Abstract
Pyrazole are potent medicinal scaffolds and exhibit a full spectrum of biological activities. This review throws light on the detailed synthetic approaches which have been applied for the synthesis of pyrazole. This has been followed by an in depth analysis of the pyrazole with respect to their medical significance. This follow-up may help the medicinal chemists to generate new leads possessing pyrazole nucleus with high efficacy.
KEY WORDS: Anti-microbial, hetrocyclic and biological activity, pyrazole
Pyrazole is a five-membered ring structure composed of three carbon atoms and two nitrogen atoms in adjacent positions as represented by the molecular formula C3H4N2. It is a weak base, with pKb 11.5 (pKa of the conjugated acid 2.49 at 25°C). The term pyrazole was first coined by Ludwig Knorr in 1883. Due to its composition and unique pharmacological effects on human beings, they are classified as alkaloids. 1-pyrazolyl-alanine was the first natural pyrazole isolated from watermelon seeds in the year 1959.[1,2]
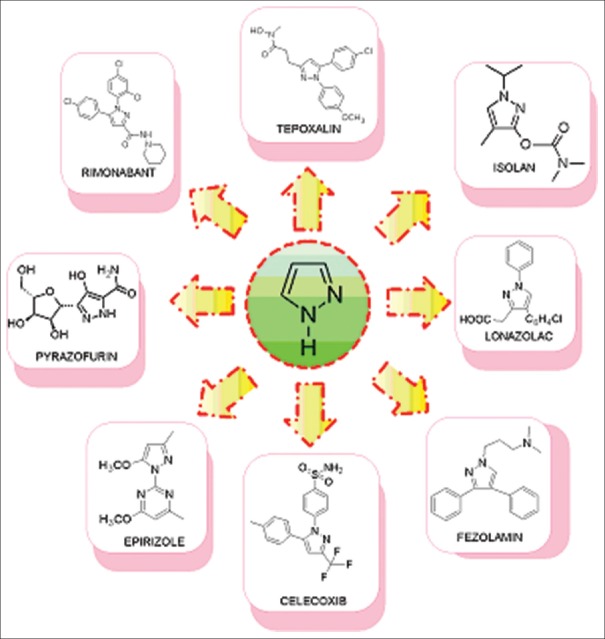
Pyrazoles are reported to possess a wide range of biological activities in literature such as anti-microbial, anti-fungal, anti-tubercular, anti-inflammatory, anti-convulsant, anticancer, anti-viral, angiotensin converting enzyme (ACE) inhibitory, neuroprotective, cholecystokinin-1 receptor antagonist, and estrogen receptor (ER) ligand activity, etc. which are as shown in Figure 1.[3] Many pyrazole derivatives has already found their application as nonsteroidal anti-inflammatory drugs clinically, such as anti-pyrine or phenazone (analgesic and antipyretic), metamizole or dipyrone (analgesic and antipyretic), aminopyrine or aminophenazone (anti-inflammatory, antipyretic, and analgesic), phenylbutazone (anti-inflammatory, antipyretic mainly used in osteoarthritis, rheumatoid arthritis, spondylitis, Reiter's disease), sulfinpyrazone (chronic gout), and oxyphenbutazone (antipyretic, analgesic, anti-inflammatory, mild uricosuric).[4] In this review, our main intention is to emphasize on the different biological activities exhibited by pyrazole moiety. Marketed drugs containing pyrazole moiety are as shown in Figure 2.
Figure 1.
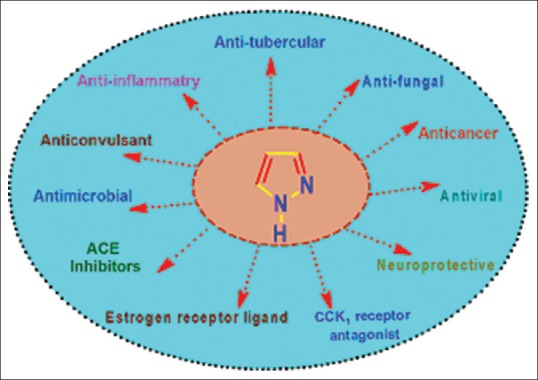
Different biological activities depicted by pyrazole moiety
Figure 2.
Pyrazole containing drugs
Synthetic Aspects of Pyrazole
-
Synthesis of 1, 3-substituted pyrazoles
An iron-catalyzed route for the regioselective synthesis of 1,3- and 1,3,5-substituted pyrazoles from the reaction of diarylhydrazones and vicinal diols [Figure 3][5]
-
Synthesis of tri- and tetra-substituted pyrazoles
A ruthenium (II)-catalyzed intramolecular oxidative CN coupling method for the facile synthesis of a tri- and tetra-substituted pyrazoles. Dioxygen gas is employed as the oxidant in this transformation and the reaction demonstrates excellent reactivity, functional group tolerance, and high yields [Figure 4][6]
-
Synthesis of 3,5-substituted-1H-pyrazole
A novel approach to the synthesis of pyrazole derivatives from tosylhydrazones of α, β-unsaturated carbonyl compounds possessing a β-hydrogen is proposed, exploiting microwave activation coupled with solvent free reaction conditions [Figure 5][7]
-
Synthesis of 3-benzofuran-2-yl-1-p-tolyl-1H-pyrazole
The 2-acyl benzofurohydrazones subjected to Vilsmeier–Haack reaction that is reaction with N, N-dimethylformamide (DMF)/POCl3 at an appropriate molar ratio, which underwent smooth cyclization followed by formylation afforded 3-(1-benzofuran-2-yl)-1-(4-fluorophenyl)-1H-pyrazole-4-carbaldehyde [Figure 6][8]
-
Synthesis of 1-(4,5-disubstitutedpyrazol-1-yl)-ethanone
A novel one-pot synthesis of pyrazoles has been accomplished by the reaction of β-formyl enamides with hydroxylamine hydrochloride catalyzed by potassium dihydrogen phosphate in acidic medium [Figure 7][9]
-
Synthesis of 1,3,5-trisubstituted-1H-pyrazole
The reaction of the easily accessible 1, 3-bisaryl-monothio-1,3-diketone or 3-(methylthio)-1,3-bisaryl-2-propenones with arylhydrazines gives 1-aryl-3,5-bisarylpyrazoles with complementary regioselectivity at position 3 and 5 [Figure 8].[10]
Figure 3.
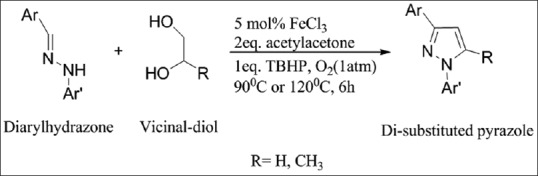
Synthesis of 1,3- and 1,3,5-substituted pyrazoles
Figure 4.
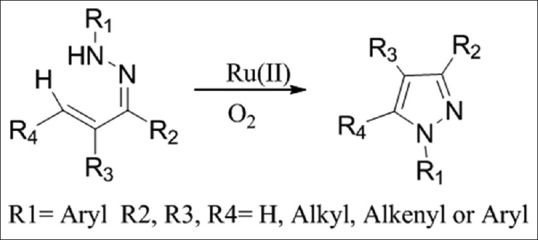
Synthesis of tri- and tetra-substituted pyrazoles
Figure 5.
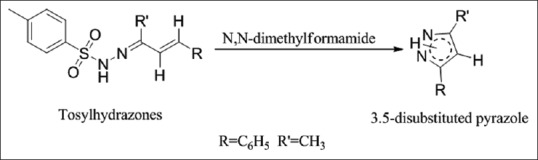
Synthesis of 3,5-substituted-1H-pyrazole
Figure 6.
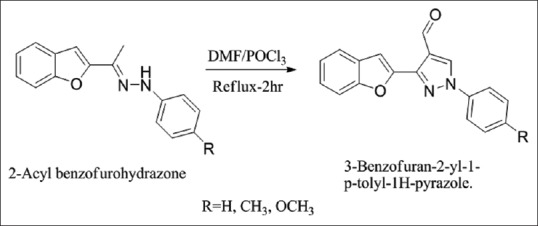
Synthesis of 3-benzofuran-2-yl-1-p-tolyl-1H-pyrazole derivatives
Figure 7.
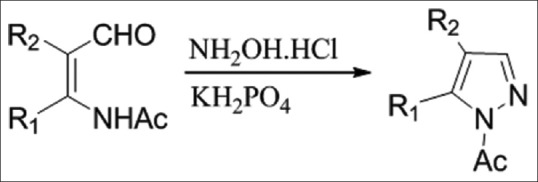
Synthesis of 1-(4,5-disubstitutedpyrazol-1-yl)-ethanone
Figure 8.
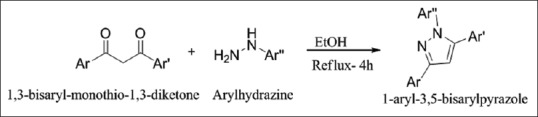
Synthesis of 1,3,5-trisubstituted-1H-pyrazole
Pharmacological activity
For a very long time, the usefulness and great therapeutic value of pyrazole nucleus have been recognized and widest range of activities of this nucleus evaluated. However, as the first synthetic organic compound having pyrazoline-5-one nucleus, to find use as an important drug. Phenylbutazone as a prototype of pyrazolidinedione is a very potent anti-inflammatory agent, but its use is now banned in some countries. Later on, many modifications of pyrazole nucleus were attempted and several compounds have been synthesized which serves as the basis for the treatment of different diseases like-inflammation, pain, cancer, tuberculosis, and diseases caused by bacteria.
Anti-inflammatory activity
Kendre et al.,[11] have synthesized a new series of pyrazole, isoxazole, benzoxazepine, benzothiazepine, and benzodiazepine derivatives by the multi-component cyclo-condensation reaction of 1-phenyl-3-(2-(tosyloxy)phenyl)propane-1,3-dione, DMF dimethyl acetal, and hydrazine or hydroxylamine hydrochloride or 2-aminothiophenol or 2-aminophenol or benzene-1,2-diamine by MW technique in aqueous media. Compound 3a screened for their anti-inflammatory activity using indomethacin as the standard drug was found to be potent [Figure 9].
Figure 9.

Synthesis of substituted pyrazole derivatives
Tewari et al.,[12] have synthesized a novel series of pyrazole derivatives and evaluated in vivo for their anti-inflammatory activity in carrageenan-induced rat paw edema model using nimesulide as the standard drug. Molecular modeling studies showed that pyrazole analogs interact with cyclooxygenase-2 (COX-2) active site by forming classical hydrogen bonding, π-π interaction, and cation–π interaction which increases the residence of ligand in the active site consequently augmenting anti-inflammatory activity of compounds. Compound 5b was found to be most potent [Figure 10].
Figure 10.
