells are potent players in maintaining immune tolerance and the regulation of lymphocyte activation (1).
One of the latest additions to the list of CD4+ T-cell subsets is the Th9 cell. This cell type develops in the presence of TGF-β and IL-4 and is characterized by the secretion of IL-9 (5, 6). Besides both being activated in the presence of IL-4, Th2 and Th9 cells also share functional features. Similar to Th2 cells, Th9 cells play an important role in promoting allergic responses and are involved in intestinal responses to helminths (7-9). Moreover, the expression of IL-9 was shown to be increased in asthmatic patients and atopic individuals compared to healthy subjects (10-12). These data indicate that IL-9 and Th2-derived cytokines may either cooperate or complement each other.
Another cytokine that is tightly associated with allergic inflammation is IL-31. This type-I cytokine was identified several years ago as a ligand for a heterodimeric receptor complex composed of the IL-31 receptor alpha (IL-31RA) chain and Oncostatin M receptor β (OSMRB) (13). IL-31 is predominantly secreted by activated CD4+ T cells (13). In particular, IL-31+ skin-homing, cutaneous lymphocyte antigen (CLA)-positive T cells (14) are thought to be the main source of enhanced IL-31 expression, which has been observed in patients suffering from atopic dermatitis (AD) and acute allergic contact dermatitis (15). In line with these findings, it was reported that over-expression of IL-31 in a transgenic mouse model induced a severe skin phenotype resembling AD in humans (13). In addition, high IL-31 levels have been detected in sera and PBMCs from patients with allergic asthma, and IL-31 expression was positively correlated with severity of the disease (16). Together, these data indicate that IL-31 is associated with type-2 inflammation. A recent study clearly showed that IL-4 is the critical factor stimulating the release of IL-31. In the presence of IL-4, not only Th2 cells, but also Th1 cells, were able to release IL-31 (17). Because IL-4 is essential not only for the differentiation of Th2 cells, but also plays a critical role in Th9 development, the present study investigated IL-31 expression in different human in vitro-polarized T helper cell subsets, including Th9. We show that, although Th9 cells secrete IL-31 during early T-cell development, fully differentiated Th9 cells are not able to release IL-31. Down-regulation of IL-31 was linked to the Th9-promoting cytokine TGF-β. Yet, the release of IL-31 by Th2 cells can be enhanced by addition of IL-33 and is mediated by the transcription factors STAT6 and NF-κB.
Materials and Methods
Th cell polarization and ELISA
All studies involving human cells were conducted in accordance with the guidelines of the World Medical Association’s Declaration of Helsinki. Human PBMCs were isolated from buffy coats of healthy donors by means of density gradient centrifugation using Lymphocyte separation medium (PAA, Pasching, Austria). Naïve CD4+ T cells were isolated from PBMCs using the Naive CD4+ T cell isolation kit II (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured in IMDM, containing 5% heat-inactivated FCS, 2mM L-glutamine, 100U/ml penicillin and 100μg/ml streptomycin (all purchased from PAA, Pasching, Austria) at a density of 2·106 cells/ml in 48-well plates pre-coated with α-CD3 (clone OKT3, eBioscience, Vienna, Austria, coating concentration 1μg/ml in 0.1M Tris HCl pH 9.5) and in the presence of soluble α-CD28 (2.5 μg/ml; BD Pharmingen, Heidelberg, Germany). For Th1 differentiation, 20ng/ml IL-12 (Immnotools, Friesoythe, Germany) and 1μg/ml α-IL-4 (eBioscience, Vienna, Austria) were added to the cells. Th2 development was induced via the addition of 50ng/ml IL-4 (kind gift from Novartis, Vienna, Austria) and 1μg/ml α-IL-12/α-IL-23 (eBioscience, Vienna, Austria). For Th9 cell generation, additionally 10ng/ml TGF-β1 (Peprotech, London, UK) was used. Th17 cells were generated by adding 10ng/ml IL-1β (Immnotools, Friesoythe, Germany), 30ng/ml IL-6 (Immnotools, Friesoythe, Germany), 0.5ng/ml TGF-β1 (Peprotech, London, UK), 50ng/ml IL-21 (Immnotools, Friesoythe, Germany) and α-IL-4. Tregs were induced by 10ng/ml TGF-β1 and 10ng/ml IL-10 (Immnotools, Friesoythe, Germany) with blocking antibodies directed against IL-12/IL-23 and IL-4. Cells were cultured for 7 days, re-stimulated under the same conditions for an additional 7 days and then re-activated by addition of α-CD3/α-CD28 for 48 hours. Supernatants for cytokine secretion analyses by means of sandwich Enzyme-linked immuno sorbent assay (ELISA) were taken after each stimulation period. ELISAs for the detection of IL-31, IL-17A, IFN-γ, IL-5, IL-13 (Peprotech, London, UK), and IL-10 and IL-9 (eBioscience, Vienna, Austria) were carried out according to the manufacturers’ instructions.
Allergen-specific T-cell clones
Supernatants from T-cell clones (TCC) specific for the major birch pollen allergen Bet v 1 were used for the assessment of IL-31. TCC were generated according to published protocols (18) and stimulated with autologous irradiated PBMCs and 5μg/ml Bet v 1 (Biomay, Vienna, Austria) for 48 hours. Cultures containing TCC and PBMCs in medium alone served as negative controls. Supernatants were kept at −20°C until cytokine measurements. Levels of IL-4, IL-13, IL-9 and IFN-γ were determined using the Luminex System 100 (Luminex, Texas, USA). Eight TCC belonged to the Th2 subset, producing IL-4/IFN-γ >5, and five TCC belonged to the Th0 subset, producing IL-4/IFN-γ = 0.2-5. IL-31 secretion was determined by sandwich ELISA (Peprotech, London, UK).
Preparation of nuclear extracts and electrophoretic mobility shift assays
Nuclear extracts from untreated and IL-4-induced CD4+ T cells or uninduced and IL-33-treated Th2 cells were prepared according to the method of Andrews and Faller (19). EMSAs were carried out as described previously (20, 21). Double-stranded oligonucleotide probes corresponding to the sequences −437 to −400, −282 to −241 and −160 to −118 relative to the start ATG from the human IL31 gene were generated by annealing synthetic sense and anti-sense oligonucleotides for 2 hours during which the temperature was gradually reduced from 95°C to 25°C, followed by radioactive 3′-end-labeling with [32P]dCTP (Hartmann Analytic, Braunschweig, Germany) using Klenow fragment (Fermentas, St. Leon-Roth, Germany). Labeled oligonucleotides were purified using Illustra Micro-Spin G-25 columns (GE Healthcare, Vienna, Austria). For oligonucleotide competition assays, non-labeled oligonucleotide was added in 50-molar excess to the binding reaction 30 minutes prior to addition of the radiolabeled probe. Super-shifting was achieved by adding 500 ng/μl antibody (α-STAT6 M20×, α-STAT5 N20×, α-NF-κB p50 N19×, α-NF-κB p52 K27×, α-NF-κB p65 F6×, Santa Cruz Biotechnology, Heidelberg, Germany) to the binding reaction. The samples were separated in 5% non-reducing polyacrylamide gels in 1 × TBE buffer. Radioactivity signals were assessed by exposing X-ray films to the dried gels. Sequences of the oligonucleotides are given below (consensus nucleotides underlined, mutations in lower case).
NF-κB −437/−400 WT sense 5’-GGCTTCGCATTTCCTCCCCAGAAATTCCCTGTGGC-3’ and anti-sense 5’-GCGGCCACAGGGAATTTCTGGGGAGGAAATGCGAA-3’; NF-κB mut sense 5’-GGCTTCGCATTTCCTCCCCAGAAATTCgCTGTGGC-3’ and anti-sense 5’-GCGGCCACAGcGAATTTCTGGGGAGGAAATGCGAA-3’; STAT6 −282/−241 WT sense 5’-GATGCATTCATGTGCCTTCTTGTGAAGTATGTGTGTGTCTGA-3’ and anti-sense 5’-GGGTCAGACACACACATACTTCACAAGAAGGCACATGAATG-3’; STAT6 −282/−241 mut sense 5’-GATGCATTCATGTGCCTatTTGTGAAGTATGTGTGTGTCTGA-3’ and anti-sense 5’-GGGTCAGACACACACATACTTCACAAatAGGCACATGAATG-3’; STAT6 −160/−118 WT sense 5’-GAGTGTTTTCTGGAGAAAAGCTGAGTAAATGGTTTTGCCATGG-3’ and anti-sense 5’-GGGCCATGGCAAAACCATTTACTCAGCTTTTCTCCAGAAAACA-3’; STAT6 −160/−118 mut sense 5’-GAGTGTTTatTGGAGAAAAGCTGAGTAAATGGTTTTGCCATGG-3’ and anti-sense 5’-GGGCCATGGCAAAACCATTTACTCAGCTTTTCTCCAatAAACA-3’.
Cloning of Il31 promoter constructs
A 474-bp fragment comprising the sequence −535 to −62 relative to the transcriptional start site of the human Il31 promoter was amplified from human genomic DNA (Roche, Vienna, Austria) using Pfu polymerase with appropriate buffer (Fermentas, St. Leon-Roth, Germany) and the primers with attached restriction sites for MluI (forward primer) and XhoI (reverse primer) listed below. The PCR tube contained 36μl H2O, 5μl 10 × Pfu buffer with MgSO4, 4μl DMSO, 1μl dNTPs (10mM each), 2μl genomic DNA, 1μl forward and reverse primer (10μM each) and 1μl Pfu polymerase. PCR – 5 minutes initial denaturation at 95°C followed by 37 cycles of 15 seconds 95°C, 30 seconds annealing at 60°C and 5 minutes elongation at 72°C, and a final elongation step of 10 minutes at 72°C – was run on an Eppendorf Mastercycler (Eppendorf, Vienna, Austria). The PCR product was cloned into the pGL3 Basic Luciferase reporter-gene vector (Promega, Mannheim, Germany). Site-directed mutagenesis of STAT6 sites and the NF-κB-binding site was carried out by inverse PCR using the 5’-phosphorylated primers listed below. The sequences of all constructs were verified by sequencing at MWG (Ebersberg, Germany). The plasmids were used to transform chemo-competent E. coli TG1 and purified using an EndoFree Plasmid Maxi Kit from Qiagen (Vienna, Austria).
Sequences of the primers are as follows (restriction sites underlined, mutated nucleotides in lower case):
IL31 474bp MluI sense 5’-AGTCACGCGTCGCCACATTCACAGCAGTTA-3’; IL31 474bp XhoI anti-sense 5’-AGTCCTCGAGCTGCCTGGAGGTATATAAAGGGC-3’; IL31 STAT6 −153/−144 mut sense 5’-atTGGAGAAAAGCTGAGTAAATGGTT-3’ and anti-sense 5’-AAACACTCAAAAGTTCTACTGGCCACGGC-3’; IL31 STAT6 −266/−257 mut sense 5’-atTTGTGAAGTATGTGTGTGTCTGAGTCAGG-3’and anti-sense 5’-AGGCACATGAATGCATCTTTGCCATTC-3’; IL31 NF-κB-418/−409 mut sense 5’-gCTGTGGCCGCTGGCCTTG-3’ and anti-sense 5’-GAATTTCTGGGGAGGAAATGCGAAG-3’
Reporter gene assays
The day before transfection, 1.25 · 105 HEKblue IL-4/IL-13 cells (Invivogen, Eubio, Vienna, Austria) were seeded into 24-well cell-culture plates in 1ml DMEM medium supplemented with 10% FCS, 2mM L-glutamine, 100U/ml penicillin and 100μg/ml streptomycin, 1× nonessential amino acids (all purchased from PAA, Pasching, Austria), 100μg/ml zeocin and 10μg/ml blasticidin and incubated at 37°C in a humidified atmosphere containing 5% CO2. Cells were transfected with 1μg luciferase reporter plasmid, 0.125μg ST2L expression construct (22) (kindly provided by Prof. SJ Martin, Dublin, Ireland) or empty pEF-Bos vector (23) (generous gift from Prof. S Nagata, Kyoto, Japan) by means of calcium phosphate co-precipitation as described previously (24). The day after the transfection the medium was changed and cells were induced with 50ng/ml IL-4 and/or 30ng/ml IL-33 (Peprotech, London, UK) or left unstimulated for 24 hours, before luciferase activity was assessed.
siRNA-based silencing
Naïve CD4+ T cells were isolated and differentiated toward a Th2 phenotype as described above. After 8 days of differentiation, cells were transfected with 100pmol siRNA targeting STAT6 (Invitrogen stealth RNA, forward 5’-CCAAAGCCACUAUCCUGUGGGACAA-3’, reverse 5’-UUGUCCCACAGGAUAGUGGCUUUGG-3’) or control oligonucleotide (AllStars Negative Control siRNA, Qiagen, Hilden, Germany) using an Amaxa Nucleofector Device I and a Human T cell nucleofector kit (Lonza, Szabo Scandic, Vienna, Austria) as described before (25), and then left incubating for three days in medium containing 100U/ml IL-2 (Immunotools, Friesoythe, Germany). Three days post-transfection, cells were transferred into fresh medium and either restimulated under Th2-conditions or left untreated for 24 hours, before they were lysed in 2× Laemmli SDS sample buffer (Bio-Rad, Vienna, Austria) for Western blot analysis or in TRI Reagent (Sigma, Vienna, Austria) for mRNA extraction and subsequent q-RT-PCR.
RNA isolation and quantitative real-time RT-PCR
Total RNA from cells was isolated using TRI Reagent (Sigma, Vienna, Austria) and reverse transcribed with RevertAid H Minus M-MulV reverse transcriptase (MBI Fermentas, St. Leon-Roth, Germany) according to the manufacturer’s instructions. Quantitative real-time PCR was carried out on a Rotorgene 3000 (Corbett Research, Mortlake, Australia) using 2 × iQ SYBR Green Supermix (Bio-Rad, Vienna, Austria) and the primers listed below. The transcript for the large ribosomal protein P0 (RPL P0) was used as a reference. The specificity of the PCRs was checked by recording a melting curve for the PCR products. Relative mRNA expression levels were calculated using the formula x=2−ΔCt, where Ct represents the threshold cycle of a given gene and ΔCt signifies the difference between the Ct values of the gene in question and the Ct value of the reference gene RPL P0. Primer sequences are as follows:
RPL P0 sense 5’-GGCACCATTGAAATCCTGAGTGATGTG-3’ and anti-sense 5’-TTGCGGACACCCTCCAGGAAG-3’;
TGFB1 sense 5’-CTGGACACGCAGTACAGCAAG-3’ and anti-sense 5’-TCATGTTGG ACAGCTGCTCCAC-3’;
TGFBR I sense 5’-ACCTTCCAAGATTCAACGTGGCTAAAACAT-3’ and anti-sense 5’-CAGGGACCAGCAAGCAGGAGAGCA-3’; TGFBR II sense 5’- GTGCTCTGGGAAATGACATCTCGCTG-3’ and anti-sense 5’-TCTCACACACCATCTGGATGCCCTG-3’; STAT6 sense 5’-CCTGGGTCAGGAGGAAAAGACTAACAGGAGAATGC-3’ and anti-sense 5’-TGGAAGGAGGTGGGCAGGGGAATGATAGAAAG-3’; GATA-3 sense 5’-GAAGAAGGAAGGCATCCAGACCAGAAACC-3’ and anti-sense 5’-AAAGGACAGGCTGGATGGCGGGT-3’; Spi1/PU.1 sense 5’-TGACGGCGAGGCGGATGGC-3’ and anti-sense 5’-TTGGACGAGAACTGGAAGGTGCCC-3’; IL31 sense 5’-TTGAAAGATGTGGAGGAAGAGAAGGG-3’ and anti-sense 5’-CTGTTGAGAAATAGTCAGGATGAAGCGTT-3’; IL13 5’-TGTGCCTCCCTCTACAGCCCTCAG-3’ and anti-sense 5’-TCAGCATCCTCTGGGTCTTCTCG-3’; IL9 sense 5’-CGGGGATCCTGGACATCAACTTCCTCATC and anti-sense 5’-CAGTGGGTATCTTGTTTGCATGGTGGTATTGGTC-3’; IL5 sense 5’-CCTTGGCACTGCTTTCTACTCATCG-3’ and anti-sense 5’-GGTTTACTCTCCGTCTTTCTTCTCCACA-3’; IL4 5’-ACCTCCCAACTGCTTCCCCCTCTGTT-3’ and anti-sense 5’-GCACCCAGGCAGCGAGTGTCCTT-3’.