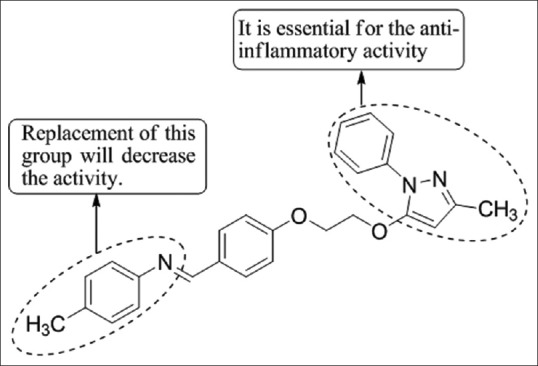
Syntheis of novel pyrazole derivatives
Alegaon et al.,[13] have synthesized 22 1,3,4-trisubstituted pyrazole derivatives and structure of newly synthesized compounds were characterized by infrared (IR), 1H nuclear magnetic resonance (NMR), 13C NMR, and mass spectral analysis. These compounds were screened for the anti-inflammatory activity by carrageenan-induced paw edema method. Compounds 5a showed excellent anti-inflammatory activity (≥84.2% inhibition) as compared to that of the standard drug diclofenac (86.72%) when measured 3 h after the carrageenan injection [Figure 11].
Figure 11.
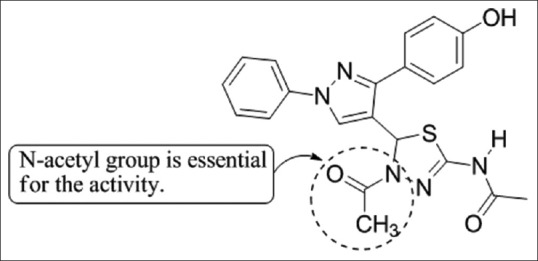
Synthesized 22 1,3,4-trisubstituted pyrazole derivatives
Sharath et al.,[14] have synthesized substituted indole based scaffolds having a pyrazole ring and evaluate for their anti-inflammatory activity and antioxidant. The structures of newly synthesized compounds were elucidated by spectroscopic methods such as IR, 1H NMR, 13C NMR, mass, and elemental analysis. Compound 7c exhibits a promising anti-inflammatory activity using indomethacin as the standard drug [Figure 12].
Figure 12.
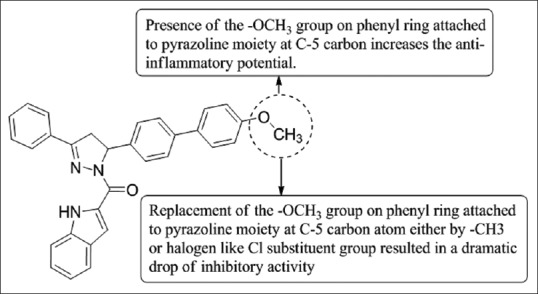
Synthesized substituted indole based scaffolds having a pyrazole ring
Thore et al.,[15] have reported the synthesis of a novel series of ethyl-5-amino-3-methylthio-1H-pyrazole-4-carboxylates from the condensation of various hydrazides with ketene dithioacetal. Compounds 3a exhibited significant anti-inflammatory activity at a dose of 25 mg/kg using diclofenac sodium as the standard drug [Figure 13].
Figure 13.
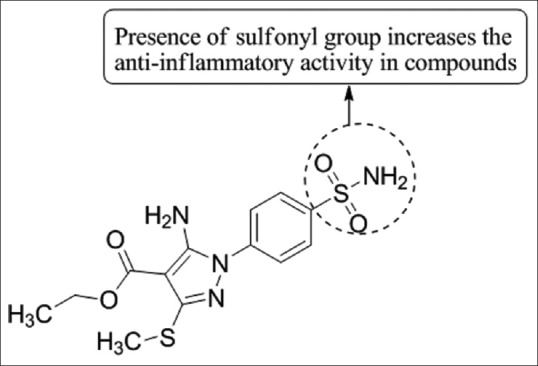
Synthesis of a novel series of ethyl-5-amino-3-methylthio-1H-pyrazole-4-carboxylates
Bandgar et al.,[16] have synthesized a series of novel pyrazole integrated benzophenones from 1-methyl-5-(2, 4, 6-trimethoxy-phenyl)-1H-pyrazole. All the compounds were proved as marked anti-inflammatory potential against various inflammatory mediators. Among the synthesized compounds, 9b, 9d, and 9f, were found to be active anti-inflammatory agents using indomethacin as the standard drug [Figure 14].
Figure 14.
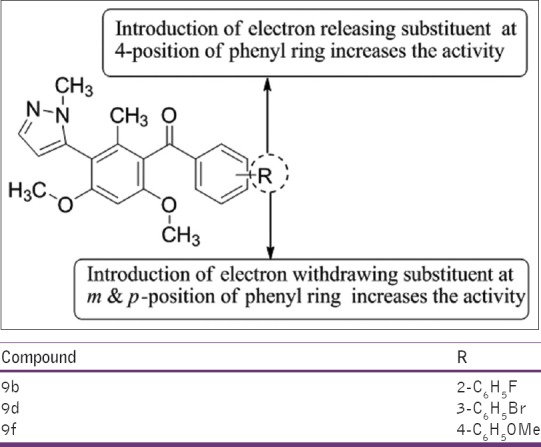
Synthesis of benzophenones from 1-methyl-5-(2, 4, 6-trimethoxyphenyl)-1H-pyrazole
Keche et al.,[17] have synthesized a series of novel 1-acetyl-3-(3,4-dimethoxypheny)-5-(4-(3-(arylureido/arylthioureido/arylsulfonamido) phenyl)-4,5-dihydropyrazole derivatives by sequential cyclization of 1-(4-nitrophenyl)-3-(3,4-dimethoxyphenyl)-pro-2-ene-1 with hydrazine hydrate, reduction followed by reaction of resulting amine with different aryl isocyanates or arylisothiocyanates or aryl sulfonyl chlorides. Compounds 4 and 16 found to have promising anti-inflammatory activity (up to 61–85% tumor necrosis factor [TNF-α] and 76–93% interleukin-6 [IL-6] inhibitory activity) at concentration of 10 lM with reference to the standard drug dexamethasone (76% TNF-α and 86% IL-6 inhibitory activity at 1 lM) [Figure 15].
Figure 15.
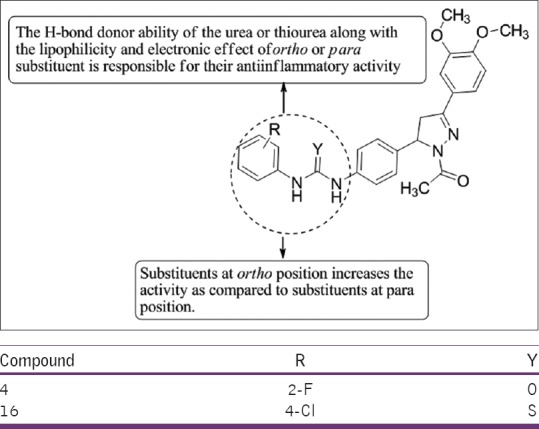
Synthesized a series of novel 1-acetyl-3-(3,4-dimethoxypheny)-5-(4-(3-(arylureido/arylthioureido/arylsulfonamido) phenyl)-4,5-dihydropyrazole derivatives
Selvam et al.,[18] have synthesized a series of 1-(4-substitutedphenyl)-3-phenyl-1H-pyrazole-4-carbaldehydes and tested for their anti-inflammatory activities. 4g, 4i, and 4k exhibited maximum activity as compared to the standard drug diclofenac sodium [Figure 16].
Figure 16.
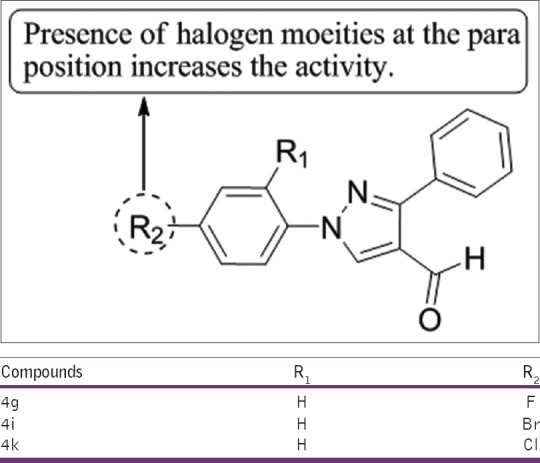
Synthesis of 1-(4-substitutedphenyl)-3-phenyl-1H-pyrazole-4-carbaldehydes
El-Sayed et al.,[19] have synthesized new pyrazole derivative characterized as N-((5-(4-chlorophenyl)-1-phenyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)methylene)-3,5 bis(trifluoromethyl)aniline (8d) which exhibited optimal anti-inflammatory activity as comparable with reference drugs diclofenac sodium and celecoxib [Figure 17].
Figure 17.

Synthesis of N-((5-(4-chlorophenyl)-1-phenyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)methylene)-3,5bis(trifluoromethyl)aniline
Nagarapu et al.,[20] have synthesized a new series of 3-phenyl-N-[3-(4-phenylpiperazin-1yl) propyl]-1H-pyrazole-5-carboxamide derivatives. All compounds were investigated using carrageenan-induced rat paw edema model in vivo using ibuprofen as the standard. Four derivatives 10a, 10e, 10f, and 10g were found to be more potent at 3 h (75%, 70%, 76%, and 78%, respectively) [Figure 18].
Figure 18.
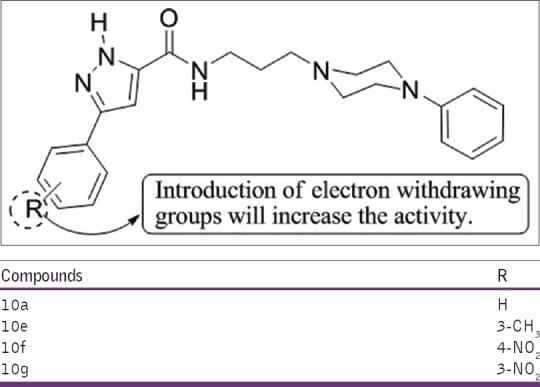
Synthesis of 3-phenyl-N-[3-(4-phenylpiperazin-1yl) propyl]-1H-pyrazole-5-carboxamide derivatives
Bandgar et al.,[21] have synthesized a series of 3,5-diaryl pyrazole derivatives characterized as 2-[5-(2-chloro-phenyl)-1H-pyrazol-3-yl]-6-(2-hydroxymethyl-1-methyl-pyrrolidin-3-yl)-3,5-dimethoxy-phenol using 8-(2-(hydroxymethyl)-1-methylpyrrolidin-3-yl)-5,7-dimethoxy-2-phenyl-4H-chromen-4-one (1) and hydrazine hydrate. The compounds 2a, 2c, 2d, and 2i give IL-6 inhibitory activity while 2a and 2d inhibit TNF-α actively [Figure 19].
Figure 19.
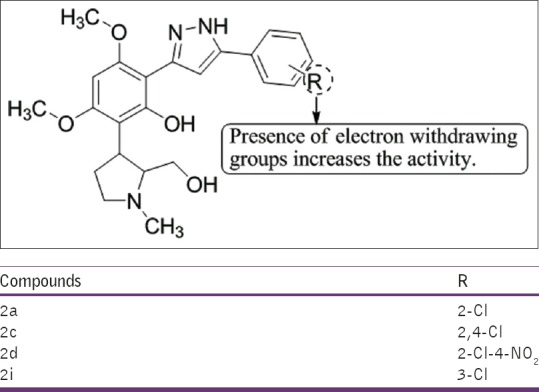
2-[5-(2-chloro-phenyl)-1H-pyrazol-3-yl]-6-(2-hydroxymethyl-1-methyl-pyrrolidin-3-yl)-3,5-dimethoxy-phenol
Bekhit et al.,[22] have synthesized a series of 4-thiazolyl pyrazolyl and studied there COX-1, COX-2 ulcerogenic effect, and acute toxicity, in vitro anti-microbial activity against Escherichia coli, Staphylococcus aureus, and Candida albicans. All compounds were examined for their anti-inflammatory activity using cotton pellet-induced granuloma and carrageenan-induced rat paw edema bioassays. Compounds 10a and 10b were found to be the most potent anti-inflammatory agents using indomethacin as the standard drug [Figure 20].
Figure 20.
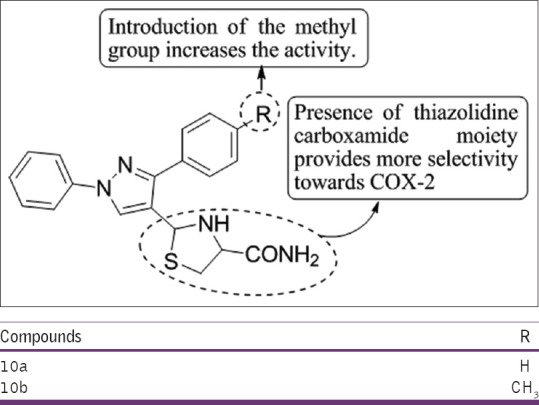
Synthesis of 4-thiazolyl pyrazolyl derivatives
Chandra et al.,[23] have reported a series of compounds with anti-inflammatory and analgesic activities. The compound (24) 1-(2, 4-Chloroacridine-9-yl)-3-(5-pyridine-4-yl)-(1,3,4-oxadiazol-2-yl-thiomethyl)-pyrazole-5-one showed better anti-inflammatory and analgesic activities at the three graded doses of 25, 50, and 100 mg/kg p.o using phenylbutazone as the standard drug [Figure 21].
Figure 21.

Synthesis of 1-(2, 4-Chloroacridine-9-yl)-3-(5-pyridine-4-yl)-(1,3,4-oxadiazol-2-yl-thiomethyl)-pyrazole-5-one
Bandgar et al.,[24] have synthesized novel series of 1-(2,4-dimethoxy-phenyl)-3-(1,3-diphenyl-1H-pyrazol-4-yl)-propenone by the Claisen–Schmidt condensation of 1-(2,4-dimethoxy-phenyl)-ethanone and substituted 1,3-diphenyl-1H-pyrazole-4-carbaldehydes. All the synthesized compounds were evaluated for anti-inflammatory activity by TNF-α and IL-6 inhibition assays. Compounds 3a, 3c, and 3g exhibited promising IL-6 inhibitory activity using dexamethasone as the standard drug [Figure 22].
Figure 22.

Synthesis of 1-(2,4-dimethoxy-phenyl)-3-(1,3-diphenyl-1H-pyrazol-4-yl)-propenone
Abdel-Hafez et al.,[25] have prepared novel pyrazole-NO hybrid molecules and evaluated them for nitric oxide release, anti-bacterial, and anti-inflammatory activities. The organic nitrate ester 10 was found to be most active as compared to the standard drug indomethacin [Figure 23].
Figure 23.
Synthesis of novel pyrazole derivatives
Barsoum and Girgis,[26] have prepared a series bis (3-aryl-4, 5-dihydro-1H-pyrazole-1-thiocarboxamides) and bis (3-aryl-4, 5-dihydro-1H-pyrazole-1-carboxamides). Synthesized compounds were tested for anti-inflammatory activity in carrageenan-induced paw edema method in rats. The compounds 2e and 2f were found to be most potent relative to indomethacin [Figure 24].
Figure 24.
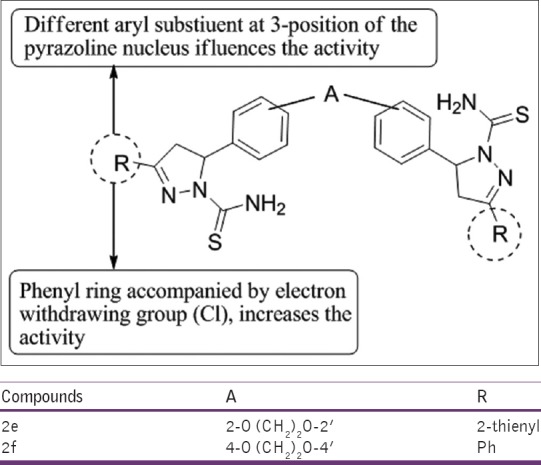
Bis (3-aryl-4,5-dihydro-1H-pyrazole-thiocarboxamides)
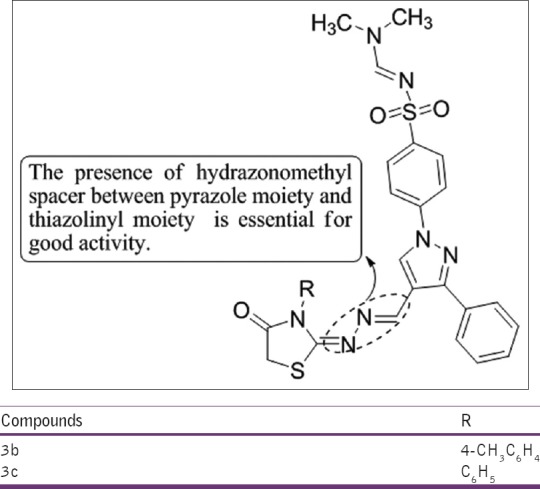
Bekhit et al.,[27] have synthesized a series of N, N-Dimethylaminomethylene-4-[3-phenyl-4-(3-substituted-4-oxothiazolidin-2-ylidenehydrazonomethyl)-1H-pyrazolyl] derivatives. All the target compounds showed good anti-inflammatory activity and 3b and 3c have surpassed that of indomethacin both locally and systemically [Figure 25].
Figure 25.

N,NDimethylaminomethylene-4-[3-phenyl-4-(3-substituted-4-oxothiazolidin-2 ylidenehydrazonomethyl)-1H-pyrazolyl] derivatives
Gökhan-Kelekçi et al.,[28] have synthesized a novel series of 1-thiocarbamoyl 3-substituted phenyl-5-(2-pyrrolyl)-4, 5-dihydro-(1H)-pyrazole derivatives as promising monoamine oxidase B (MAO-B) inhibitor. Few synthesized compounds showed high activity against both the MAO-A and MAO-B isoforms. They also tested synthesized compound for their anti-inflammatory activity against carrageenan-induced edema and an acetic acid-induced increase in capillary permeability in mice. The compound 3k exhibit anti-inflammatory (comparable to indomethacin), analgesic, and MAO-B inhibitory activity [Figure 26].
Figure 26.
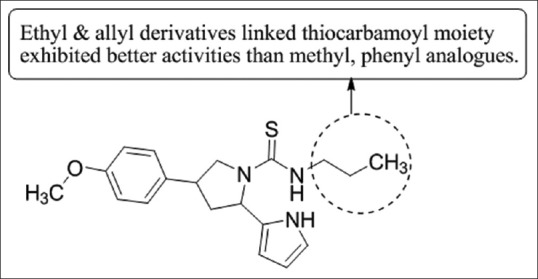
1-thiocarbamoyl 3-substituted phenyl-5-(2-pyrrolyl)-4,5-dihydro-(1H)-pyrazole derivatives
Burguete et al.,[29] has synthesized novel ring substituted 3-phenyl-1-(1,4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives and of their 4,5-dihydro-(1H)-pyrazole analogs and evaluated them for their anti-inflammatory activities. Compound 2a was found to be most potent against carrageenan induced rat paw edema test using indomethacin as the standard drug [Figure 27].
Figure 27.
Substituted 3-phenyl-1-(1,4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives
Bekhit and Abdel-Aziem[30] have synthesized 3-(5-Bromo-2-thienyl)-4-[1-phenylthiocarbamoyl-3-(4-methylphenyl)-2-pyrazolin-5-yl]-1-phenyl-1H-pyrazole from a novel series of structurally related 1H-pyrazolyl derivatives and these compounds were tested for their in vivo anti-inflammatory activity by cotton pellet-induced granuloma and sponge implantation model of inflammation in rats. Compound 12a was found to be most potent using indomethacin as the standard [Figure 28].
Figure 28.
Synthesized 3-(5-Bromo-2-thienyl)-4-[1-phenylthiocarbamoyl-3-(4-methylphenyl)-2-pyrazolin-5-yl]-1-phenyl-1H-pyrazole
Balsamo et al.,[31] have synthesized several hetero-aromatic analogs of (2-aryl-1-cyclopentenyl-1-alkylidene)-(arylmethyloxy)amine COX-2 inhibitors, in which the cyclopentene moiety was replaced by pyrazole, proved to be significantly active only toward the COX-1. Compounds 12 and 16 were found to be active [Figure 29].
Figure 29.
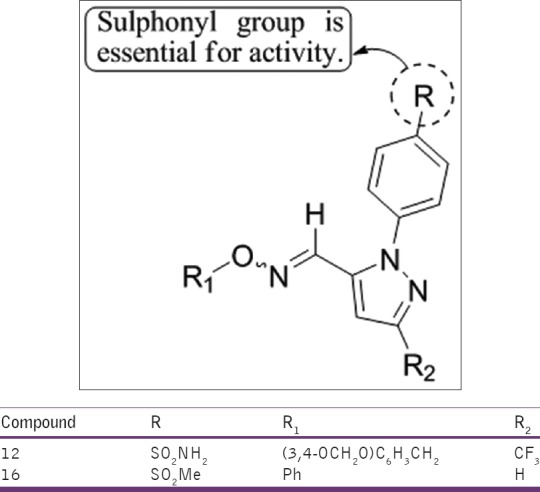
(2-aryl-1-cyclopentenyl-1-alkylidene) (arylmethyloxy) amine
Anti-microbial, anti-tubercular, and anti-fungal activity
Ahsan and Saini,[32] have designed and synthesized a series of thiocetazone based pyrazoline analogs by the condensation of 4-aminoacetophenone and p-anisidine in methanolic sodium hydroxide solution followed by the cyclization of intermediate chalcone with appropriate semicarbazide/thiosemicarbazide in glacial acetic acid. All the synthesized compounds were characterized by 1H NMR, IR, and mass spectral data and the purity of the compounds was checked by elemental analysis. Compound 4i showed maximum activity against Mycobacterium tuberculosis (MTB H37Rv) with minimum inhibitory concentration (MIC) of 7.41 mM [Figure 30].