SDS PAGE and Western blotting
Cells were harvested by centrifugation, lysed in 2 × Laemmli Sample Buffer (Bio-Rad, Vienna, Austria) and frozen at −75°C. After thawing, lysates were denaturated by 7 minutes’ incubation at 95°C and afterwards centrifuged to remove the cell debris. Protein lysates were separated on precast NuPAGE 4-12% gradient gels (Invitrogen, Lofer, Austria) and blotted onto nitrocellulose membranes in a Trans-blot semi-dry blotting chamber (both Bio-Rad, Vienna, Austria) using 2 × Transfer Buffer (Invitrogen, Lofer, Austria). The membrane was blocked by incubation in Tris-buffered saline containing 0.1% Tween 20 and 5% non-fat dry milk for 1 hour. The primary antibodies (α-pSTAT6 (Tyr 641), α-STAT6, α-STAT3, α-GATA-3, α-SMAD2 (Ser465/467)/SMAD3 (Ser423/425), α-SMAD2/SMAD3) and HRP-linked secondary antibodies were purchased from Cell Signaling Technology (Danvers, MA) and used according to the manufacturer’s instructions. Detection was carried out using Supersignal enhanced chemiluminescence substrate (West Pico, Pierce, Rockford, IL) and Biomax X-ray films and exposure cassettes with intensifying screens (Kodak, Sigma-Aldrich, Vienna, Austria) or the Immun-starTM WesternC chemiluminescence kit and the ChemiDocTM MP Imaging system with Image lab software (all Bio-Rad, Vienna, Austria). For stripping, the membrane was incubated in 50 mM Tris-HCl, pH 6.8, 2% SDS, 10 mM β-mercaptoethanol for 20 minutes at 50°C.
Results
IL-31 is secreted by developing but not by fully differentiated Th9 cells
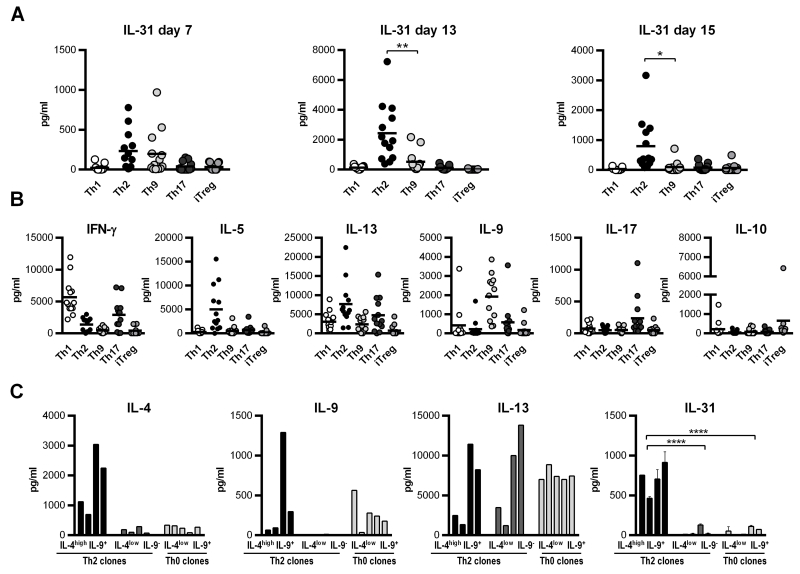
IL-31 was found to be associated with Th2-mediated diseases and hence is considered to be especially involved in Th2-driven immunity. In line with these observations, a recent study nicely showed that IL-4 is a key inducer of IL-31 expression in human CD4+ T cells (17). Because IL-4 is not only the main cytokine inducing the Th2 phenotype, but is also required for Th9 development (5, 6), we performed detailed analyses of IL-31 expression in different human T helper cell subsets, with a special focus on Th2 and Th9 cells. Naïve CD4+ T cells were isolated from buffy coats and cultured under Th1-, Th2-, Th9-, Th17- and iTreg-polarizing conditions. After one week of stimulation, cells were re-stimulated for another week under the same polarizing conditions as before. Thereafter, cells were activated for two days with antibodies directed against CD3 and CD28. Supernatants were taken after each stimulation step and analyzed for IL-31 protein by ELISA. As shown in Figure 1A, already after the first round of stimulation (day 7), IL-31 was predominantly secreted by Th2 cells, but developing Th9 cells secreted substantial amounts of IL-31 as well. Although secretion of IL-31 is generally enhanced after the second stimulation (day 13), the amount of IL-31 released by Th9 cells was clearly less than that secreted by Th2 cells. However, after the last re-stimulation (day 15), which was performed in the absence of the polarizing cytokines IL-4 and TGF-β, only Th2 cells, but not Th9 cells, released IL-31. To control for the quality of effector phenotype generation, signature cytokines of each subset were measured as well (Figure 1B).
Figure 1. IL-31 secretion by in vitro differentiating CD4+ T-cell subsets and Bet v 1-specific T-cell clones.
Naïve CD4+ T cells were isolated from human buffy coats and cultured under Th1-, Th2-, Th9-, Th17-, and iTreg-polarizing conditions for 7 days, re-stimulated under the same conditions for an additional week and thereafter re-activated for 2 days with antibodies directed against CD3 and CD28. Supernatants were taken after each stimulation period and tested for IL-31 protein by ELISA. Data represent mean values of 13 polarization experiments carried out in duplicates using cells from 13 individual donors. (A). To control for the efficiency of CD4+ subset generation, signature cytokines for each subset were measured (B). T-cell clones specific for the major birch pollen allergen Bet v 1 and classified as Th2 and Th0 were stimulated with autologous irradiated PBMCs and 5μg/ml Bet v 1 for 48 hours. Secretion of the cytokines IL-4, IL-13 and IL-9 was determined using the Luminex System 100, and IL-31 expression was measured in duplicates by ELISA (C). Statistical analyses: one-way ANOVA/Dunnett’s multiple comparisons test. * p ≤ 0.05, ** p ≤ 0.01,**** p ≤ 0.0001.
To test whether our observation on IL-31 release by in vitro-primed Th-cell subsets holds true for in vivo-primed and differentiated Th cells, we analyzed the production of IL-31 by 13 allergen-specific T-cell clones (TCC) expanded from the peripheral blood of allergic patients. TCC were classified into Th2- and Th0-like cells according to their allergen-induced production of IL-4 and IFN-γ (18). Th2 clones were further distinguished by their IL-4 expression (IL-4high Th2, IL-4low Th2). We observed IL-9 secretion by four Th2-like clones (ratio IL-4/IFN-γ >5) and five Th0-like clones (ratio IL-4/IFN-γ = 0.2-5). However, neither of the allergen-specific TCC showed a clear Th9-like phenotype. Supernatants from these nine TCC and the four IL-9-negative Th2 clones were subjected to IL-31 ELISA. The production of IL-31 correlated with the release of IL-4 but not with IL-13 or IL-9 (Figure 1C). Hence, among the Th2 clones, only those cultures which secreted high levels of IL-4 produced substantial amounts of IL-31. This clearly substantiates the observation that IL-4 is a key factor in promoting IL-31 expression.
IL-4 and IL-33 drive the secretion of IL-31 in (developing) Th2 cells in a dose-dependent fashion
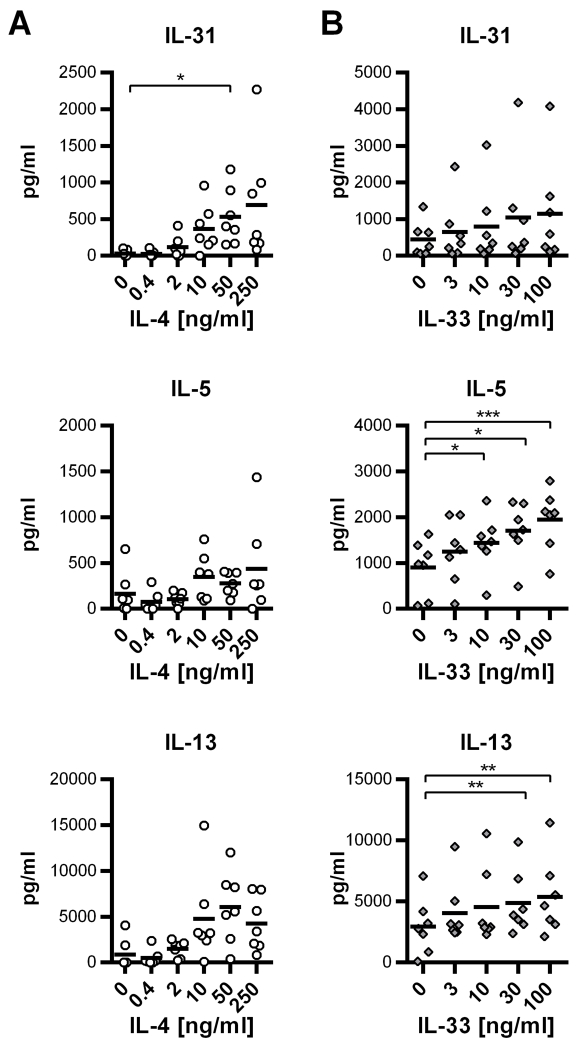
Because IL-4-dependent T helper cell subsets were the only sources of IL-31 in our experiments, we sought to analyze the effect of IL-4 on IL-31 production in more detail. Thus, we isolated naïve CD4+ T cells from human buffy coats and induced differentiation of Th2 cells by exposing the CD4+ T cells to rising concentrations of IL-4. After one week, cells were re-stimulated and IL-31 released into the supernatant was measured after 7 days of re-stimulation. As controls, the levels of the Th2 cytokines IL-5 and IL-13 were also determined. ELISA analyses revealed that IL-4 increases the expression of IL-31 along with IL-5 and IL-13 in a concentration-dependent manner (Figure 2A).
Figure 2. IL-4 and IL-33 promote the expression of IL-31 in human Th2 cells.
A. Naïve CD4+ T cells were polyclonally activated by α-CD3/α-CD28 and treated with IL-4 in rising concentrations as indicated. After one week of stimulation, cells were re-stimulated for 7 days. Secretion of the cytokines IL-31, IL-5 and IL-13 was measured by ELISA. Data represent mean values of 7 independent experiments carried out in duplicates using cells from 7 individual donors. The black horizontal bars symbolize the overall mean for each experimental group. B. Naïve CD4+ T cells were differentiated toward Th2 cells in the presence of rising concentrations of IL-33. Cytokine secretion was analyzed by ELISA. Mean values of duplicates from 7 independent experiments are shown. The black horizontal bars signify the overall means. Statistical analyses: one-way ANOVA/Dunnett’s multiple comparisons test. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
The IL-1 family member IL-33 is associated with Th2-type immune reactions and has been shown to augment the expression of the Th2-secreted cytokines IL-13 and IL-5 in murine Th2 cells (26, 27). The heterodimeric receptor for IL-33 consists of ST2L, also known as IL-1 receptor like 1 (IL-1RL1), and IL-1 receptor accessory protein (IL-1AcP) (26, 28). Whereas IL-1AcP is ubiquitously expressed, ST2L is predominantly found on the surface of mast cells and Th2 cells (29). Th2 cells acquire ST2L expression in the course of differentiation, and IL-33 itself was shown to promote the expression of ST2L by Th2 cells (27). To analyze if IL-33 impacts on IL-31 expression by human in vitro-generated Th2 cells, naïve CD4+ T cells were skewed toward a Th2 phenotype in the presence of IL-33 in rising concentrations, ranging from 3ng/ml to 100ng/ml. Analyses of IL-31, IL-5 and IL-13 expression by ELISA revealed similar data to that observed in the murine system (26, 27): production of IL-5 and IL-13 increased with rising concentrations of IL-33. Although the effect was less pronounced, the same tendency was observed for IL-31 (Figure 2B).
TGF-β attenuates IL-31 secretion by Th2-polarized cells
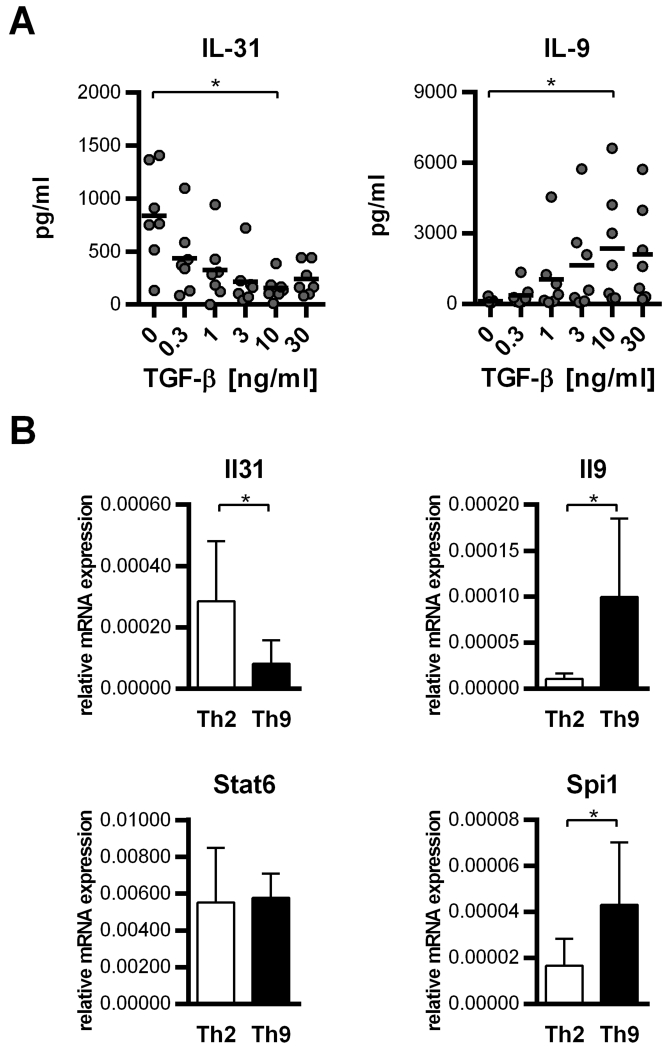
While IL-4 and IL-33 are known to promote the Th2 phenotype, CD4+ T cells cultured in the presence of IL-4 and TGF-β clearly develop toward Th9. Because we observed lower IL-31 levels in Th9- compared to Th2-skewed cells, we speculated that this is due to the addition of TGF-β. To prove this hypothesis, we stimulated naïve CD4+ T cells under Th2-polarizing conditions and treated them with TGF-β in rising concentrations to achieve a gradual switchover toward Th9. ELISA analyses showed that TGF-β increases the secretion of IL-9 while simultaneously decreasing the release of IL-31. Additionally, the release of IL-5 and IL-13 was inhibited by TGF-β, indicating that there occurred a transition from the Th2 to the Th9 phenotype (Figure 3A). One of the key transcription factors in Th9 development is PU.1, the gene product of Spi1 (30). Because expression of PU.1 is induced by TGF-β (31), we investigated whether the TGF-β-mediated suppression of IL-31 is directly associated with the induction of Spi1/PU.1. Therefore, we stimulated naïve CD4+ T cells under Th2-polarizing conditions for one week and re-stimulated them under either Th2-polarizing or Th9-inducing conditions for 6 days. In accordance with the protein data, we observed a clear down-regulation of IL-31 mRNA expression in cells cultured in the presence of TGF-β, whereas IL-9 and Spi/PU.1 mRNA expression was strongly up-regulated in Th9 cells. As expected, STAT6 expression was only moderately affected by TGF-β treatment (Figure 3B).
Figure 3. TGF-β blocks the release of IL-31 by Th2 cells.
A. Naïve CD4+ T cells were cultured with α-CD3/α-CD28 + IL-4 and treated with TGF-β1 in concentrations as indicated to induce a gradual switchover from the Th2-phenotype toward the Th9-phenotype. After one week in culture, cells were re-stimulated under the same conditions. Protein expression of IL-31 and IL-9 after one week of re-stimulation was assessed by ELISA. Mean values of duplicates from 7 experiments using cells from individual donors are shown (black horizontal bars signify the overall means; statistical analyses: one-way ANOVA/Dunnett’s multiple comparisons test. * p ≤ 0.05.). B. Naïve CD4+ T cells were cultured under Th2-polarzing conditions and re-stimulated under Th2- or Th9-promoting conditions for 6 days. Expression of Il31, Il9, Stat6 and Spi1 was analyzed by q-RT-PCR. Data show mean values of at least 6 experiments; error bars indicate standard deviations. Statistical analyses: t-test. * p ≤ 0.05.