Figure 30.
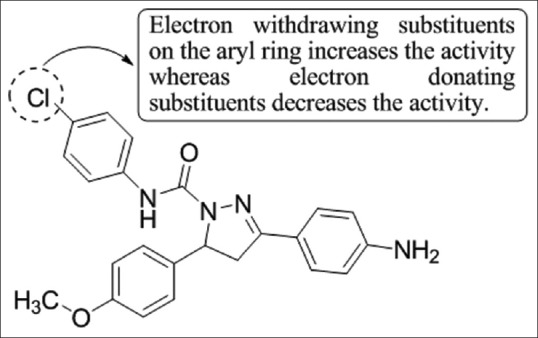
Synthesis of pyrazoline analogs
Pathak et al.,[33] have synthesized various substituted 4,6-diarylpyrimidin-2-amine (4), 4,6-diaryl-2-(heteroaryl)pyrimidine and 1-(3,5-diaryl-4,5-dihydro-1H-pyrazol-1-yl)ethanone in good yields and evaluated them for their in vitro anti-tubercular activity against MTB H37Rv strain. Compounds 7b and 7c were found to be active [Figure 31].
Figure 31.
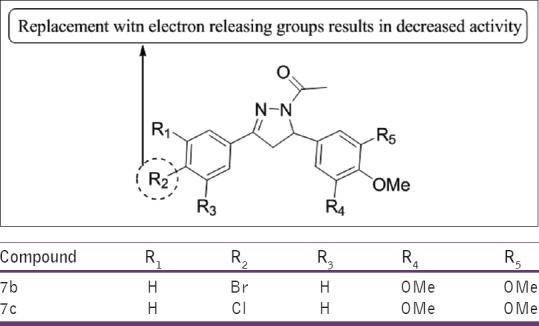
Substituted 4,6-diarylpyrimidin-2-amine (4), 4,6-diaryl-2-(heteroaryl) pyrimidine
Maurya et al.,[34] have synthesized various substituted 5,6-dihydro-8-methoxybenzo[h]quinazolin-2-amine, 1-(3-(4-alkoxyphenyl)-7-methoxy-3,3a,4,5-tetrahydro-2H benzo[g]indazol-2 yl)ethanone, pyrazole, and 2,6-diarylpyridine derivatives in good yields and evaluated them for their in vitro anti-tubercular activity against MTB H37Rv strain. Compound 19a was found to be potent and safe [Figure 32].
Figure 32.
Substituted 5,6-dihydro-8-methoxybenzo[h]quinazolin-2-amine
Ahsan et al.,[35] has synthesized a series of 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide/carbothioamide analogs and evaluated them for anti-tubercular activity. Compound 4o showed low to high inhibitory activities against MTB H37Rv and INH resistant MTB with MIC 3.12 lM and 6.25 lM, respectively [Figure 33].
Figure 33.
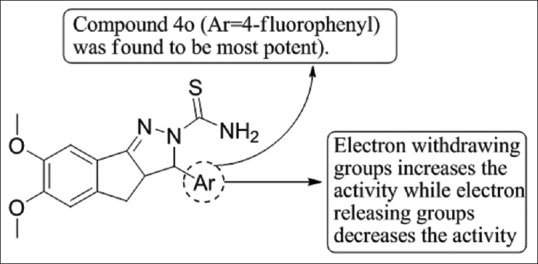
4-dihydro-3Hindeno[ 1,2-c]pyrazole-2-carboxamide
Bondock et al.,[36] have synthesized a series of fused pyrazole-pyrimidine derivatives. The compound 21b was found to exhibit the most potent in-vitro anti-fungal activity with MICs (6.25 μ/ml) against Aspergillus fumigatus and Fusarium oxysporum comparable with chloroamphenicol [Figure 34].
Figure 34.
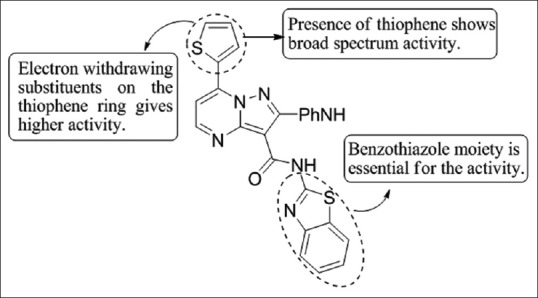
Fused pyrazolepyrimidine derivatives
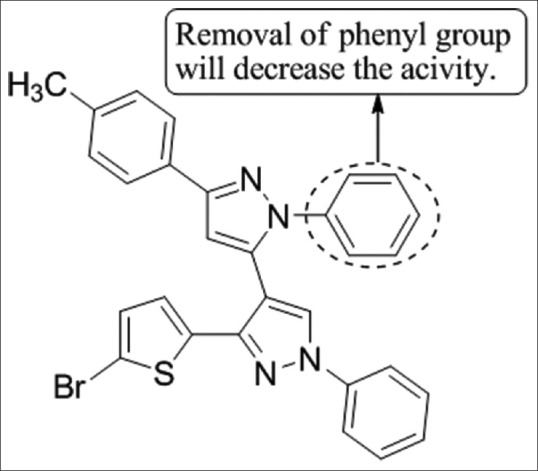
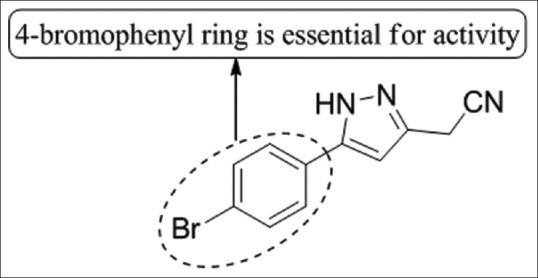
Ragavan et al.,[37] have synthesized a group of novel 1,5-diaryl pyrazole by altering the active part (amide linkage) and tested for anti-bacterial activity against E. coli (American Type Culture Collection [ATTC] - 25922), S. aureus (ATTC-25923), Pseudomonas aeruginosa (ATTC-27853), Klebsiella pneumonia. Compound 11 showed good activity and further studies revealed that the aliphatic amide pharmacophore is important for the anti-microbial activities of studied pyrazole; especially the presence of 4-piperidine moiety enhances the activity [Figure 35].
Figure 35.
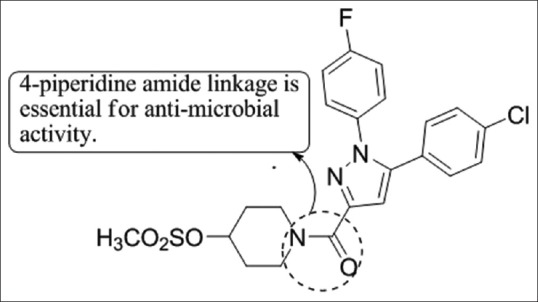
1,5-diaryl pyrazole
Argadeet al.,[38] have reported the synthesis of pyrazole containing 2,4-disubstituted oxazol-5-one as a new class of anti-microbial compounds. Compound 3d showed the highest activity against ampicillin and ketoconazole as standard drugs [Figure 36].
Figure 36.
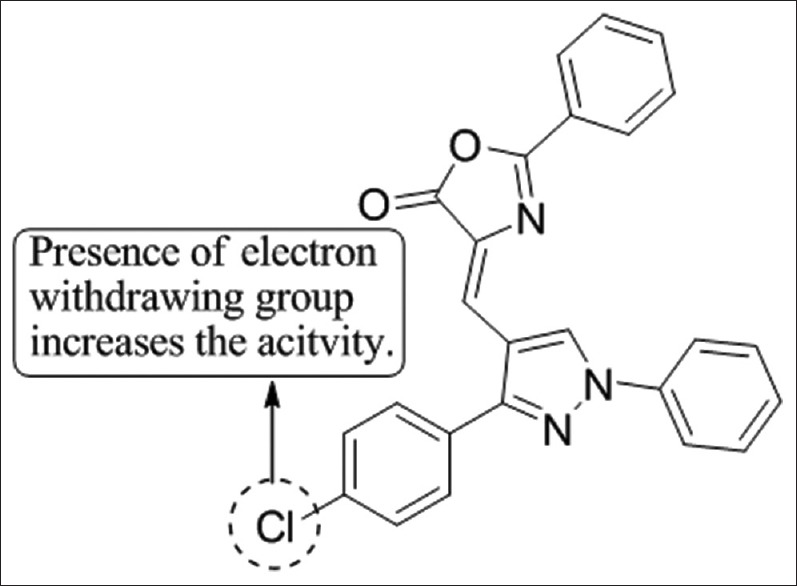
Synthesis of 2,4-disubstituted oxazol-5-one
Chovatia et al.,[39] have reported the synthesis of 1-acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives and these compounds were tested in vitro for their anti-tubercular and anti-microbial properties. All the compounds were screened against MTB strain H37Rv at a concentration of 6.25 µg/mL in BACTEC 12B medium using the ALAMAR radiometric system and were compared against the standard drug rifampin at 0.25 µg/mL concentrations, which showed 98% inhibition. The anti-microbial activity was done against the bacterial strains Bacillus coccus, Bacillus subtilis, E. coli, Proteus vulgaris, and the fungi Aspergillus niger at a concentration of 40 µg/mL using ampicillin, amoxicillin, norfloxacin, benzyl penicillin, and griseofulvin as standard drugs. Compound 4b showed promising results [Figure 37].
Figure 37.
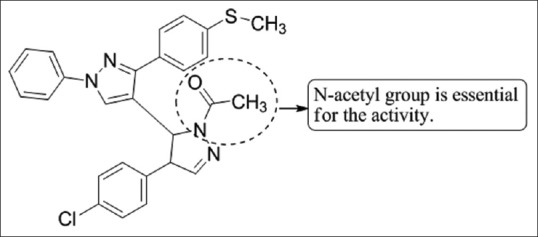
1-acetyl- 3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives
Rai and Kalluraya[40] have reported novel series of nitrofuran containing 1, 3, 4, 5 tetrasubstituted pyrazole derivatives. The compound showed good anti-bacterial and anti-fungal activity. The anti-bacterial activity was studied on E. coli, P. aeruginosa, S. aureus, and B. subtilis using furacin as the standard drug. The anti-fungal activity was studied on C. albicans using fluconazole as the standard drug. Compound 3b showed promising results [Figure 38].
Figure 38.

1, 3, 4, 5 tetrasubstituted pyrazole derivatives
Neuroprotective activity
Cocconcelli et al.,[41] have described the parallel synthesis of aryl azoles. Here substituted hydrazine is made to react with α-β-unsaturated ketones in the presence of a catalyst (acetic acid), which leads to the regioselective formation of 4,5-dihydro-1-H-pyrazole. All compounds obtained were evaluated in an in vitro assay using an N-methyl-D-aspartate toxicity paradigm showing a neuroprotective activity between 15% and 40%. Compound 3a showed good activity [Figure 39].