SMAD2/3 phosphorylation in naïve CD4+ T cells results in diminished IL-31 production
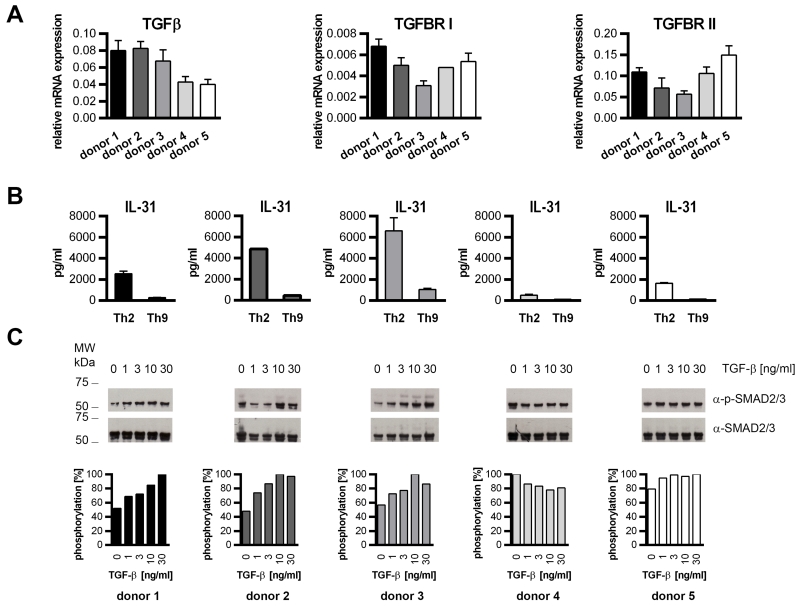
Because IL-31 production by Th2 and developing Th9 cells varied greatly from donor to donor, we analyzed mRNA expression of TGF-β and its receptor complex composed of TGF-β recetor (TGFBR) I and II in naïve human T cells (n=5) before they were subjected to Th2 and Th9 differentiation. Although the differences in mRNA expression of TGF-β and its receptor between individual donors was rather low (Figure 4A), TGFBRI and TGFBRII seemed to be negatively correlated with IL-31 production in Th2 cells (Figure 4B). Additionally, a fraction of the naïve cells was treated for 15 minutes with TGF-β1 in rising concentrations to monitor potential differences in TGF-β downstream signaling. TGF-β1 signaling is central to T-cell homeostasis and maintenance of the naïve T-cell compartment. Hence, freshly isolated human T cells exhibit active TGF-β downstream signaling, which results in the phosphorylation of the signaling molecules SMAD2 and SMAD3 (32). In agreement with this, phospho-SMAD2/3 was detected in samples from untreated cells. However, the SMAD-phosphorylation level in untreated cells did vary between the donors and seemed to determine the ability of the cells to produce IL-31. We found that cells which had shown moderate SMAD-phosphorylation right after isolation secreted more IL-31 than cells with strong SMAD-phosphorylation (Figure 4C).
Figure 4. Phosphorylation of SMAD2 and SMAD3 correlates with limited IL-31 production.
A. Naïve human CD4+ T cells derived from 5 individual donors were analyzed for the expression of TGFβ, TGFBRI and TGFBRII mRNA by real-time q-RT-PCR. B. A fraction of the cells was in vitro-differentiated into Th2 and Th9 cells. IL-31 secretion was analyzed by ELISA on day 13. Data represent mean values of duplicates, error bars indicate standard deviations. C. Naïve CD4+ T cells were stimulated for 15 minutes with TGF-β1 at the concentrations indicated. Phosphorylation of SMAD2 and SMAD3 (p-SMAD2/3) and total SMAD2/3 was monitored by Western blotting. Phosphorylation levels relative to total SMAD2/3 and normalized to the signal maximum are shown.
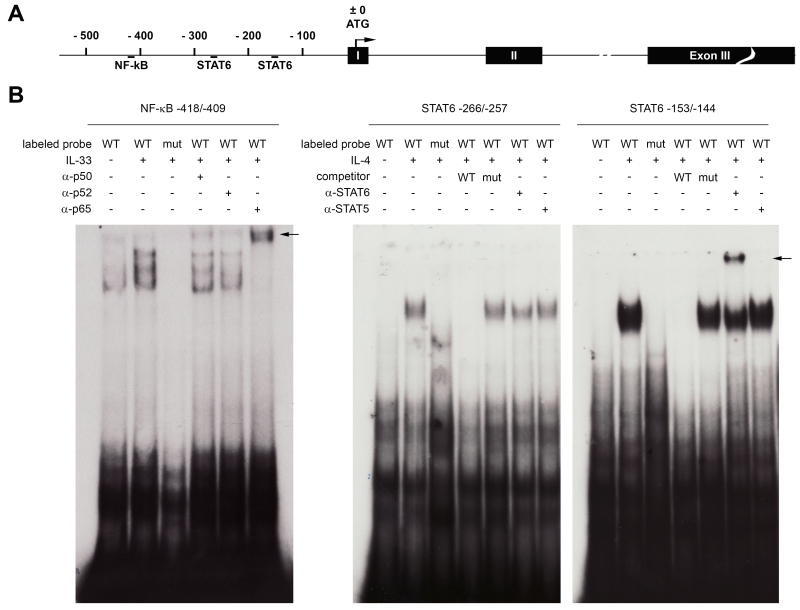
STAT6 and NF-κB bind to the human Il31 genomic locus
IL-4-induced gene expression is mainly mediated by the transcription factor STAT6, whereas IL-33 activates NF-κB (26). Analysis of the human Il31 promoter sequence revealed the presence of two STAT6 consensus sequences, located 257 and 144 bp upstream of the translation start site (TSS) and one NF-κB consensus motif at position −409 relative to the TSS (Figure 5A). These motifs were tested in gel-shift assays for the ability to bind STAT6 or NF-κB, respectively. Gel-shift assays using 32P-labeled oligonucleotides comprising the putative STAT6-binding sites and nuclear extracts derived from IL-4-induced naïve human CD4+ T cells revealed that IL-4 stimulates the formation of nucleoprotein-DNA complexes with both STAT6-consensus motifs. The mutation TTC to TAT in the STAT consensus motif has been previously shown to abrogate STAT6-binding (24, 25, 33). Introducing this mutation into the putative STAT6-motifs of the human Il31 promoter prevented IL-4-induced complex formation. For competition assays, a 50-fold molar excess of unlabeled oligonucleotide was added to the binding reaction prior to the addition of the labeled probes. Pre-incubation of the nuclear extracts with an antibody directed against STAT6 led to the formation of a super-shifted complex (motif −144) or at least partly inhibited formation of the complex (motif −257). Addition of α-STAT5 had no effect on the IL-4-induced nucleoprotein-DNA complexes (Figure 5B, right panel).
Figure 5. STAT6 and NF-κB bind to response elements within the human Il31 promoter.
A. Schematic representation of the human Il31 genomic locus. Positions of the two STAT6-binding sites and the NF-κB response element are indicated relative to the translational start site (ATG). B. Gel-shift assays using radiolabeled probes comprising the NF-κB-response element (left panel) and the two STAT6-binding sites (right panel) were carried out. Nuclear extracts from untreated or IL-33-induced in vitro-generated Th2 cells, or from uninduced or IL-4-stimulated naïve human T cells were incubated with double-stranded oligonucleotide harboring the wild-type (WT) transcription factor binding motifs or mutated versions thereof (mut) and the formed nucleoprotein-DNA complexes were resolved by native PAGE. For competition assays, 50-fold molar excess of unlabeled oligonucleotide was added to the binding reaction. In super-shift experiments, nuclear extracts were pre-incubated with antibodies directed against the NF-κB subunits p50, p52, and p65 or antibodies specific for STAT6 or STAT5. Black arrows indicate the positions of the super-shifted bands.
To prove binding of NF-κB to the Il31 promoter, a radioactively labeled oligonucleotide comprising the putative NF-κB-response element was incubated with nuclear extracts derived from in vitro-polarized Th2 cells that were either left untreated or stimulated with IL-33. We observed IL-33-dependent formation of nucleoprotein-DNA complexes. These complexes were not observed when we introduced a C to G point mutation into the NF-κB consensus sequence. To identify which subunits of NF-κB contribute to IL-33-induced binding to the Il31 promoter, nuclear extracts were pre-incubated with antibodies specific for p50, p52 and p65. The addition of α-p50 reduced the amount of IL-33-induced complexes and induced a slight super-shifted band, whereas α-p52 seemed to specifically inhibit formation of only one of the three induced complexes. The strongest effects were observed for α-p65, which induced a super-shift of all three IL-33-induced bands (Figure 5B, left panel).