Figure 39.
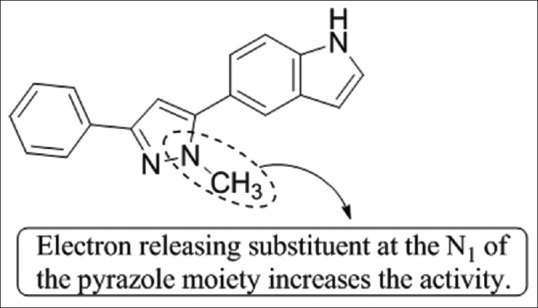
4,5-dihydro-1-H-pyrazole
Estrogen receptor ligands
Naoum et al.,[42] has synthesized novel tetra-substituted pyrazole derivatives bearing a nitro substituent on their phenol ring and their binding affinity toward the ER subtypes ERα and ERβ was determined. Compound 5c was found to be active [Figure 40].
Figure 40.
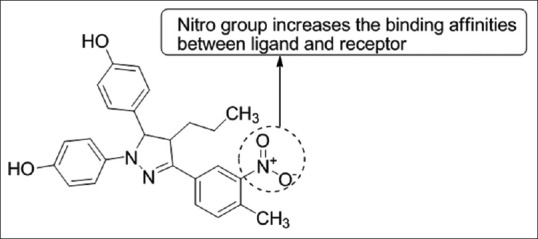
Tetra-substituted pyrazole derivatives
Cholecystokinin-1 receptor antagonists
Gomez et al.,[43] have reported the synthesis and structure-activity relationship studies of 1,5-diarylpyrazoles analogs with various structural modifications of the alkane side chain of the molecule and concluded that compound showed good oral bioavailability and could be used for the potential treatments of irritable bowel syndrome and other gastrointestinal disorders. Compound 19 showed good results [Figure 41].
Figure 41.
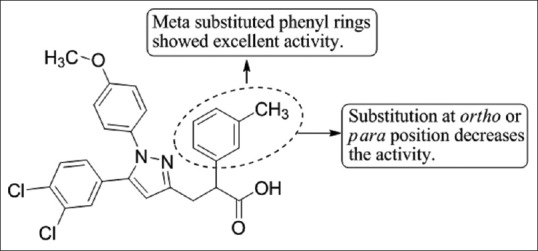
1,5-diarylpyrazoles analogs
Anticonvulsant and antidepressant activity
Ahsan[44] has synthesized 3-substituted-N-aryl-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide and the anticonvulsant activity and neuroprotection assay were done according to Antiepileptic Drug Development Programme protocol. Compound 4b showed neuroprotection activity with 26.2 ± 1.9% of total propidium iodide uptake at 100 µM and inhibitory concentration 50 (IC50) of the compound was found to be 159.20 ± 1.21 µM [Figure 42].
Figure 42.
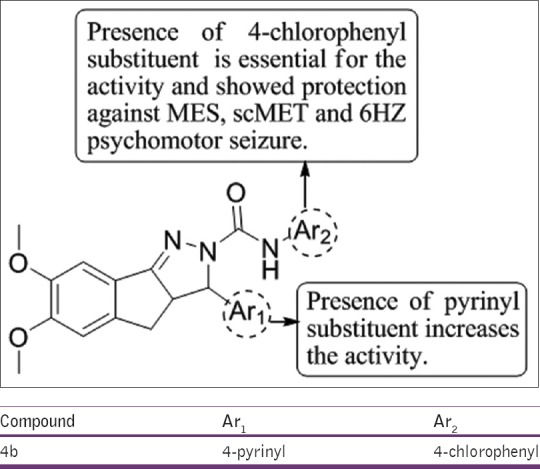
3-substituted-N-aryl-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide
Abdel-Aziz et al.,[45] have described two synthetic paths for the formation of diacylhydrazines, 5amino-1-substiuted pyrazole-3,3,4-tricarbonitriles and oxadiazole, pyrazolone derivatives, showing antidepressant activity using tail suspension behavioral despair test and anticonvulsant activity against pentylenetetrazol induced seizures in mice. Compounds 4a and 4b showed good activity as compared to imipramine at a dose of 10 mg/kg dose level [Figure 43].
Figure 43.
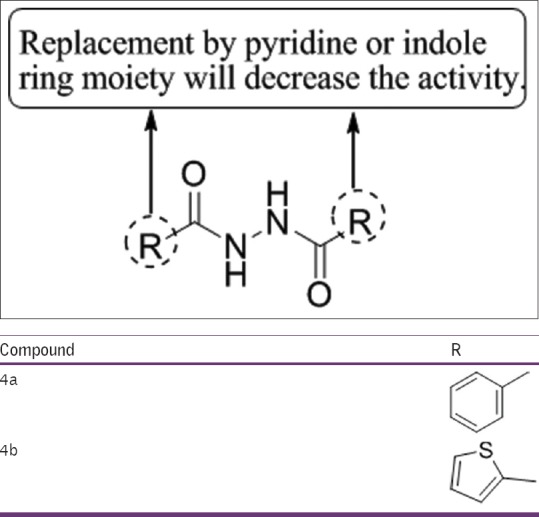
5amino-1-substiuted pyrazole-3,3,4-tricarbonitriles
Chimenti et al.,[46] have synthesized a novel series of 1-acetyl-3-(4-hydroxy- and 2,4-dihydroxyphenyl)-5-phenyl-4,5-dihydro-(1H)-pyrazole derivatives and investigated their ability to selectively inhibit the activity of the isoforms of MAO. The newly synthesized compound proved to be more reversible, potent, and selective inhibitors of MAO-A than of MAO-B. Compounds 6 and 11 were found to be most potent [Figure 44].
Figure 44.
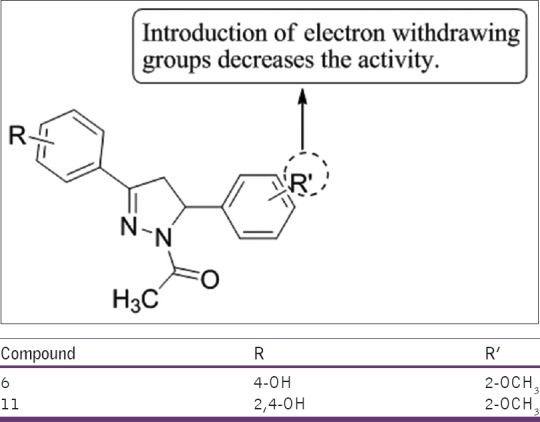
1-acetyl-3-(4-hydroxy- and 2,4-dihydroxyphenyl)-5-phenyl-4,5-dihydro-(1H)-pyrazole derivatives
Antiviral activity
el-Sabbagh et al.,[47] have synthesized 4, 5-disubstituted pyrazole derivatives. The derivative containing R=Cl group showed the potent antiviral activity against a broad panel of viruses in different cell culture (HEL cell cultures). Compound 7 was found to be most potent [Figure 45].
Figure 45.
4, 5-disubstituted pyrazole derivatives
Rashad et al.,[48] have synthesized substituted pyrazole derivatives. These derivatives showed promising antiviral activity against hepatitis A virus and Herpes simplex virus type-1 using plaque infective assay. Compounds 1, 2, and 3 showed good activity against amantadine and acyclovir (used as controls) [Figure 46].
Figure 46.
Substituted pyrazole derivatives
Angiotensin converting enzyme-inhibitory activity
Bonesi et al.,[49] have synthesized a series of pyrazole derivatives and investigated their potential activity as ACE inhibitory activity by performing the assay. One of the derivatives of pyrazole (15) showed effective ACE-inhibitory activity with 0.123 mM IC50 value [Figure 47].
Figure 47.
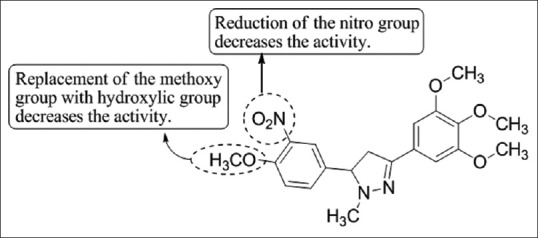
Synthesis of pyrazole derivatives
Anticancer activity
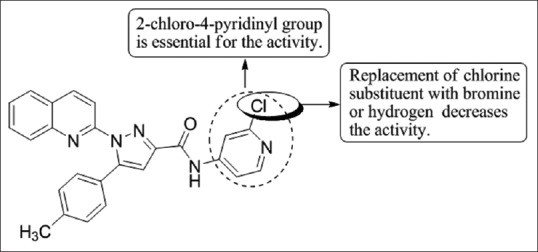
Cankara Pirol et al.,[50] have synthesized a series of novel amide derivatives of 5-(p-tolyl)-1-(quinolin-2-yl)pyrazole-3-carboxylic acid and determined their anti-proliferative activities against three human cancer cell lines (Huh7, human liver; MCF7, breast and HCT116, colon carcinoma cell lines). Compound 4j with 2-chloro-4-pyridinyl group in the amide part showed good cytotoxic activity against all cell lines with IC50 values of 1.6 mM, 3.3 mM, and 1.1 mM for Huh7, MCF7, and HCT116 cells [Figure 48].
Figure 48.
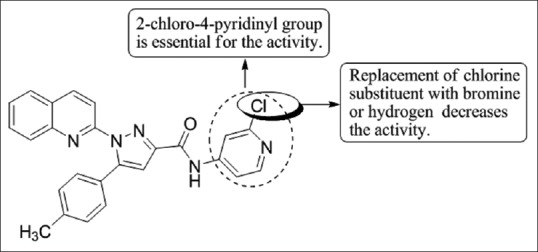
5-(p-tolyl)-1-(quinolin-2-yl)pyrazole-3-carboxylic acid
Ali et al.,[51] have synthesized a series of imidazo[2,1-b]thiazoles having pyrazole moieties through the reaction of 6-hydrazinylimidazo[2,1-b]thiazoles with different dicarbonyl compounds. The compounds were screened at the National Cancer Institute, USA for anticancer activity and 5a showed promising results [Figure 49].
Figure 49.
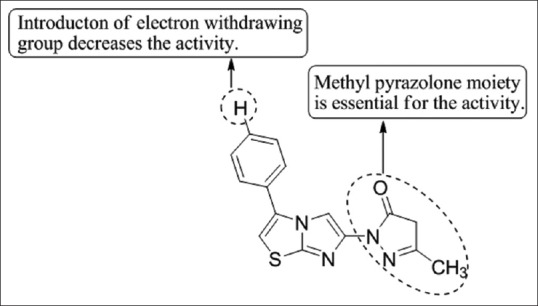
Synthesis of imidazo[2,1-b]thiazoles having pyrazole moieties
Sangani et al.,[52] have synthesized a series of pyrazole quinolone-pyridine hybrids based on molecular hybridization technique and synthesized by a base-catalyzed cyclocondensation reaction through one-pot multicomponent reaction. All compounds were tested for in vitro anti-bacterial and anticancer activities of which 7k showed promising results [Figure 50].
Figure 50.
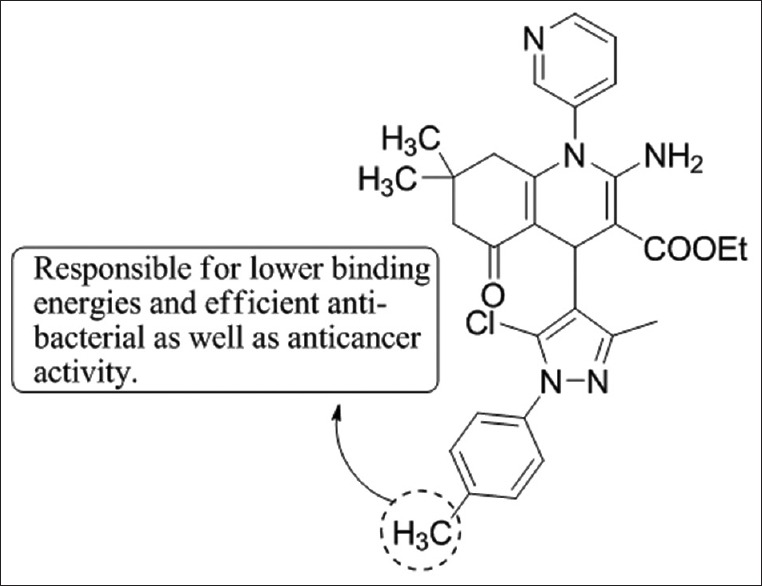
Synthesis of pyrazole quinolonepyridine hybrids
Dawood et al.,[53] have synthesized N-(4-(Pyrazol-4-yl)thiazol-2-yl)-N0-phenylthiourea derivative and then treated with a variety of hydrazonoyl chlorides under basic condition (refluxed) to afford the corresponding 2-(4-(pyrazol- 4-yl)thiazol-2-ylimino)-1,3,4-thiadiazole derivatives. Compound 23d was found to be most potent as compared to the doxorubicin as the reference drug [Figure 51].
Figure 51.
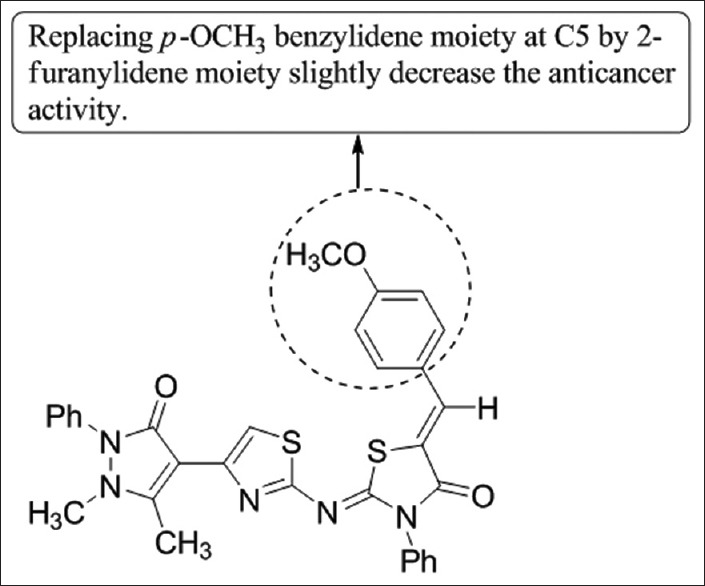
N-(4-(Pyrazol-4-yl)thiazol-2- yl)-N0-phenylthiourea derivative
Puthiyapurayil et al.,[54] have designed and synthesized a novel combinatorial library of S-substituted-1,3,4-oxadiazole bearing N-methyl-4-(trifluoromethyl) phenyl pyrazole moiety and then tested it for in-vitro cytotoxic activity by 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide assay. Compound 5e showed good anticancer activity with IC50 value of 15.54 mM in MCF-7 cells, compared to doxorubicin as a standard drug [Figure 52].
Figure 52.
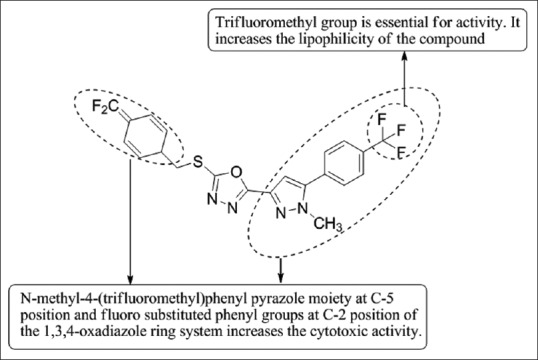
S-substituted-1,3,4-oxadiazole bearing N-methyl-4-(trifluoromethyl) phenyl pyrazole moiety
Christodoulou et al.,[55] have synthesized a series of trisubstituted pyrazole derivatives and (Bis(trifluoroacetoxy)iodo)benzene-mediated conversion of molecules bearing the fused pyrazolo [4,3-c] quinolone ring system and evaluated them for anti-angiogenic activity by using in vitro assays for endothelial cell proliferation and migration, and the chicken chorioallantoic membrane assay. Compounds having fused pyrazolo [4,3-c] quinoline motifs emerged as potent anti-angiogenic compounds and inhibits the growth of human breast (MCF-7) and cervical (Hela) carcinoma cells in vitro. Compound 8b was found to be most potent [Figure 53].
Figure 53.
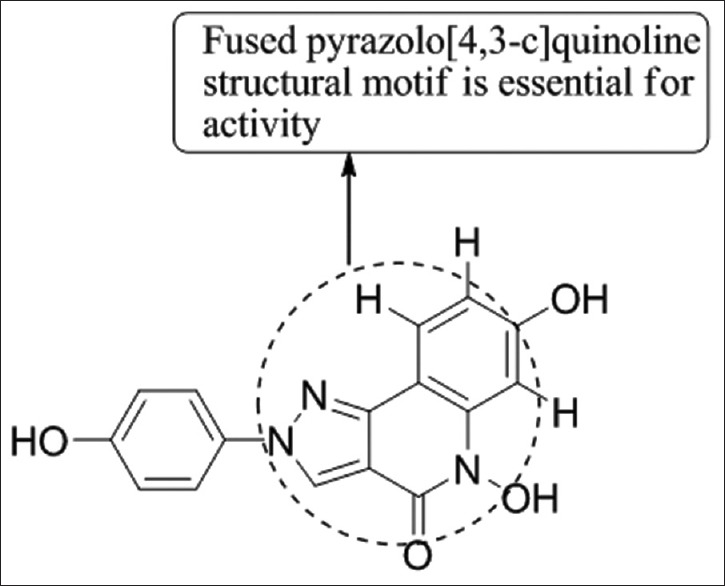
Trisubstituted pyrazole derivatives
Lv et al.,[56] have designed two series of pyrazole derivatives and evaluated them for their potential epidermal growth factor receptor kinase inhibitors activity. Compound 3-(3, 4-dimethylphenyl)-5-(4-methoxy phenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (C5) is most potent with IC50 of 0.07 μM, as compared to positive control erlotinib [Figure 54].
Figure 54.
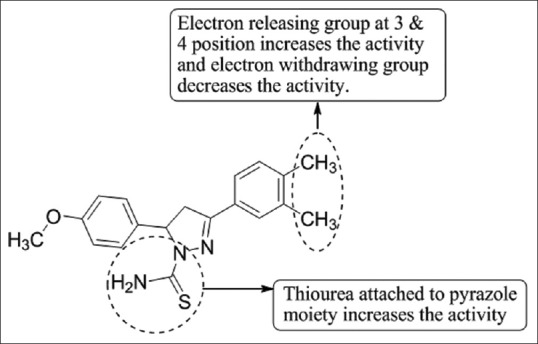
Synthesis of 3-(3,4-dimethylphenyl)-5-(4-methoxy phenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide
Niculescu-Duvaz et al.,[57] have synthesized a series of analogs leading to the discovery of 6-{2-[4-(4 methyl piperazin-1-yl)-phenyl]-5-pyridin-4-yl-3H-imidazol-4-yl}-2,4-dihydro-indeno [1,2-c] pyrazole and carried out bioassay inhibition of purified mutant BRAF activity in vitro. Compound 1j was found to be most potent [Figure 55].
Figure 55.
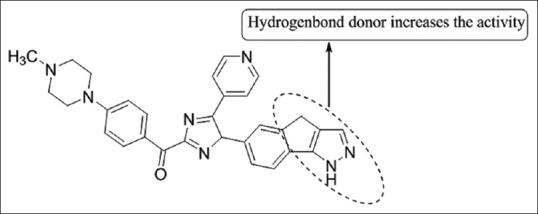
6-{2-[4-(4 methyl piperazin-1-yl)-phenyl]-5-pyridin-4-yl-3H-imidazol-4-yl}-2,4-dihydro-indeno [1,2-c] pyrazole
Insuasty et al.,[58] have synthesized novel (E)-1-aryl-3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-ones (pyrazolicchalcones), among them some compound showed potent activity against leukemia (K-562 and SR), renal cancer (UO-31), and non-small cell lung cancer (HOP-92) cell lines, with the most important GI50 values ranging from 0.04 μ to 11.4 lμ, from the in vitro assays. Compound 5c was found to be most potent [Figure 56].
Figure 56.

(E)-1-aryl-3-(3-aryl-1- phenyl-1H-pyrazol-4-yl)prop-2-en-1-one(pyrazolicchalcones)
Zheng et al.,[59] have synthesized a series of novel 3-aryl-1-(4-tert-butylbenzyl)-1H-pyrazole-5-carbohydrazide hydrazone derivatives and investigated their effects on A549 cell growth, the compound (E)-1-(4-tert-butylbenzyl)-NO-(1-(5-chloro-2-hydroxyphenylethylidene)-3-(4-chlorophenyl)-1H-pyrazole-5-carbohydrazide
(3e) possessed the highest growth inhibitory effect and induced apoptosis of A549 lung cancer cells [Figure 57].
Figure 57.
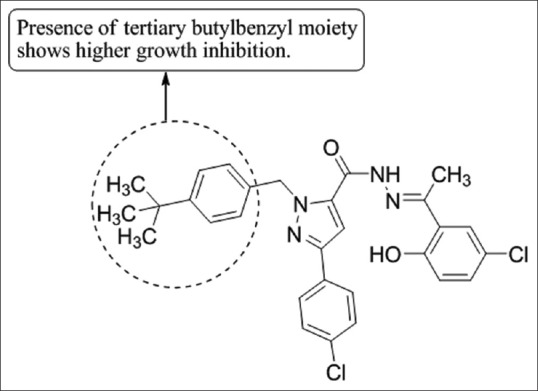
3-aryl-1-(4- tert-butylbenzyl)-1H-pyrazole-5-carbohydrazide hydrazone derivatives
Bruno et al.,[60] have reported the synthesis and the chemotaxis inhibitory activity of a number of 1H pyrazole-4-carboxylic acid ethyl esters. Few compounds have been reported as potent inhibitors of IL-8- and N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLPOMe) - stimulated Olga neutrophil chemotaxis. Most active compound (2e) in the fMLP-OMe induced chemotaxis test showed IC50 0.19–2 nM against dexamethasone as the standard compound [Figure 58].