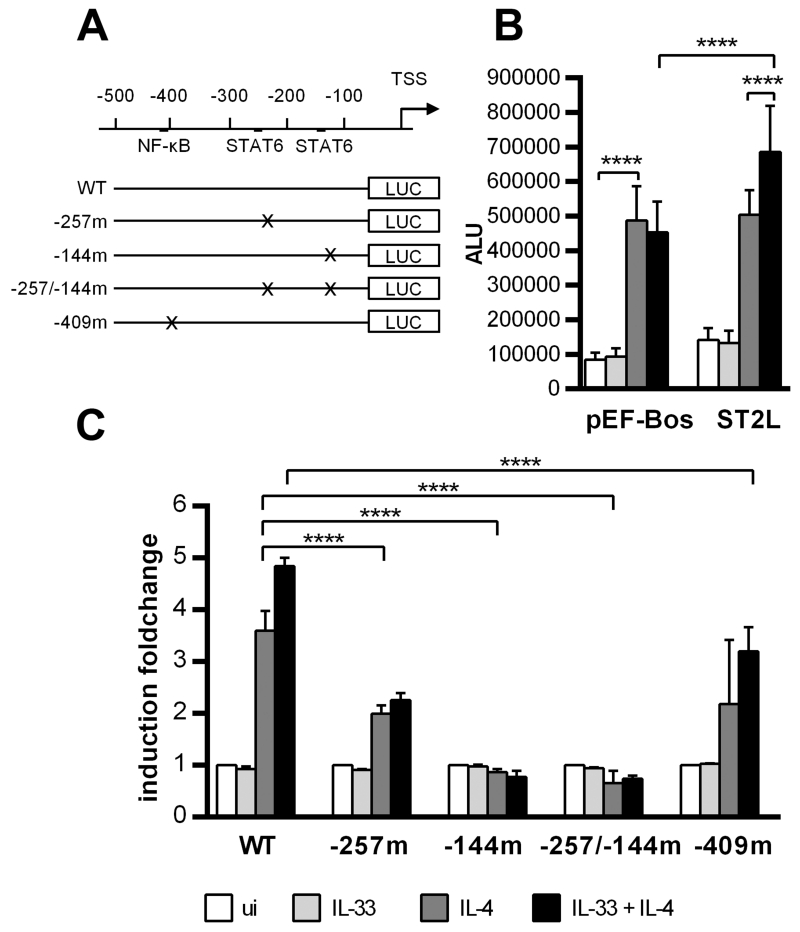
The human Il31 promoter is cooperatively activated via the proximal STAT6 binding site and an NF-κB motif
To determine the contributions of the three transcription factor binding sites identified by EMSA to regulating the human Il31 promoter, we amplified a 474-bp fragment comprising the Il31 promoter sequence −535 to −62 relative to the start ATG from human genomic DNA and cloned it into the pGL3Basic luciferase reporter-vector. By site-directed mutagenesis, the same mutations that abrogated complex formation in the EMSA were introduced into one or both STAT6-binding sites. Additionally, the NF-κB-binding site was mutated (Figure 6A). The resulting constructs were transfected into the HEKblue IL-4/IL-13 cell line. These cells stably express STAT6 and hence are IL-4 responsive. To be able to study the effects of IL-33 on the Il31 promoter, cells also have to be transfected with the IL-33 receptor subunit ST2L. We therefore co-transfected the reporter plasmid containing the wild-type Il31 promoter with an expression plasmid encoding human ST2L (22) or an empty pEF-Bos vector (23) and induced the cells with 50ng/ml IL-4, 30ng/ml IL-33 or a combination of both stimuli. Our data clearly show that the wild-type promoter activates luciferase expression when stimulated with IL-4, independent of the presence of ST2L. IL-33 alone had no effect, but it increased IL-4-mediated luciferase activity in cells overexpressing ST2L (Figure 6B). Mutation of the NF-κB-response element abolished the synergistic effect of IL-33 and IL-4. The Il31 promoter construct harboring a mutation in the distal STAT6 motif (−257m) remained to some extent IL-4 responsive. In contrast, mutation of the proximal STAT6-binding site (constructs −144m and −257/−144m) abolished promoter activity completely (Figure 6C). This indicates that the proximal STAT6-motif within the human Il31 promoter is indispensable for IL-4-mediated IL-31 expression, whereas the distal STAT6 motif and the NF-κB site further enhance IL-31 expression.
Figure 6. Mutation of STAT6-binding elements within the Il31 promoter impairs promoter activity.
A. Schematic representation of reporter constructs from the human Il31 promoter. A 474-bp fragment of the human Il31 promoter was cloned into the pGL3 Basic luciferase reporter plasmid. Mutations were introduced by site-directed mutagenesis into the NF-κB-binding site or into one or both STAT6-binding elements. B. Reporter gene assay of HEKblue IL-4/IL-13 cells transfected with the wild-type (WT) Il31 promoter construct and either an expression vector encoding the IL-33 receptor subunit ST2L or empty pEF-Bos vector. The day after the transfection, cells were induced with 50 ng/ml IL-4 and/or 30 ng/ml IL-33. After 24 hours of stimulation, luciferase activity was assessed. Data represent mean values of three independent experiments carried out in duplicates; error bars indicate standard deviations. ALU arbitrary light units, ui uninduced. C. Reporter gene assay of HEKblue IL-4/IL-13 cells transfected with the wild-type (WT) Il31 promoter construct or mutated versions thereof. The expression vector encoding human ST2L was co-transfected. Data were normalized to the corresponding uninduced sample. Mean values of two independent experiments carried out in duplicates are shown. Error bars indicate standard deviations. Statistical analyses: one-way ANOVA/Tukey’s multiple comparisons test. **** p ≤ 0.0001.
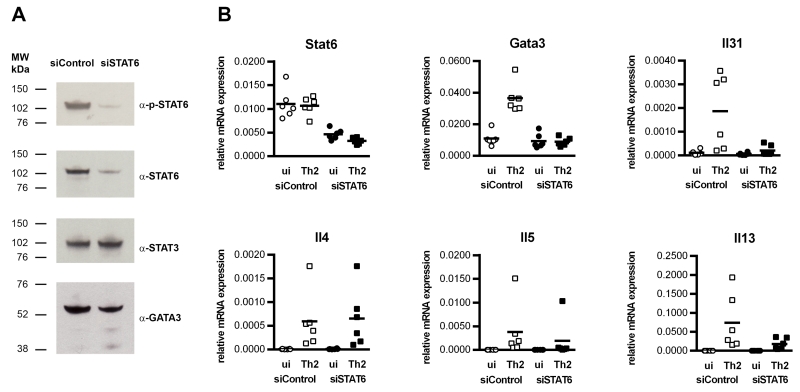
STAT6 is required for IL-31 expression by human Th2 cells
To confirm the crucial role of STAT6 for IL-31 expression in human Th2 cells, mRNA expression in STAT6-silenced, in vitro-generated Th2 cells was analyzed. Silencing of STAT6 was accomplished by transfecting the cells with small interfering (si)RNAs that target STAT6 mRNA for degradation. Three days after transfection, STAT6-silenced cells were re-stimulated for 24 hours under Th2-polarizing conditions. The efficacy of STAT6-silencing was monitored by immuno-blotting. Transfection of the cells with siRNA specific for STAT6 resulted in a radical reduction of STAT6 protein, observed by Western blot detection of phospho-STAT6 and STAT6 (Figure 7A). To test the specificity of the silencing RNA, we also measured STAT3. STAT3, like STAT6, has been shown to contribute to the Th2-phenotype (34), but STAT3 levels were not affected by the STAT6-interfering oligonucleotide (Figure 7A). When we detected GATA-3, the master regulator of Th2 cells, we found it to be present not only in control cells, but also in STAT6-silenced cells (Figure 7A). Real-time q-RT-PCR analyses revealed that, after transfection, both samples showed similar expression of GATA-3, but upon re-stimulation, only the control-transfected cells were able to up-regulate GATA-3 mRNA (Figure 7B). Analyses of mRNA expression of the Th2 cytokine genes Il4, Il5 and Il13 showed that silencing of STAT6 reduced the levels of Il5 and Il13 transcripts in re-stimulated cells, whereas Il4 expression was not significantly impaired (Figure 6B). Re-stimulation under Th2-conditions clearly induced the expression of Il31 mRNA in control cells, but not in STAT6-silenced cells (Figure 7B), which demonstrates that STAT6 is absolutely essential for IL-31 expression.
Figure 7. STAT6 is crucial for IL-31 expression in Th2 cells.
Human in vitro-polarized Th2 cells were transfected with siRNA targeting STAT6 (siSTAT6) or control oligonucleotide (siControl). Four days post-transfection, cells were replated in fresh medium and re-stimulated under Th2 conditions (α-CD3/α-CD28 + IL-4 + α-IL-12/α-IL-23) for 24 hours. A. Western blot analyses showing reduced levels of phospho-STAT6 (p-STAT6), STAT6 and GATA-3 in STAT6-silenced Th2 cells. B. Real-time q-RT-PCR analyses of mRNA expression of Stat6, Gata3, Il4, Il5, Il13 and Il31. Data represent 6 experiments carried out in duplicates using cells from 6 individual donors.