Figure 58.
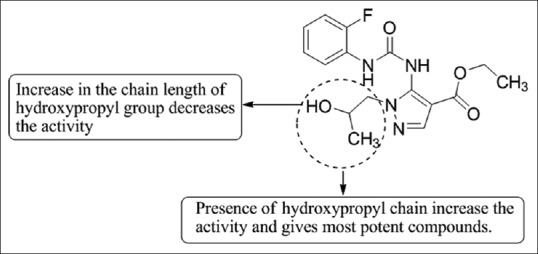
1H pyrazole-4-carboxylic acid ethyl esters
Xia et al.,[61] have synthesized a series of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide derivatives. Nine compounds of the series are reported to inhibit the growth of A549 cells and induced the cell apoptosis. Compounds with logP values in the range of 3.12–4.94 have been found to show more inhibiting effect on the growth of A549 cells. Compound 4b was found to be most potent [Figure 59].
Figure 59.
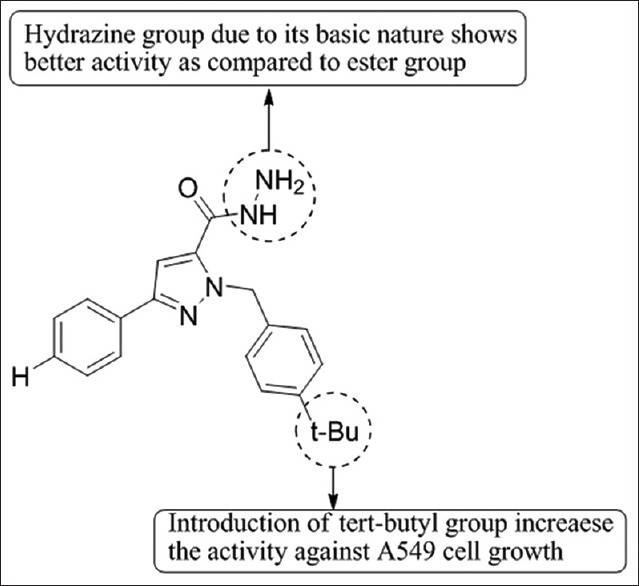
Novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide derivatives
Conclusion
Literature survey shows that pyrazole derivatives are found to be pharmacologically more potent and hence their design and synthesis are the potential area of research. This has been noticed so far, that modification on pyrazole moiety displayed valuable biological activities. It will be interesting to observe that these modifications can be utilized as potent therapeutic agents in future. The biological profiles of these new generations of pyrazole would represent a fruitful matrix for the further development of better medicinal agents. Recent observations suggest that substituted pyrazole and heterocycles, which are the structural isosters of nucleotides owing fused heterocyclic nuclei in their structures that allow them to interact easily with the biopolymers, possess potential activity with lower toxicities in the chemotherapeutic approach in humans. For now, researchers have been attracted to designing more potent pyrazole derivatives having a wide diversity of biological activity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Perrin DD. London: Butterworths; 1972. Dissociation Constants of Organic Bases in Aqueous Solution. [Google Scholar]
- 2.Eicher T, Hauptmann S. 2nd ed. New York: Wiley-VCH; 2003. The Chemistry of Heterocycles: Structure, Reactions, Synthesis and Applications. [Google Scholar]
- 3.Katz AM, Pearson CM, Kennedy JM. A clinical trial of indomethacin in rheumatoid arthritis. Clin Pharmacol Ther. 1965;6:25–30. doi: 10.1002/cpt19656125. [DOI] [PubMed] [Google Scholar]
- 4.Ilango K, Valentina P. 1st ed. India: Keerthi Publishers; 2007. Textbook of Medicinal Chemistry; pp. 327–33. [Google Scholar]
- 5.Panda N, Jena AK. Fe-catalyzed one-pot synthesis of 1,3-di- and 1,3,5-trisubstituted pyrazoles from hydrazones and vicinal diols. J Org Chem. 2012;77:9401–6. doi: 10.1021/jo301770k. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Chen S, Sun Y, Yang J, Rao Y. Synthesis of tri- and tetrasubstituted pyrazoles via Ru(II) catalysis: Intramolecular aerobic oxidative C-N coupling. Org Lett. 2012;14:5030–3. doi: 10.1021/ol3022353. [DOI] [PubMed] [Google Scholar]
- 7.Corradi A, Leonelli C, Rizzuti A, Rosa R, Veronesi P, Grandi R, et al. New “green” approaches to the synthesis of pyrazole derivatives. Molecules. 2007;12:1482–95. doi: 10.3390/12071482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goudarshivannanavar BC, Jayadevappa H, Mahadevan KM. A convenient synthesis of 2 (2-benzo [b] furo) indoles and benzofuropyrazoles. Indian J Chem. 2009;48B:1419–23. [Google Scholar]
- 9.Saikia A, Barthakur MG, Borthakur M, Saikia CJ, Bora U, Boruah RC. Conjugate base catalysed one-pot synthesis of pyrazoles from β-formyl enamides. Tetrahedron Letts. 2006;47:43–6. [Google Scholar]
- 10.Kumar SV, Yadav SK, Raghava B, Saraiah B, Ila H, Rangappa KS, et al. Cyclocondensation of arylhydrazines with 1,3-bis(het)arylmonothio-1,3-diketones and 1,3-bis(het)aryl-3-(methylthio)-2-propenones: Synthesis of 1-aryl-3,5-bis(het)arylpyrazoles with complementary regioselectivity. J Org Chem. 2013;78:4960–73. doi: 10.1021/jo400599e. [DOI] [PubMed] [Google Scholar]
- 11.Kendre BV, Landge MG, Bhusare SR. Synthesis and biological evaluation of some novel pyrazole, isoxazole, benzoxazepine, benzothiazepine and benzodiazepine derivatives bearing an aryl sulfonate moiety as antimicrobial and anti-inflammatory agents. Arab J Chem. 2015 ARABJC 1561. [Google Scholar]
- 12.Tewari AK, Singh VP, Yadav P, Gupta G, Singh A, Goel RK, et al. Synthesis, biological evaluation and molecular modeling study of pyrazole derivatives as selective COX-2 inhibitors and anti-inflammatory agents. Bioorg Chem. 2014;56:8–15. doi: 10.1016/j.bioorg.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Alegaon SG, Alagawadi KR, Garg MK, Dushyant K, Vinod D. 1,3,4-Trisubstituted pyrazole analogues as promising anti-inflammatory agents. Bioorg Chem. 2014;54:51–9. doi: 10.1016/j.bioorg.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Sharath V, Kumar HV, Naik N. Synthesis of novel indole based scaffolds holding pyrazole ring as anti-inflammatory and antioxidant agents. J Pharm Res. 2013;6:785–90. [Google Scholar]
- 15.Thore SN, Gupta SV, Baheti KG. Novel ethyl-5-amino-3-methylthio-1H-pyrazole- 4-carboxylates: Synthesis and pharmacological activity. J Saudi Chem Soc. 2012 in press. [Google Scholar]
- 16.Bandgar BP, Chavan HV, Adsul LK, Thakare VN, Shringare SN, Shaikh R, et al. Design, synthesis, characterization and biological evaluation of novel pyrazole integrated benzophenones. Bioorg Med Chem Lett. 2013;23:912–6. doi: 10.1016/j.bmcl.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Keche AP, Hatnapure GD, Tale RH, Rodge AH, Kamble VM. Synthesis, anti-inflammatory and antimicrobial evaluation of novel 1-acetyl-3,5-diaryl-4,5-dihydro (1H) pyrazole derivatives bearing urea, thiourea and sulfonamide moieties. Bioorg Med Chem Lett. 2012;22:6611–5. doi: 10.1016/j.bmcl.2012.08.118. [DOI] [PubMed] [Google Scholar]
- 18.Selvam TP, Kumar PV, Saravanan G, Prakash CR. Microwave-assisted synthesis, characterization and biological activity of novel pyrazole derivatives. J Saudi Chem Soc. 2014;18:1015–21. [Google Scholar]
- 19.El-Sayed MA, Abdel-Aziz NI, Abdel-Aziz AA, El-Azab AS, El Tahir KE. Synthesis, biological evaluation and molecular modelling study of pyrazole and pyrazoline derivatives as selective COX-2 inhibitors and anti-inflammatory agents. Part 2. Bioorg Med Chem. 2012;20:3306–16. doi: 10.1016/j.bmc.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 20.Nagarapu L, Mateti J, Gaikwad HK, Bantu R, Sheeba Rani M, Prameela Subhashini NJ. Synthesis and anti-inflammatory activity of some novel 3-phenyl-N-[3-(4-phenylpiperazin-1yl) propyl]-1H-pyrazole-5-carboxamide derivatives. Bioorg Med Chem Lett. 2011;21:4138–40. doi: 10.1016/j.bmcl.2011.05.105. [DOI] [PubMed] [Google Scholar]
- 21.Bandgar BP, Totre JV, Gawande SS, Khobragade CN, Warangkar SC, Kadam PD. Synthesis of novel 3,5-diaryl pyrazole derivatives using combinatorial chemistry as inhibitors of tyrosinase as well as potent anticancer, anti-inflammatory agents. Bioorg Med Chem. 2010;18:6149–55. doi: 10.1016/j.bmc.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 22.Bekhit AA, Fahmy HT, Rostom SA, El-Din A Bekhit A. Synthesis and biological evaluation of some thiazolylpyrazole derivatives as dual anti-inflammatory antimicrobial agents. Eur J Med Chem. 2010;45:6027–38. doi: 10.1016/j.ejmech.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Chandra T, Garg N, Lata S, Saxena KK, Kumar A. Synthesis of substituted acridinyl pyrazoline derivatives and their evaluation for anti-inflammatory activity. Eur J Med Chem. 2010;45:1772–6. doi: 10.1016/j.ejmech.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Bandgar BP, Gawande SS, Bodade RG, Gawande NM, Khobragad CN. Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agents. Bioorg Med Chem. 2009;17:8168–73. doi: 10.1016/j.bmc.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Hafez el-SM, Abuo-Rahma Gel-D, Abdel-Aziz M, Radwan MF, Farag HH. Design, synthesis and biological investigation of certain pyrazole-3-carboxylic acid derivatives as novel carriers for nitric oxide. Bioorg Med Chem. 2009;17:3829–37. doi: 10.1016/j.bmc.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 26.Barsoum FF, Girgis AS. Facile synthesis of bis (4,5-dihydro-1H-pyrazole-1-carboxamides) and their thio-analogues of potential PGE(2) inhibitory properties. Eur J Med Chem. 2009;44:2172–7. doi: 10.1016/j.ejmech.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Bekhit AA, Ashour HM, Abdel Ghany YS, Bekhit Ael-D, Baraka A. Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H-pyrazole as anti-inflammatory antimicrobial agents. Eur J Med Chem. 2008;43:456–63. doi: 10.1016/j.ejmech.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 28.Gökhan-Kelekçi N, Yabanoglu S, Küpeli E, Salgin U, Ozgen O, Uçar G, et al. A new therapeutic approach in Alzheimer disease: Some novel pyrazole derivatives as dual MAO-B inhibitors and antiinflammatory analgesics. Bioorg Med Chem. 2007;15:5775–86. doi: 10.1016/j.bmc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Burguete A, Pontiki E, Hadjipavlou-Litina D, Villar R, Vicente E, Solano B, et al. Synthesis and anti-inflammatory/antioxidant activities of some new ring substituted 3-phenyl-1-(1,4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives and of their 4,5-dihydro-(1H)-pyrazole analogues. Bioorg Med Chem Letts. 2007;17:6439–43. doi: 10.1016/j.bmcl.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Bekhit AA, Abdel-Aziem T. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg Med Chem. 2004;12:1935–45. doi: 10.1016/j.bmc.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Balsamo A, Coletta I, Guglielmotti A, Landolfi C, Mancini F, Martinelli A, et al. Synthesis of heteroaromatic analogues of (2-aryl-1-cyclopentenyl-1-alkylidene)-(arylmethyloxy) amine COX-2 inhibitors: Effects on the inhibitory activity of the replacement of the cyclopentene central core with pyrazole, thiophene or isoxazole ring. Eur J Med Chem. 2003;38:157–68. doi: 10.1016/s0223-5234(02)01448-4. [DOI] [PubMed] [Google Scholar]