Discussion
Although numerous in vitro and in vivo studies have aimed at investigating the role of IL-31, its biological functions are still not fully understood. Human studies and overexpression studies in IL-31 transgenic mice clearly indicate that high IL-31 concentrations are tightly associated with allergic asthma and inflammatory skin diseases, including AD. Thus, IL-31 is regarded as novel player in type 2 inflammation. This assumption was substantiated by the discovery that Th2 cells are the most important producers of IL-31. Indeed, in the initial study that identified the four-helix bundle cytokine IL-31 as a unique ligand for IL-31RA, it was reported that in vitro-generated murine Th2 cells, rather than Th1 cells, release IL-31 (13). More recently, this finding was confirmed in human Th cell clones derived from grass pollen allergic donors (17). IL-4, which is the main inducer of Th2 polarization, was reported to act as key factor for the induction of IL-31 expression. Neutralizing IL-4 in Th2 cell clones significantly reduced IL-31 secretion. In addition, upon treatment with IL-4, even Th1 cell clones were able to release moderate amounts of IL-31 (17). Because IL-4 is also highly important for Th9-cell development, we sought to investigate IL-31 release by in vitro-differentiated Th9 cells compared to Th2, Th1, Th17 and iTreg cells. Whereas after the first round of stimulation, which was carried out in the presence of polarizing cytokines, Th9 and Th2 cells produced similar amounts of IL-31, the production of IL-31 by Th9 cells decreased over time. Finally, after the last re-stimulation in the absence of polarizing cytokines, almost no IL-31 was detectable in Th9 cells. When we analyzed IL-31 secretion by human allergen-specific TCC, we also found it to be dependent on the cells’ ability to produce IL-4. These observations indicate that only cells that produce IL-4 in an autocrine fashion are able to secrete considerable amounts of IL-31, which substantiates previous findings showing that IL-4 is indispensable for the expression of this cytokine (35). In addition, we clearly showed that TGF-β, which is essential for the development of IL-9-secreting cells (5, 6), strongly inhibits IL-31 expression at both the mRNA and protein levels. The effect of TGF-β on IL-31 synthesis was consistently observed, but with high inter-individual variability. Analysis of TGF-β signaling revealed that freshly isolated naïve CD4+ T cells show moderate to strong phosphorylation of SMAD2 and SMAD3, which is in line with an earlier study showing SMAD2 and SMAD3 phosphorylation in freshly isolated human T cells (32). We found SMAD activation to be negatively correlated with the potential of the cells to produce IL-31, which indicates that SMAD2 and SMAD3 might play a role in IL-31 suppression. It is well known that in Th2 effector cells, TGF-β induces the expression of PU.1, which interferes with GATA-3 DNA binding (36). Consequently, PU.1 negatively regulates the Th2 phenotype by inhibiting the expression of IL-5 and IL-13, both of which depend on GATA-3 (37, 38). Because IL-31 expression is mainly mediated by STAT6, it is unlikely that TGF-β-dependent suppression of GATA-3 binding contributes to the observed decrease in IL-31 expression. Rather, one could assume that TGF-β directly compromises IL-4-mediated STAT6 activation either by diminishing STAT6 binding activity or by down-regulating IL-4 receptor expression as shown previously (39). In contrast to TGF-β, IL-33, an IL-1 family member that is tightly associated with the activation of numerous diseases, including asthma and AD (40), was shown to promote IL-4-driven IL-31 secretion in a concentration-dependent fashion. This observation substantiates a recent study that reports enhanced Il31 mRNA expression in IL-33-treated memory T cells from grass pollen allergic patients (17). In addition, the classical Th2 cytokines IL-5 and IL-13 are increased in a similar way, which is in agreement with a previous study showing that murine in vitro-generated Th2 cells, but not Th1 cells, respond to IL-33 treatment with enhanced production of IL-5 and IL-13 (26, 27). Thus, based on its activation by IL-4 and IL-33 and its suppression by TGF-β, IL-31 seems to behave like a classical Th2 cytokine. However, at the transcriptional level, IL-5 and IL-13 are regulated by GATA-3 (37, 38, 41), whereas IL-31 seems to fully depend on STAT6. Because within CD4+ T-cell subsets GATA-3 expression is limited to Th2 cells, the expression of GATA-3-dependent genes is also restricted to Th2 cells. By contrast, STAT6 is widely expressed. Hence, cells other than Th2 can be stimulated to produce IL-31, as was shown for Th1 cells. Th1 clones produce IL-31 but not IL-13 in response to IL-4. Moreover, despite being exposed to IL-4, the amount of secreted IFN-γ did not alter, which indicates that the cells retain their Th1 character (35). One might assume that IL-31, due to its direct dependence on IL-4/STAT6-signaling, differs from the established Th2 cytokines IL-4, IL-5 and IL-13 also in its expression kinetics and function during allergic inflammation. Although there is convincing evidence that IL-31 induces pro-inflammatory cytokines, and there is a clear correlation between IL-31 level and disease-severity in atopic skin disorders (13, 15, 42, 43), further observations are needed to elucidate the definite role of IL-31 in allergic inflammation.
It is well established that IL-4-induced gene-regulation is mediated by the transcription factor STAT6, whereas IL-33 activates NF-κB. In order to identify the molecular mechanisms underlying IL-4-/IL-33-induced IL-31 production, we analyzed the human Il31 locus for the presence of candidate transcription factor binding motifs and found two STAT6-consensus sites and one putative NF-κB-binding motif. EMSAs showed that STAT6 present in nuclear extracts from IL-4-treated primary human T cells can associate with both STAT6-response elements, but especially the proximal STAT6 response element binds STAT6 with high affinity. Moreover, we showed for the first time binding of IL-33-activated NF-κB to the NF-κB response element within the human Il31 promoter. To evaluate the role of the transcription factor binding sites for Il31 promoter activity, reporter gene assays were carried out. HEKblue IL-4/IL-13 cells co-transfected with a luciferase reporter under the control of the Il31 promoter and an expression plasmid encoding the IL-33 receptor subunit ST2L showed IL-4-dependent activation of the reporter, which was augmented in the presence of IL-33 and thus mirrored our results from human Th2 cells. Introduction of specific mutations into the NF-κB-binding site and the distal STAT6 responsive motif of the Il31 promoter lowered, but did not completely abolish, its responsiveness. Of note, mutation of the NF-κB-site and the STAT-consensus sequence both seemed to impair enhancement of luciferase activity following IL-33 treatment. In contrast, mutation of the proximal STAT6-motif resulted in complete loss of inducibility. This demonstrates that the proximal STAT6-site is indispensible for IL-4-mediated Il31 transcription, whereas the distal STAT6 motif and the NF-κB-site instead enhance Il31 gene expression. To define the role of STAT6 in IL-31 expression by human Th2 cells in more detail, we performed siRNA-mediated silencing of STAT6 in in vitro-generated human Th2 cells. Re-stimulation of control-transfected cells under Th2 conditions resulted in an up-regulation of Il31 mRNA, but failed to induce Il31 expression in STAT6-deficient cells. STAT6-knockdown also resulted in reduced mRNA levels of the Th2 cytokines Il5 and Il13 as well, but the effect was less pronounced. This may be explained by the fact that expression of IL-5 and IL-13 in Th2 cells mainly depends on the transcription factor GATA-3 throughout all developmental stages (41). Compared to control cells, GATA-3 mRNA was not affected by silencing of STAT6. However, STAT6-silenced cells failed to up-regulate GATA-3 expression upon re-stimulation with IL-4, because GATA-3 itself is regulated by STAT6 (44, 45). Hence, STAT6-silencing might indirectly impact on the expression of Il5 and Il13 by reducing the levels of GATA-3. In contrast to IL-5 and IL-13, IL-4 production by fully differentiated Th2 cells is independent of GATA-3 (41). We found no difference in Il4 expression levels between STAT6 knockdown and control cells.
Taken together, our study gives new insights into the regulation of IL-31 expression in human Th cell subsets, with a special focus on Th2 and developing Th9 cells.
Acknowledgments
We thank Professors Shigekazu Nagata, Department of Medical Chemistry Graduate School of Medicine, Kyoto University, Japan and Seamus J. Martin, Department of Genetics, Trinity College, Dublin, Ireland for providing pEF-Bos and ST2L plasmids. We would also like to acknowledge Dr. Beatrice Jahn-Schmid, Department of Pathophysiology and Allergy Research, Medical University of Vienna, for providing supernatants from IL-9+ Th0 clones.