- 32.Ahsan MJ, Saini V. Design and synthesis of 3-(4-aminophenyl)-5-(4- methoxyphenyl)-4,5-dihydro-1H-pyrazole-1- carboxamide/carbothioamide analogues as antitubercular agents. Beni Suef Univ J Basic Appl Sci. 2015;4:41–6. [Google Scholar]
- 33.Pathak V, Maurya HK, Sharma S, Srivastava KK, Gupta A. Synthesis and biological evaluation of substituted 4,6-diarylpyrimidines and 3,5-diphenyl-4,5-dihydro-1H-pyrazoles as anti-tubercular agents. Bioorg Med Chem Lett. 2014;24:2892–6. doi: 10.1016/j.bmcl.2014.04.094. [DOI] [PubMed] [Google Scholar]
- 34.Maurya HK, Verma R, Alam S, Pandey S, Pathak V, Sharma S, et al. Studies on substituted benzo [h] quinazolines, benzo [g] indazoles, pyrazoles, 2,6-diarylpyridines as anti-tubercular agents. Bioorg Med Chem Lett. 2013;23:5844–9. doi: 10.1016/j.bmcl.2013.08.101. [DOI] [PubMed] [Google Scholar]
- 35.Ahsan MJ, Samy JG, Soni S, Jain N, Kumar L, Sharma LK, et al. Discovery of novel antitubercular 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide/carbothioamide analogues. Bioorg Med Chem Lett. 2011;21:5259–61. doi: 10.1016/j.bmcl.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 36.Bondock S, Fadaly W, Metwally MA. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur J Med Chem. 2010;45:3692–701. doi: 10.1016/j.ejmech.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Ragavan RV, Vijayakumar V, Kumari NS. Synthesis and antimicrobial activities of novel 1,5-diaryl pyrazoles. Eur J Med Chem. 2010;45:1173–80. doi: 10.1016/j.ejmech.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 38.Argade ND, Kalrale BK, Gill CH. Microwave assisted improved method for the synthesis of pyrazole containing 2,4-disubstituted oxazole-5-one and their anti-microbial activity. Eur J Chem. 2008;5:120–9. [Google Scholar]
- 39.Chovatia PT, Akabari JD, Kacchadia PK, Zalavadia PD, Joshi S. Synthesis and selective anti-tubercular and antimicrobial inhibitor activity of 1-acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives. J Serbian Chem Soc. 2007;71:713–20. [Google Scholar]
- 40.Rai NS, Kalluraya B. A novel series of nitrofuran containing 1,3,4,5-tetrasubstituted pyrazole via 1,3 dipolar addition reaction. Indian J Chem. 2007;46B:375–8. [Google Scholar]
- 41.Cocconcelli G, Diodato E, Caricasole A, Gaviraghi G, Genesio E, Ghiron C, et al. Aryl azoles with neuroprotective activity – Parallel synthesis and attempts at target identification. Bioorg Med Chem. 2008;16:2043–52. doi: 10.1016/j.bmc.2007.10.090. [DOI] [PubMed] [Google Scholar]
- 42.Naoum F, Kasiotis KM, Magiatis P, Haroutounian SA. Synthesis of novel nitro-substituted tri-aryl pyrazole derivatives as potential estrogen receptor ligands. Molecules. 2007;12:1259–73. doi: 10.3390/12071259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez L, Hack MD, McClure K, Sehon C, Huang L, Morton M, et al. SAR studies of 1,5-diarylpyrazole-based CCK1 receptor antagonists. Bioorg Med Chem Lett. 2007;17:6493–8. doi: 10.1016/j.bmcl.2007.09.093. [DOI] [PubMed] [Google Scholar]
- 44.Ahsan MJ. Anticonvulsant activity and neuroprotection assay of 3-substituted-N-aryl-6,7-dimethoxy-3a,4-dihydro-3 H-indeno [1,2-c] pyrazole-2-carboxamide analogues. Arab J Chem. 2013;3:644–50. [Google Scholar]
- 45.Abdel-Aziz M, Abuo-Rahma Gel-D, Hassan AA. Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. Eur J Med Chem. 2009;44:3480–7. doi: 10.1016/j.ejmech.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 46.Chimenti F, Bolasco A, Manna F, Secci D, Chimenti P, Befani O, et al. Synthesis and selective inhibitory activity of 1-acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives against monoamine oxidase. J Med Chem. 2004;47:2071–4. doi: 10.1021/jm031042b. [DOI] [PubMed] [Google Scholar]
- 47.el-Sabbagh OI, Baraka MM, Ibrahim SM, Pannecouque C, Andrei G, Snoeck R, et al. Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur J Med Chem. 2009;44:3746–53. doi: 10.1016/j.ejmech.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 48.Rashad AE, Hegab MI, Abdel-Megeid RE, Micky JA, Abdel-Megeid FM. Synthesis and antiviral evaluation of some new pyrazole and fused pyrazolopyrimidine derivatives. Bioorg Med Chem. 2008;16:7102–6. doi: 10.1016/j.bmc.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 49.Bonesi M, Loizzo MR, Statti GA, Michel S, Tillequin F, Menichini F. The synthesis and angiotensin converting enzyme (ACE) inhibitory activity of chalcones and their pyrazole derivatives. Bioorg Med Chem Lett. 2010;20:1990–3. doi: 10.1016/j.bmcl.2010.01.113. [DOI] [PubMed] [Google Scholar]
- 50.Cankara Pirol S, Çaliskan B, Durmaz I, Atalay R, Banoglu E. Synthesis and preliminary mechanistic evaluation of 5-(p-tolyl)-1-(quinolin-2-yl) pyrazole-3-carboxylic acid amides with potent antiproliferative activity on human cancer cell lines. Eur J Med Chem. 2014;87:140–9. doi: 10.1016/j.ejmech.2014.09.056. [DOI] [PubMed] [Google Scholar]
- 51.Ali AR, El-Bendary ER, Ghaly MA, Shehata IA. Synthesis, in vitro anticancer evaluation and in silico studies of novel imidazo [2,1-b] thiazole derivatives bearing pyrazole moieties. Eur J Med Chem. 2014;75:492–500. doi: 10.1016/j.ejmech.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 52.Sangani CB, Makawana JA, Zhang X, Teraiya SB, Lin L, Zhu HL. Design, synthesis and molecular modeling of pyrazole-quinoline-pyridine hybrids as a new class of antimicrobial and anticancer agents. Eur J Med Chem. 2014;76:549–57. doi: 10.1016/j.ejmech.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 53.Dawood KM, Eldebss TM, El-Zahabi HS, Yousef MH, Metz P. Synthesis of some new pyrazole-based 1,3-thiazoles and 1,3,4-thiadiazoles as anticancer agents. Eur J Med Chem. 2013;70:740–9. doi: 10.1016/j.ejmech.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 54.Puthiyapurayil P, Poojary B, Chikkanna C, Buridipad SK. Design, synthesis and biological evaluation of a novel series of 1,3,4-oxadiazole bearing N-methyl-4-(trifluoromethyl)phenyl pyrazole moiety as cytotoxic agents. Eur J Med Chem. 2012;53:203–10. doi: 10.1016/j.ejmech.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 55.Christodoulou MS, Liekens S, Kasiotis KM, Haroutounian SA. Novel pyrazole derivatives: Synthesis and evaluation of anti-angiogenic activity. Bioorg Med Chem. 2010;18:4338–50. doi: 10.1016/j.bmc.2010.04.076. [DOI] [PubMed] [Google Scholar]
- 56.Lv PC, Li HQ, Sun J, Zhou Y, Zhu HL. Synthesis and biological evaluation of pyrazole derivatives containing thiourea skeleton as anticancer agents. Bioorg Med Chem. 2010;18:4606–14. doi: 10.1016/j.bmc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 57.Niculescu-Duvaz D, Niculescu-Duvaz I, Suijkerbuijk BM, Ménard D, Zambon A, Nourry A, et al. Novel tricyclic pyrazole BRAF inhibitors with imidazole or furan central scaffolds. Bioorg Med Chem. 2010;18:6934–52. doi: 10.1016/j.bmc.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Insuasty B, Tigreros A, Orozco F, Quiroga J, Abonía R, Nogueras M, et al. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg Med Chem. 2010;18:4965–74. doi: 10.1016/j.bmc.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 59.Zheng LW, Wu LL, Zhao BX, Dong WL, Miao JY. Synthesis of novel substituted pyrazole-5-carbohydrazide hydrazone derivatives and discovery of a potent apoptosis inducer in A549 lung cancer cells. Bioorg Med Chem. 2009;17:1957–62. doi: 10.1016/j.bmc.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 60.Bruno O, Brullo C, Bondavalli F, Schenone S, Spisani S, Falzarano MS, et al. 1-Methyl and 1-(2-hydroxyalkyl)-5-(3-alkyl/cycloalkyl/phenyl/naphthylureido)-1H-pyrazole-4-carboxylic acid ethyl esters as potent human neutrophil chemotaxis inhibitors. Bioorg Med Chem. 2009;17:3379–87. doi: 10.1016/j.bmc.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 61.Xia Y, Dong ZW, Zhao BX, Ge X, Meng N, Shin DS, et al. Synthesis and structure-activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide derivatives as potential agents against A549 lung cancer cells. Bioorg Med Chem. 2007;15:6893–9. doi: 10.1016/j.bmc.2007.08.021. [DOI] [PubMed] [Google Scholar]