This work was supported by the Austrian Science Fund, FWF – Fonds zur Förderung der wissenschaftlichen Forschung, grant number P 22202
Abbreviations
- ALU
arbitrary light units
- CLA
cutaneous lymphocyte antigen
- IL-1RL1
IL-1 receptor like 1
- IL-1RAcP
IL-1 receptor accessory protein
- q-RT-PCR
quantitative reverse transcriptase PCR
- ST2L
transmembrane form of ST2
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan YY, Flavell RA. How diverse--CD4 effector T cells and their functions. Journal of molecular cell biology. 2009;1:20–36. doi: 10.1093/jmcb/mjp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. Journal of immunology. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 4.Annunziato F, Romagnani S. Heterogeneity of human effector CD4+ T cells. Arthritis research & therapy. 2009;11:257. doi: 10.1186/ar2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nature immunology. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature immunology. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 7.Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF-beta promote T(H)9 cell-mediated pulmonary allergic pathology. The Journal of allergy and clinical immunology. 2012;129:1000–1010. e1003. doi: 10.1016/j.jaci.2011.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Temann UA, Laouar Y, Eynon EE, Homer R, Flavell RA. IL9 leads to airway inflammation by inducing IL13 expression in airway epithelial cells. International immunology. 2007;19:1–10. doi: 10.1093/intimm/dxl117. [DOI] [PubMed] [Google Scholar]
- 9.Khan WI, Richard M, Akiho H, Blennerhasset PA, Humphreys NE, Grencis RK, Van Snick J, Collins SM. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infection and immunity. 2003;71:2430–2438. doi: 10.1128/IAI.71.5.2430-2438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erpenbeck VJ, Hohlfeld JM, Volkmann B, Hagenberg A, Geldmacher H, Braun A, Krug N. Segmental allergen challenge in patients with atopic asthma leads to increased IL-9 expression in bronchoalveolar lavage fluid lymphocytes. The Journal of allergy and clinical immunology. 2003;111:1319–1327. doi: 10.1067/mai.2003.1485. [DOI] [PubMed] [Google Scholar]
- 11.Yao W, Tepper RS, Kaplan MH. Predisposition to the development of IL-9-secreting T cells in atopic infants. The Journal of allergy and clinical immunology. 2011;128:1357–1360. e1355. doi: 10.1016/j.jaci.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bullens DM, Kasran A, Dilissen E, De Swert K, Coorevits L, Van Snick J, Ceuppens JL. In vivo maturation of T(H) cells in relation to atopy. The Journal of allergy and clinical immunology. 2011;128:234–237. e237. doi: 10.1016/j.jaci.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 13.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, Bort S, Mudri S, Kuijper JL, Bukowski T, Shea P, Dong DL, Dasovich M, Grant FJ, Lockwood L, Levin SD, LeCiel C, Waggie K, Day H, Topouzis S, Kramer J, Kuestner R, Chen Z, Foster D, Parrish-Novak J, Gross JA. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 14.Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, Storey H, LeCiel C, Harder B, Gross JA. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117:418–425. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 15.Neis MM, Peters B, Dreuw A, Wenzel J, Bieber T, Mauch C, Krieg T, Stanzel S, Heinrich PC, Merk HF, Bosio A, Baron JM, Hermanns HM. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. 2006;118:930–937. doi: 10.1016/j.jaci.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Lei Z, Liu G, Huang Q, Lv M, Zu R, Zhang GM, Feng ZH, Huang B. SCF and IL-31 rather than IL-17 and BAFF are potential indicators in patients with allergic asthma. Allergy. 2008;63:327–332. doi: 10.1111/j.1398-9995.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- 17.Stott B, Lavender P, Lehmann S, Pennino D, Durham S, Schmidt-Weber CB. Human IL-31 is induced by IL-4 and promotes TH2-driven inflammation. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.03.050. doi:10.1016/j.jaci.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Schulten V, Radakovics A, Hartz C, Mari A, Vazquez-Cortes S, Fernandez-Rivas M, Lauer I, Jahn-Schmid B, Eiwegger T, Scheurer S, Bohle B. Characterization of the allergic T-cell response to Pru p 3, the nonspecific lipid transfer protein in peach. J Allergy Clin Immunol. 2009;124:100–107. doi: 10.1016/j.jaci.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohler I, Rieber EP. Allergy-associated I epsilon and Fc epsilon receptor II (CD23b) genes activated via binding of an interleukin-4-induced transcription factor to a novel responsive element. Eur J Immunol. 1993;23:3066–3071. doi: 10.1002/eji.1830231204. [DOI] [PubMed] [Google Scholar]
- 21.Hoeck J, Woisetschlager M. Activation of eotaxin-3/CCLl26 gene expression in human dermal fibroblasts is mediated by STAT6. J Immunol. 2001;167:3216–3222. doi: 10.4049/jimmunol.167.6.3216. [DOI] [PubMed] [Google Scholar]
- 22.Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, Lavelle EC, Martin SJ. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maier E, Wirnsberger G, Horejs-Hoeck J, Duschl A, Hebenstreit D. Identification of a distal tandem STAT6 element within the CCL17 locus. Hum Immunol. 2007;68:986–992. doi: 10.1016/j.humimm.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maier E, Hebenstreit D, Posselt G, Hammerl P, Duschl A, Horejs-Hoeck J. Inhibition of suppressive T cell factor 1 (TCF-1) isoforms in naive CD4+ T cells is mediated by IL-4/STAT6 signaling. J Biol Chem. 2011;286:919–928. doi: 10.1074/jbc.M110.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali S, Huber M, Kollewe C, Bischoff SC, Falk W, Martin MU. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc Natl Acad Sci U S A. 2007;104:18660–18665. doi: 10.1073/pnas.0705939104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, Nguyen ET, Robertson MJ, Perumal NB, Tepper RS, Nutt SL, Kaplan MH. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nature immunology. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goswami R, Jabeen R, Yagi R, Pham D, Zhu J, Goenka S, Kaplan MH. STAT6-dependent regulation of Th9 development. Journal of immunology. 2012;188:968–975. doi: 10.4049/jimmunol.1102840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Classen S, Zander T, Eggle D, Chemnitz JM, Brors B, Buchmann I, Popov A, Beyer M, Eils R, Debey S, Schultze JL. Human resting CD4+ T cells are constitutively inhibited by TGF beta under steady-state conditions. J Immunol. 2007;178:6931–6940. doi: 10.4049/jimmunol.178.11.6931. [DOI] [PubMed] [Google Scholar]
- 33.Hoeck J, Woisetschlager M. STAT6 mediates eotaxin-1 expression in IL-4 or TNF-alpha-induced fibroblasts. Journal of immunology. 2001;166:4507–4515. doi: 10.4049/jimmunol.166.7.4507. [DOI] [PubMed] [Google Scholar]
- 34.Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, Nguyen ET, Levy DE, Kaplan MH. The transcription factor STAT3 is required for T helper 2 cell development. Immunity. 2011;34:39–49. doi: 10.1016/j.immuni.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stott B, Lavender P, Lehmann S, Pennino D, Durham S, Schmidt-Weber CB. Human IL-31 is induced by IL-4 and promotes TH2-driven inflammation. J Allergy Clin Immunol. 2013;132:446–454. e445. doi: 10.1016/j.jaci.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 36.Chang HC, Zhang S, Thieu VT, Slee RB, Bruns HA, Laribee RN, Klemsz MJ, Kaplan MH. PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity. 2005;22:693–703. doi: 10.1016/j.immuni.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Pai SY, Truitt ML, Ho IC. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc Natl Acad Sci U S A. 2004;101:1993–1998. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Jr., Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 39.Heath VL, Murphy EE, Crain C, Tomlinson MG, O’Garra A. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. European journal of immunology. 2000;30:2639–2649. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 41.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16:3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 42.Raap U, Wichmann K, Bruder M, Stander S, Wedi B, Kapp A, Werfel T. Correlation of IL-31 serum levels with severity of atopic dermatitis. J Allergy Clin Immunol. 2008;122:421–423. doi: 10.1016/j.jaci.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 43.Cornelissen C, Luscher-Firzlaff J, Baron JM, Luscher B. Signaling by IL-31 and functional consequences. European journal of cell biology. 2012;91:552–566. doi: 10.1016/j.ejcb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Asnagli H, Afkarian M, Murphy KM. Cutting edge: Identification of an alternative GATA-3 promoter directing tissue-specific gene expression in mouse and human. Journal of immunology. 2002;168:4268–4271. doi: 10.4049/jimmunol.168.9.4268. [DOI] [PubMed] [Google Scholar]
- 45.Scheinman EJ, Avni O. Transcriptional regulation of GATA3 in T helper cells by the integrated activities of transcription factors downstream of the interleukin-4 receptor and T cell receptor. J Biol Chem. 2009;284:3037–3048. doi: 10.1074/jbc.M807302200. [DOI] [PubMed] [Google Scholar]